Abstract
Stress can impair T cell-mediated immunity. To determine if infants with high stress responses had deficits in T-cell mediated immunity, we examined the association of pain-induced cortisol responsiveness with thymic function and vaccine responses in infants. This study was performed among 306 (male=153 and female=153) participants of a randomized, controlled trial examining the effect of neonatal vitamin A supplementation on immune function in Bangladesh (NCT01583972). Salivary cortisol was measured before and 20 min after a needle stick (vaccination) at 6 w of age. The thymic index (TI) was determined by ultrasonography at 1, 6, 10 and 15 w. T-cell receptor excision circle (TREC) and blood T-cell concentrations were measured at 6 and 15 w. Responses to Bacillus Calmette–Guérin (BCG), tetanus toxoid, hepatitis B virus and oral poliovirus vaccination were assayed at 6 and 15 w. Cortisol responsiveness was negatively associated with TI at all ages (p<0.01) in boys only, was negatively associated with naïve helper T-cell concentrations in both sexes at both 6 (p=0.0035) and 15 w (p=0.0083), and was negatively associated with the delayed-type hypersensitivity (DTH) skin test response to BCG vaccination at 15 w (p=0.034) in both sexes. Infants with a higher cortisol response to pain have differences in the T-cell compartment and a lower DTH response to vaccination. Sex differences in the immune system were seen as early as 6 w of age in these healthy infants.
Keywords: Stress, cortisol, thymus, T-cell, vaccine, vitamin A
Lay summary:
T-cells develop in the thymus and are an important component of immunologic memory. This study reports that in infants below 4 m of age a high cortisol stress response to pain is associated with a smaller thymus size in boys, and with lower levels of naïve T-cells and a poorer skin test response to immunization in both boys and girls. These findings suggest that infants with a higher cortisol response to stress have impaired thymic function and immunologic memory, compared to infants with a lower response.
Introduction
Cortisol, a glucocorticoid hormone, affects the health and functioning of multiple organ systems, including the immune system, and is one of the mediators of stress-induced immune-suppression (Webster Marketon and Glaser, 2008). Such immune-suppression affects both the innate and adaptive components of the immune system. The thymus, a key component of adaptive immunity, is particularly affected by cortisol, which causes apoptotic death of thymocytes (Cohen, 1992; Nieto, Gonzalez, Gambon, Diaz-Espada, & Lopez-Rivas, 1992). This apoptosis presumably accounts for the negative association seen between elevated cortisol and lower thymic output of naïve T-cells (measured by analysis of T-cell receptor excision circles [TREC] in blood) in adults (Benjamin et al., 2016) and between elevated cortisol and smaller thymus size in very-low birthweight infants (de Felice et al., 2008) suggesting a constant, negative effect of elevated cortisol on thymic function across the lifespan. Unlike thymocytes, peripheral-blood T-cells are resistant to cortisol-induced apoptosis (Nieto, et al., 1992) but cortisol infusion transiently decreases concentrations of both naïve and memory T-cells by causing redistribution to tissue sites such as lymph nodes (Dimitrov et al., 2009).
The thymus is essential for adaptive immunity because it is the source of naïve T-cells (Majumdar and Nandi, 2018). Decreased thymic function in early infancy, when output of naïve T-cells is at its peak (den Braber et al., 2012), can decrease the ability of the immune system to recognize novel antigens later in life due a decrease in diversity of the T-cell repertoire. This decreased diversity presumably accounts for the lower vaccine response seen in young adults who underwent thymectomies at a median age of 11 m (Prelog et al., 2008). These young adults also had fewer naïve T-cells and the deficit in vaccine responsive was greatest in those whose thymectomies occurred under 6 m of age. Genetic defects in thymic function (e.g., DiGeorge syndrome) are also associated with smaller thymus size, decreased thymic output and increased risk of death from infections, presumably due to impaired adaptive immunity (Sauce and Appay, 2011). Studies of euthymic new-borns in Guinea Bissau at high risk of malaria and other infections has shown that a smaller thymus at birth is associated with an increased risk of death from infectious diseases during the first 2 y of life (Aaby et al., 2002). In a separate study in the same setting a 2-fold larger thymus measured at 6 m of age was associated with a 70% lower risk of death over the next 30 m (Garly et al., 2008). Similarly, a smaller thymus during infancy was associated with an increased risk of death from infectious diseases through 5 y of age in Bangladesh, where the overall risk of death was lower than in Guinea Bissau (Moore et al., 2014). Thus variation in thymus size early in infancy, driven by environmental factors that may include stress, has adverse effects on immune function and overall health later in life, as has been recently reviewed (Moore, Collinson, Tamba N’Gom, Aspinall, & Prentice, 2006).
In this study, we tested the hypothesis that infants with higher stress responses would have impaired T-cell-mediated immunity, based on the premise outlined above. Specifically, we determined if higher pain-induced (via a needle-stick for vaccination) cortisol responsiveness was associated with thymus size, peripheral blood naïve and memory T lymphocyte and TREC concentrations, as well as with responses to T-cell-dependent vaccines, in Bangladeshi infants between birth and 15 w of age. The study was conducted with infants recruited into a randomized, controlled trial examining the effect of vitamin A supplementation to new-born infants on vaccine responsiveness and thymic function in Dhaka, Bangladesh.
Materials and methods
Study design:
This study was an observational study nested within a block-randomized double-blind placebo-controlled clinical trial [ClinicalTrials.gov identifier: NCT01583972], conducted on healthy infants in Bangladesh, as outlined in the Figure S1. The study was approved by the Research Review Committee (RRC) and the Ethical Review Committee (ERC) of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). Written consents were obtained from the parents or legal guardian of the study participants. The detail of the inclusion-exclusion criteria, study design, laboratory methodology, and baseline characteristics of the study participant have been described earlier (Ahmad, Raqib, Qadri, & Stephensen, 2014).
Study subjects and sample collection:
The study subjects were enrolled at the Maternal and Child Health Training Institute (MCHTI). The study was conducted at International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) and MCHTI in collaboration with the USDA Western Human Nutrition Research Center located at the University of California, Davis. The detail of the parent study design and baseline data have been described earlier (Ahmad, et al., 2014). In brief, a total of 306 infants were enrolled in the parent study (male=153) for 50,000 IU vitamin A supplementation within 48h of birth. All the infants received standard vaccines according to Bangladesh Ministry of Health’s guidelines. Vaccine administration and sample collection are described in Figure S1. Collected saliva and blood samples were stored in an ice chest with a gel pack and then transported to the Immunobiology, Nutrition and Toxicology (INT) Laboratory at icddr,b within 3–4 h of collection for analysing, processing, and storing at −80°C. We could not collect saliva for cortisol measurement from all 306 infants because of a lack of availability of the saliva collection kits during two periods of the study, each about one month in duration, during the months June, July, and August of enrolment period January 2012 to May 2013. A total of 229 pre-vaccine cortisol and 231 post vaccine cortisol samples were collected. Thus, in this paper, we are presenting data from 229 infants (male=107). Two study participants withdrew from the study at 10 w and, thus, no follow-up data were available from those two after 10 w. All anthropometric and laboratory data were securely stored on using the RedCap database (Harris et al., 2009) provided by the UC Davis Clinical and Translational Sciences Center.
