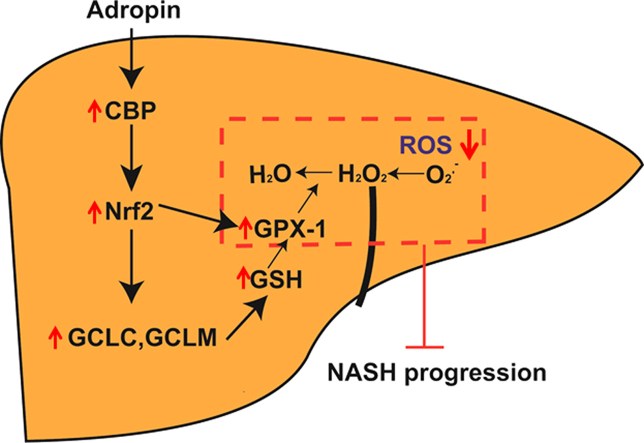
Abstract
Adropin, a secretory signal peptide, has shown beneficial effects on improving glucose homeostasis and dyslipidemia. However, whether this peptide affects nonalcoholic steatohepatitis (NASH) has remained unclear. In this study, the serum adropin levels, liver injury and oxidative stress were measured in diet-induced NASH mice. Adropin knock-out mice and palmitate treated primary hepatic cells were used to investigate the influence of adropin on liver injury. Our results show that serum adropin levels were decreased and negatively correlated with liver injury in NASH mice. Knockout of adropin significantly exacerbated hepatic steatosis, inflammatory responses and fibrosis in mice after either methionine-choline deficient diet (MCD) or western diet (WD) feeding. And the treatment with adropin bioactive peptides ameliorated NASH progression in mice. Adropin alleviated hepatocyte injury by upregulating the expression of Gclc, Gclm, and Gpx1 in a manner dependent on Nrf2 transcriptional activity and by increasing the glutathione (GSH) levels. And adropin significantly increased CBP expression and promoted its binding with Nrf2, which enhanced Nrf2 transcriptional activity. Furthermore, AAV8-mediated overexpression of hepatic Nrf2 expression functionally restored the liver injury induced by adropin-deficiency MCD-fed mice. These findings provide evidence that adropin activates Nrf2 signaling and plays a protective role in liver injury of NASH and therefore might represent a novel target for the prevention and treatment of NASH.
Abbreviations: NASH, nonalcoholic steatohepatitis; MCD, methionine-choline deficient; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase regulatory subunit; GPX, glutathione peroxidase; Nrf2, nuclear factor erythroid 2-related factor 2; GSH, glutathione; CBP, CREB-binding protein; NAFLD, nonalcoholic fatty liver disease; SS, simple steatosis; HCC, hepatocellular carcinoma; ROS, reactive oxygen species; TG, triglyceride; FFA, free fatty acid; Enho, energy homeostasis associated; VEGFR, vascular endothelial growth factor receptor; PA, palmitate; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KO, knock out; WT, wild type; Col1a1, alpha-1 type I collagen; IL, interleukin; TNF, tumor necrosis factor; ALT, alanine amino transferase; AST, aspartate amino transferase; MMP, mitochondrial membrane potential; NOX, NADPH oxidase; SOD, superoxide dismutase; CAT, catalase; PRDX, peroxiredoxin; γ-GCS, γ-glutamylcysteine synthase; Keap1, kelch-like ECH-associated protein 1; ARE, antioxidant response element; IR, insulin resistance; GST, Glutathione S-transferase; NF-κB, nuclear factor κB, CCR, C-C chemokine receptor
Keywords: NASH, ROS, Lipotoxicity, Nrf2, GSH
Graphical abstract
Highlights
-
•
The serum adropin levels were lower in the NASH model mice and negatively correlated with oxidative damage and liver injury.
-
•
Mice lacking adropin (Adropin-KO) exhibited more severe liver injury and accelerated NASH progression.
-
•
Treatment with adropin alleviated hepatocyte injury by upregulating CBP/Nrf2 mediated antioxidant activity.
-
•
AAV8-mediated overexpression of hepatic Nrf2 expression ameliorated the NASH progression in Adropin-KO mice.
1. Introduction
Currently, nonalcoholic fatty liver disease (NAFLD) has become one of the most common causes of chronic liver diseases linked to insulin resistance, obesity and related disorders, such as type 2 diabetes and metabolic syndrome. The disease spectrum of NAFLD involves a spectrum of conditions ranging from simple steatosis (SS), steatohepatitis, cirrhosis and hepatocellular carcinoma (HCC) [1]. Nowadays, the pure hepatic steatosis has been regarded as a benign clinical course, but nonalcoholic steatohepatitis (NASH), the more aggressive form of the disease, is responsible for a higher risk of cirrhosis, HCC and the increased liver-related mortality in NAFLD patients [2], [3].
The mechanism of SS to NASH progression is largely unknown. Currently, an increasing number of studies have shown that reactive oxygen species (ROS) plays a vital role in this process [4]. In the SS state, the hepatocytes are overwhelmed by triglycerides (TGs), which are now considered a benign compensatory host response. However, when the hepatic capacity to oxidize, store and export free fatty acids (FFAs) as TGs are overwhelmed by their flux from the periphery or hepatic de novo lipogenesis, the excess FFAs in liver may cause lipotoxicity. Chronic FFAs overload induces liver injury and hepatocyte cell death, which triggers hepatic inflammation and fibrogenesis and drives the progression of SS to NASH [5]. When hepatocytes are overwhelmed by FFAs, dysfunctional mitochondria produce a large amount of ROS, which can directly cause cell apoptosis and death [6]. In addition, some antioxidants have been shown to ameliorate the lipotoxicity in NASH [7]. Therefore, how to decrease ROS production to alleviate liver injury is an important target for the treatment of NASH.
Adropin which is encoded by the energy homeostasis-associated gene (Enho), has been proposed to be a secreted protein [8]. Studies have shown that dietary nutrients or energy intake can affect the gene expression and circulating levels of adropin [9], [10], [11]. In metabolic homeostasis, adropin can improve glucose homeostasis and dyslipidemia in obesity mice [12]. Furthermore, adropin has been found to downregulate PDK4 expression and increase the glucose utilization [13]. Since the metabolic disorders and NAFLD are closely related and ROS plays an important role in the progression of SS to NASH, we hypothesized that adropin may exert protective effects on ROS in NASH. Therefore, the purpose of this study was to investigate whether adropin can activate antioxidant reaction to facilitate the ROS clearance and prevent the NASH progression.
