Abstract
Stroke is the fifth leading cause of death in the U.S., with more than 100,000 deaths annually. There are a multitude of risks associated with stroke, including aging, cardiovascular disease, hypertension, Alzheimer’s disease (AD), and immune suppression. One of the many challenges, which has so far proven to be unsuccessful, is the identification of a cost-effective diagnostic or prognostic biomarker for stroke. Alkaline phosphatase (AP), an enzyme first discovered in the 1920s, has been evaluated as a potential biomarker in many disorders, including many of the co-morbidities associated with stroke. This review will examine the basic biology of AP, and its most common isoenzyme, tissue nonspecific alkaline phosphatase (TNAP), with a specific focus on the central nervous system. It examines the preclinical and clinical evidence which supports a potential role for AP in stroke and suggest potential mechanism(s) of action for AP isoenzymes in stroke. Lastly, the review speculates on the clinical utility of AP isoenzymes as potential blood biomarkers for stroke or as AP-targeted treatments for stroke patients.
Keywords: stroke, alkaline phosphatase, biomarker, tissue nonspecific alkaline phosphatase, blood-brain barrer
1.0. Stroke: Current Biomarkers and Therapeutics
1.1. Stroke
Stroke is one of the leading causes of death and disability in the U.S., accounting for approximately 1 in every 19 death (Benjamin et al. 2018). When stroke occurs, oxygen and glucose perfusion are restricted in specific brain regions, leading to cell death and the subsequent loss of memory and motor function. The size of the stroke often correlates with the extent of disability, assessed through a simplified modified Rankin scale questionnaire (Bruno et al. 2013). There are two different types of stroke. Hemorrhagic strokes occur when a weakened blood vessel leaks out into the brain tissue. The second, and most common type of stroke, is known as ischemic stroke, where blood flow to a certain area of the brain is blocked from a blood clot or plaque lesion. Ischemic stroke can be embolic, where a clot or plaque lesion forms in another area within the body then travels and gets stuck in the brain, or thrombotic, where a clot forms within one of the vessels that supplies blood to the brain (Dirnagl et al. 1999).
Strokes commonly occur in the elderly and occur more often in women than men. Risk factors for stroke include: hypertension, high cholesterol, atherosclerosis, smoking, excessive drinking, and diabetes. Although reperfusion of the ischemic brain is the goal for treatment, intense inflammation and further tissue damage occurs during the reperfusion process. Clinical evidence shows that an evolution of brain injury often occurs in the hours to days following a stroke, which allows only a small time-frame for successful therapeutic intervention. Within minutes of a stroke, the neurons at the core of the infarct that are closest to the region of oxygen or glucose deprivation undergo necrotic cell death. The necrotic core is surrounded by an area of tissue, the penumbra, that is less severely impacted by the lack of blood flow and remains metabolically active; functionality of the penumbra may be lost but is salvageable. Clinicians focus on restoring the functionality of the penumbra region as part of their stroke treatment (Dirnagl et al. 1999; Dirnagl and Endres 2014; Benjamin et al. 2018).
1.2. Stroke Biomarkers and Therapeutics
Although numerous biomarkers have been assessed for their use in stroke, none so far have proven to be reliable enough to use as a standard in the clinic. Several current or emerging stroke biomarkers are described in Table 1. There is a critical need to develop faster and less expensive diagnostic testing tools for stroke, such as the use of blood biomarker panels. Despite growing efforts to identify blood biomarkers that may be useful for the determination and differentiation of stroke, there are no current specific biomarker recommendations for use in the clinic. Likewise, stroke therapeutics are limited. Since the approval of recombinant tissue plasminogen activator (rtPA) in 1996, no other drug has received FDA approval to treat stroke (The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group 1995; Adams et al. 1996; Report of the Quality Standards Subcommittee of the American Academy of Neurology 1996). The quest for new stroke therapeutics has been plagued by numerous clinical trial failures, due in large part to the discrepancy between the positive results in preclinical animal models of stroke, and the subsequent negative findings when the same therapeutics are tested in human clinical trials (Bushnell et al. 2006; Jickling and Sharp 2015). One possible candidate for use as a diagnostic and prognostic marker for stroke is alkaline phosphatase (AP). Several studies have indicated that AP may be actively beneficial or detrimental in many inflammatory and ischemic settings (Heemskerk et al. 2009; Davidson et al. 2012; Shimizu et al. 2013a; Liu et al. 2016; Gdara et al. 2018). This review will discuss the practicality of exploring AP as a potential marker for stroke and how AP can be manipulated for therapeutic purposes.
Table 1.
Current or potential biomarkers as diagnostic or prognostic tools for stroke.
| Biomarker Group | Molecule or Cell | Diagnostic Ability | Prognostic Ability | Reference |
|---|---|---|---|---|
| Neurotropic Factors | Brain-derived neurotropic factor (BDNF) |
↑at stroke onset | ↓ = poor prognosis | (Gandolfi et al., 2017) |
| Myokines | Irisin | ↑ = good prognosis | (Gandolfi et al., 2017) | |
| Myostatin | ↑ = muscle wasting | (Gandolfi et al., 2017) | ||
| Follistatin-like 1 (FSTL1) | ↑ = good prognosis | (Gandolfi et al., 2017) | ||
| Cytokines | IL-6, TNF-α, IL-10, IL- 4, IL-17, IL-23, TGF-α, IL-15, IL-19, IL-33, IL- 1β |
↑ at stroke onset | Varies | (Beghetti et al. 2003; Sonderer and Katan Kahles 2015; Bonaventura et al. 2016; Gandolfi et al. 2017) |
| Chemokines | C-X-C motif chemokine (CXCL)12; C-X3-C motif chemokine ligand (CX3CL)1; and monocyte chemoattractant protein (MCP)-1 |
↑ at stroke onset | Varies | (Bonaventura et al., 2016) |
| Neuropeptides | Neuropeptide Y | ↑ = good prognosis | (Gandolfi et al., 2017) | |
| Proenkephalin | ↑ = poor prognosis | (Gandolfi et al., 2017) | ||
| Growth Factors | Vascular endothelial growth factor (VEGF) |
↑ at stroke onset | (Gandolfi et al., 2017) | |
| Immune Cells | CD4+CD28− T cells | ↑ = poor prognosis | (Gandolfi et al., 2017) | |
| Regulatory T cells (Tregs) |
Peripheral pattern changes after stroke |
↑ = good prognosis | (Gandolfi et al., 2017) | |
| Natural Killer (NK) cells |
↑ in brain at stroke onset |
(Gandolfi et al., 2017) | ||
| T and B Lymphocytes; CD4+ & CD8+ T cells; γδ-T cells |
↑ at stroke onset; peripheral pattern changes after stroke |
(Gandolfi et al., 2017; Bonaventura et al., 2016) | ||
| Microglia | ↑ at stroke onset | (Bonaventura et al., 2016) | ||
| Neutrophils | ↑ at stroke onset | (Bonaventura et al., 2016) | ||
| Dendritic cells | ↓ during stroke | (Gandolfi et al., 2017) | ||
| Protein & Enzyme | CRP, GTT, GPT, bilirubin |
↑ at stroke onset | Varies; typically ↑ = poor prognosis |
(Beghetti etal. 2003; Pineda et al. 2008; Tang et al. 2013; Luo et al. 2013; Muscari et al. 2014; Sonderer and Katan Kahles 2015; Bonaventura et al. 2016) |
| AP | Usually ↑ at stroke onset, but some variation |
↑ = poor prognosis | (Cheung et al. 2008; Metwalli et al. 2014; Muscari et al. 2014; Ryu et al. 2014; Lee et al. 2015; Schiff et al. 2016) | |
| MicroRNAs | miRNA-320b | ↓ = ↑ risk factor of carotid atherosclerosis |
(Gandolfi et al., 2017) | |
| miRNA-146a, −181b, and −30a |
Varies | ↓ = ↑ neuroprotection | (Martinez and Peplow 2016; Gandolfi et al. 2017; Khoshnam et al. 2017) |
|
| miRNA-107, −128b, and −153 | ↑ at stroke onset | (Martinez and Peplow, 2016; Khoshnam et al., 2017) | ||
| Reactive Oxygen Species |
Antioxidant enzymes | ↑ at stroke onset = redox imbalance |
(Bonaventura et al., 2016; Khoshnam et al., 2017) | |
| Damage Associated Molecular Patterns (DAMPS) |
Toll-like receptors (TLRs); neutrophil calcium influx |
↑ at stroke onset | (Bonaventura et al., 2016) |
Key: ↑ = increase; ↓ = decrease
2.0. Alkaline Phosphatase
2.1. Alkaline Phosphatase Genetics and Cell Biology
Alkaline phosphatase (AP) was first discovered in 1923, when Dr. Robert Robison described the presence of an enzyme abundant in animal bone that rapidly hydrolyzed hexosemonophosphoric acid into phosphoric acid (Robison 1923). AP has since been shown to play a significant role in human bone mineralization, confirmed by many cases of hypophosphatasia, a rare metabolic inherited disease caused by a mutation in the ALPL gene (Moore et al. 1990; Whyte et al. 2009; Barvencik et al. 2011). There are four isoenzymes of AP in humans, i.e. intestinal (IAP), placental (PLAP), germinal (GCAP), and tissue nonspecific (TNAP) (Millan 1986; Weiss et al. 1986; Berger et al. 1987; Millan and Manes 1988), which can be reviewed in (Van Hoof and De Broe 1994; Buchet et al. 2013). The first three isoenzymes are expressed in the tissues for which they are named and each is encoded by a unique homologous gene loci in humans: ALPI, ALPP, ALPPL2, and ALPL (Harris 1990; Buchet et al. 2013). The TNAP protein, also known as bone/liver/kidney AP, is expressed by a variety of tissues including multiple cell types in the brain. TNAP is the most abundant isoenzyme collected from blood, where approximately 50-60% is derived from bone, 30% from the intestines, and 10-20% from the liver (Moss 1982).
APs belong to the ectophosphatase enzyme family and are localized in multiple mammalian cells and tissues (Bannister and Romanul 1963; Kang and West 1982; Paiva et al. 1983; Mori and Nagano 1985; Van Hoof and De Broe 1994; Champion et al. 2003). This class of enzymes is anchored on the cell plasma membrane surface by a glycosylphosphatidylinositol (GPI) moiety which allows them to act on substrates in the extracellular space. APs can be localized in the lipid rafts of the plasma membrane outer leaflet via the C-terminus to the GPI, found as a soluble protein in the serum, or as a vesicle-associated protein in the extracellular space. GPI-anchored AP proteins can be shed from the plasma membrane by cleavage from phosphatidylinositol-phospholipases to take on the soluble form in blood (Low and Zilversmit 1980; Low 1987).