Salivary cortisol analysis:
Infant saliva was collected by a Salimetrics infant’s saliva collection kit (Salimetrics, Carlsbad, CA, U.S.A.) before and 20 min after a single intramuscular immunization with pentavalent vaccine at 6 w of age to determine cortisol responsiveness. Pre-vaccine saliva was collected between 9:39 AM and 1:34 PM with a median of 11:34 AM. During saliva collection, the infant’s behavior (asleep, awake, awake and crying), saliva collection time, body temperature, anthropometry, breastfeeding status, and other health-related information were recorded. After completion of the enrolment, the salivary concentration was determined by enzyme immunoassay (EIA) kit (Salimetrics) as per the manufacturer’s instruction. Appropriate low and high-quality standards were measured with every EIA plate assay. The intra-assay coefficient of variation (CV) was 4.25% and inter-assay CV was 4.30%. We successfully collected saliva and measured pre- and post-vaccine salivary cortisol from 229 infants’ (male=107). Venipuncture blood collection occurred during the same visit and the sequence of blood collection and vaccination varied due to scheduling issues beyond the control of the study. Pre-saliva collection occurred before blood collection in 66 infants. All other saliva collections occurred from 1–184 min after blood collection. Post-vaccine saliva collection occurred 23.4 ± 0.47 min after the vaccination. Based on the time between blood collection and pre-vaccine saliva collection, infants were divided into 4 time groups; G1 = obtained saliva collection before blood collection (n=66), G2 = obtained saliva collection 1–30 min after blood collection (n=67), G3 = obtained saliva collection 31–60 min after blood collection (n=80), G4 = more than 60 min after blood collection (n=16) to evaluate the effect of the venipuncture on salivary cortisol.
Thymus size:
Thymus size was measured by ultrasound at 1, 6, 10 and 15 w of age. Thymic index (TI) was obtained by multiplying the transverse diameter and the sagittal area of the largest lobe (Hasselbalch, Nielsen, Jeppesen, Pedersen, & Karkov, 1996).
Blood cells counts:
Naïve T cell counts in per µL of peripheral blood at 6 and 15 w were determined by commercially available Multitest four-color reagent kits (CD45RA-FITC/CD45RO-PE/CD3-PerCP/CD4-APC) with Trucount Tubes (BD Biosciences, San Jose, CA, U.S.A.) on flow cytometer FACSCalibur (BD Biosciences, San Jose, CA, U.S.A.). Flow data were analyzed by FlowJo software (FlowJo, LLC., OR, U.S.A.). The gating strategy for memory and naïve T-cells assay is shown in Figure S5. TREC copy number was per million PBMCs was determined by sybergreen based qPCR method (Raqib et al., 2007). Additionally, complete blood count was performed by hematology analyzer Sysmex XT1800i (Sysmex, IL, U.S.A.). Details of these methods have been described previously (Ahmad, et al., 2014).
Vaccine responses:
To determine vaccine specific memory T-cell (CD45RO+) response we cultured 1X106/mL isolated PBMC in the presence of 5.0 μg/mL of PPD of tuberculin (NIBSC, Potters Bar, UK), 7.5 μg/mL of rHBsAg subtype ADW (Fitzgerald, MA, U.S.A.), 5.0 Lf/mL of tetanus toxoid (NIBSC, Potters Bar, UK) and 10% of inactivated trivalent polio vaccine (IPOL, Sanofi Pasteur S.A., Lyon, France). The superantigen staphylococcus enterotoxin B (SEB; 0.25 μg/mL; Sigma Chemicals, MO, U.S.A.) and phosphate buffered saline (PBS) were used as positive and negative controls, respectively. On the 6th day of culture, the cells were harvested for staining with CD3, CD4, and CD45RO markers and analyzed by FACSAria-III (BD Biosciences, San Jose, CA, U.S.A.). Flow data were analyzed by FlowJo software (FlowJo, LLC., OR, U.S.A.). The gating strategy for the T-cell stimulation index assay is shown in Figure S6. The proliferation index was calculated by dividing the ratio of antigen-stimulated memory T-cells to total T-cells by the ratio of control memory T-cells to total T-cells. To determine active secretion of antibodies from PBMC in response to in vivo vaccination, we used ALS (antibodies from lymphocyte secretions) assay method. In brief, 1×107 isolated PBMC was cultured with standard Russ-10 and 10% heat-inactivated FBS for 48h. Commercially available kits were used to determine anti-TT IgG (Binding Site, CA, U.S.A.), anti-HBsAg IgG (Alpha Diagnostic, San Antonio, TX, U.S.A.), and anti-Polio IgG (IBL international, NC, U.S.A.) antibodies in culture media according to manufacturers’ instruction. Tuberculin skin tests response to BCG immunization was assessed at 15 w, by using the intradermal injection of PPD. Induration area was determined by the formula “0.8 X tuberculin skin tests long diameter (TSTLD) X tuberculin skin tests short diameter (TSTSD)”. Tuberculin skin tests long diameter (TSTLD) ≥10mm was considered as a positive response to categorize the PPD skin test response. Details of this method have been described earlier (Ahmad, et al., 2014).
Nutritional Status:
Breastfeeding was characterized by using World Health Organization criteria(WHO/UNICEF, 2008) with some modifications. “Breastfeeding + non–human milk” and “Breastfeeding + solid or semi-solid food” were combined to category “Other”, which provided us with 3 categories of breastfeeding status, exclusively breastfeed, predominant breastfeed and other. Other nutritional status was measured by widely used instruments following standard procedures as was described in our previous publication (Huda et al., 2014).
Gestational age:
Gestational age was calculated from ultrasound data or from the date of the first day of the mother’s last menstrual period as was described earlier (Ahmad, et al., 2014).