2. Materials and methods
2.1. Reagents and antibodies
Adropin(34-76) for animal injections was purchased from ChinaPeptides. Adropin(34-76) used for cell treatment was obtained from Phoenix Pharmaceuticals. Antibodies against CD45 (ab10558), F4/80 (ab6640), MCP1 (ab7202) and Nrf2 (ab62352) were purchased from Abcam. Antibodies against acetyl-lysine (9681), cleaved Caspase-3 (9661) and Bax (2772) were obtained from Cell Signaling Technology. Antibodies against CBP (sc-369), Complex IV (sc-23983), cytochrome c (sc-13561) and GAPDH (sc-293335) were purchased from Santa Cruz Biotechnology. Lipofectamine transfection reagent was obtained from Invitrogen.
2.2. Animal models
All mice were maintained in a specific pathogen-free facility with constant temperature and humidity under a 12 h dark/light cycle, with free access to food and water. All animal procedures were conducted in accordance with the guidelines of the Animal Care and Protection Committee of Sun Yat-sen University. Eight-week-old male C57BL/6J mice were purchased from the Experimental Animal Center of Guangdong Province (Guangzhou, China). Adropin knock-out mice (C57BL/6J background) were constructed using the CRISPR/Cas9 technique by the Shanghai Biomodel Organism Science & Technology Development Co., Ltd.
2.3. Primary cell culture
Primary hepatocytes were isolated from the liver of C57BL/6J mice by the two step collagenase perfusion method. And primary murine hepatocytes were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 μg/ml streptomycin and 100 units/ml penicillin. Cells at 70–80% confluence were starved overnight in serum free medium before exposure to various treatments.
2.4. Statistical analysis
The data was shown as the means ± SD. Unpaired Student's t-test was used to test the difference between two groups. And one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test was applied for comparisons between multiple experimental groups. SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Differences were considered significant at P < 0.05.
An expanded methods section is available in the Online Data Supplement.
3. Results
3.1. Adropin expression decreased in NASH mice and was negatively correlated with oxidative damage and liver injury
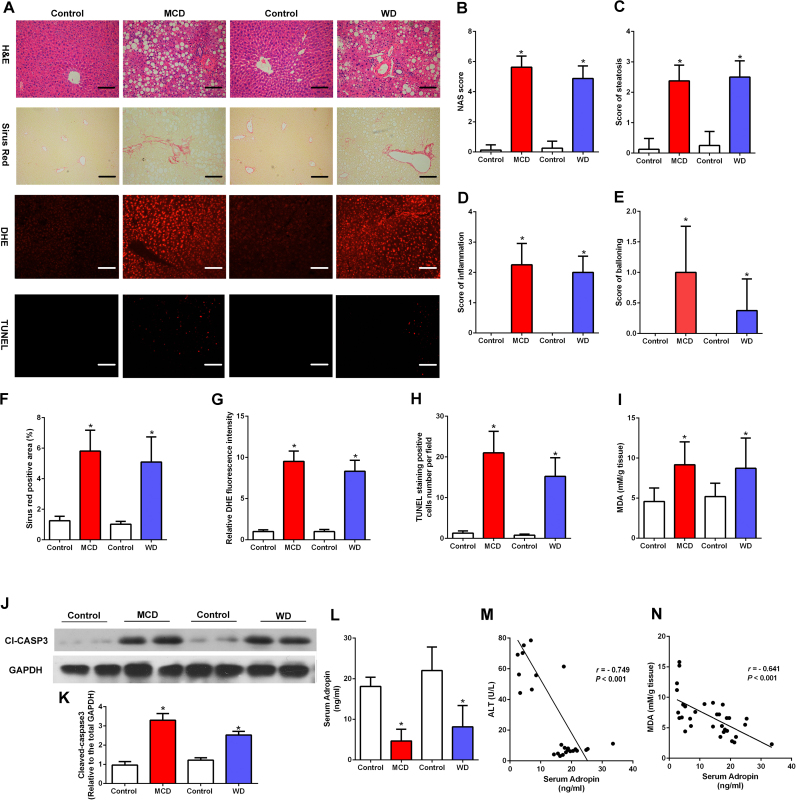
There were significant characteristic features of NASH in mice fed either methionine-choline deficient diet (MCD) for 8 weeks or western diet (WD) for 16 weeks compared with those of control mice. The body weight change, liver weight, adipose tissue weight and food intake information during the experiment period were shown in Table 1. The liver sections of NASH mice exhibited a combination of macrosteatosis and microsteatosis, inflammation, ballooning and severe fibrosis (Fig. 1A–F). Furthermore, serum ALT, AST levels and liver free fatty acids were elevated by NASH diet feeding (Fig. S1A–C). We also investigated the oxidative and antioxidative balance in NASH mice. Remarkably, the DHE staining showed higher hepatic ROS levels in NASH mice (Fig. 1A, G). And TUNEL-positive hepatic cells were increased in mice fed with MCD or WD (Fig. 1A, H). Notably, the liver malondialdehyde (MDA) levels were significantly higher while GSH levels were lower in NASH model (Fig. 1I, Fig. S1D). Immunoblotting of liver lysates revealed that caspase-3 cleavage was induced by MCD or WD feeding (Fig. 1J–K). Furthermore, serum adropin levels were significantly decreased in NASH model (Fig. 1L) and negatively associated with the serum ALT levels and liver MDA levels (Fig. 1M–N). And we measured mRNA levels of adropin in different tissues of NASH mice. As shown in Fig. S1E, the serum adropin levels decreased in accordance with the downregulated liver and adipose Enho expression. These results indicate that adropin may be involved in the oxidative damage and lipotoxicity in the development of NASH.
Table 1.
General parameters evaluated in C57BL/6 J male mice fed with MCD diet or WD diet for 8 or 16 weeks.
| Control | MCD | Control | WD | |
|---|---|---|---|---|
| Initial body weight (g) | 21.70 ± 2.05 | 22.11 ± 2.28 | 22.04 ± 2.07 | 21.58 ± 2.45 |
| Final body weight (g) | 26.36 ± 1.76 | 15.06 ± 1.23* | 26.24 ± 1.84 | 36.51 ± 2.97* |
| Liver weight (g) | 0.93 ± 0.22 | 0.50 ± 0.14* | 1.03 ± 0.17 | 1.38 ± 0.20* |
| Subcutaneous fat weight (g) | 0.65 ± 0.13 | 0.13 ± 0.05* | 0.65 ± 0.15 | 3.14 ± 0.47* |
| Epididymal fat weight (g) | 0.54 ± 0.12 | 0.18 ± 0.06* | 0.57 ± 0.12 | 2.97 ± 0.37* |
| Food intake (g/day/mouse) | 2.80 ± 0.38 | 2.44 ± 0.13 | 2.88 ± 0.14 | 2.96 ± 0.15 |
All values are mean ± SD, n = 8. Statistical analysis of the data for multiple comparisons was performed by ANOVA.
P < 0.05 versus the control group.
Fig. 1.