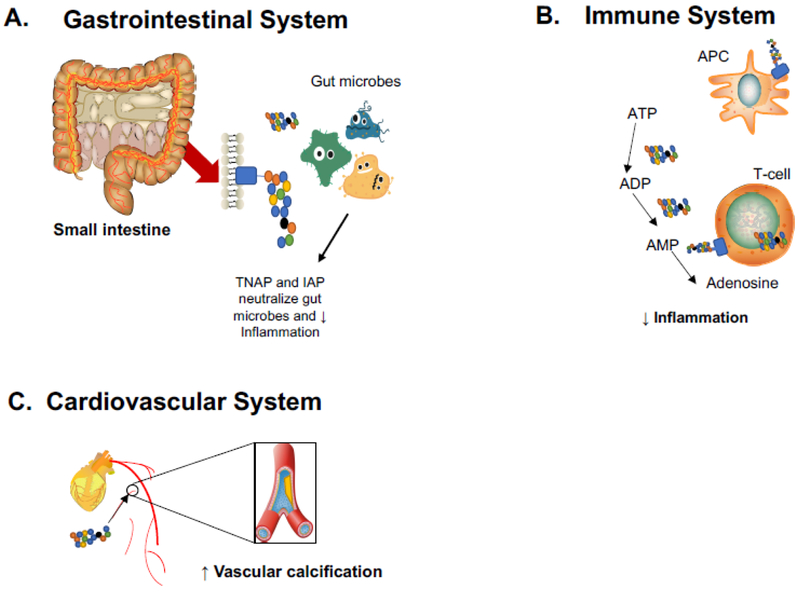
Abnormal levels of AP can result in hypophosphatasia (Waymire et al. 1995; Razazizan et al. 2013; Sebastian-Serrano et al. 2016). Thus, AP has a well-characterized role in skeletal mineralization, and speculation into other physiological function(s) of AP enzymes has generated the most interest with regard to host defense. The role in inflammation is due, in part, to its ability to neutralize endotoxins through dephosphorylation of the lipid-A moiety converting it to the non-toxic monophosphoryl product, and it may target bacterial components like CpG DNA and flagellin (Poelstra et al. 1997a, b; Chen et al. 2010). Similarly, AP also deactivates ATP, which when upregulated can act as an immunological danger signal, while maintaining homeostasis of gut bacteria (Poelstra et al. 1997a; Malo et al. 2010; Peters et al. 2015). Figure 1 summarizes AP’s actions in the periphery.
Figure 1. Role of alkaline phosphatase isoenzymes in the periphery:
AP plays an important role in the physiology and pathophysiology of many organ systems. AP’s actions in the gastrointestinal, immune, and cardiovascular systems are most relevant to the systemic immune response in ischemic stroke. A.) In the gastrointestinal system, both TNAP and intestinal AP play an important anti-inflammatory role by neutralizing gut microbes. B.) Immune system: Numerous peptides, lipids, and other molecules are recognized by antigen-presenting cells (APC) to activate T-cells in the periphery. During T-cell activation adenosine triphosphate (ATP) is released, contributing to the inflammatory environment. TNAP can convert ATP to the anti-inflammatory molecule adenosine through stepwise conversion of ATP to adenosine diphosphate (ADP) and adenosine monophosphate (AMP). C.) Cardiovascular system: An excess of bone AP contributes to vascular calcification, leading to stiff muscle walls and, eventually, atherosclerosis.
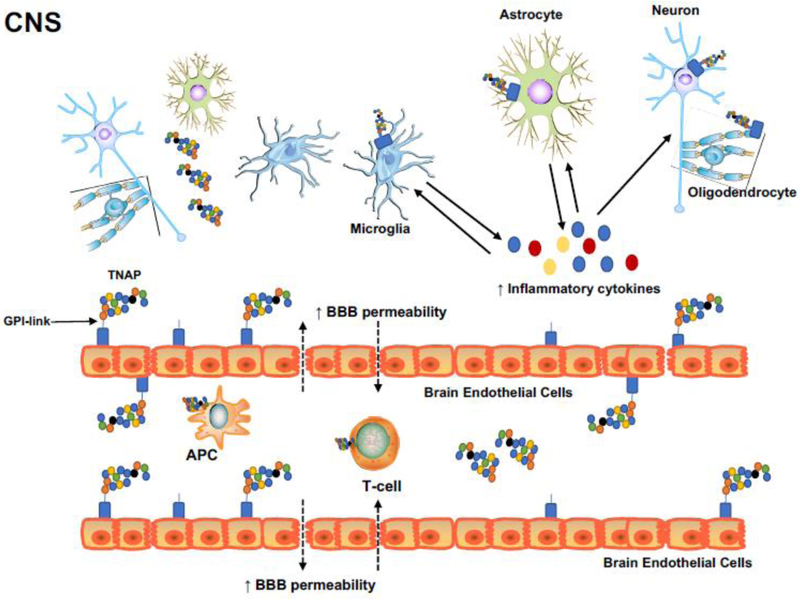
Another complication of abnormal AP serum levels in Akp2 (mouse TNAP gene) null mice is epilepsy. Nearly three decades after its initial discovery, Shimizu showed histochemical evidence of TNAP in the nervous system of several animal models (Shimizu 1950). However, the roles of TNAP in neurological disorders remains poorly understood. Since TNAP has been shown to interact with multiple substrates and molecules, it is highly likely that TNAP exhibits multiple functions in the brain (Waymire et al. 1995; Whyte et al. 1995; Ermonval et al. 2009). Depending on the cell type, TNAP can be transiently or constitutively expressed within the central nervous system, which suggests multiple mechanisms of gene expression across the many cell types in the brain and spinal cord (Narisawa et al. 1994; MacGregor et al. 1995; Fonta 2004; Langer et al. 2008). Some data suggest that TNAP may play a role in neurotransmitter metabolism (Fonta 2004; Fonta et al. 2005; Balasubramaniam et al. 2010), and Hanics et al. used TNAP null mice to show that TNAP deficiency leads to decreased brain myelination and synaptogenesis. These findings suggest that TNAP plays an important role in brain development and that TNAP deficiency can contribute to many forms of neurological dysfunction, including epilepsy (Hanics et al. 2012). Figure 2 depicts numerous presumed functions of AP in the CNS.
Figure 2. Role of tissue nonspecific alkaline phosphatase (TNAP) in the central nervous system:
TNAP is expressed in neurons, microglia, astrocytes, oligodendrocytes, and is highly expressed in brain endothelial cells. TNAP is localized in the lipid rafts of the plasma membrane outer leaflet via its C-terminus to the GPI. It can also be found as a soluble protein in the serum or as a vesicle-associated protein in the extracellular space. TNAP may have a role in blood-brain barrier (BBB) breakdown, neuroinflammation, and vascular dysfunction in stroke and other neurological disorders. Inflammatory mediators such as reactive oxygen species (ROS), proteases, and inflammatory cytokines, promote the breakdown of junctional proteins at the BBB. The loss of junctional proteins weakens the BBB and allows activated T-cells, antigen-presenting cells (APC), other leukocytes, and pro-inflammatory mediators to traverse the BBB, with bidirectional movement between the brain parenchyma and cerebral circulation. These mechanisms play an important role in the pathophysiology of ischemic stroke and other neuroinflammatory disorders.
3.0. Alkaline Phosphatase as a Biomarker for Stroke
3.1. Serum Alkaline Phosphatase as a Diagnostic Tool
Normal blood AP levels vary depending on sex and age, although AP levels typically display a wide range within these respective groups (Fenuku and Foli 1975; Lester 1977; Molla et al. 1990; Magnusson et al. 1995; Zierk et al. 2017; Wanjian et al. 2017; Li et al. 2018). For example, naturally high levels of AP are seen in children because their bones are still growing. Thus, clinicians and researchers rely on an average range of “normal” AP levels rather than a specific number. In general, abnormal AP levels are indicative there is some condition or disorder that has disrupted homeostasis. Some temporary conditions may also affect AP levels, including pregnancy, bone fractures, and taking specific medications (Herbeth et al. 1981; Rodin et al. 1989; Okesina et al. 1995; Choi and Pai 2000; Sadighi et al. 2008). For example, pregnancy can cause AP levels to be elevated 2-3 times that of normal due to an increase in placental AP (Okesina et al. 1995; Choi and Pai 2000). Alternatively, a patient’s serum total AP levels can also be increased following a meal due to an increase in the intestinal isoenzyme; however, this is typically a very transient elevation that quickly returns to normal levels if the patient is otherwise healthy (Sukumaran and Bloom 1953; Khan et al. 2016).
Elevated levels of AP are also indicative of numerous disorders including excessive skeletal mineralization, Paget’s disease, tumors, and, potentially, Alzheimer’s disease (AD) (Naik etal. 1977; Lampl et al. 1990; Vardy et al. 2012). The magnitude of AP elevation tends to reflect the extent of dysfunction. Typically, AP tends to be most markedly elevated due to hepatic obstruction, hepatitis, and other liver diseases (Schlaeger 1975; Paritpokee et al. 1999). During certain conditions, AP levels can increase greater than 3-fold and are measured at 10-12 times the upper limit of normal, particularly in bone disorders (Mayo Clinic; Moss 1982; Jassam et al. 2009; Teitelbaum et al. 2011). Interestingly, AP levels are generally normal during osteoporosis (Kelly et al. 1967). Although less clinically explored, observations of abnormal AP serum levels have also been associated with neurological disorders such as AD (Kellett et al. 2011; Vardy et al. 2012).
The standard clinical chemistry AP tests used in laboratory medicine report total AP enzyme activity rather than the amount of total AP protein. For simplicity, this review will use AP levels to refer to AP activity, as is commonly done in clinical practice. Thus, when used as part of a typical comprehensive metabolic panel, this test represents the sum of all soluble AP isoenzyme activity in serum or plasma. Clinicians often interpret elevated levels of AP with pathological conditions related to bone and liver disorders because the majority of AP detected in the blood comes from the liver and bone; however, this test can also indicate intestinal or parathyroid disease (Mayo Clinic; Posen et al. 1967; Yoneda et al. 1988; Tuin et al. 2009). Additional testing can also be performed to determine the source of possible dysregulation, including liver (L1 and L2), bone (B1, B2, B/I, and B1x), intestinal, and placental tissues. Normal AP ranges for these various isoenzymes also differ. Clinicians can continue to monitor elevated AP levels as the patient undergoes treatment to indicate that a treatment may or may not be working (Wolf 1978; Van Hoof and De Broe 1994).
3.2. Alkaline Phosphatase as a Putative Biomarker for Neurological Injury
Researchers have used AP activity as a brain microvessel marker in primates for decades, and its presence has been described in the cerebral parenchyma of both young and old monkeys (Friede 1966; Bell and Ball 1985; Anstrom et al. 2002; Fonta and Imbert 2002; Fonta 2004; Fonta et al. 2005). Primate, mouse, rat, and human brains have been used to demonstrate that expression of TNAP activity can be found in brain endothelial cells and neurons (Charegaonkar and Rindani 1961; Meyer Peter 1963; Friede 1966; Narisawa et al. 1994; Nishihara et al. 1994; Fonta 2004; Ermonval et al. 2009; Brun-Heath et al. 2011). Numerous studies indicate that AP RNA expression, protein levels, or enzyme activity may vary significantly between primates and rodents. In primate brains, AP activity can be detected in one or a few cortical layers, but not in all areas, while in rodent brains, AP activity is more scattered with variable levels of intensity (Brun-Heath et al., 2011).
Several studies have demonstrated that TNAP is elevated in many neurological disorders, including some types of brain injury (Yamashita et al. 1989) and AD (Gong et al. 1993; Kellett et al. 2011; Vardy et al. 2012). TNAP has been shown to play a role in tau phosphorylation. Vardy et al. showed that TNAP is increased in both human brain tissue and plasma from patients with familial and sporadic AD (Vardy et al. 2012). Also, in patients with various brain injuries, the concentration of serum AP correlated with functional outcome and increased TNAP had an inverse correlation with cognitive function (Yamashita et al. 1989; Kellett et al. 2011; Vardy et al. 2012). Among patients with brain tumors, those that also had pulmonary carcinomatous meningitis were found to have elevated AP levels in their cerebrospinal fluid (CSF), while AP concentrations in control patients with epilepsy and stroke were decreased in comparison to the brain tumor group (Lampl et al. 1990). Increased liver AP levels were described in patients with nontraumatic intracranial hemorrhages (Meythaler et al. 1998).