Statistical analysis:
Paired t-test was performed to determine the difference between pre- and post-vaccine cortisol. Student’s t-test was performed to compare between sex and supplemental groups. Two-way ANOVA was used to compare pre- and post-vaccine cortisol concentration among time groups based on the time between the blood draw and saliva collection. Associations between cortisol responsiveness and thymic index or blood cell counts were determined by multiple regression modelling. Pre-vaccine cortisol concentration was used as a confounding factor to control the effect of known (venipuncture blood collection) and unknown baseline stress on cortisol responsiveness. Additionally, the analysis we statistically controlled for the following possible confounding factors: infant’s behavior during saliva collection, salivary collection time, sex, mode of delivery, birthweight, length for age Z-score, weight for length Z-score, and breastfeeding status. A p-value of ≤0.05 was considered statistically significant for all analyses. Data are presented as means ± SD unless otherwise indicated. Statistical analysis was performed using R version 3.4.2. Graphs were produced by GraphPad Prism (La Jolla, CA, U.S.A.).
Results
Characteristics of the study population:
The age range of the mothers participating in this study was 18–37 y. Most of the participating mother had some elementary or secondary education (3–17 y) with <5% having no education. More than half of the infants were delivered by C-section (Table 1), 6.11% were born preterm (<37 w of gestation) and 27.04% were born with a low birth-weight (<2500 g). Most infants were exclusively breastfeeding at 6 w of age with the rate progressively decreasing with age (Table 1). The prevalence of wasting (weight-for-length below “−2” Z-scores) was between 4.80 and 7.42% during the study while the prevalence of stunting (length-for-age below “−2” z-scores) was between 17.5 and 11.9% (Table 1).
Table 1:
Characteristics of the participants
| Characteristics | n1 | Statistics2 |
|---|---|---|
| Mother age, y | 229 | 23.3 ± 4.07 |
| Gestational age, w | 227 | 39.3 ± 1.58 |
| Birth weight, kg | 229 | 2.73 ± 0.375 |
| Birth length, cm | 229 | 46.5 ± 2.17 |
| Sex, Boys | 229 | 46.7% |
| Type of delivery, C-section | 229 | 59.8% |
| WHZ-score | ||
| 6 w | 229 | −0.028 ± 1.16 |
| 10 w | 227 | −0.215 ± 1.290 |
| 15 w | 227 | −0.310 ± 1.175 |
| WHZ-score < −2 WHZ | ||
| 6 w | 229 | 4.80% |
| 10 w | 227 | 7.42% |
| 15 w | 227 | 7.42% |
| HAZ-score | ||
| 6 w | 229 | −1.07 ± 1.06 |
| 10 w | 227 | −0.834 ± 0.984 |
| 15 w | 227 | −0.853 ± 1.02 |
| HAZ-score < −2 HAZ | ||
| 6 w | 229 | 17.5% |
| 10 w | 227 | 10.0% |
| 15 w | 227 | 11.9% |
| Exclusive breastfeeding | ||
| at 1 w | 229 | 82.5% |
| up to 6 w | 229 | 59.4% |
| up to 10 w | 227 | 40.1% |
| up to 15 w | 227 | 28.2% |
Gestational age was not available from 2 subjects. 2 subjects withdraw consent at 10 w thus total subjects were 227 at 10 and 15 w of age.
Mean ± SD or frequency
Post-vaccine salivary cortisol was higher than pre-vaccine salivary cortisol:
The post-vaccine cortisol concentration increased significantly (t = −15.029, DF = 227, p <0.001) above the pre-vaccine concentration (Figure 1), as expected, and this increase was seen regardless of the timing of the vaccination relative to blood collection at the same visit (Figure S2 A). Group 2 had significantly higher pre-vaccine cortisol concentration than group 1 (p < 0.001) and group 4 (p = 0.0020). However, post-vaccine cortisol concentration did not differ by timing groups (Figure S2 B).
Figure 1:
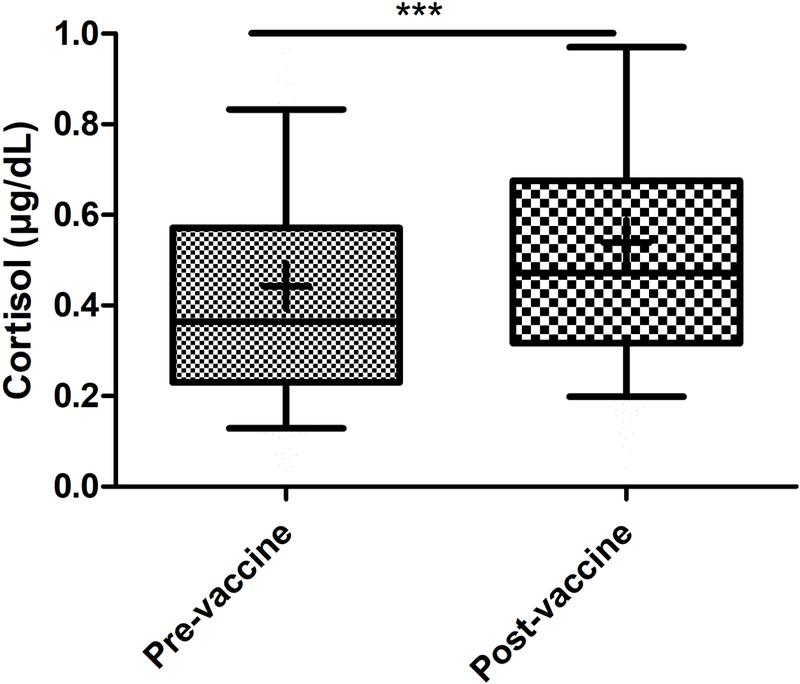
Post-vaccine salivary cortisol was significantly higher than pre-vaccine cortisol (n = 229). Comparison was calculated by independent two group t-test. Box = 25th 75th percentile, whiskers = 10th - 90th percentile, + = mean, horizontal bar = median, *** = p<0.001.
Vitamin A supplementation had no effect on cortisol responsiveness:
Cortisol responses were 0.143 ± 0.45 and 0.0541 ± 0.358, respectively for vitamin A and placebo groups. The cortisol responsiveness did not differ between vitamin A and placebo groups when compared by t-test (t = −0.76264, DF = 224.93, p=0.45), nor was a treatment effect seen (p=0.21) after adjusting for covariates including pre-vaccine cortisol, infant’s behavior during saliva collection, saliva collection time, sex, mode of delivery, and nutritional status (Table S1).
Cortisol responsiveness was negatively associated with thymus size in boys only:
The association of cortisol responsiveness with thymus size was evaluated using multiple regression analysis with adjustment for covariates infant’s behavior during saliva collection, pre-vaccine cortisol, saliva collection time, sex, mode of delivery, birthweight, length for age Z-score, weight for length Z-score, and breastfeeding status. In this analysis, a significant interaction was seen for cortisol responsiveness and sex (p<0.05) at all study visits, thus results are reported separately for males and females. The cortisol responsiveness was negatively associated with thymus size at all time points, but the association was seen only in boys (Table 2). Male infants with cortisol responsiveness in the highest quartile consistently had a 35.4%–37.7% lower predicted TI at all-time points compared to the lowest quartile for cortisol responsiveness, standardizing for exclusive breastfeeding, vaginal delivery, infants asleep during saliva collection, and the median saliva collected time [11:34 AM] (Figure S3).