Dynamics of serum adropin levels changed in mice fed with NASH diet. Eight-week-old male C57BL/6J mice were fed with MCD diet or WD diet for 8 or 16 weeks. (A) Respresentative liver H&E, sirus red, DHE and TUNEL staining (magnification, ×200), scale bar: 200 µm. (B-E) Hepatic histological analysis of H&E staining. (F) Quantitative analysis of Sirius Red staining. (G) Quantitative analysis of DHE staining. (H) Quantitative analysis of TUNEL staining. (I) The liver MDA contents. (J-K) Cleaved caspase-3 expression of total liver lysates from NASH diet–fed mice. (L) Serum Adropin levels. (M) Correlation of serum ALT with serum adropin levels. (N) Correlation of liver MDA contents with serum adropin levels. (G, K) Control diet group was set as 1. The data are expressed as the mean ± SD, n = 8, * P < 0.05 versus the control diet group.
3.2. Adropin deficiency accelerated NASH progression and aggravated oxidative stress
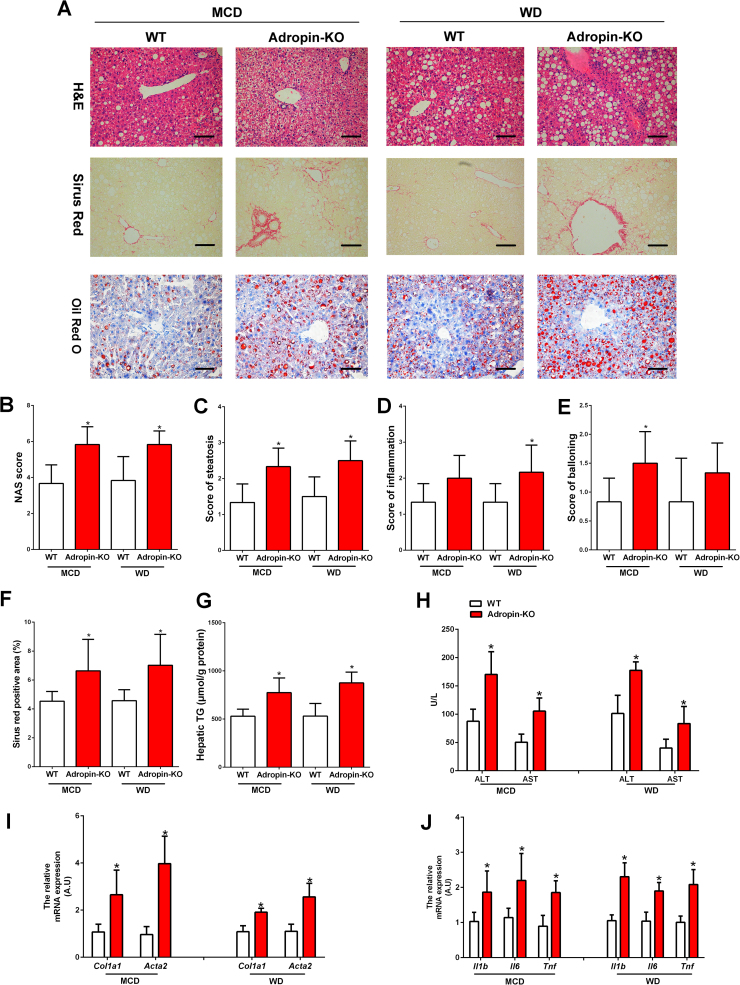
Mice lacking adropin (Adropin-KO) were used to assess the role of adropin in the pathogenesis of NASH. There was no significantly difference between Adropin-KO mice and WT mice in body weight change, liver weight, adipose tissue weight and daily food intake (Table 2). After MCD feeding for 4 weeks or WD feeding for 16 weeks, compared to wild type (WT) mice, Adropin-KO mice exhibited the more severe hepatic macrosteatosis, inflammation and ballooning, with a significantly higher NAS score (Fig. 2A–E). And the fibrosis areas were increased in the Adropin-KO mice (Fig. 2F). Notably, Adropin-KO mice had pronounced perisinusoidal fibrosis (Fig. 2A), a pattern characteristic of fibrosis with a metabolic etiology such as NASH. Oil Red O staining and hepatic triglyceride (TG) measurement also demonstrated the more lipid accumulation in Adropin-KO mice (Fig. 2A, G). In MCD-fed mice, adropin-deficiency had no significantly effects on the fasting glucose and insulin levels. However, in WD-fed mice, adropin-deficiency exacerbated the insulin resistance (Fig. S2A–B). Furthermore, serum ALT and AST levels were notably higher in the Adropin-KO mice than WT mice (Fig. 2H). Staining of F4/80, CD45 and MCP1 were performed to evaluate the extent of inflammatory responses in the liver. Compared to WT mice, more inflammatory responses proceeded in the liver tissues of the Adropin-KO mice (Fig. S3A–D). There was also a substantial induction of genes related to fibrosis (Col1a1, Acta2) and inflammation (Il1b, Il6 and Tnf) in the Adropin-KO livers (Fig. 2D–E).
Table 2.
General parameters evaluated in WT or adropin-KO mice fed with MCD diet or WD diet for 4 or 16 weeks.
| MCD |
WD |
|||
|---|---|---|---|---|
| WT | Adropin-KO | WT | Adropin-KO | |
| Initial body weight (g) | 24.25 ± 1.47 | 24.09 ± 1.36 | 23.98 ± 1.05 | 23.73 ± 1.37 |
| Final body weight (g) | 16.46 ± 1.42 | 16.73 ± 1.52 | 35.54 ± 2.50 | 36.01 ± 4.17 |
| Liver weight (g) | 0.67 ± 0.10 | 0.65 ± 0.14 | 1.43 ± 0.12 | 1.47 ± 0.10 |
| Subcutaneous fat weight (g) | 0.21 ± 0.06 | 0.22 ± 0.07 | 3.37 ± 0.41 | 3.34 ± 0.30 |
| Epididymal fat weight (g) | 0.23 ± 0.06 | 0.26 ± 0.10 | 3.24 ± 0.25 | 3.02 ± 0.42 |
| Food intake (g/day/mouse) | 2.51 ± 0.16 | 2.48 ± 0.23 | 3.14 ± 0.18 | 3.10 ± 0.26 |
All values are mean ± SD, n = 6. Statistical analysis of the data for multiple comparisons was performed by ANOVA.
Fig. 2.
NASH pathological changes were exacerbated by knock-out of adropin in mice. Adropin-KO mice and the wild type (WT) littermate were fed with MCD or WD for 4 or 16 weeks. (A) H&E, Sirius Red and Oil Red O staining of liver sections (magnification, ×200), scale bar: 200 µm. (B-E) Hepatic histological analysis of H&E staining. (F) Quantitative analysis of Sirius Red staining. (G) Hepatic TG contents. (H) Serum ALT and AST levels. (I) The mRNA expression of Col1a1 and Acta2 in the liver. (J) The mRNA expression of Il1b, Il-6 and Tnf in the liver. (I, J) WT control group was set as 1. The data are expressed as the mean ± SD, n = 6, * P < 0.05 versus WT control.