Elevated serum AP levels may also underscore brain-peripheral immune interactions during stroke, as Muscari et al. found evidence that the liver participates in the response to acute ischemic stroke by releasing enzymes (Muscari et al., 2014). AP levels have been shown to increase in relation to large-volume cerebral white matter hyperintensities and may be associated with multi-cerebral microbleeds in ischemic stroke patients (Ryu et al. 2014; Lee et al. 2015; Liu et al. 2016). While Lee et al. suggest that increased serum AP may be a marker for impaired cerebral microcirculation, Liu et al. were unable to replicate these results in their study (Lee et al. 2015; Liu et al. 2016). The authors state these differences may be due to the small number and clinical characteristics of their subjects, as well as the statistical methods used in both studies (Liu et al. In contrast, a smaller number of studies have reported a decrease in serum TNAP in other neurological disorders. For example, TNAP levels were found to be significantly decreased in traumatic brain injury (Arun et al. 2015). Additionally, AP activity was found to be neither significantly elevated nor reduced in aging patients alone (Vardy et al. 2012) or in patients with multiple sclerosis (Hanna et al. 1997; Tremlett et al. 2006). These findings underscore the importance of continued research to elucidate the mechanisms under which AP or TNAP levels are elevated or reduced in neurological disorders.
3.3. Alkaline Phosphatase as a Diagnostic and Prognostic Stroke Biomarker
Prior clinical studies have explored AP as a diagnostic and prognostic marker to assess stroke risk by correlating indices of AP activity such as: dysfunctional bone metabolism (Barnadas et al. 2014; Namba et al., 2017), cardiovascular disease (Webber et al. 2010; Kim et al. 2017; Makil et al. 2017), cancer (Giessen et al. 2014; Barnadas et al. 2014; Hammerich et al. 2017) and periodontitis (Kunjappu et al. 2012). More importantly, elevated AP levels in stroke patients have been correlated with stroke severity and hypertension (Pratibha et al. 2014; Tan et al. 2017). While the underlying molecular mechanisms to support this association are unclear, other studies have provided additional support for this observation. In patients with coronary artery disease who underwent percutaneous coronary intervention, those with the highest AP levels had the greatest risk of 3-year mortality or secondary outcomes (cardiac mortality, nonfatal myocardial infarction, stent thrombosis or stroke) (Ndrepepa et al. 2017). A study using a cohort of patients with preserved kidney function reported AP as an independently useful tool for predicting mortality and stroke recurrence (Zong et al. 2018). Another study of acute ischemic stroke patients revealed that patients within the highest serum AP quartile had the highest incidence of early mortality (Zhong et al. 2018). Lastly, another cohort of patients who experienced a transient ischemic attack showed that those with increased serum AP levels on admission were more likely to have subsequent ischemic stroke events (Uehara et al. 2018). A smaller minority of studies do not support any associations between AP serum and stroke. For example, AP levels did not correlate with extracranial or intracranial arterial stenosis patients with ischemic stroke (Kim et al. 2013). Overall, evidence from most reported studies suggests a significant association between AP and various stroke outcomes. Table 2 describes the major direct and indirect mechanisms that support a role for elevated AP levels in the pathophysiology of stroke.
Table 2.
Direct and indirect effects of stroke on alkaline phosphatase levels
| Condition | Mechanism Related to Stroke | Reference |
|---|---|---|
| Ischemia/hypoxia | ↑ cellular stress → ↑ ATP release →↑ tissue damage | (Beghetti et al. 2003; Kunutsor et al. 2014; Peters et al. 2014; Khoshnam et al. 2017) |
| Inflammation | ↑ cellular stress → ↑ ATP release →↑ tissue damage | (Kunutsor et al. 2014; Peters et al. 2014; Khoshnam et al. 2017) |
| ↑ inflammatory cytokines →↑ tissue damage | (Beghetti et al. 2003; Kunutsor et al. 2014; Peters et al. 2014; Bonaventura et al. 2016; Khoshnam et al. 2017) | |
| ↑ NO levels →↑ tissue damage | (Kunutsor et al. 2014; Peters et al. 2014; Bonaventura et al. 2016; Khoshnam et al. 2017) | |
| Liver damage | ↑ enzyme release | (Muscari et al., 2014) |
The majority of AP biomarker studies have explored the use of AP as a prognostic tool. Nearly 30 years ago, Yamashita et al. suggested AP may be useful in predicting prognosis of brain damage in patients with postresuscitation encephalopathy, ruptured cerebral aneurysms, acute subdural hematoma and contusion, and non-traumatic intracerebral hemorrhage (Yamashita et al. 1989). A large retrospective study assessing serum AP levels in primary sclerosing cholangitis patients at the time of diagnosis and one year after diagnosis demonstrated that AP may hold prognostic value in prediction of endpoint-free survival (de Vries et al. 2016). Clinical studies have shown that increased AP is associated with risk of cardiovascular disease, one of the many risk factors for stroke (Tonelli et al. 2009; Park et al. 2013; Wannamethee et al. 2013; Kunutsor et al. 2014). Elevated AP levels are correlated with more vascular deaths and recurrent vascular events, suggesting that AP may be a predictor for mortality in stroke patients (Pratibha et al. 2014; Tan et al. 2017). Abnormal AP has been associated with many of the risk factors leading to stroke, including heavy drinking (Ebuehi and Asonye 2007; Shimizu et al. 2013a), obesity (Menahan et al. 1985; Golik et al. 1991), and hypertension (Shimizu et al. 2013b). Elevated AP has been shown to correlate with poor functional outcome and mortality in cardiovascular disease (Park et al. 2013; Wannamethee et al. 2013; Karabulut et al. 2014; Kunutsor et al. 2014) and in stroke patients (Ryu et al. 2010; Kim et al. 2013; Tan et al. 2016). Investigators theorize that the negative association between AP levels and stroke outcome may be linked to the fact that increased AP levels are associated with increased inflammation and enhanced vascular calcification leading to atherosclerosis (Tonelli et al. 2009; Kim et al. 2013; Ryu et al. 2014). Preclinical and clinical studies can be designed to address this question.
4.0. Alkaline Phosphatase-Based Therapeutics in Stroke
4.1. Therapeutic Administration of Alkaline Phosphatase
Exogenous administration of AP has been shown to have beneficial effects on the outcome of numerous inflammatory disorders in humans and animal models of the associated disease, including sepsis (Ebrahimi et al., 2011; Heemskerk et al., 2009; Verweij et al., 2004; Pickkers et al., 2009), ulcerative colitis (Lukas et al., 2010; Tuin et al., 2009), necrotizing enterocolitis (Whitehouse et al. 2010), and multiple sclerosis (Huizinga et al. 2012). These studies and a number of others which have shown positive disease outcomes using AP therapeutics are described in Table 3. For example, pre-symptomatic AP administration reduced signs of neurological distress in experimental autoimmune encephalomyelitis (EAE) mice (Huizinga et al. 2012). Systemic inflammation is also common after surgery and often complicates surgical outcomes. In some cases, exogenous administration of AP may be useful to reduce post-operative care requirements. Davidson et al. showed that AP’s ability to neutralize inflammatory substrates illustrates that it may be protective against systemic inflammation in post-operative patients (Davidson et al., 2012). Oral administration of AP to ulcerative colitis patients for one week also improved clinical response scores and decreased CRP levels (Lukas et al., 2010). Alternatively, intravenous administration of AP decreased plasma creatinine levels in patients with renal complications from severe sepsis or septic shock (Heemskerk et al. 2009; Pickkers et al. 2012). Phase II clinical trials have already shown the benefits of using bovine-derived intestinal AP in a subset of critically ill patients (Heemskerk et al. 2009). Administration of a human recombinant AP (recAP) consisting of a placental/intestinal AP hybrid resulted in positive phase I clinical trial outcome in a subset of septic patients (Kiffer-Moreira et al. 2014; Peters et al. 2016b), that is supported by effective recAP therapeutic efficacy from preclinical models (Peters et al. 2016a, 2017). Thus, the use of TNAP or recombinant AP molecules may provide a viable therapeutic option for patients as well as provide insights on AP’s mechanism(s) of action in cerebrovascular disease.
Table 3:
Positive Therapeutic Outcomes in Preclinical and Clinical AP Studies
| Type of AP Therapy | Study Population |
Injury or Disease Model | Outcome | Ref. |
|---|---|---|---|---|
| AP Administration | ||||
| PLAP | Mouse | LPS | PLAP administration improved sepsis survival, possibly by halting its’ development | (Bentala et al. 2002) |
| PLAP | Mouse | LPS | PLAP treatment improved survival and lowered NO levels in septic mice | (Verweij et al. 2004) |
| IAP | Mouse & pig | LPS | IAP administration attenuates LPS toxicity up to 80%, resulting in increased survival and inhibits differentiation of white blood cell and thrombocyte counts | (Beumer2003) |
| IAP | Mouse | Sepsis | IAP treatment reduced local and systemic inflammatory responses, as well as distant damage in the liver and lungs | (van Veen et al. 2005) |
| IAP | Sheep | Sepsis | Administration of IAP in fecal peritonitis-induced septic shock improved gas exchange, decreased blood IL-6 levels, and increased survival time | (Su et al. 2006) |
| IAP | Phase IIa clinical trial | Sepsis | Infusion of IAP in severe sepsis and septic shock patients inhibits the upregulation of renal iNOS, leading to reduction of NO metabolite production and attenuated tubular enzymuria, resulting in overall improved renal function | (Heemskerk et al. 2009) |
| IAP | Randomized, double-blind, placebo-controlled clinical study | Sepsis | IAP administration significantly improved renal function in septic patients | (Pickkers et al. 2009) |
| IAP | Rat | Inflammatory bowel disease | IAP treatment alleviates epithelial layer damage associated with DSS in rat intestines | (Tuin et al. 2009) |
| IAP | Open-label, first-in-patient exploratory trial | Ulcerative colitis | IAP administration was associated with short-term improvement in UC disease activity | (Lukas et al. 2010) |
| IAP | Rat | NEC | Supplemental IAP has a protective role in experimental NEC | (Whitehouse et al. 2010) |
| IAP | Mouse | Antibiotic treatment |
IAP supplementation increased growth of commensal bacteria leading to restored gut microbiota lost to antibiotic treatment | (Malo et al. 2010) |
| IAP | Mouse | Sepsis | IAP treatment enhanced survival and reduced organ damage in septic mice | (Ebrahimi et al. 2011) |
| IAP | Phase IIa prospective randomized, double-blind, placebo-controlled clinical trial | Sepsis and AKI | Overall, IAP treatment improves renal function in patients with severe sepsis or sepsis shock with AKI | (Pickkers et al. 2012) |
| IAP | Rat | Inflammatory bowel disease | Intrarectally administered IAP in models of rats colitis resulted in a lower colonic weight and tissue damage score; normalized expression of neutrophil markers and IL-1β; and counteracted bacterial translocation | (Martinez-Moya et al. 2012) |
| IAP | Mouse | Multiple Sclerosis | Pre-symptomatic treatment of EAE with IAP reduces neurological symptoms | (Huizinga et al. 2012) |
| IAP | Rat | NEC | IAP supplementation decreased histologic injury scores and barrier permeability in the ileum of rat pups with NEC | (Rentea et al. 2012) |
| IAP | Mouse | Metabolic syndrome |
IAP supplementation inhibited absorption of endotoxins and improved the lipid profile in mice, resulting in prevention or reversal of metabolic syndrome | (Kaliannan et al. 2013) |
| IAP | Rat | NEC | IAP treatment decreased iNOS and TNF-α expression, and decreased LPS translocation into the serum of infant rats | (Rentea et al. 2013) |
| IAP | Rat | NEC | IAP supplementation decreased intestinal injury and inflammation, including TNF-α, IL-6 and iNOS by LPS in preterm rat intestine | (Heinzerling et al. 2014) |
| IAP | Mouse | Antibiotic- associated infections |
Antibiotics+IAP oral supplementation resulted in weight maintenance, reduced clinical severity, reduced gut inflammation, and improved survival following infection | (Alam et al. 2014) |
| recAP | Rat | LPS | recAP treatment has renal protective effects from LPS-induced damage | (Peters et al. 2015) |
| recAP | Rat | Renal ischemia and reperfusion; LPS | recAP exerted a clear renal protective anti-inflammatory effect | (Peters et al. 2016a) |
| AP Inhibition | ||||
| Levamisole | Prospective clinical trial | Colon cancer | The addition of levamisole to 5FU-adjuvant therapy improved survival in stage II and III colon cancer patients | (Taal et al. 2001) |
| L-Phen | Rat | LPS | Suggest that lAPs in the gastrointestinal tract reduce LPS content in serum | (Koyama et al. 2002) |
| SBI-425 | Mouse | Medial vascular calcification | TNAP inhibition significantly reduced aortic calcification and cardiac hypertrophy, and extended lifespan over vehicle-treated controls | (Sheen et al. 2015) |
| SBI-425 | Mouse | Hypophosphatasia | TNAP inhibition reduces calcium and lipid levels to improve the course of coronary atherosclerosis | (Romanelli et al. 2017) |
| SBI-425 | Mouse | Pseudoxanthoma elasticum (PXE) | TNAP inhibition attenuated calcification, altering disease development and progression in vivo | (Ziegler et al. 2017) |
PLAP: placental alkaline phosphatase; IAP: intestinal alkaline phosphatase; LPS: lipopolysaccharide; NO: nitric oxide; iNOS: inducible nitric oxide synthase; DSS: dextran sodium sulfate; NEC: Necrotizing enterocolitis; AKI: acute kidney injury; EAE: experimental autoimmune encephalomyelitis; recAP: human recombinant alkaline phosphatase; L-Phen: L-phenylalanine
4.2. AP Therapeutic Inhibition of Alkaline Phosphatase
In contrast, a smaller set of studies have proposed a therapeutic approach that inhibits TNAP. An emerging concept which supports a role for AP in vascular disease is that elevated serum AP, most likely due to elevated TNAP activity, promotes vascular calcification and leads to increased risk of cardiovascular disease and stroke. To prevent these outcomes, administration of an AP inhibitor has been suggested as one potential therapeutic option. Research from Pratibha and colleagues suggests that administration of AP inhibitors could be used to prevent cerebral ischemia in high-risk populations (Pratibha et al. 2014). A study in stroke-prone rats found beneficial effects against cardiovascular disease complications using a traditional fungal medicine and found that the number of capillaries that expressed AP in the heart was significantly decreased compared to untreated rats. Typically, TNAP is minimally localized to cardiac microvessels in comparison to its levels in cerebral microvessels (Koyama et al. 2006). Some of the better-known AP inhibitors, including tetramisole and levamisole, are no longer used in the clinic due to lack of AP isoenzyme specificity and serious neurological side effects associated with chronic treatment (Nowak et al. 2015). Sheen et al. have made efforts towards screening for promising TNAP inhibitors. One of their more-promising candidates, SBI-425, has been shown to reduce aortic calcification and prolong life in TNAP-overexpressing mice (Sheen et al. 2015). Overall, AP inhibitors have proven to be useful for reducing vascular calcification, an important risk factor for the development of stroke. Thus, it is imperative that we identify the circumstances when AP, and more specifically TNAP, is beneficial and when it is harmful. The identification of these mechanisms will prove crucial in the development of AP-based therapeutics to prevent or to treat cerebrovascular disorders such as stroke.