Table 2:
Associations between thymic indices and 6 wk vaccination-induced cortisol responsiveness (∆Cortisol) in infants determined by multivariate regression modelling
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Regression model |
∆Cortisol (µg/dL) |
Regression model |
∆Cortisol (µg/dL) |
|||||
| R2 | p | β (SE) | p | R2 | P | β (SE) | p | |
| Thymic index | ||||||||
| 1 w | 0.243 | 0.016 | −5.50 (1.81) | 0.0032 | 0.346 | <0.001 | 1.60 (1.46) | 0.28 |
| 6 w | 0.315 | <0.001 | −10.4 (2.95) | <0.001 | 0.216 | 0.0085 | −1.278 (2.97) | 0.67 |
| 10 w | 0.286 | 0.0028 | −11.6 (3.21) | <0.001 | 0.172 | 0.021 | −2.309 (2.92) | 0.43 |
| 15 w | 0.222 | 0.034 | −12.5 (3.27) | <0.001 | 0.204 | 0.014 | −1.99 (3.04) | 0.51 |
Regression models determined association between thymic index and cortisol response (∆Cortisol) controlling the factors: infant’s behaviour during saliva collection, saliva collection time, pre-vaccine cortisol, mode of delivery, birthweight, length for age Z-score, weight for length Z-score, and breastfeeding status. 1 w regression model was determined by using birthweight and birth length instead of weight for length and length for age Z-scores, respectively.
Cortisol responsiveness was negatively associated with peripheral blood T-cell concentrations:
Consistent with the results for TI, the concentration of total naïve T-cells (CD3+CD45RA+) at 6 w (β (SE) = −2225 (819), p=0.0072) and of naïve helper T-cells (CD3+CD4+CD45RA+) at both 6 w (β (SE) = −1671 (565), p=0.0035) and 15 w (β (SE) = −1800 (675), p=0.0085) were negatively associated with the cortisol responsiveness, though no sex interaction was seen (Table 3). The associations remained significant even after adjusting with corresponding thymic indices (data not shown; though the absolute value of β-coefficients decreased by 3% – 25%) suggesting an independent effect of cortisol responsiveness on peripheral blood T-cells not mediated through an effect on thymus size. The predicted naïve CD4+ T-cell concentrations in infants with cortisol responsiveness in the highest quartile were 23.1% and 22.8% lower compared to lowest quartile at 6 w and 15 w, respectively, representing differences of 609 and 640 cells/uL blood (Figure S4; standardized for vaginal-delivered, exclusively breastfeed, infants who were asleep during saliva collection, with saliva collected at 11:34 AM [median saliva collection time]). Similar differences were seen for total CD4+ T-cells (Table 3, Figure S4) but not for total memory and CD4+ memory T-cells (Table 3).
Table 3:
Associations between T cell concentrations and TREC concentrations (per million PBMC) and 6 w vaccination-induced cortisol responsiveness in infants determined by multivariate regression modelling
| Regression model |
∆Cortisol (µg/dL) |
||||
|---|---|---|---|---|---|
| R2 | p | β (SE) | p | ||
| Total T cells | |||||
| 6 w | 0.135 | 0.0064 | −1974 (904) | 0.030 | |
| 15 w | 0.0512 | 0.63 | −1889 (1192) | 0.12 | |
| CD4+ T cells | |||||
| 6 w | 0.189 | <0.001 | −1685 (619) | 0.0071 | |
| 15 w | 0.132 | 0.0067 | −1773 (720) | 0.015 | |
| Total Naïve T cells | |||||
| 6 w | 0.143 | 0.0034 | −2225 (819) | 0.0072 | |
| 15 w | 0.064 | 0.41 | −2032 (1078) | 0.061 | |
| Naïve CD4+ T cells | |||||
| 6 w | 0.209 | <0.001 | −1671 (565) | 0.0035 | |
| 15 w | 0.134 | 0.0056 | −1800 (675) | 0.0083 | |
| Total Memory T cells | |||||
| 6 w | 0.0652 | 0.41 | 0.300 (0.484) | 0.54 | |
| 15 w | 0.0683 | 0.34 | 0.208 (0.395) | 0.60 | |
| Memory CD4+ cells | |||||
| 6 w | 0.0461 | 0.74 | 0.193 (0.370) | 0.60 | |
| 15 w | 0.0599 | 0.48 | −0.988 (0.295) | 0.74 | |
| TREC levels | |||||
| 6 w | 0.0903 | 0.26 | 225.3 (309) | 0.47 | |
| 15 w | 0.0583 | 0.66 | 4.59 (295.7) | 0.99 | |
Regression models determined association between immune cells and cortisol response (∆Cortisol) controlling the factors: infant’s behaviour during saliva collection, saliva collection time, pre-vaccine cortisol, sex, mode of delivery, birthweight, length for age Z-score, weight for length Z-score, and breastfeeding status.
Cortisol responsiveness was negatively associated with PPD skin test:
Infants received vaccines at birth, 6, 10, and 14 w of age (Figure 4). Three types of vaccine responses were analyzed: (1) T-cell proliferation; (2) antibody response; and (3) the delayed-type hypersensitivity (DTH) response to purified protein derivative (PPD) antigen as an index of the response to BCG immunization. None of the proliferation responses showed a statistically significant association with salivary cortisol (Table 4). However, the odds ratio of having a positive PPD skin test at 15 w of age was lower (β (SE) = −5.31 (2.51), p = 0.034) with a higher cortisol response (Table 4) and infants had a 23.7% lower predicted probability of having a positive PPD skin test if they were in the highest versus lowest quartile of cortisol responsiveness (Figure 2). In addition, the PPD skin test area analyzed as a continuous variable was also negatively associated with cortisol responsiveness (Table 4; p = 0.049).