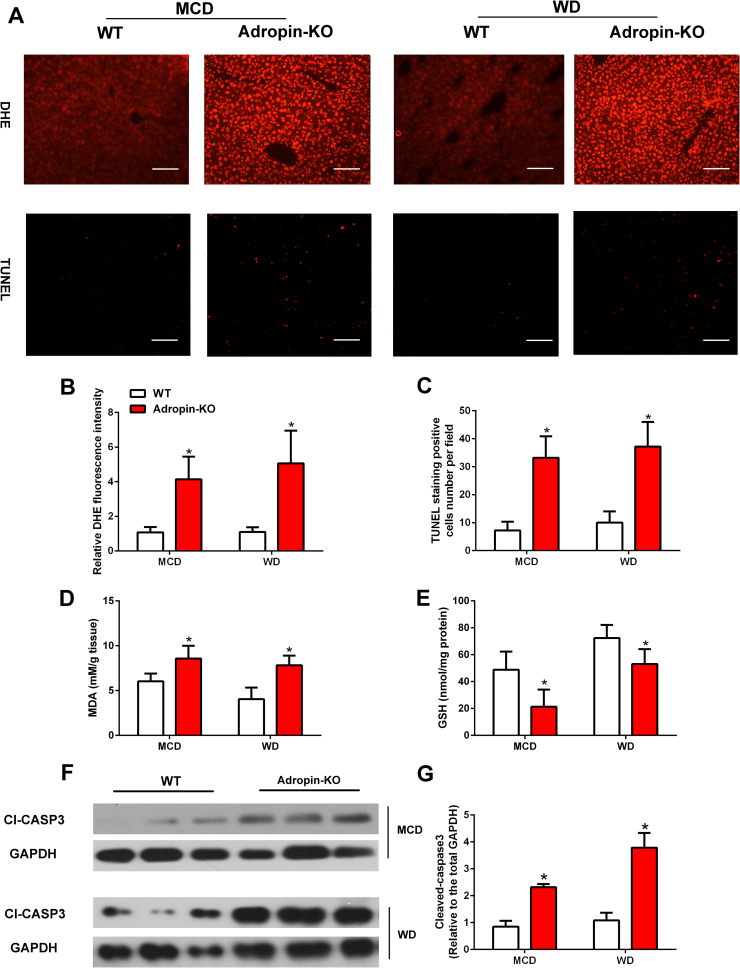
As for oxidative stress, ROS levels were significantly increased in the liver of Adropin-KO mice as compared to WT mice (Fig. 3A–B). And TUNEL-positive cells increased in Adropin-KO mice (Fig. 3A, C). Moreover, MDA levels, a product of lipid peroxidation, were notably higher in the livers of Adropin-KO mice (Fig. 3D). The GSH levels in liver were lower in Adropin-KO mice than WT mice (Fig. 3E). In addition, increased expression of cleaved caspase-3 was observed in Adropin-KO mice (Fig. 3F–G). Collectively, these data suggests that adropin plays an important role in the process of NASH in mice and its deficiency exacerbates key pathogenic events of NASH.
Fig. 3.
NASH diet induced liver oxidative stress was exaggerated in adropin-KO mice. Adropin-KO mice and the wild type (WT) littermate were fed with MCD or WD for 4 or 16 weeks. (A) DHE and TUNEL staining of liver sections (magnification, ×200), scale bar: 200 µm. (B) Quantitative analysis of DHE staining. (C) Quantitative analysis of TUNEL staining. (D) The liver MDA contents. (E) The liver GSH levels. (F-G) Cleaved caspase-3 expression of total liver lysates. (B, G) WT control group was set as 1. The data are expressed as the mean ± SD, n = 6, * P < 0.05 versus WT control.
3.3. Treatment with adropin alleviated hepatocyte injury in NASH mice and hepatocytes
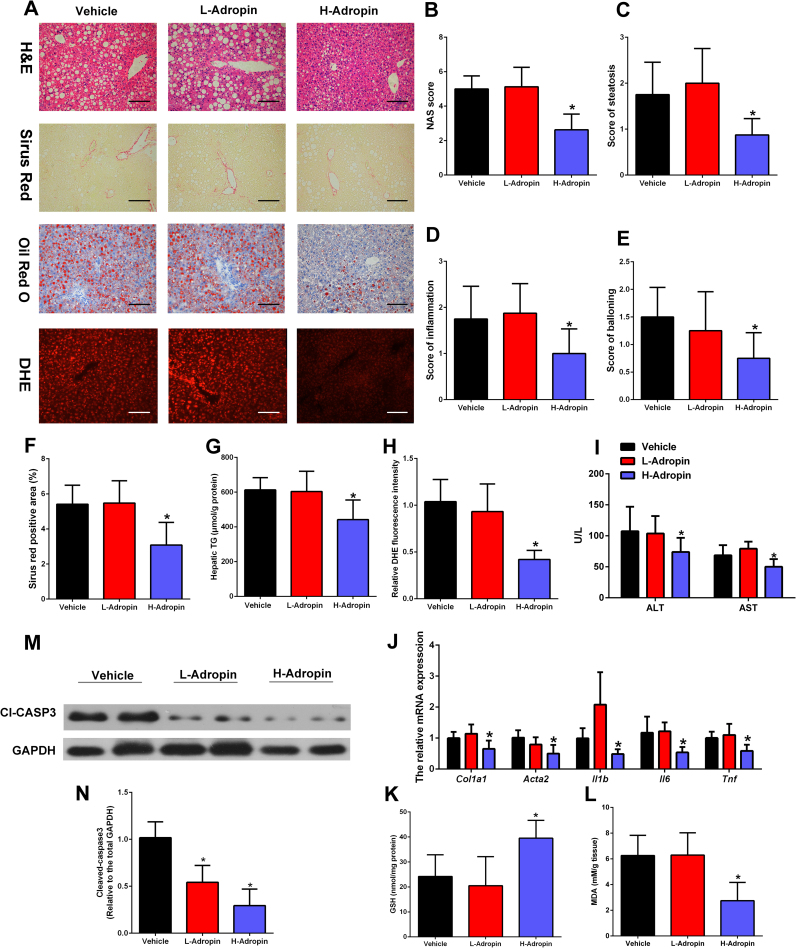
To investigate the therapeutic effects of adropin on NASH, mice were fed MCD for 4 weeks and then were randomly divided into 3 groups, which were administered daily intraperitoneal (i.p.) injections of vehicle, low-dose adropin peptide (34−76) (50 nmol/kg/i.p.) or high-dose adropin (500 nmol/kg/i.p.) for one week. Injections of adropin had no influence on the body weight, liver weight, adipose tissue weight and daily food intake in MCD-fed mice (Table 3). And high-dose adropin treatment attenuated the liver steatosis, inflammation and ballooning in MCD-fed mice (Fig. 4A–E). The H&E and Oil Red O staining showed high-dose adropin treatment shifted the liver steatosis from macrosteatosis to microsteatosis (Fig. 4A). Furthermore, compared with those in MCD control mice, liver fibrosis areas (Fig. 4F), hepatic TG (Fig. 4G), hepatic ROS (Fig. 4H) and serum ALT, AST levels (Fig. 4I) were attenuated in high-dose adropin treated mice. Moreover, the mRNA expression of genes related to fibrosis (Col1a1, Acta2) and inflammation (Il1b, Il6 and Tnf) was downregulated by high-dose adropin treatment (Fig. 4J). While the liver MDA levels were lower in the group with high-dose adropin treatment than in the vehicle group, the GSH levels were significantly higher (Fig. 4K–L). Furthermore, the injections of adropin decreased the hepatic levels of cleaved caspase-3 (Fig. 4M–N). These results suggest that the injections of the bioactive peptide adropin (34−76) can ameliorate NASH and oxidative stress in mice.