5.0. Potential Mechanistic Targets for Alkaline Phosphatase in Stroke
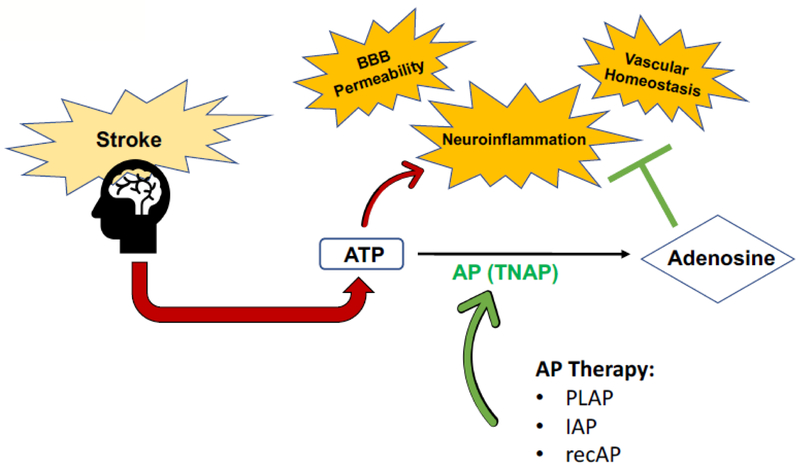
The proposed mechanism of action for AP’s therapeutic utility in stroke include enhancement of BBB integrity, relief of inflammation, and promotion of vascular homeostasis, as shown in Figure 3. ATP release is an important consequence of stroke-associated inflammation, induced by multiple brain cell types, especially BBB endothelial cells. AP enhancement with exogenous PLAP, IAP, or recombinant alkaline phosphatase (recAP) may help to decrease inflammation by catalyzing the reaction of pro-inflammatory, toxic ATP to anti-inflammatory, nontoxic adenosine. This in turn activates neuroprotective signaling cascades which limit inflammation, enhance BBB integrity, and promote vascular homeostasis.
Figure 3. Proposed mechanism of action for implementation of alkaline phosphatase-based therapeutics in stroke:
An important consequence that results from the induction of stroke-associated inflammatory pathways is increased ATP release in multiple brain cell types, particularly in BBB endothelial cells. AP administration, via placental alkaline phosphatase (PLAP), intestinal alkaline phosphatase (IAP), or recombinant alkaline phosphatase (recAP) may help to decrease inflammation by facilitating the hydrolysis of pro-inflammatory, toxic ATP to anti-inflammatory, nontoxic adenosine. Binding of adenosine to its receptors has been shown to activate neuroprotective signaling cascades which limit inflammation, enhance BBB integrity, and promote vascular homeostasis.
5.1. Blood-Brain Barrier Permeability
Increased BBB permeability is likely to have a role in the putative association between AP and stroke. TNAP is expressed in brain endothelial cells and may play an important role in BBB maintenance and integrity (El Hafny et al. 1996; Deracinois et al. 2012), as well as transportation of proteins, including insulin, across the barrier (Calhau et al. 2002). The cerebral microvasculature is protected by astrocytes, pericytes, and the extracellular matrix that surrounds the vessels (ladecola and Nedergaard 2007). The primary proteins involved in keeping the cerebral environment separate from the rest of the body are tight junctions, and the dysfunction of these proteins leads to BBB breakdown and neuronal cell death. Following stroke, toxic proteases, cytokines, and free radicals form to aid in the removal of dead cells, however these molecules also cause tissue damage and participate in BBB disruption (Yang and Rosenberg 2011; Jiang et al. 2018). Rabbits with diabetic ketoacidosis exhibited increased inducible nitric oxide synthase (NOS) activity and decreased AP activity levels in brain endothelium (Zhu et al. 2004). One emerging concept suggests that TNAP may be released into the circulation to neutralize inflammation produced in response to damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). The release of membrane-bound TNAP as soluble TNAP into the circulation may result in a compromised BBB (Pike et al. 2015). In vitro evidence for a direct role for TNAP in the regulation of BBB integrity was demonstrated by Deracinois and colleagues (Deracinois et al. 2012, 2015). Treatment of cultured brain capillary endothelial cells with the nonspecific AP inhibitor, levamisole, increased permeability to a fluorescent tracer and reorganized the actin cytoskeleton; removal of levamisole reversed the increase in permeability and cytoskeletal disruption (Deracinois et al. 2015). These results support a critical role for TNAP in the integrity of the cerebral microvasculature. TNAP’s mechanism(s) of action at the BBB are also summarized in Figure 2.
5.2. Neuroinflammation
Elevated serum AP is a highly characteristic part of the inflammatory response in multiple disorders. Prior studies have suggested that abnormal AP levels contribute to the development of cerebral small vessel disease and cardiovascular disease (Tonelli et al. 2009; Park et al. 2013; Wannamethee et al. 2013; Kunutsor et al. 2014; Lee et al. 2015). Huizinga et al. proposed that AP has a role in limiting neuroinflammation by interfering with immune activation through neutralization of LPS and endogenous substrates such as ATP. They found that AP administration during the priming phases of EAE reduced neurological signs of multiple sclerosis (MS) (Huizinga et al. 2012). Post-stroke infections are very common in stroke patients, particularly respiratory and urinary tract infections (Langhorne et al. 2000; Vernino et al. 2003). Thus, it is likely that mechanisms related to infection-induced inflammation are also involved in the initiation of AP upregulation in stroke patients. Increasing evidence suggests that infection often precedes or triggers chronic neurological diseases such as MS (Buljevac et al. 2002). AP’s natural ability to neutralize inflammatory substrates may prove that it has a beneficial role in slowing neuroinflammation. Recent studies have shown the importance of the gut-brain axis in MS, and demonstrate that microbial infection can activate myelin-reactive T cells in the CNS (Nogai et al. 2005; Berer et al. 2011). ATP, an endogenous damage associated molecular pattern (DAMP) signal, is upregulated by multiple cells in response to stress, and has been shown to activate microglia in response to brain injury (Davalos et al. 2005), and can also trigger oligodendrocyte excitotoxicity (Matute et al. 2007). In contrast, chronic liver disease also increased AP activity in rat brains (Dhanda et al. 2018). Elevated brain AP levels found in both CNS and systemic examples of infection-induced inflammation strongly suggests that similar neuroinflammatory mechanisms are also present in the pathophysiology of stroke.
5.3. Altered Vascular Homeostasis
The disruption of cerebral microvascular homeostasis is another common feature of stroke. In contrast to other studies which suggest that serum AP levels are elevated post-stroke, Shimizu et al. speculated that altered vascular homeostasis plays a role in the association between lower AP levels and increased risk of stroke (Shimizu et al. 2013a). Endothelial progenitor cells (EPCs) have been shown to circulate in peripheral blood and contribute to maintenance of the vasculature (Asahara et al. 1997). Previous research has shown that reduced levels of EPCs predict atherosclerotic disease progression (Schmidt-Lucke et al. 2005). Another study showed an association between reduced EPCs and an increased number of infarctions, but no significant correlation with atherosclerosis; this finding is likely due to the multiple risk factors and cell types that contribute to vascular dysfunction (Taguchi et al. 2004). Since EPCs also contribute to vascular repair, it is likely that a reduction in these cells may lead to increased risk of stroke (Shimizu et al. 2013a). Thickening of the cerebral vessel walls via increased collagen represents a normal feature of aging. In leukoaraiosis, which is caused by chronic ischemia, venous collagenosis is further increased and may be due, in part, to altered TNAP activity in cerebral microvessels (Brown et al. 2002). Lee et al. also speculate that the association between AP and indicators of cerebral small vessel disease includes issues with vascular calcification and microcirculation impairment (Lee et al. 2015). It is hypothesized that vascular calcification leads to stiff vessel walls and the resultant microcirculatory dysfunction may lead to myocardial ischemia, and by extension, cerebrovascular dysfunction and the onset of cerebral ischemia (Sigrist and McIntyre 2008). Alternatively, other studies have suggested that elevated bone AP levels may accelerate the development of cardiovascular disease through vascular calcification, presumably due to impaired vascular homeostasis (Shioi et al. 2002; Shimizu et al. 2013a). Thus, there are multiple putative mechanisms through which altered TNAP activity in endothelial cells and EPCs could impact cerebrovascular homeostasis to increase stroke risk or negatively impact post-stroke outcomes.
6.0. Conclusion
Although AP is a ubiquitous enzyme expressed in numerous tissues, a comprehensive understanding of its molecular and cellular mechanisms of action remains elusive. Emerging clinical evidence supports the potential utility of AP as a rapid, cost-effective blood biomarker to be used singly or as part of a biomarker panel in stroke patients. In light of the proposed mechanism in Figure 3, the complex cell biology of AP represents is an important consideration for the development of targeted AP-based biomarkers and therapeutics for stroke. It will be critically important to understand the circumstances under which AP administration or AP inhibition are beneficial as well as detrimental. Harnessing this knowledge may represent an important milestone in the development of novel therapeutic agents for stroke.