Table 4:
Associations between vaccine responses and 6-week vaccination induced cortisol responsiveness in infants
| Regression model |
∆Cortisol (µg/dL) |
|||
|---|---|---|---|---|
| R2 | p | β (SE) | p | |
| CD4+ T-cell SI at 6 w | ||||
| PPD | 0.119 | 0.23 | −0.286 (0.958) | 0.77 |
| SEB | 0.0947 | 0.46 | −1.22 (1.18) | 0.30 |
| CD4+ T-cell SI at 15 w | ||||
| PPD | 0.0718 | 0.48 | 0.428 (1.08) | 0.69 |
| SEB | 0.0676 | 0.68 | −0.517 (1.01) | 0.61 |
| TT | 0.0786 | 0.36 | 40.5 (58.2) | 0.49 |
| HBV | 0.141 | 0.024 | 34.1(55.7) | 0.54 |
| ALS antibody at 15 w | ||||
| TT IgG, mIU/mL | 0.0592 | 0.564 | −1.26 (1.33) | 0.35 |
| Polio IgG, mIU/mL | 0.0933 | 0.14 | 1.26e-06 (6.75e-07) | 0.065 |
| HBV IgG, mIU/mL | 0.157 | 0.13 | −0.0197 (0.0245) | 0.43 |
| PPD skin test at 15 w | ||||
| Area (cm2) | 0.0659 | 0.37 | −110.4 (55.8) | 0.049 |
| Positive (n=187), Negative = 0 (n=37) |
-- | −5.31 (2.51) | 0.034 | |
Regression models determined association between vaccine response and cortisol response (∆Cortisol) controlling the factors: infant’s behaviour during saliva collection, pre-vaccine cortisol, saliva collection time, sex, mode of delivery, birthweight, length for age Z-score, weight for length Z-score, and breastfeeding status.
Figure 2:
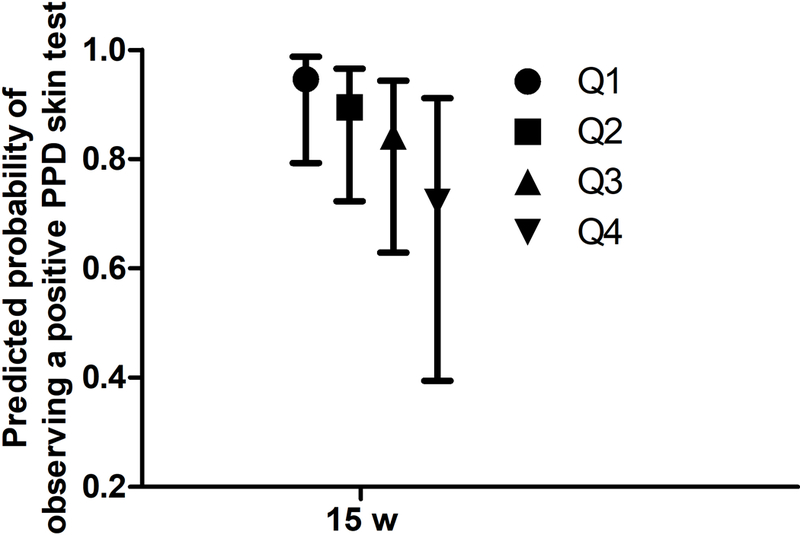
Predicted probability of observing positive PPD skin test at 15 w by cortisol responsiveness quartiles in normally delivered exclusively breastfeed infants having median baseline cortisol, cortisol collection time, HAZ and WHZ scores. Bar represents 95% CI. Mean and CI were predicted from the regression model described in the Table 4.
Discussion
In the present study, we assessed cortisol responsiveness by measuring pre- and post-vaccine salivary cortisol at 6 w of age and characterized associations with the T-cell compartment of the immune system, and with T-cell mediated vaccine responses. The salivary cortisol levels from infants in the present study (Figure 1) were very similar to those recently reported (Albers, Beijers, Riksen-Walraven, Sweep, & de Weerth, 2015) for infants at 14 w of age, who had morning salivary cortisol concentrations of 0.27 ± 0.18 µg/dL and 0.31 ± 0.23 µg/dL at home and a child care facility, respectively. Thus the data from our study is consistent with recent literature.
A principal finding of our study was that thymus size between 1 and 15 w of age was consistently smaller in boys (but not in girls) with higher cortisol responsiveness, suggesting a negative effect of elevated cortisol on thymic function in boys. To the best of our knowledge, this is the first report showing associations between thymus size and challenge-induced cortisol responsiveness in healthy infants, and it is not clear why this effect would be seen only in boys. The cortisol response does not seem to differ between male and female infants (unlike adults), though data are sparse, as recently reviewed (Rao and Androulakis, 2017). Work on prenatal stress indicates that male and female infants respond differently with regard to emotional development (Braithwaite, Murphy, Ramchandani, & Hill, 2017), suggesting that sex differences might be seen in other systems as well. One possibility in the thymus, based on data from mice, is that testosterone can act in the thymus to produce cortisol which then acts locally to cause thymocyte apoptosis (Chen, Qiao, Tuckermann, Okret, & Jondal, 2010). Since infants in our study are in the period of “mini-puberty”, when estrogen and testosterone differ between the sexes (Lamminmaki et al., 2012), it is possible that the additive effect of stress-induced and testosterone-induced cortisol combine to a decrease in thymus size in boys that is not seen in girls. Though we have measured cortisol responsiveness at just a single time point, 6 w of age, the inverse association between cortisol responsiveness and thymus size from 1–15 w of age suggests that high responsiveness at 6 w may be representative of cortisol responsiveness throughout early infancy.
Consistent with the observations on thymus size, we also found that peripheral blood levels of naïve T-cells were also negatively associated with the cortisol responsiveness, though TREC levels were not. The association with naïve T-cells was not sex-specific. The association remains significant even after adjusting for thymus size which suggests a direct effect of cortisol responsiveness on peripheral-blood naïve T-cells independent of an effect on the thymus. It is possible that this association could result from a redistribution of T-cells away from the blood but this redistribution effect is also seen for memory T cells (Dimitrov, et al., 2009), which we did not observe in the present study. Other studies also report stress-related reductions in peripheral blood levels of naïve T-cells, which could be related either to thymic function or redistribution. For example, one study found that reductions of urinary cortisol resulting from a stress-management intervention were associated with increases of naïve T-cell levels in blood (Antoni et al., 2005) and other studies have reported decreased helper T-cells in peripheral blood during exam stress (Glaser et al., 1985; Kiecolt-Glaser et al., 1986).