Table 3.
General parameters evaluated in C57BL/6 J male mice with intraperitoneal injections of vehicle, low-dose adropin or high-dose adropin.
| Vehicle | L-Adropin | H-Adropin | |
|---|---|---|---|
| Initial body weight (g) | 21.90 ± 1.81 | 21.75 ± 1.79 | 22.47 ± 1.90 |
| Final body weight (g) | 14.46 ± 1.51 | 14.64 ± 1.50 | 14.78 ± 1.55 |
| Liver weight (g) | 0.54 ± 0.07 | 0.57 ± 0.14 | 0.60 ± 0.11 |
| Subcutaneous fat weight (g) | 0.25 ± 0.09 | 0.28 ± 0.06 | 0.29 ± 0.10 |
| Epididymal fat weight (g) | 0.25 ± 0.11 | 0.30 ± 0.10 | 0.31 ± 0.11 |
| Food intake (g/day/mouse) | 2.41 ± 0.21 | 2.38 ± 0.30 | 2.40 ± 0.23 |
All values are mean ± SD, n = 8. Statistical analysis of the data for multiple comparisons was performed by ANOVA.
Fig. 4.
The bioactive adropin peptide ameliorated the liver injury in MCD-fed mice. C57BL/6 J mice were fed an MCD for four weeks and administered intraperitoneal (i.p.) injections of vehicle, low-dose adropin (34−76) (50 nmol/kg/i.p.), or high-dose adropin (500 nmol/kg/i.p.) for one week. (A) Representative liver histology (H&E staining, Sirius Red, Oil Red O and DHE staining) (magnification, ×200), scale bar: 200 µm. (B-E) Hepatic histological analysis of H&E staining. (F) Quantitative analysis of Sirius Red staining. (G) Hepatic TG contents. (H) Quantitative analysis of DHE staining. (I) Serum ALT and AST levels. (J) The mRNA expression of Col1a1, Acta2, Il1b, Il-6 and Tnf in the liver. (K) The liver MDA levels. (L) The liver GSH levels. (M-N) Cleaved caspase-3 expression of total liver lysates. (H, J, N) Vehicle control group was set as 1. The data are expressed as the mean ± SD, n = 8, * P < 0.05 versus control group.
In accordance with the findings in mice, palmitate (PA)-treated cells showed a substantial increase in the ROS levels, which were reduced by adropin treatment in a dose- and time-dependent manner (Fig. S4A–B). And adropin can significantly lower the O2·- and H2O2 levels induced by PA treatment (Fig. S4C–D). Furthermore, adropin treatment elevated the mitochondrial membrane potential (MMP) in a dose- and time-dependent manner (Fig. S4E–F). Cleaved caspase-3, which was elevated by PA treatment, was maintained at a normal level by adropin (Fig. S4G–H). In addition, analysis of cytosol and mitochondrial fractions showed that adropin can abrogate the cytochrome c release and Bax mitochondrial translocation that were caused by palmitate treatment (Fig. S4I–N). Overall, these results demonstrate treatment with adropin can alleviate hepatocyte injury of NASH in vivo and in vitro.
3.4. Adropin elicited antioxidant reaction through the Nrf2 pathway
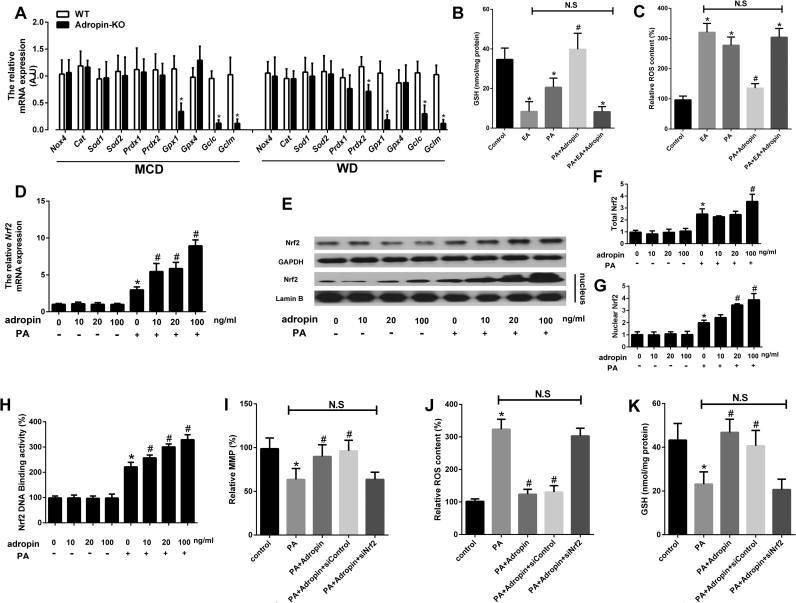
In adropin-KO mice, there was no difference of Nox4, superoxide dismutase (Sod1, Sod2), catalase (Cat) and peroxiredoxin (Prdx1) expression as compared to the control, but the mRNA expression of glutathione peroxidase-1 (Gpx1) and glutamate-cysteine ligase subunits (Gclc, Gclm) was much lower (Fig. 5A). In hepatocytes, adropin treatment also elevated the GSH levels and the expression of Gclc, Gclm and Gpx1 in a dose-dependent manner (Fig. S5A–B). Ethacrynic acid (EA) incubation can significantly decrease the GSH levels (Fig. 5B). And adropin administration failed to decrease ROS levels by EA incubation (Fig. 5C). Therefore, it could be inferred that adropin can increase the expression of γ-glutamylcysteine synthase (γ-GCS), which is the first rate-limiting enzyme in GSH synthesis to increase the amount of GSH. Furthermore, adropin can elevate GPX1, which can convert H2O2 to H2O to reduce the ROS levels in liver. These results suggest that adropin enhances the antioxidant reaction to protect against NASH progression.
Fig. 5.