Acknowledgements
The project was also supported by NIH T32AG052375 (ALB) and K01NS081014, U54GM104942, P20 GM109098 (CMB). We appreciate insightful feedback from Dr. Ashley B. Petrone.
References
- Adams HP, Brott TG, Furlan AJ, et al. (1996) Guidelines for Thrombolytic Therapy for Acute Stroke: A Supplement to the Guidelines for the Management of Patients with Acute Ischemic Stroke. Circulation 94:1167–1174. doi: 10.1161/01.CIR.94.5.1167 [DOI] [PubMed] [Google Scholar]
- Alam SN, Yammine H, Moaven O, et al. (2014) Intestinal Alkaline Phosphatase Prevents Antibiotic-Induced Susceptibility to Enteric Pathogens: Annals of Surgery 259:715–722. doi: 10.1097/SI_A.0b013e31828fae14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom JA, Brown WR, Moody DM, et al. (2002) Temporal expression pattern of cerebrovascular endothelial cell alkaline phosphatase during human gestation. J Neuropathol Exp Neurol 61:76–84 [DOI] [PubMed] [Google Scholar]
- Arun P, Oguntayo S, Albert SV, et al. (2015) Acute decrease in alkaline phosphatase after brain injury: A potential mechanism for tauopathy. Neuroscience Letters 609:152–158. doi: 10.1016/j.neulet.2015.10.036 [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967. doi: 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S, Bowling F, Carpenter K, et al. (2010) Perinatal hypophosphatasia presenting as neonatal epileptic encephalopathy with abnormal neurotransmitter metabolism secondary to reduced co-factor pyridoxal-5’-phosphate availability. Journal of Inherited Metabolic Disease 33:25–33. doi: 10.1007/s10545-009-9012-y [DOI] [PubMed] [Google Scholar]
- Bannister RG, Romanul FCA (1963) The localization of alkaline phosphatase activity in cerebral blood vessels. Journal of Neurology, Neurosurgery & Psychiatry 26:333–340. doi: 10.1136/jnnp.26.4.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnadas A, Manso L, de la Piedra C, et al. (2014) Bone turnover markers as predictive indicators of outcome in patients with breast cancer and bone metastases treated with bisphosphonates: Results from a 2-year multicentre observational study (ZOMAR study). Bone 68:32–40. doi: 10.1016/j.bone.2014.07.036 [DOI] [PubMed] [Google Scholar]
- Barvencik F, Beil FT, Gebauer M, et al. (2011) Skeletal mineralization defects in adult hypophosphatasia—a clinical and histological analysis. Osteoporosis International 22:2667–2675. doi: 10.1007/s00198-011-1528-y [DOI] [PubMed] [Google Scholar]
- Beghetti M, Rimensberger PC, Kalangos A, et al. (2003) Kinetics of procalcitonin, interleukin 6 and C-reactive protein after cardiopulmonary-bypass in children. Cardiology in the Young 13:161–7 [DOI] [PubMed] [Google Scholar]
- Bell MA, Ball MJ (1985) Laminar variation in the microvascular architecture of normal human visual cortex (area 17). Brain Research 335:139–143. doi: 10.1016/0006-8993(85)90284-7 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, et al. (2018) Heart Disease and Stroke Statistics 2018 Update: A Report From the American Heart Association. Circulation, doi: 10.1161/cir.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Bentala H, Verweij WR, der Vlag AH-V, et al. (2002) Removal of Phosphate from Lipid A as a Strategy to Detoxify Lipopolysaccharide: Shock 18:561–566. doi: 10.1097/00024382-200212000-00013 [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, et al. (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479:538–541. doi: 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- Berger J, Garattini E, Hua JC, Udenfriend S (1987) Cloning and sequencing of human intestinal alkaline phosphatase cDNA. Proceedings of the National Academy of Sciences 84:695–698. doi: 10.1073/pnas.84.3.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer C (2003) Calf Intestinal Alkaline Phosphatase, a Novel Therapeutic Drug for Lipopolysaccharide (LPS)-Mediated Diseases, Attenuates LPS Toxicity in Mice and Piglets. Journal of Pharmacology and Experimental Therapeutics 307:737–744. doi: 10.1124/jpet.103.056606 [DOI] [PubMed] [Google Scholar]
- Bonaventura A, Liberale L, Vecchie A, et al. (2016) Update on Inflammatory Biomarkers and Treatments in Ischemic Stroke. International Journal of Molecular Sciences 17:. doi: 10.3390/ijms17121967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Challa VR, et al. (2002) Venous collagenosis and arteriolar tortuosity in leukoaraiosis. Journal of the Neurological Sciences 203:159–163. doi: 10.1016/s0022-510x(02)00283-6 [DOI] [PubMed] [Google Scholar]
- Brun-Heath I, Ermonval M, Chabrol E, et al. (2011) Differential expression of the bone and the liver tissue nonspecific alkaline phosphatase isoforms in brain tissues. Cell and Tissue Research 343:521–536. doi: 10.1007/s00441-010-1111-4 [DOI] [PubMed] [Google Scholar]
- Bruno A, Shah N, Akinwuntan AE, et al. (2013) Stroke Size Correlates with Functional Outcome on the Simplified Modified Rankin Scale Questionnaire. Journal of Stroke and Cerebrovascular Diseases 22:781–783. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Buchet R, Millan JL, Magne D (2013) Multisystemic Functions of Alkaline Phosphatases In: Millan J (ed) Phosphatase Modulators. Methods in Molecular Biology (Methods and Protocols). Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- Buljevac D, Flach HZ, Hop WCJ, et al. (2002) Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125:952–960. doi: 10.1093/brain/awf098 [DOI] [PubMed] [Google Scholar]
- Bushnell CD, Hurn P, Colton C, et al. (2006) Advancing the Study of Stroke in Women: Summary and Recommendations for Future Research From an NINDS-Sponsored Multidisciplinary Working Group. Stroke 37:2387–2399. doi: 10.1161/01.STR.0000236053.37695.15 [DOI] [PubMed] [Google Scholar]
- Calhau C, Martel F, Pinheiro-Silva S, et al. (2002) Modulation of insulin transport in rat brain microvessel endothelial cells by an ecto-phosphatase activity. Journal of Cellular Biochemistry 84:389–400. doi: 10.1002/jcb.10027 [DOI] [PubMed] [Google Scholar]
- Champion EE, Glazier JD, Greenwood SL, et al. (2003) Localization of Alkaline Phosphatase and Ca2+-ATPase in the Cat Placenta. Placenta 24:453–461. doi: 10.1053/plac.2002.0952 [DOI] [PubMed] [Google Scholar]
- Charegaonkar PM, Rindani TH (1961) Effect of age, sex and gonadal hormone on alkaline phosphatase activity of rat brain tissue. Proceedings of the Indian Academy of Sciences - Section B 54:113–116 [Google Scholar]
- Chen KT, Malo MS, Moss AK, et al. (2010) Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. American Journal of Physiology - Gastrointestinal and Liver Physiology 299:G467–75. doi: 10.1152/ajpgi.00364.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BM, Ong KL, Cheung RV, et al. (2008) Association between plasma alkaline phosphatase and C-reactive protein in Hong Kong Chinese. Clinical Chemistry and Laboratory Medicine 46:523–7. doi: 10.1515/cclm.2008.111 [DOI] [PubMed] [Google Scholar]
- Choi J, Pai SH (2000) Serum lipid concentrations change with serum alkaline phosphatase activity during pregnancy. Annals o f Clinical & Laboratory Science 30:422–428 [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, et al. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience 8:752–758. doi: 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- Davidson J, Tong S, Hauck A, et al. (2012) Alkaline phosphatase activity after cardiothoracic surgery in infants and correlation with post-operative support and inflammation: a prospective cohort study. Critical Care 16:R160. doi: 10.1186/cc11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries EMG, Wang J, Leeflang MMG, et al. (2016) Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver International 36:1867–1875. doi: 10.1111/liv.13110 [DOI] [PubMed] [Google Scholar]
- Deracinois B, Duban-Deweer S, Pottiez G, et al. (2012) TNAP and EHD1 Are Over-Expressed in Bovine Brain Capillary Endothelial Cells after the Re-Induction of Blood-Brain Barrier Properties. PLOS ONE 7:e48428. doi: 10.1371/journal.pone.0048428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deracinois B, Lenfant A-M, Dehouck M-P, Flahaut C (2015) Tissue Non-specific Alkaline Phosphatase (TNAP) in Vessels of the Brain In: Fonta C, Negyessy L (eds) Neuronal Tissue-Nonspecific Alkaline Phosphatase (TNAP). Springer; Netherlands, Dordrecht, pp 125–151 [DOI] [PubMed] [Google Scholar]
- Dhanda S, Gupta S, Haider A, et al. (2018) Systemic inflammation without gliosis mediates cognitive deficits through impaired BDNF expression in bile duct ligation model of hepatic encephalopathy. Brain, Behavior, and Immunity 70:214–232. doi: 10.1016/j.bbi.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Endres M (2014) Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 45(5):1510–8. doi: 10.1161/strokeaha.113.004075 [DOI] [PubMed] [Google Scholar]
- Dirnagl U, ladecola C, Moskowitz M (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–7 [DOI] [PubMed] [Google Scholar]
- Ebrahimi F, Malo MS, Alam SN, et al. (2011) Local peritoneal irrigation with intestinal alkaline phosphatase is protective against peritonitis in mice. Journal of Gastrointestinal Surgery 15:860–9. doi: 10.1007/s11605-010-1405-6 [DOI] [PubMed] [Google Scholar]
- Ebuehi OAT, Asonye CL (2007) Gender and Alcohol Consumption Affect Human Serum Enzymes, Protein and Bilirubin. Asian Journal of Biochemistry 2:330–336 [Google Scholar]
- El Hafny B, Bourre J-M, Roux F (1996) Synergistic stimulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities by retinoic acid and astroglial factors in immortalized rat brain microvessel endothelial cells. Journal of Cellular Physiology 167:451–460. doi: [DOI] [PubMed] [Google Scholar]
- Ermonval M, Baudry A, Baychelier F, et al. (2009) The Cellular Prion Protein Interacts with the Tissue Non-Specific Alkaline Phosphatase in Membrane Microdomains of Bioaminergic Neuronal Cells. PLOS ONE 4:e6497. doi: 10.1371/journal.pone.0006497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenuku Rl, Foli AK (1975) Variations in total serum alkaline phosphatase activity with age and sex in adult and adolescent Ghanaians. Clin Chim Acta 60:303–306 [DOI] [PubMed] [Google Scholar]
- Fonta C (2004) Areal and Subcellular Localization of the Ubiquitous Alkaline Phosphatase in the Primate Cerebral Cortex: Evidence for a Role in Neurotransmission. Cerebral Cortex 14:595–609. doi: 10.1093/cercor/bhh021 [DOI] [PubMed] [Google Scholar]
- Fonta C, Imbert M (2002) Vascularization in the primate visual cortex during development. Cereb Cortex 12:199–211 [DOI] [PubMed] [Google Scholar]
- Fonta C, Negyessy L, Renaud L, Barone P (2005) Postnatal development of alkaline phosphatase activity correlates with the maturation of neurotransmission in the cerebral cortex. The Journal of Comparative Neurology 486:179–196. doi: 10.1002/cne.20524 [DOI] [PubMed] [Google Scholar]
- Friede RL (1966) A quantitative mapping of alkaline phosphatase in the brain of the rhesus monkey. Journal of Neurochemistry 13:197–203 [DOI] [PubMed] [Google Scholar]