The DTH skin test response to PPD antigen, which involves the development of T cell-mediated induration (Siegrist, 2013), was negatively associated with cortisol responsiveness, though our ex vivo measures of T-cell responsiveness were not. It is possible that the in vivo DTH response is more directly affected by plasma cortisol than are T-cell responses measured over a period of 6 d in media containing only 10% autologous plasma. Lower DTH responses have previously been associated with elevated stress. A study involving preterm infants reported that those with high morning cortisol had lower DTH skin-test responses to a variety of recall antigens (Buske-Kirschbaum et al., 2007). It is true that other studies have reported effects on ex vivo T cell responses as well. A study in non-human primates found decreased T-lymphocyte proliferation during peer-separation stress (Friedman, Coe, & Ershler, 1991) while similar results were seen for T-cell proliferative responses to Epstein-Barr virus antigen as a result of exam stress in students (Glaser et al., 1993). Lower Hepatitis B (HBV) vaccine-specific T-cell proliferation and antibody responses were also seen in healthy adults reporting higher stress (Glaser et al., 1992). However, none of these studies evaluated cortisol responsiveness, which may be influenced by factors, such as prior stress (Herman et al., 2016), that alter cortisol responsiveness and also impact immune function.
Our study had several strengths, including its relatively large sample size (n=229), the examination of multiple aspects of adaptive immunity, the direct observation of immunizations by study personnel, and the unique population of healthy infants. A weakness of our study was the timing of the pre-vaccine saliva collection, which was not consistently coordinated with the venepuncture blood collection, though adequate statistical adjustment was made for this additional variable.
In summary, our findings show a sex-specific association of elevated stress-induced cortisol with smaller thymus size, though other negative associations of cortisol with CD4 T-cell concentrations and the DTH response to BCG vaccination were observed in both boys and girls. Demonstration of sex differences in immune function, including response to vaccination, is not new (Garly et al., 2004). Differential stress responses between boys and girls may provide mechanistic explanations for other sex-specific differences in immune function; this phenomenon warrants further study.
Supplementary Material
Figure S1: Study procedures. The top portion of the figure indicates the 15-w timeline of the overall study. “I” indicates the event occurred mentioned at the corresponding right side in the figure. A total 306 infants were enrolled in the parent study. Salivary cortisol responsiveness was measured in 229 saliva infants. The inset describes the day of saliva collection, focusing on the four groupings (G1 – G4) described in the Methods Section that categorize the timing of the pre-vaccination saliva collection for cortisol relative to a venepuncture that occurred on the same day: G1, saliva collection before blood collection; G2, saliva collection 1–30 min after blood collection; G3, saliva collection 31–60 min after blood collection; and G4, saliva collection >60 min after blood collection.
Figure S2: Comparison between pre- and post- vaccine cortisol concentration within pre-saliva collection time groups (A). Comparison among pre- and post- vaccine cortisol concentration within pre-saliva collection time groups (B). Two-way ANOVA with TukeyHSD post hoc analysis was used to compare between groups and time point. G1 = obtained saliva collection before blood collection (n = 66), G2 -G4 = obtained pre-vaccine saliva collection 1–30 min (n=67), 31–60 min (n=80), and >60 min (n=16); respectively. Mean ± SEM, ***=p<0.001.
Figure S3: Predicted thymic index by cortisol responsiveness quartiles in normally delivered exclusively breastfeed male infants having median baseline cortisol, cortisol collection time, HAZ and WHZ scores. Bar represents 95% CI. Mean and CI for male infants were predicted from the regression models described in the Table 2.
Figure S4: Predicted CD4+ cell numbers by cortisol responsiveness quartiles in normally delivered exclusively breastfeed infants having median baseline cortisol, cortisol collection time, HAZ and WHZ scores. Bar represents 95% CI. Mean and CI were predicted from the regression models described in the Table 4.
Figure S5: Flow cytometric analysis of total, naïve, and memory T-cells counts per µL of peripheral blood was determined by commercially available Multitest four-color reagent kits (CD45RA-FITC/CD45RO-PE/CD3-PerCP/CD4-APC) with Trucount Tubes. Absolute counts were determined by comparing with bead counts.
Figure S6: Flow cytometric analysis of vaccine specific proliferation index of CD4+ T-cells. PBMC was isolated from obtained blood samples and stimulated with vaccine specific antigens or PBS control for 6 days. Harvested cells were staining with live/death, CD3, CD4, and CD45RO markers and analyzed by flow-cytometry. The proliferation index was calculated by dividing the ratio of antigen-stimulated memory T-cells (eg; CD4+CD45RO+ in large lymphocyte) to total T-cells (large lymphocyte CD4+ + small lymphocyte CD4+) by the ratio of PBS controlled memory T-cells to total T-cells.
Acknowledgements
We also thankfully acknowledge all the study participants. The parent study was supported by the World Health Organization via a Bill and Melinda Gates Foundation grant and Thrasher Research Fund. We respectfully acknowledge Janet M Peerson, Senior Statistician, WHNRC for her selfless guiding on statistical analysis. We also give thanks to all the research staff, data entry staff, hospital nurses, and physicians at the collaborating clinic MCHTI.
Funding details
This work was funded by World Health Organization Project Number 2010168947 which was funded from the Bill and Melinda Gates Foundation, Thrasher Research Fund grant number 11488 and USDA-ARS Project Number: 2032–53000-001–00-D. RedCap database usage was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860.
Abbreviations used in this manuscript
- ALS
Antibodies from lymphocyte secretions
- BCG
Bacillus Calmette–Guérin
- DTH
Delayed-type hypersensitivity
- HBV
Hepatitis B
- PPD
Purified protein derivative
- SEB
Staphylococcus Enterotoxin B
- TREC
T-cell receptor rearrangement excision circles
- TI
Thymic index
- W
Week
- Y
Year
Footnotes
Geolocation information
The study subjects were recruited in Dhaka, Bangladesh (Latitude =23.7243, Longitude = 90.3875).