Adropin induced antioxidant reaction and activated the Nrf2 pathway. (A) Adropin-KO mice and the wild type (WT) littermate were fed with MCD or WD for 4 or 16 weeks. The mRNA expression of antioxidant related genes were measured. WT control group was set as 1. The data are expressed as the mean ± SD, n = 6, * P < 0.05 versus WT control. Primary murine hepatocytes preloaded with PA (400 µM) were treated with or without adropin (100 ng/ml) or ethacrynic acid (EA) (2 mg/ml) for 24 h. The intracellular GSH levels (B) and the relative ROS content (C) were measured. Primary murine hepatocytes pretreated with PA (400 µM) were treated with adropin at different dosages (0–100 ng/ml) for 24 h. The mRNA expression of Nrf2 (D), the protein expression of Nrf2 (E-G) and the Nrf2 transcription activity (H) were measured. Primary murine hepatocytes preloaded with PA (400 µM) were treated with or without adropin (100 ng/ml) and transfected with or without Nrf2 siRNA for 24 h. And the relative MMP (I), intracellular ROS content (J) and GSH levels (K) were measured. (C, D, F, G, H, I, J) Blank control group was set as 1. The data are expressed as the mean ± SD (n = 3–5, * P < 0.05 versus blank control group; # P < 0.05 versus PA-treatment group).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important endogenous transcription factor for cells to defend against oxidative stress. Under physiological conditions, Nrf2 binds to Keap1 in the cytoplasm. Under stress conditions, Nrf2 disaggregates from Nrf2/Keap1 complex and then translocates into the nucleus where it binds to the antioxidant response element (ARE) to initiate the transcription of antioxidant related genes. The qPCR and western blot results showed that PA can relatively elevate Nrf2 expression and induce its nuclear translocation, while adropin caused the greater elevation of Nrf2 expression, which was further confirmed by immunofluorescence measurement (Fig. 5D–G, Fig. S6A). Furthermore, adropin can significantly enhance Nrf2 transcriptional activity (Fig. 5H). To verify that Nrf2 activation participated in adropin cytoprotection, we transfected cells with siRNA to knock down the expression of Nrf2. And under Nrf2 knockdown conditions, adropin failed to protect hepatocytes from palmitate-induced MMP loss and ROS increase (Fig. 5I–J). In addition, adropin cannot upregulate Gclc, Gclm or Gpx1 expression or the GSH levels when Nrf2 was knocked down (Fig. 4K, Fig. S6B–D).
3.5. Adropin upregulated Nrf2 transcription activity through CBP
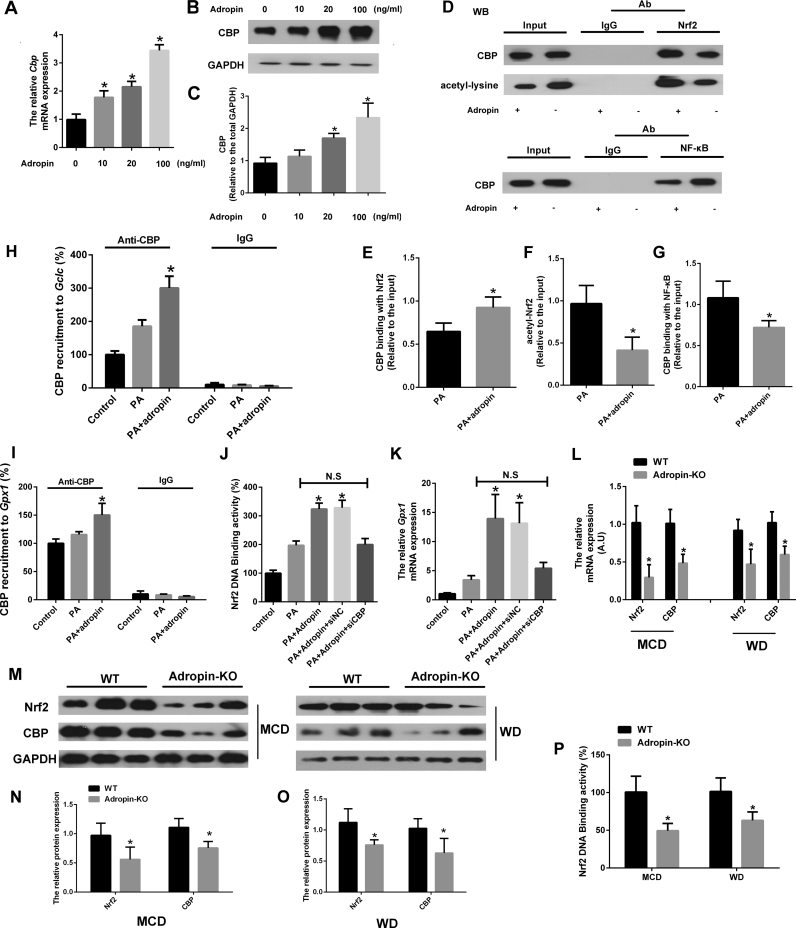
Since CBP-induced acetylation of Nrf2 was found to increase the binding of Nrf2 to ARE and increase Nrf2-dependent transcription, we further examined its role in adropin upregulation of Nrf2 transcriptional activity. Fig. 6A–C showed that adropin enhanced CBP expression at both the mRNA and protein levels. And we immunoprecipitated nuclear extracts of treated cells by anti-Nrf2 or anti-NF-κB antibody, increases in acetylated Nrf2 protein levels and the binding of Nrf2 with CBP and a reduction in the binding of NF-κB with CBP were found by adropin administration (Fig. 6D–G). In addition, ChIP analysis indicated that adropin-induced increase in CBP levels significantly upregulated the transactivation of Gclc and Gpx1 (Fig. 6H–I). When CBP was knocked down by siRNA, adropin administration failed to increase Nrf2 transcriptional activity (Fig. 6J–K). Furthermore, Nrf2 and CBP expression were remarkably downregulated in Adropin-KO mice fed with NASH diet (Fig. 6L–O). And the Nrf2 DNA binding activity was also impaired in the liver of Adropin-KO mice (Fig. 6P). Collectively, these results show that adropin alleviates oxidative stress through the induction of Nrf2 activity and CBP plays a vital role in adropin-induced Nrf2 transcriptional activity.
Fig. 6.
