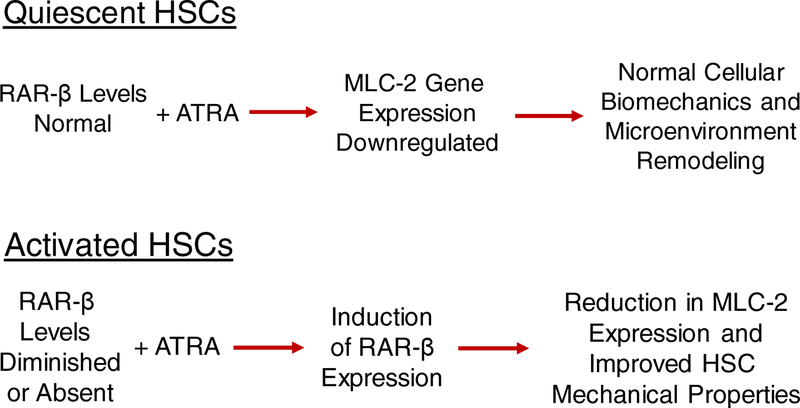
The role of hepatic stellate cells (HSCs) in the development of fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) has long been the focus of considerable research interest. There also has been longstanding interest in understanding the molecular processes underlying how HSCs store retinoids (vitamin A and its natural and synthetic analogs) and how the availability of these transcriptional regulators may influence HSC biology. The very elegant studies of Cortes et al. reported in this issue of Hepatology provide new insight into the actions of all-trans-retinoic acid (ATRA), mediated specifically by one of its nuclear hormone receptors, retinoic acid receptor-β (RAR-β), to promote the downregulation of myosin light chain 2 (MLC-2) expression and HSC deactivation (1). The new findings of Cortes et al. are summarized in Figure 1 and considered in more detail below. Collectively, these new data suggest that RAR-β may be a useful target for new therapies aimed at HSCs and suppression of the desmoplastic reaction associated with liver disease.
Figure 1.
Pictorial summation of the actions of all-trans-retinoic acid (ATRA) and retinoic acid receptor-β (RAR-β) in quiescent (Upper) and activated hepatic stellate cells (HSCs) (Lower) as related to reversing or preventing hepatic disease.
Retinoids, especially the ATRA metabolite, are very potent transcriptional regulators that help control expression of more than 500 diverse genes (2). A role for retinoids in preventing or blocking hepatic disease development has long been recognized. Some highlights of this published research need to be briefly mentioned to facilitate understanding of these new findings. Work from Yanagitani et al. (3), published move than a decade ago, convincingly established that normal RAR signaling is required for maintaining a healthy liver. When these authors expressed a transgene encoding a dominant/negative RAR mutant form that inhibits the activities of all three RARs (RAR-α, -β, and –γ), specifically in hepatocytes, these transgenic mice spontaneously developed steatosis by 4 months-of-age.
Progressively worsening liver disease was observed so that by 12 months-of-age half of the transgenic mice had developed liver tumors (3). Studies by Mezaki et al. (4) showed that RAR-α protein is localized within the cytoplasm as an insoluble protein in HSCs activated in vitro. However, these authors did not establish whether this extra-nuclear localization was a cause or an effect of HSC activation. Mukhopadhyay et al. showed that expression of cannabinoid receptor-1 (CB1R) in the liver is regulated by RAR-γ and proposed that the actions of this retinoid nuclear receptor, acting through its effects on CB1R expression, contributes to fatty liver development (5). More recently, published work from Trasino et al. demonstrated that treatment of cultured HSCs with a highly selective RAR-β agonist reduced their activation, as evidenced by decreased HSC expression of α-smooth muscle actin (6). Livers of mice fed a high fat diet to induce steatosis and treated with the same synthetic RAR-β agonist exhibited reduced steatosis, oxidative stress, and expression of pro-inflammatory markers in the liver (6).
Through immunohistochemical study of liver tissue obtained from 22 patients with cirrhosis and 10 matched healthy individuals, Cortes et al. were able to show that RAR-β protein expression levels are markedly reduced in cirrhotic liver compared to tissue from health individuals. In silico analysis of gene expression data from 372 HCC patients and 50 controls also demonstrated that RAR-β expression in HCC patients is significantly lower than in normal subjects. The loss of RAR-β protein expression in tissue sections was associated with upregulation of MLC-2 protein levels, as well as an increase in phosphorylated-MLC-2 (pMLC-2), the active form of MLC-2. In vitro studies of activated primary human HSCs established that ATRA treatment of HSCs increases RAR-β mRNA and protein expression in these cells. Furthermore, RAR-β exhibited very high nuclear localization in ATRA treated HSCs compared to vehicle treated HSCs. It has been known for many years that the gene encoding RAR-β possesses a retinoic acid response element and consequently RAR-β expression is inducible upon ATRA treatment (2,7). ATRA treatment also significantly decreased MLC-2 levels in HSCs, but levels comparable to controls were observed following ATRA treatment in the presence of siRNA against RAR-β. Combining ATRA treatment with a synthetic RAR-β antagonist also prevented the effect of ATRA on RAR-β and MC-2 levels, suggesting that ATRA downregulates MLC-2 levels through RAR-β mediated transactivation. Collectively, the authors’ data establish that MLC-2 and pMLC-2 are upregulated in cirrhotic and HCC tissue and that in vivo ATRA negatively regulates MLC-2 via RAR-β.