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Aaby P, Marx C, Trautner S, Rudaa D, Hasselbalch H, Jensen H, & Lisse I (2002). Thymus size at birth is associated with infant mortality: a community study from Guinea-Bissau. Acta Paediatr, 91(6), pp. 698–703. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12162605 [DOI] [PubMed] [Google Scholar]
- Ahmad SM, Raqib R, Qadri F, & Stephensen CB (2014). The effect of newborn vitamin A supplementation on infant immune functions: trial design, interventions, and baseline data. Contemp Clin Trials, 39(2), pp. 269–279. doi: 10.1016/j.cct.2014.09.004 Retrieved from 10.1016/j.cct.2014.09.004http://www.ncbi.nlm.nih.gov/pubmed/25269669 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25269669 [DOI] [PubMed] [Google Scholar]
- Albers EM, Beijers R, Riksen-Walraven JM, Sweep FC, & de Weerth C (2015). Cortisol levels of infants in center care across the first year of life: links with quality of care and infant temperament. Stress, pp. 1–10. doi: 10.3109/10253890.2015.1089230 Retrieved from 10.3109/10253890.2015.1089230http://www.ncbi.nlm.nih.gov/pubmed/26455788 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26455788 [DOI] [PubMed]
- Antoni MH, Cruess DG, Klimas N, Carrico AW, Maher K, Cruess S, Lechner SC, Kumar M, Lutgendorf S, Ironson G, Fletcher MA, & Schneiderman N (2005). Increases in a marker of immune system reconstitution are predated by decreases in 24-h urinary cortisol output and depressed mood during a 10-week stress management intervention in symptomatic HIV-infected men. J Psychosom Res, 58(1), pp. 3–13. doi: 10.1016/j.jpsychores.2004.05.010 Retrieved from 10.1016/j.jpsychores.2004.05.010http://www.ncbi.nlm.nih.gov/pubmed/15771864 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15771864 [DOI] [PubMed] [Google Scholar]
- Benjamin CL, Stowe RP, St John L, Sams CF, Mehta SK, Crucian BE, Pierson DL, & Komanduri KV (2016). Decreases in thymopoiesis of astronauts returning from space flight. JCI Insight, 1(12), p e88787 doi: 10.1172/jci.insight.88787 Retrieved from 10.1172/jci.insight.88787https://www.ncbi.nlm.nih.gov/pubmed/27699228 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27699228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite EC, Murphy SE, Ramchandani PG, & Hill J (2017). Associations between biological markers of prenatal stress and infant negative emotionality are specific to sex. Psychoneuroendocrinology, 86, pp. 1–7. doi: 10.1016/j.psyneuen.2017.09.004 Retrieved from 10.1016/j.psyneuen.2017.09.004https://www.ncbi.nlm.nih.gov/pubmed/28888992 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28888992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Krieger S, Wilkes C, Rauh W, Weiss S, & Hellhammer DH (2007). Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. J Clin Endocrinol Metab, 92(9), pp. 3429–3435. doi: 10.1210/jc.2006-2223 Retrieved from 10.1210/jc.2006-2223http://www.ncbi.nlm.nih.gov/pubmed/17566098 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17566098 [DOI] [PubMed] [Google Scholar]
- Chen Y, Qiao S, Tuckermann J, Okret S, & Jondal M (2010). Thymus-derived glucocorticoids mediate androgen effects on thymocyte homeostasis. FASEB J, 24(12), pp. 5043–5051. doi: 10.1096/fj.10-168724 Retrieved from 10.1096/fj.10-168724https://www.ncbi.nlm.nih.gov/pubmed/20798244 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20798244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JJ (1992). Glucocorticoid-induced apoptosis in the thymus. Semin Immunol, 4(6), pp. 363–369. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1286164 [PubMed] [Google Scholar]
- de Felice C, Toti P, Musaro M, Peruzzi L, Paffetti P, Pasqui L, Magaldi R, Bagnoli F, Rinaldi M, Rinaldi G, Grilli G, Tonni G, & Latini G (2008). Early activation of the hypothalamic-pituitary-adrenal-axis in very-low-birth-weight infants with small thymus at birth. J Matern Fetal Neonatal Med, 21(4), pp. 251–254. doi: 10.1080/14767050801927871 Retrieved from 10.1080/14767050801927871https://www.ncbi.nlm.nih.gov/pubmed/18330821 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18330821 [DOI] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, & Tesselaar K (2012). Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity, 36(2), pp. 288–297. doi: 10.1016/j.immuni.2012.02.006 Retrieved from 10.1016/j.immuni.2012.02.006https://www.ncbi.nlm.nih.gov/pubmed/22365666 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22365666 [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, & Lange T (2009). Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood, 113(21), pp. 5134–5143. doi: 10.1182/blood-2008-11-190769 Retrieved from 10.1182/blood-2008-11-190769http://www.ncbi.nlm.nih.gov/pubmed/19293427 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19293427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Coe CL, & Ershler WB (1991). Time-dependent effects of peer separation on lymphocyte proliferation responses in juvenile squirrel monkeys. Dev Psychobiol, 24(3), pp. 159–173. doi: 10.1002/dev.420240303 Retrieved from 10.1002/dev.420240303http://www.ncbi.nlm.nih.gov/pubmed/1936580 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1936580 [DOI] [PubMed] [Google Scholar]
- Garly ML, Jensen H, Martins CL, Bale C, Balde MA, Lisse IM, & Aaby P (2004). Hepatitis B vaccination associated with higher female than male mortality in Guinea-bissau: an observational study. Pediatr Infect Dis J, 23(12), pp. 1086–1092. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15626943 [PubMed] [Google Scholar]
- Garly ML, Trautner SL, Marx C, Danebod K, Nielsen J, Ravn H, Martins CL, Bale C, Aaby P, & Lisse IM (2008). Thymus size at 6 months of age and subsequent child mortality. J Pediatr, 153(5), pp. 683–688, 688 e681–683. doi: 10.1016/j.jpeds.2008.04.069 Retrieved from 10.1016/j.jpeds.2008.04.069https://www.ncbi.nlm.nih.gov/pubmed/18589444 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18589444 [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Bonneau RH, Malarkey W, Kennedy S, & Hughes J (1992). Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med, 54(1), pp. 22–29. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1553399 [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Stout JC, Tarr KL, Speicher CE, & Holliday JE (1985). Stress-related impairments in cellular immunity. Psychiatry Res, 16(3), pp. 233–239. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2935896 [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Bonneau RH, Esterling BA, Atkinson C, & Kiecolt-Glaser JK (1993). Stress and the memory T-cell response to the Epstein-Barr virus in healthy medical students. Health Psychol, 12(6), pp. 435–442. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8293726 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 42(2), pp. 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbalch H, Nielsen MB, Jeppesen D, Pedersen JF, & Karkov J (1996). Sonographic measurement of the thymus in infants. Eur Radiol, 6(5), pp. 700–703. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8934137 [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, & Myers B (2016). Regulation of the Hypothalamic‐Pituitary‐Adrenocortical Stress Response. Comprehensive Physiology [DOI] [PMC free article] [PubMed]