- Gandolfi M, Smania N, Vella A, et al. (2017) Assessed and Emerging Biomarkers in Stroke and Training-Mediated Stroke Recovery: State of the Art. Neural Plasticity 2017:15 pages, doi: 10.1155/2017/1389475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdara NB, Belgacem A, Khemiri I, et al. (2018) Protective effects of phycocyanin on ischemia/reperfusion liver injuries. Biomedicine & Pharmacotherapy 102:196–202. doi: 10.1016/j.biopha.2018.03.025 [DOI] [PubMed] [Google Scholar]
- Giessen C, Nagel D, Glas M, et al. (2014) Evaluation of preoperative serum markers for individual patient prognosis in stage l-lll rectal cancer. Tumor Biology 35:10237–10248. doi: 10.1007/s13277-014-2338-6 [DOI] [PubMed] [Google Scholar]
- Golik A, Rubio A, Weintraub M, Byrne L (1991) Elevated serum liver enzymes in obesity: a dilemma during clinical trials. Int J Obes 15:797–801 [PubMed] [Google Scholar]
- Gong C-X, Singh TJ, Grundke-lqbal I, Iqbal K (1993) Phosphoprotein Phosphatase Activities in Alzheimer Disease Brain. Journal of Neurochemistry 61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x [DOI] [PubMed] [Google Scholar]
- Hammerich KH, Donahue TF, Rosner IL, et al. (2017) Alkaline phosphatase velocity predicts overall survival and bone metastasis in patients with castration-resistant prostate cancer. Urologic Oncology: Seminars and Original Investigations 35:460.e21–460.e28. doi: 10.1016/j.urolonc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Hanics J, Janos B, Xiao J, et al. (2012) Ablation of TNAP function compromises myelination and synaptogenesis in the mouse brain. Cell and Tissue Research 349:459–71. doi: 10.1007/s00441-012-1455-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna AN, Waldman WJ, Lott JA, et al. (1997) Increased alkaline phosphatase isoforms in autoimmune diseases. Clinical Chemistry 43:1357–1364 [PubMed] [Google Scholar]
- Harris H (1990) The human alkaline phosphatases: What we know and what we don’t know. Clinica Chimica Acta 186:133–150. doi: 10.1016/0009-8981(90)90031-M [DOI] [PubMed] [Google Scholar]
- Heemskerk S, Masereeuw R, Moesker O, et al. (2009) Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Critical Care Medicine 32:417–23. doi: 10.1097/CCM.0b013e31819598af [DOI] [PubMed] [Google Scholar]
- Heinzerling NP, Liedel JL, Welak SR, et al. (2014) Intestinal alkaline phosphatase is protective to the preterm rat pup intestine. Journal of Pediatric Surgery 49:954–960. doi: 10.1016/j.jpedsurg.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeth B, Bagrel A, Dalo B, et al. (1981) Influence of oral contraceptives of differing dosages on a-1-antitrypsin, γ-glutamyltransferase and alkaline phosphatase. Clinica Chimica Acta 112:293–299. doi: 10.1016/0009-8981(81)90452-6 [DOI] [PubMed] [Google Scholar]
- Huizinga R, Kreft KL, Onderwater S, et al. (2012) Endotoxin- and ATP-neutralizing activity of alkaline phosphatase as a strategy to limit neuroinflammation. Journal of Neuroinflammation 9:266–266. doi: 10.1186/1742-2094-9-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ladecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nature Neuroscience 10:1369–1376. doi: 10.1038/nn2003 [DOI] [PubMed] [Google Scholar]
- Jassam NJ, Horner J, Marzo-Ortega H, et al. (2009) Transient rise in alkaline phosphatase activity in adults. BMJ Case Reports 2009:bcr0920092250–bcr0920092250. doi: 10.1136/bcr.09.2009.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Andjelkovic AV, Zhu L, et al. (2018) Blood-brain barrier dysfunction and recovery after ischemic stroke. Progress in Neurobiology 163–164:144–171. doi: 10.1016/j.pneurobio.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Sharp FR (2015) Improving the translation of animal ischemic stroke studies to humans. Metabolic Brain Disease 30:461–467. doi: 10.1007/s11011-014-9499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliannan K, Hamarneh SR, Economopoulos KP, et al. (2013) Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proceedings of the National Academy of Sciences 110:7003–7008. doi: 10.1073/pnas.1220180110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, West WL (1982) Ultrastructural localization of glucose-6-phosphatase and alkaline phosphatase in the vaginal epithelium of rat. J Morphol 171:1–10. doi: 10.1002/jmor.1051710102 [DOI] [PubMed] [Google Scholar]
- Karabulut A, Sahin I, Avci II, et al. (2014) Impact of serum alkaline phosphatase level on coronary collateral circulation. Kardiologia Polska 72:1388–93. doi: 10.5603/KP.a2014.0114 [DOI] [PubMed] [Google Scholar]
- Kellett KAB, Williams J, Vardy ERLC, et al. (2011) Plasma alkaline phosphatase is elevated in Alzheimer’s disease and inversely correlates with cognitive function. International journal of molecular epidemiology and genetics 2:114–121 [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ, Jowsey J, Riggs BL, Elveback LR (1967) Relationship between serum phosphate concentration and bone resorption in osteoporosis. J Lab Clin Med 69:110–115 [PubMed] [Google Scholar]
- Khan MJ, Ahmed B, Ahmed S, Khan M (2016) Increase in serum alkaline phosphatase due to fatty meal in undergraduate students of Khyber Medical University, Khyber Pakhtunkhwa. J Pak Med Assoc 66:378–379 [PubMed] [Google Scholar]
- Khoshnam SE, Winlow W, Farbood Y, et al. (2017) Emerging Roles of microRNAs in Ischemic Stroke: As Possible Therapeutic Agents. Journal of Stroke 19:166–187. doi: 10.5853/jos.2016.01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffer-Moreira T, Sheen CR, Gasque KC da S, et al. (2014) Catalytic Signature of a Heat-Stable, Chimeric Human Alkaline Phosphatase with Therapeutic Potential. PLoS ONE 9:e89374. doi: 10.1371/journal.pone.0089374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GS, Im E, Rhee JH (2017) Association of physical activity on body composition, cardiometabolic risk factors, and prevalence of cardiovascular disease in the Korean population (from the fifth Korea national health and nutrition examination survey, 2008–2011). BMC Public Health 17:. doi: 10.1186/s12889-017-4126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Song T-J, Song D, et al. (2013) Serum Alkaline Phosphatase and Phosphate in Cerebral Atherosclerosis and Functional Outcomes After Cerebral Infarction. Stroke 44:3547–9. doi: 10.1161/strokeaha.113.002959 [DOI] [PubMed] [Google Scholar]
- Koyama I, Matsunaga T, Harada T, et al. (2002) Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clinical Biochemistry 35:455–461. doi: 10.1016/S0009-9120(02)00330-2 [DOI] [PubMed] [Google Scholar]
- Koyama T, Taka A, Togashi H (2006) Cardiovascular effects produced by a traditional fungal medicine, Fuscoporia obliqua extract, and microvessels in the left ventricular wall of stroke-prone spontaneously hypertensive rat (SHRSP). Clinical Hemorheology & Microcirculation 35:491–498 [PubMed] [Google Scholar]
- Kunjappu JJ, Mathew VB, Hegde S, et al. (2012) Assessment of the alkaline phosphatase level in gingival crevicular fluid, as a biomarker to evaluate the effect of scaling and root planing on chronic periodontitis: An in vivo study. Journal of Oral and Maxillofacial Pathology 16:54–57. doi: 10.4103/0973-029x.92974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunutsor SK, Apekey TA, Khan H (2014) Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis 236:7–17. doi: 10.1016/j.atherosclerosis.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Lampl Y, Paniri Y, Eshel Y, Sarova-Pincha I (1990) Alkaline phosphatase level in CSF in various brain tumors and pulmonary carcinomatous meningitis. Journal of Neuro-Oncology 9:35–40. doi: 10.1007/bf00167066 [DOI] [PubMed] [Google Scholar]
- Langer D, Hammer K, Koszalka P, et al. (2008) Distribution of ectonucleotidases in the rodent brain revisited. Cell and Tissue Research 334:199–217. doi: 10.1007/s00441-008-0681-x [DOI] [PubMed] [Google Scholar]
- Langhorne P, Stott DJ, Robertson L, et al. (2000) Medical Complications After Stroke: A Multicenter Study. Stroke 31:1223–1229. doi: 10.1161/01.STR.31.6.1223 [DOI] [PubMed] [Google Scholar]
- Lee H-B, Kim J, Kim S-H, et al. (2015) Association between Serum Alkaline Phosphatase Level and Cerebral Small Vessel Disease. PLOS ONE 10:e0143355. doi: 10.1371/journal.pone.0143355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester E (1977) Serum Enzyme Levels in Healthy Old People. Annals of Clinical Biochemistry: An international journal of biochemistry and laboratory medicine 14:118–119. doi: 10.1177/000456327701400121 [DOI] [PubMed] [Google Scholar]
- Li X, Wang D, Yang C, et al. (2018) Establishment of age-and gender-specific pediatric reference intervals for liver function tests in healthy Han children. World Journal of Pediatrics 14:151–159. doi: 10.1007/s12519-018-0126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang D, Li J, et al. (2016) High Serum Alkaline Phosphatase Levels in Relation to Multi-Cerebral Microbleeds in Acute Ischemic Stroke Patients with Atrial Fibrillation and/or Rheumatic Heart Disease. Current Neurovascular Research 13:303–308. doi: 10.2174/1567202613666160817095623 [DOI] [PubMed] [Google Scholar]
- Low MG (1987) Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochemical Journal 244:1–13. doi: 10.1042/bj2440001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low MG, Zilversmit DB (1980) Role of phosphatidylinositol in attachment of alkaline phosphatase to membranes. Biochemistry 19:3913–3918. doi: 10.1021/bi00558a004 [DOI] [PubMed] [Google Scholar]
- Lukas M, Drastich P, Konecny M, et al. (2010) Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflammatory Bowel Diseases 16:1180–6. doi: 10.1002/ibd.21161 [DOI] [PubMed] [Google Scholar]
- Luo Y, Li J, Zhang J, Xu Y (2013) Elevated bilirubin after acute ischemic stroke linked to the stroke severity. International Journal of Developmental Neuroscience 31:634–638. doi: 10.1016/j.ijdevneu.2013.08.002 [DOI] [PubMed] [Google Scholar]
- MacGregor GR, Zambrowicz BP, Soriano P (1995) Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development 121:1487–1496 [DOI] [PubMed] [Google Scholar]
- Magnusson P, Hager A, Larsson L (1995) Serum Osteocalcin and Bone and Liver Alkaline Phosphatase Isoforms in Healthy Children and Adolescents. Pediatric Research 38:955–961. doi: 10.1203/00006450-199512000-00021 [DOI] [PubMed] [Google Scholar]
- Makil ES, Tang X, Frazier EA, Collins RT 2nd (2017) Alkaline Phosphatase: A Biomarker of Cardiac Function in Pediatric Patients. Pediatric Cardiology 38:762–9. doi: 10.1007/s00246-017-1577-x [DOI] [PubMed] [Google Scholar]
- Malo MS, Alam SN, Mostafa G, et al. (2010) Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 59:1476. doi: 10.1136/gut.2010.211706 [DOI] [PubMed] [Google Scholar]
- Martinez B, Peplow PV (2016) Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury. Neural Regeneration Research 11:1375–1378. doi: 10.4103/1673-5374.191196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moya P, Ortega-Gonzalez M, Gonzalez R, et al. (2012) Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacological Research 66:144–153. doi: 10.1016/j.phrs.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Matute C, Torre I, Perez-Cerda F, et al. (2007) P2X7 Receptor Blockade Prevents ATP Excitotoxicity in Oligodendrocytes and Ameliorates Experimental Autoimmune Encephalomyelitis. The Journal of Neuroscience 27:9525–33. doi: 10.1523/jneurosci.0579-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Clinic Test ID: ALKI Alkaline Phosphatase, Total and Isoenzymes, Serum. 2017: [Google Scholar]