Adropin increased CBP expression and its binding with Nrf2 to enhance Nrf2 transcriptional activity. Primary murine hepatocytes pretreated with PA (400 µM) were treated with adropin at different dosages (0–100 ng/ml) for 24 h. The mRNA expression of Cbp (A) and the protein expression of CBP (B-C) were measured. CBP-Nrf2 and CBP-NFκB interactions were studied by immunoprecipitation (IP) in the hepatocytes treated with or without adropin (100 ng/ml) (D-G). Chromatin immunoprecipitation (ChIP) assays were performed to investigate the presence of CBP at the antioxidant response element (ARE) sites on the promoters of Gclc (H) and Gpx1 (I) in the hepatocytes treated with or without adropin (100 ng/ml). Primary murine hepatocytes pretreated with PA (400 µM) were treated with or without adropin (100 ng/ml) and transfected with CBP siRNA or control for 24 h. And the DNA-binding activity of Nrf2 (J) and the mRNA expression of Gpx1 (K) were measured. (A, C, E, F, G) PA-treated group was set as 1. (H, I, J, K) Blank control group was set as 1. The data are expressed as the mean ± SD, n = 3–5, *P < 0.05 versus PA-treatment group. NC indicates negative control. N.S indicates no significance. Adropin-KO mice and the wild type (WT) littermate were fed with MCD or WD for 4 or 16 weeks. The mRNA (L) and protein expression (M-O) of Nrf2 and Cbp were measured. And the liver lysates DNA-binding activity of Nrf2 was detected (P). (L, N, O, P) WT control group was set as 1. The data are expressed as the mean ± SD, n = 6, *P < 0.05 versus WT control.
3.6. Overexpression of hepatic Nrf2 expression functionally restored the liver injury induced by adropin-deficiency in mice
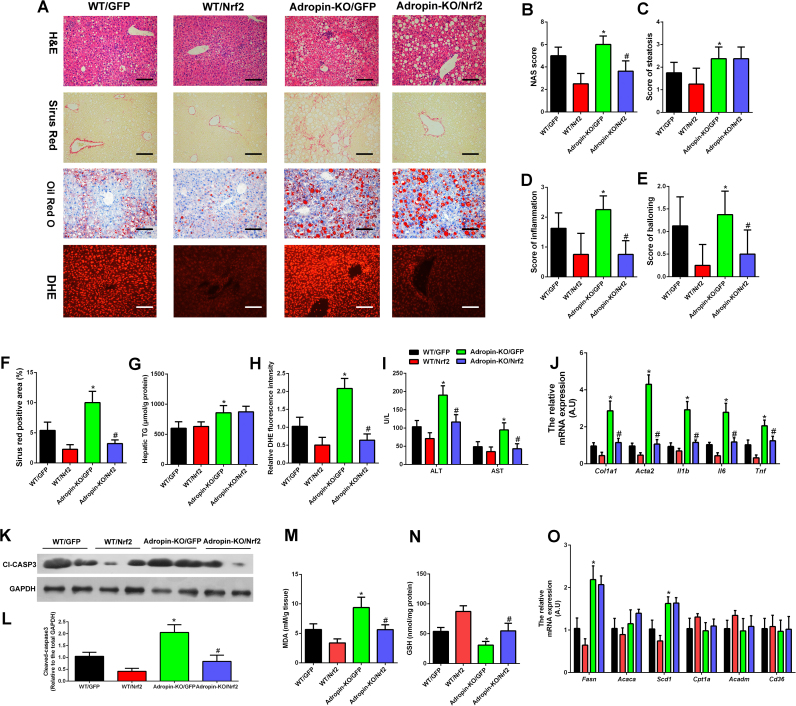
The above studies illustrated the peptide adropin enhanced Nrf2 transcription activity to alleviate the oxidative stress and liver injury. To further test this in vivo, we generated Nrf2 overexpression adeno-associated virus (AAV) type 8 under control of the Tbg promoter. Tail vein injection of AAV-Nrf2 increased the Nrf2 expression specifically in the liver (Fig. S5). We transduced WT and Adropin-KO mice with AAV-GFP or AAV-Nrf2 and fed these mice with MCD for 4 weeks. The body weight change, liver weight, adipose tissue weight and food intake information during the experiment period were shown in Table 4. As what we observed above, adropin deficiency exacerbated steatosis, inflammation and fibrosis in the NASH progression. Overexpression of Nrf2 in liver improved the NASH in WT mice and eliminated worsening of inflammation and fibrosis but had no effect on steatosis in Adropin-KO mice (Fig. 7A–F). Oil Red O staining and hepatic triglyceride (TG) measurement also demonstrated overexpression of Nrf2 in the liver failed to decrease the lipid accumulation in Adropin-KO mice (Fig. 7A, G). DHE staining showed the ROS levels were lowered by AAV-Nrf2 (Fig. 7A, H). Furthermore, the serum ALT and AST levels were decreased by AAV-Nrf2 treatment in adropin-KO mice (Fig. 7I). CD45 and MCP1 staining showed that less inflammatory responses were found within the liver tissues of the AAV-Nrf2 treated-mice (Fig. S8A–C). And the induction of genes related to inflammation and fibrosis in Adropin-KO mice was attenuated by AAV-Nrf2 (Fig. 7J). Accordingly, caspase 3 cleavage (Fig. 7K–L) and MDA levels (Fig. 7M) returned to control levels by AAV-Nrf2 administration and the liver GSH levels (Fig. 7N) were increased by AAV-Nrf2 in Adropin-KO mice. Notably, the genes involved in the lipogenesis were upregulated in adropin-KO mice, which could not be rescued by overexpression of Nrf2 pathway (Fig. 7O). This may partly explain the Nrf2 overexpression could not improve the steatosis in Adropin-KO mice. Overall, these results support the important functional role of Nrf2 activity in mediating adropin's effects on NASH progression.
Table 4.
General parameters evaluated in WT or adropin-KO mice with administration of AAV8-GFP or AAV8–Nrf2 vectors.
| WT |
Adropin-KO |
|||
|---|---|---|---|---|
| AAV8-GFP | AAV8–Nrf2 | AAV8-GFP | AAV8–Nrf2 | |
| Initial body weight (g) | 22.28 ± 2.34 | 21.91 ± 2.64 | 22.88 ± 3.06 | 21.75 ± 3.31 |
| Final body weight (g) | 15.10 ± 1.64 | 14.89 ± 1.46 | 15.55 ± 1.34 | 15.38 ± 1.40 |
| Liver weight (g) | 0.57 ± 0.10 | 0.60 ± 0.15 | 0.57 ± 0.10 | 0.63 ± 0.12 |
| Subcutaneous fat weight (g) | 0.29 ± 0.13 | 0.24 ± 0.08 | 0.26 ± 0.10 | 0.28 ± 0.12 |
| Epididymal fat weight (g) | 0.29 ± 0.11 | 0.30 ± 0.14 | 0.27 ± 0.13 | 0.28 ± 0.10 |
| Food intake (g/day/mouse) | 2.46 ± 0.29 | 2.49 ± 0.33 | 2.44 ± 0.38 | 2.39 ± 0.43 |
All values are mean ± SD, n = 8. Statistical analysis of the data for multiple comparisons was performed by ANOVA.
Fig. 7.