- Huda MN, Lewis Z, Kalanetra KM, Rashid M, Ahmad SM, Raqib R, Qadri F, Underwood MA, Mills DA, & Stephensen CB (2014). Stool microbiota and vaccine responses of infants. Pediatrics, 134(2), pp. e362–372. doi: 10.1542/peds.2013-3937 Retrieved from 10.1542/peds.2013-3937http://www.ncbi.nlm.nih.gov/pubmed/25002669 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25002669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Strain EC, Stout JC, Tarr KL, Holliday JE, & Speicher CE (1986). Modulation of cellular immunity in medical students. J Behav Med, 9(1), pp. 5–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2939253 [DOI] [PubMed] [Google Scholar]
- Lamminmaki A, Hines M, Kuiri-Hanninen T, Kilpelainen L, Dunkel L, & Sankilampi U (2012). Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm Behav, 61(4), pp. 611–616. doi: 10.1016/j.yhbeh.2012.02.013 Retrieved from 10.1016/j.yhbeh.2012.02.013https://www.ncbi.nlm.nih.gov/pubmed/22373494 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22373494 [DOI] [PubMed] [Google Scholar]
- Majumdar S, & Nandi D (2018). Thymic Atrophy: Experimental Studies and Therapeutic Interventions. Scand J Immunol, 87(1), pp. 4–14. doi: 10.1111/sji.12618 Retrieved from 10.1111/sji.12618https://www.ncbi.nlm.nih.gov/pubmed/28960415 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28960415 [DOI] [PubMed] [Google Scholar]
- Moore SE, Collinson AC, Tamba N’Gom P, Aspinall R, & Prentice AM (2006). Early immunological development and mortality from infectious disease in later life. Proc Nutr Soc, 65(3), pp. 311–318. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16923314 [DOI] [PubMed] [Google Scholar]
- Moore SE, Fulford AJ, Wagatsuma Y, Persson LA, Arifeen SE, & Prentice AM (2014). Thymus development and infant and child mortality in rural Bangladesh. Int J Epidemiol, 43(1), pp. 216–223. doi: 10.1093/ije/dyt232 Retrieved from 10.1093/ije/dyt232https://www.ncbi.nlm.nih.gov/pubmed/24366492 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24366492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Gonzalez A, Gambon F, Diaz-Espada F, & Lopez-Rivas A (1992). Apoptosis in human thymocytes after treatment with glucocorticoids. Clin Exp Immunol, 88(2), pp. 341–344. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1572099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelog M, Wilk C, Keller M, Karall T, Orth D, Geiger R, Walder G, Laufer G, Cottogni M, Zimmerhackl Lothar B, Stein J, Grubeck-Loebenstein B, & Wuerzner R (2008). Diminished response to tick-borne encephalitis vaccination in thymectomized children. Vaccine, 26(5), pp. 595–600. doi: 10.1016/j.vaccine.2007.11.074 Retrieved from 10.1016/j.vaccine.2007.11.074https://www.ncbi.nlm.nih.gov/pubmed/18178293 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18178293 [DOI] [PubMed] [Google Scholar]
- Rao RT, & Androulakis IP (2017). Modeling the Sex Differences and Interindividual Variability in the Activity of the Hypothalamic-Pituitary-Adrenal Axis. Endocrinology, 158(11), pp. 4017–4037. doi: 10.1210/en.2017-00544 Retrieved from 10.1210/en.2017-00544https://www.ncbi.nlm.nih.gov/pubmed/28938475 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28938475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M, Moore SE, & Fuchs G (2007). Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am J Clin Nutr, 85(3), pp. 845–852. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17344508 [DOI] [PubMed] [Google Scholar]
- Sauce D, & Appay V (2011). Altered thymic activity in early life: how does it affect the immune system in young adults? Curr Opin Immunol, 23(4), pp. 543–548. doi: 10.1016/j.coi.2011.05.001 Retrieved from 10.1016/j.coi.2011.05.001https://www.ncbi.nlm.nih.gov/pubmed/21752618 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21752618 [DOI] [PubMed] [Google Scholar]
- Siegrist C-A (2013). Vaccine immunology - Plotkin, Stanley A. In Orenstein WA & Offit PA (Eds.), Vaccines (Sixth Edition) (pp. 14–32). London: W.B. Saunders. [Google Scholar]
- Webster Marketon JI, & Glaser R (2008). Stress hormones and immune function. Cell Immunol, 252(1–2), pp. 16–26. doi: 10.1016/j.cellimm.2007.09.006 Retrieved from 10.1016/j.cellimm.2007.09.006http://www.ncbi.nlm.nih.gov/pubmed/18279846 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18279846 [DOI] [PubMed] [Google Scholar]
- WHO/UNICEF. (2008). Indicators for Assessing Infant and Young Child Practices Geneva, Switzerland: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Study procedures. The top portion of the figure indicates the 15-w timeline of the overall study. “I” indicates the event occurred mentioned at the corresponding right side in the figure. A total 306 infants were enrolled in the parent study. Salivary cortisol responsiveness was measured in 229 saliva infants. The inset describes the day of saliva collection, focusing on the four groupings (G1 – G4) described in the Methods Section that categorize the timing of the pre-vaccination saliva collection for cortisol relative to a venepuncture that occurred on the same day: G1, saliva collection before blood collection; G2, saliva collection 1–30 min after blood collection; G3, saliva collection 31–60 min after blood collection; and G4, saliva collection >60 min after blood collection.
Figure S2: Comparison between pre- and post- vaccine cortisol concentration within pre-saliva collection time groups (A). Comparison among pre- and post- vaccine cortisol concentration within pre-saliva collection time groups (B). Two-way ANOVA with TukeyHSD post hoc analysis was used to compare between groups and time point. G1 = obtained saliva collection before blood collection (n = 66), G2 -G4 = obtained pre-vaccine saliva collection 1–30 min (n=67), 31–60 min (n=80), and >60 min (n=16); respectively. Mean ± SEM, ***=p<0.001.
Figure S3: Predicted thymic index by cortisol responsiveness quartiles in normally delivered exclusively breastfeed male infants having median baseline cortisol, cortisol collection time, HAZ and WHZ scores. Bar represents 95% CI. Mean and CI for male infants were predicted from the regression models described in the Table 2.
Figure S4: Predicted CD4+ cell numbers by cortisol responsiveness quartiles in normally delivered exclusively breastfeed infants having median baseline cortisol, cortisol collection time, HAZ and WHZ scores. Bar represents 95% CI. Mean and CI were predicted from the regression models described in the Table 4.
Figure S5: Flow cytometric analysis of total, naïve, and memory T-cells counts per µL of peripheral blood was determined by commercially available Multitest four-color reagent kits (CD45RA-FITC/CD45RO-PE/CD3-PerCP/CD4-APC) with Trucount Tubes. Absolute counts were determined by comparing with bead counts.
Figure S6: Flow cytometric analysis of vaccine specific proliferation index of CD4+ T-cells. PBMC was isolated from obtained blood samples and stimulated with vaccine specific antigens or PBS control for 6 days. Harvested cells were staining with live/death, CD3, CD4, and CD45RO markers and analyzed by flow-cytometry. The proliferation index was calculated by dividing the ratio of antigen-stimulated memory T-cells (eg; CD4+CD45RO+ in large lymphocyte) to total T-cells (large lymphocyte CD4+ + small lymphocyte CD4+) by the ratio of PBS controlled memory T-cells to total T-cells.