- Menahan LA, Sobocinski KA, Austin BP (1985) Characterization of elevated plasma alkaline phosphatase activity in genetically obese mice. Metabolism - Clinical and Experimental 34:272–277. doi: 10.1016/0026-0495(85)90012-5 [DOI] [PubMed] [Google Scholar]
- Metwalli AR, Rosner IL, Cullen J, et al. (2014) Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urologic Oncology: Seminars and Original Investigations 32:761–768. doi: 10.1016/j.urolonc.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter Meyer (1963) HISTOCHEMISTRY OF THE DEVELOPING HUMAN BRAIN I. Alkaline Phosphatase, Acid Phosphatase, and AS Esterase in the Cerebellum. Acta Neurologica Scandinavica 39:123–138. doi: 10.1111/j.1600-0404.1963.tb05314.x [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Hazlewood J, DeVivo MJ, Rosner M (1998) Elevated liver enzymes after nontraumatic intracranial hemorrhages. Archives of Physical Medicine and Rehabilitation 79:766–771. doi: 10.1016/s0003-9993(98)90354-9 [DOI] [PubMed] [Google Scholar]
- Millan JL (1986) Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem 261:3112–5 [PubMed] [Google Scholar]
- Millan JL, Manes T (1988) Seminoma-derived Nagao isozyme is encoded by a germ-cell alkaline phosphatase gene. Proceedings of the National Academy of Sciences 85:3024–3028. doi: 10.1073/pnas.85.9.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A, Lalani R, Khurshid M, et al. (1990) Serum alkaline phosphatase in apparently healthy karachi population. Journal of Pakistan Medical Association 182–184 [PubMed] [Google Scholar]
- Moore CA, Ward JC, Rivas ML, et al. (1990) Infantile hypophosphatasia: Autosomal recessive transmission to two related sibships. American Journal of Medical Genetics 36:15–22. doi: 10.1002/ajmg.1320360105 [DOI] [PubMed] [Google Scholar]
- Mori S, Nagano M (1985) Electron-microscopic cytochemistry of alkaline-phosphatase activity in endothelium, pericytes and oligodendrocytes in the rat brain. Histochemistry 82:225–231. doi: 10.1007/BF00501399 [DOI] [PubMed] [Google Scholar]
- Moss DW (1982) Alkaline Phosphatase Isoenzymes. Clinical Chemistry 28:2007–2016 [PubMed] [Google Scholar]
- Muscari A, Collini A, Fabbri E, et al. (2014) Changes of liver enzymes and bilirubin during ischemic stroke: mechanisms and possible significance. BMC Neurology 14:. doi: 10.1186/1471-2377-14-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik RB, Gosling P, Price CP (1977) Comparative study of alkaline phosphatase isoenzymes, bone histology, and skeletal radiography in dialysis bone disease. British Medical Journal 1:1307–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba S, Yamaoka-Tojo M, Kakizaki R, et al. (2017) Effects on bone metabolism markers and arterial stiffness by switching to rivaroxaban from warfarin in patients with atrial fibrillation. Heart Vessels 32:977. doi: 10.1007/S00380-017-0950-2 [DOI] [PubMed] [Google Scholar]
- Narisawa S, Hasegawa H, Watanabe K, Millan JL (1994) Stage-specific expression of alkaline phosphatase during neural development in the mouse. Developmental Dynamics 201:227–235. doi: 10.1002/aja.1002010306 [DOI] [PubMed] [Google Scholar]
- Ndrepepa G, Xhepa E, Braun S, et al. (2017) Alkaline phosphatase and prognosis in patients with coronary artery disease. European Journal of Clinical Investigation 47:378–387. doi: 10.1111/eci.12752 [DOI] [PubMed] [Google Scholar]
- Nishihara Y, Hayashi Y, Fujii T, et al. (1994) The alkaline phosphatase in human plexus chorioideus. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 1209:274–278. doi: 10.1016/0167-4838(94)90196-1 [DOI] [PubMed] [Google Scholar]
- Nogai A, Siffrin V, Bonhagen K, et al. (2005) Lipopolysaccharide Injection Induces Relapses of Experimental Autoimmune Encephalomyelitis in Nontransgenic Mice via Bystander Activation of Autoreactive CD4+ Cells. The Journal of Immunology 175:959–966. doi: 10.4049/jimmunol.175.2.959 [DOI] [PubMed] [Google Scholar]
- Nowak LG, Rosay B, Czege D, Fonta C (2015) Tetramisole and Levamisole Suppress Neuronal Activity Independently from Their Inhibitory Action on Tissue Non-specific Alkaline Phosphatase in Mouse Cortex In: Fonta C, Negyessy L (eds) Neuronal Tissue-Nonspecific Alkaline Phosphatase (TNAP). Springer; Netherlands, Dordrecht, pp 239–281 [DOI] [PubMed] [Google Scholar]
- Okesina AB, Donaldson D, Lascelles PT, Morris P (1995) Effect of gestational age on levels of serum alkaline phosphatase isoenzymes in healthy pregnant women. International Journal of Gynecology & Obstetrics 48:25–29. doi: 10.1016/0020-7292(94)02248-8 [DOI] [PubMed] [Google Scholar]
- Paiva J, Damjanov I, Lange PH, Harris H (1983) Immunohistochemical Localization of Placental-like Alkaline Phosphatase in Testis and Germ-Cell Tumors Using Monoclonal Antibodies. Am J Pathol 111:156–165 [PMC free article] [PubMed] [Google Scholar]
- Paritpokee N, Tangkijvanich P, Teerasaksilp S, et al. (1999) Fast liver alkaline phosphatase isoenzyme in diagnosis of malignant biliary obstruction. J Med Assoc Thai 82:1241–1246 [PubMed] [Google Scholar]
- Park J-B, Kang D, Yang H-M, et al. (2013) Serum alkaline phosphatase is a predictor of mortality, myocardial infarction, or stent thrombosis after implantation of coronary drug-eluting stent. European Heart Journal 34:920–931. doi: 10.1093/eurheartj/ehs419 [DOI] [PubMed] [Google Scholar]
- Peters E, Ergin B, Kandil A, et al. (2016a) Effects of a human recombinant alkaline phosphatase on renal hemodynamics, oxygenation and inflammation in two models of acute kidney injury. Toxicology and Applied Pharmacology 313:88–96. doi: 10.1016/j.taap.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Peters E, Geraci S, Heemskerk S, et al. (2015) Alkaline phosphatase protects against renal inflammation through dephosphorylation of lipopolysaccharide and adenosine triphosphate. British Journal of Pharmacology 172:4932–4945. doi: 10.1111/bph.13261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Heuberger JAAC, Tiessen R, et al. (2016b) Pharmacokinetic Modeling and Dose Selection in a Randomized, Double-Blind, Placebo-Controlled Trial of a Human Recombinant Alkaline Phosphatase in Healthy Volunteers. Clinical Pharmacokinetics 55:1227–1237. doi: 10.1007/s40262-016-0399-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Masereeuw R, Pickkers P (2014) The Potential of Alkaline Phosphatase as a Treatment for Sepsis-Associated Acute Kidney Injury. Nephron Clinical Practice 127:144–148. doi: 10.1159/000363256 [DOI] [PubMed] [Google Scholar]
- Peters E, Schirris T, van Asbeck AH, et al. (2017) Effects of a human recombinant alkaline phosphatase during impaired mitochondrial function in human renal proximal tubule epithelial cells. European Journal of Pharmacology 796:149–157. doi: 10.1016/j.ejphar.2016.12.034 [DOI] [PubMed] [Google Scholar]
- Pickkers P, Heemskerk S, Schouten J, et al. (2012) Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Critical Care 16:R14. doi: 10.1186/cc11159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickkers P, Snellen F, Rogiers P, et al. (2009) Clinical pharmacology of exogenously administered alkaline phosphatase. European Journal of Clinical Pharmacology 65:393–402. doi: 10.1007/s00228-008-0591-6 [DOI] [PubMed] [Google Scholar]
- Pike AF, Kramer Nl, Blaauboer BJ, et al. (2015) An alkaline phosphatase transport mechanism in the pathogenesis of Alzheimer’s disease and neurodegeneration. Chemico-Biological Interactions 226:30–39. doi: 10.1016/j.cbi.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Pineda S, Bang OY, Saver JL, et al. (2008) Association of Serum Bilirubin with Ischemic Stroke Outcomes. Journal of stroke and cerebrovascular diseases 17:147–152. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelstra K, Bakker WW, Klok PA, et al. (1997a) A physiologic function for alkaline phosphatase: Endotoxin detoxification. Laboratory Investigation 76:319–327 [PubMed] [Google Scholar]
- Poelstra K, Bakker WW, Klok PA, Hardonk MJ (1997b) Dephosphorylation of Endotoxin by Alkaline Phosphatase in Vivo. Am J Pathol 151:1163–1169 [PMC free article] [PubMed] [Google Scholar]
- Posen S, Neale FC, Birkett DJ, Brudenell-Woods J (1967) Intestinal Alkaline Phosphatase in Human Serum. American Journal of Clinical Pathology 48:81–86. doi: 10.1093/ajcp/48.1.81 [DOI] [PubMed] [Google Scholar]
- Pratibha S, Praveen-Kumar S, Agadi JB (2014) Increased serum alkaline phosphatase and serum phosphate as predictors of mortality after stroke. Journal of Clinical Diagnostic Research 8:CC01–CC03. doi: 10.7860/jcdr/2014/8350.4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razazizan N, Mirmoeini M, Daeichin S, Ghadiri K (2013) Comparison of 25-Hydroxy Vitamin D, Calcium and Alkaline Phosphatase Levels in Epileptic and Non-Epileptic Children. Acta Neurol Taiwan 22:112–116 [PubMed] [Google Scholar]
- Rentea RM, Liedel JL, Fredrich K, et al. (2013) Enteral intestinal alkaline phosphatase administration in newborns decreases iNOS expression in a neonatal necrotizing enterocolitis rat model. Journal of Pediatric Surgery 48:124–128. doi: 10.1016/j.jpedsurg.2012.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentea RM, Liedel JL, Welak SR, et al. (2012) Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. Journal of Pediatric Surgery 47:1135–1142. doi: 10.1016/j.jpedsurg.2012.03.018 [DOI] [PubMed] [Google Scholar]
- Report of the Quality Standards Subcommittee of the American Academy of Neurology (1996) Practice Advisory: Thrombolytic therapy for acute ischemic Stroke-Summary Statement. Neurology 47:835–839. doi: 10.1212/WNL.47.3.835 [DOI] [PubMed] [Google Scholar]
- Robison R (1923) The possible significance of hexosephosphoric esters in ossification. Biochemical Journal 17:286–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin A, Duncan A, Quartero HWP, et al. (1989) Serum Concentrations of Alkaline Phosphatase Isoenzymes and Osteocalcin in Normal Pregnancy*. The Journal of Clinical Endocrinology & Metabolism 68:1123–1127. doi: 10.1210/jcem-68-6-1123 [DOI] [PubMed] [Google Scholar]
- Romanelli F, Corbo A, Salehi M, et al. (2017) Overexpression of tissue-nonspecific alkaline phosphatase (TNAP) in endothelial cells accelerates coronary artery disease in a mouse model of familial hypercholesterolemia. PLOS ONE 12:e0186426. doi: 10.1371/journal.pone.0186426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu W-S, Lee S-H, Kim CK, et al. (2014) High serum alkaline phosphatase in relation to cerebral small vessel disease. Atherosclerosis 232:313–318. doi: 10.1016/j.atherosclerosis.2013.11.047 [DOI] [PubMed] [Google Scholar]