Cortes et al. further investigated the effects of ATRA treatment on HSC mechanical properties. ATRA treatment of HSCs was found to abrogate mechanically driven migration towards stiffer substrates and to generate significantly reduced traction forces compared to control HSCs. But these beneficial effects were not observed when ATRA was administered combined with an RAR-β antagonist. Moreover, durotaxis and cell migration by HSCs was found to be sensitive to ATRA and RAR-β signaling. The authors conclude that RAR-β, acting to regulate MLC-2 expression, is a transcriptional regulator of cellular biomechanics and microenvironmental remodeling in HSCs and that the effects of retinoid signaling on a variety of cell behaviors may regulate hepatic disease and cancer development through mechanical, positive feedback loops.
Given the role of HSCs in propagating the development of fibrosis in both the early and later stages of liver disease and the urgent need to find new therapies that target this action of activated HSCs, the investigations by Cortes et al. point to a new approach for targeting mechanotransduction in HSCs by regulating MLC-2 transcription and reprogramming mechanically HSCs through RAR-β. This hypothesis merits further investigation with the aim of targeting RAR-β signaling in HSCs to block liver disease development and progression. It should be noted that that in earlier published work, this same group of investigators reported similar findings that point to a role for ATRA and RAR-β in blocking pancreatic stellate cell (PSC) activation (8). This earlier work established that ATRA restores mechanical quiescence to PSCs through RAR-β dependent downregulation of MLC-2 expression. Thus, not only is RAR-β an attractive target for undertaking therapeutic interventions aimed at hepatic disease it is also an attractive target for interventions aimed at blocking pancreatic disease.
Although the literature is now unequivocal that ATRA acting through its nuclear receptors is integrally involved in the maintenance of a healthy liver, it remains to be definitively established if and how this protective effect is related to the retinoid storage capacity of HSCs. Greater than half of all retinoid present within the body of a healthy well-nourished individual is stored within HSCs (9). Thus, it is tempting to speculate that these stores, ie ones that are rapidly lost in the early stages of HSC activation, are present solely to protect against HSC activation. However, this notion is likely incorrect for at least two reasons. First, HSC levels of retinyl ester and retinol, which are precursors for ATRA synthesis, are present at concentrations that are four orders of magnitude greater than those of ATRA. Secondly, the levels of HSC retinoid stores are modulated directly by dietary retinoid intake, being released into the circulation in times of dietary insufficiency and replenished in times of dietary sufficiency. Consequently, it seems unlikely that these HSC stores are present solely to prevent activation of the cell upon insult to the liver. Rather, it is more plausible that HSC stores are needed additionally to buffer against retinoid insufficiency in all tissues.
In summary, the new findings of Cortes et al. reported in this issue of Hepatology extend molecular understanding of the actions of ATRA and RAR-β in HSC biology, establishing that their actions to downregulate MLC-2 gene expression directly affects the mechanical properties of the cells. Importantly, the new data being reported also point to RAR-β as a potential target for therapeutic interventions aimed at blocking or reversing hepatic fibrosis, cirrhosis and HCC.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
REFERENCES
- 1.Cortes E, Lachowski D, Rice A, Chronopoulos A, Robinson B, Thorpe S, et al. RAR-β is downregulated in HCC & cirrhosis and its expression inhibits myosin-driven activation and durotaxis in hepatic stellate cells. Hepatology 2018. July 28 Doi: 10.1002/hep. 30193 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 2002;43:1773–808. [DOI] [PubMed] [Google Scholar]
- 3.Yanagitani A, Yamada S, Yasui S, Shimomura T, Murai R, Murawaki Y, et al. Retinoic acid receptor α dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology 2004;40:366–375. [DOI] [PubMed] [Google Scholar]
- 4.Mezaki Y, Yamaguchi N, Yoshikawa K, Miura M, Imai K, Itoh H, Senoo H. Insoluble, speckled cytosolic distribution of retinoic acid receptor alpha protein as a marker for hepatic stellate cell activation in vitro. J Histochem Cytochem 2009;57:687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay B, Liu J, Osei-Hyiaman D, Godlewski G, Makhopadhyay P, Wang L, et al. Tanscriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting by retinoic acid receptor-γ. J Biol Chem 2010;285:19002–19011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trasino SE, Tang X-H, Jessurun J, Gudas LJ. A retinoic acid receptor β2 agonist reduces hepatic stellate cell activation in nonalcoholic fatty liver disease. J Mol Med.2016;94:1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudas JM, Sporn MB, Roberts AB. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. New York: Raven Press; 1994. p. 443–520. [Google Scholar]
- 8.Chronopoulos A, Robins B, Sarper M, Cortes E, Auernheimer V, Lachowski D, et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodeling and inhibit cancer cell invasion. Nature Commun 2016;7:12630 Doi: 10.1038/ncomms12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DD, Jiang H, et al. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochem Biophys Acta 2009;1791:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]