- Ryu W-S, Lee S-H, Kim CK, et al. (2010) Increased serum alkaline phosphatase as a predictor of long-term mortality after stroke. Neurology 75:1995–2002. doi: 10.1212/WNL.0b013e3181ff966a [DOI] [PubMed] [Google Scholar]
- Sadighi A, Roshan MM, Moradi A, Ostadrahimi A (2008) The effects of zinc supplementation on serum zinc, alkaline phosphatase activity and fracture healing of bones. Saudi Medical Journal 29:1276–1279 [PubMed] [Google Scholar]
- Schiff A, Yang J, Winner LH, Schwartz MC (2016) Bone-Specific Alkaline Phosphatase in Patients Who Have Undergone the Fontan Operation. Pediatric Cardiology 37:1370–1376. doi: 10.1007/s00246-016-1443-2 [DOI] [PubMed] [Google Scholar]
- Schlaeger R (1975) The mechanism of the increase in the activity of liver alkaline phosphatase in experimental cholestasis: measurement of an increased enzyme concentration by immunochemical titration. Z Klin Chem Klin Biochem 13:277–281 [DOI] [PubMed] [Google Scholar]
- Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. (2005) Reduced Number of Circulating Endothelial Progenitor Cells Predicts Future Cardiovascular Events. Circulation 111:2981–2987. doi: 10.1161/circulationaha.104.504340 [DOI] [PubMed] [Google Scholar]
- Sebastian-Serrano A, Engel T, de Diego-Garcia L, et al. (2016) Neurodevelopmental alterations and seizures developed by mouse model of infantile hypophosphatasia are associated with purinergic signaling deregulation. Human Molecular Genetics 25:4143–4156. doi: 10.1093/hmg/ddw248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen CR, Kuss P, Narisawa S, et al. (2015) Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. Journal of Bone and Mineral Research 30:824–836. doi: 10.1002/jbmr.2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N (1950) Histochemical studies on the phosphatase of the nervous system. The Journal of Comparative Neurology 93:201–217. doi: 10.1002/cne.900930203 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Imano H, Ohira T, et al. (2013a) Alkaline Phosphatase and Risk of Stroke Among Japanese: The Circulatory Risk in Communities Study (CIRCS). Journal of stroke and cerebrovascular diseases 22:1046–1055. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nakazato M, Sekita T, et al. (2013b) Association between alkaline phosphatase and hypertension in a rural Japanese population: The Nagasaki Islands study. Journal of Physiological Anthropology 32:10. doi: 10.1186/1880-6805-32-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi A, Katagi M, Okuno Y, et al. (2002) Induction of Bone-Type Alkaline Phosphatase in Human Vascular Smooth Muscle Cells. Circulation Research 91:9–16. doi: 10.1161/01.res.0000026421.61398.f2 [DOI] [PubMed] [Google Scholar]
- Sigrist MK, McIntyre CW (2008) Vascular calcification is associated with impaired microcirculatory function in chronic haemodialysis patients. Nephron Clinical Practice 108:121–6. doi: 10.1159/000114202 [DOI] [PubMed] [Google Scholar]
- Sonderer J, Katan Kahles M (2015) Aetiological blood biomarkers of ischaemic stroke. Swiss Medical Weekly 145:w14138. doi: 10.4414/smw.2015.14138 [DOI] [PubMed] [Google Scholar]
- Su F, Brands R, Wang Z, et al. (2006) Beneficial effects of alkaline phosphatase in septic shock. Critical Care Medicine 34:2182–2187. doi: 10.1097/01.CCM.0000229887.70579.29 [DOI] [PubMed] [Google Scholar]
- Sukumaran M, Bloom WL (1953) Influence of Diet on Serum Alkaline Phosphatase in Rats and Men. Experimental Biology and Medicine 84:631–634. doi: 10.3181/00379727-84-20735 [DOI] [PubMed] [Google Scholar]
- Taal B, Tinteren HV, Zoetmulder F (2001) Adjuvant 5FU plus levamisole in colonic or rectal cancer:improved survival in stage II and III. British Journal of Cancer 85:1437–1443. doi: 10.1054/bjoc.2001.2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Matsuyama T, Moriwaki H, et al. (2004) Circulating CD34-Positive Cells Provide an Index of Cerebrovascular Function. Circulation 109:2972–2975. doi: 10.1161/01.cir.0000133311.25587.de [DOI] [PubMed] [Google Scholar]
- Tan G, Hao Z, Lei C, et al. (2016) Subclinical change of liver function could also provide a clue on prognosis for patients with spontaneous intracerebral hemorrhage. Neurological Sciences 37:1693–1700. doi: 10.1007/si0072-016-2656-0 [DOI] [PubMed] [Google Scholar]
- Tan L-M, Wang L, Chen J-J, et al. (2017) Diagnostic performance of bone metabolic indexes for the detection of stroke. Saudi Medical Journal 37:30–35. doi: 10.15537/smj.2017.1.15813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WK, Liang H, Chu WCW, et al. (2013) Association between high serum total bilirubin and post-stroke depression. Psychiatry Clinical Neurosciences 67:259–64. doi: 10.1111/pcn.12051 [DOI] [PubMed] [Google Scholar]
- Teitelbaum JE, Laskowski A, Barrows FP (2011) Benign transient hyperphosphatasemia in infants and children: a prospective cohort. J Pediatr Endocrinol Metab 24:351–353 [PubMed] [Google Scholar]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue Plasminogen Activator for Acute Ischemic Stroke. The New England Journal of Medicine 333:1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- Tonelli M, Curhan G, Pfeffer M, et al. (2009) Relation Between Alkaline Phosphatase, Serum Phosphate, and All-Cause or Cardiovascular Mortality. Circulation 120:1784–92. doi: 10.1161/circulationaha.109.851873 [DOI] [PubMed] [Google Scholar]
- Tremlett H, Seemuller S, Zhao Y, et al. (2006) Liver test abnormalities in multiple sclerosis: Findings from placebo-treated patients. Neurology 67:1291–1293. doi: 10.1212/01.wnl.0000238515.27055.62 [DOI] [PubMed] [Google Scholar]
- Tuin A, Poelstra K, de Jager-Krikken A, et al. (2009) Role of alkaline phosphatase in colitis in man and rats. Gut 58:379–87. doi: 10.1136/gut.2007.128868 [DOI] [PubMed] [Google Scholar]
- Uehara T, Ohara T, Minematsu K, et al. (2018) Predictors of Stroke Events in Patients with Transient Ischemic Attack Attributable to Intracranial Stenotic Lesions. Internal Medicine 57:295–300. doi: 10.2169/internalmedicine.9447-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof V, De Broe M (1994) Interpretation and clinical significance of alkaline phosphatase isoenzyme patterns. Crit Rev Clin Lab Sci 31:197–293. doi: 10.3109/10408369409084677 [DOI] [PubMed] [Google Scholar]
- van Veen SQ, van Vliet AK, Wulferink M, et al. (2005) Bovine Intestinal Alkaline Phosphatase Attenuates the Inflammatory Response in Secondary Peritonitis in Mice. Infection and Immunity 73:4309–4314. doi: 10.1128/IAI.73.7.4309-4314.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy ER, Kellett KA, Cocklin SL, Hooper NM (2012) Alkaline phosphatase is increased in both brain and plasma in Alzheimer’s disease. Neurodegenerative Diseases 9:31–37. doi: 10.1159/000329722 [DOI] [PubMed] [Google Scholar]
- Vernino S, Brown RD, Sejvar J J, et al. (2003) Cause-Specific Mortality After First Cerebral Infarction: A Population-Based Study. Stroke 34:1828–1832. doi: 10.1161/01.STR.0000080534.98416.A0 [DOI] [PubMed] [Google Scholar]
- Verweij WR, Bentala H, Huizinga-van der Vlag A, et al. (2004) Protection against an Escherichia coli-induced sepsis by alkaline phosphatase in mice. Shock 22:174–9 [DOI] [PubMed] [Google Scholar]
- Wanjian G, Jie H, Liang G, et al. (2017) Establishment of Reference Interval for Alkaline Phosphatase in Healthy Children of Various Ethnicities, Aged 0–12 Years. Laboratory Medicine 48:166–171. doi: 10.1093/labmed/lmx017 [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Sattar N, Papcosta O, et al. (2013) Alkaline Phosphatase, Serum Phosphate, and Incident Cardiovascular Disease and Total Mortality in Older Men. Arteriosclerosis, Thrombosis, and Vascular Biology 33:1070–6. doi: 10.1161/atvbaha.112.300826 [DOI] [PubMed] [Google Scholar]
- Waymire KG, Mahuren JD, Jaje JM, et al. (1995) Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nature Genetics 11:45–51 [DOI] [PubMed] [Google Scholar]
- Webber M, Krishnan A, Thomas NG, Cheung BM (2010) Association between serum alkaline phosphatase and C-reactive protein in the United States National Health and Nutrition Examination Survey 2005-2006. Clinical Chemistry and Laboratory Medicine 48:167–73. doi: 10.1515/cclm.2010.052 [DOI] [PubMed] [Google Scholar]
- Weiss MJ, Henthorn PS, Lafferty MA, et al. (1986) Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proceedings of the National Academy of Sciences 83:7182–7186. doi: 10.1073/pnas.83.19.7182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse JS, Riggle KM, Purpi DP, et al. (2010) The protective role of intestinal alkaline phosphatase in necrotizing enterocolitis. Journal of Surgical Research 163:79–85. doi: 10.1016/j.jss.2010.04.048 [DOI] [PubMed] [Google Scholar]
- Whyte MP, Landt IM, Ryan LM, et al. (1995) Alkaline Phosphatase: Placental and Tissue-nonspecific Isoenzymes Hydrolyze Phosphoethanolamine, Inorganic Pyrophosphate, and Pyridoxal 5’-Phosphate. J Clin Invest 95:1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte MP, Wenkert D, McAlister WH, et al. (2009) Chronic Recurrent Multifocal Osteomyelitis Mimicked in Childhood Hypophosphatasia. Journal of Bone and Mineral Research 24:1493–1505. doi: 10.1359/jbmr.090308 [DOI] [PubMed] [Google Scholar]
- Wolf P (1978) Clinical significance of an increased or decreased serum alkaline phosphatase level. Arch Pathol Lab Med 102:497–501 [PubMed] [Google Scholar]
- Yamashita M, Sasaki M, Mii K, et al. (1989) Measurement of serum alkaline phosphatase isozyme I in braindamaged patients. Neurologia Medico-Chirurgica (Tokyo) 29:995–8 [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA (2011) Blood-Brain Barrier Breakdown in Acute and Chronic Cerebrovascular Disease. Stroke 42:3323–3328. doi: 10.1161/strokeaha.110.608257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M, Takatsuki K, Yamauchi K, et al. (1988) Effect of Parathyroid Function on Serum Bone Gla Protein. Endocrinologia Japonica 35:39–45. doi: 10.1507/endocrj1954.35.39 [DOI] [PubMed] [Google Scholar]
- Zhong C, You S, Chen J, et al. (2018) Serum Alkaline Phosphatase, Phosphate, and In-Hospital Mortality in Acute Ischemic Stroke Patients. Journal of Stroke and Cerebrovascular Diseases 27:257–266. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.041 [DOI] [PubMed] [Google Scholar]
- Zhu XY, Gu XJ, Chang JB, et al. (2004) [Changes of blood-cerebrospinal fluid barrier in rabbits with diabetic ketoacidosis], Chinese Critical Care Medicine 16:175–8 [PubMed] [Google Scholar]
- Ziegler SG, Ferreira CR, MacFarlane EG, et al. (2017) Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Science Translational Medicine 9:eaal1669. doi: 10.1126/scitranslmed.aal1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierk J, Arzideh F, Haeckel R, et al. (2017) Pediatric reference intervals for alkaline phosphatase. Clinical Chemistry and Laboratory Medicine (CCLM) 55:102–110. doi: 10.1515/cclm-2016-0318 [DOI] [PubMed] [Google Scholar]
- Zong L, Wang X, Li Z, et al. (2018) Alkaline Phosphatase and Outcomes in Patients With Preserved Renal Function: Results From China National Stroke Registry. Stroke 49:1176–1182. doi: 10.1161/STROKEAHA.118.020237 [DOI] [PubMed] [Google Scholar]