Overexpression of hepatic Nrf2 expression functionally restored the liver injury induced by adropin-deficiency in mice. WT and Adropin-KO male mice fed MCD for 4 weeks. AAV8-GFP and AAV8–Nrf2 vectors were administered following the initiation of MCD feeding. (A) H&E, Sirius Red, Oil Red O, and DHE staining of liver sections (magnification, ×200), scale bar: 200 µm. (B-E) Hepatic histological analysis of H&E staining. (F) Quantitative analysis of Sirius Red staining. (G) Hepatic TG contents. (H) Quantitative analysis of DHE staining. (I) Serum ALT and AST levels. (J) The mRNA expression of Col1a1, Acta2, Il1b, Il6 and Tnf in the liver. (K-L) Cleaved caspase-3 expression of total liver lysates. (M) The liver MDA levels. (N) The liver GSH levels. (O) The mRNA expression of Fasn, Acaca, Scd1, Cpt1a, Acadm and Cd36. (H, J, L, O) WT/GFP group was set as 1. The data are expressed as the mean ± SD, n = 8, * P < 0.05 wt/GFP versus Adropin-KO/GFP; #P < 0.05 Adropin-KO/GFP versus Adropin-KO/Nrf2.
4. Discussion
Adropin was first discovered as a peptide that regulates glycolipid metabolism in 2008 [8]. It has also been shown that adropin can regulate the expression of hepatic lipid synthesis genes [8]. In addition, a large number of population studies confirmed that the serum levels of adropin were negatively correlated with the risk factors of metabolic diseases [14], [15], [16]. However, adropin's role in regulating nonalcoholic steatohepatitis was still unclear. For the first time, our study confirms that the expression of adropin decreased in NASH models of mice fed MCD or WD. Our research also elucidates its protective mechanism involved in nonalcoholic steatohepatitis. The results demonstrate that adropin can activate the Nrf2 pathway to elicit an antioxidant response, which may alleviate the progress of nonalcoholic steatohepatitis. Our study results suggest that adropin may be a new biomarker for the diagnosis of NASH and a new target for the treatment of NASH.
Nonalcoholic fatty liver disease is not a single disease entity. It describes a spectrum of liver conditions that range from simple steatosis to nonalcoholic steatohepatitis coupled with marked inflammation and fibrosis to severe liver disease such as cirrhosis and possibly hepatocellular carcinoma. The biological mechanisms causing progression through the spectrum of NAFLD stages are not well defined. The initial hypothesis proposed by Day and James was the ‘two-hit’ model [17]. Insulin resistance (IR) plays an important role in the “first hit”; IR leads to hepatic de novo lipogenesis and impairs fatty acid (FA) export, which results in hepatic steatosis. In addition, on the basis of the first hit, the liver is sensitive to injury. Furthermore, lipid peroxidation damage and oxidative stress, which were caused by the “secondary hit”, induce inflammation, hepatic cell degeneration or necrosis and even liver fibrosis and the occurrence of cirrhosis. To study the hepatic injury from SS to NASH, MCD or long-term WD diet-induced NASH is widely used animal models. And we observed that the decreased expression of adropin was associated with liver injury in the diet-induced NASH mice. In addition, in vivo studies demonstrated that adropin deficiency can accelerate the liver cells apoptosis and exacerbate NASH progression in mice. In vitro, adropin can also elevate the cell viability, which was decreased by the pretreatment of palmitate. These results suggested that adropin plays a vital role in alleviating liver injury in the course of NASH progression.
One of the mechanisms by which free fatty acids induce lipotoxicity is the production of excessive reactive oxygen species by a large number of free fatty acids in the process of oxidation, which can destroy the reactive oxygen system balance [18]. Excessive reactive oxygen production can cause lipid peroxidation [19], inflammation, destruction of protein and enzyme activity [20], and cell dysfunction and apoptosis [21]. A large number of population studies have confirmed that levels of reactive oxygen species are increased in NASH patients [22], [23] and that the levels of ROS were associated with the extent of liver damage [24]. A clinical randomized controlled double-blind experiment also confirmed that vitamin E, as a natural scavenging reactive oxygen species, can relieve liver damage in NASH patients [25]. ROS, including O2·-, H2O2 and ONOO−, are produced by almost all cell types. NADPH oxidase is major source of ROS production [26]. NADPH oxidases may contribute to hepatic oxidative injury by producing O2·- [27]. However, our results showed that adropin had no significant effect on Nox4 expression, which suggested that adropin had no effect on the production of ROS derived from NADPH oxidase. In addition to NOX, mitochondria are thought to be primary contributors to oxidative stress because the mitochondrial single-electron transport chain can produce most of the ROS, such as O2·- [28]. ROS generation is proportional to transmembrane potential [29]. We observed that the mitochondrial membrane potential was significantly decreased and that the O2·- levels increased in the NASH model, while adropin treatment significantly elevated mitochondrial membrane potential and reduced the O2·- levels. This demonstrates that adropin possesses the capability to alleviate the ROS predominately produced from liver mitochondria. In hepatocytes, O2·- can be converted into H2O2 and further eliminated to H2O by SOD or GPX and other antioxidant enzymes or compounds. In support of this, we observed that adropin increased the GSH levels and the expression of Gclc, Gclm and Gpx1. Altogether, the results of our study suggested that adropin may favor mitochondrial function and antioxidant enzymes to alleviate oxidative stress and apoptosis, resulting in protection against the course of NASH development.
Nrf2 is the most important transcription factor involved in the induction of antioxidant responses [30]. It has been well documented that Nrf2-dependent antioxidant and cytoprotective genes contain almost all antioxidant enzymes, such as heme oxygenase and some members of the GST family [31]. When the cells are exposed to oxidative stress, Nrf2 translocates to the nucleus and further activates ARE-mediated downstream gene expression [32]. It is now well accepted that complete loss of Nrf2 in liver leads to aggravate NASH progression [33]. And activation of Nrf2 pathway in the liver attenuates the liver injury in NASH [34], [35]. Similar results were found in our study. Overexpression of hepatic Nrf2 expression functionally attenuated the liver injury in MCD-fed mice. So Nrf2 is a key target in the future NASH treatment. Furthermore, Our results showed that adropin can significantly upregulate the Nrf2 activity. Adropin-KO mice exhibited lower expression and activity of Nrf2 by NASH diet feeding and adropin treatment can increase the level of Nrf2 in the nucleus and its transcriptional activity in vivo and in vitro, thereby increasing the expression of downstream antioxidant genes (Gclc, Gclm and Gpx1). However, it is worth noting that activation of Nrf2 pathway by AAV abolished worsening of inflammation and fibrosis but had no effect on steatosis in Adropin-KO mice. This suggests that systemic adropin deficiency in mice might accelerate NASH progression not only via oxidative stress in liver. Adropin can regulate glucose metabolism which seems irrelevant of Nrf2 and we indeed found adropin-deficiency exacerbated the insulin resistance in WD-fed mice. Furthermore, in accordance with previous researches [8], [36], we also found genes involved in the lipogenesis were upregulated in adropin-KO mice by NASH diet feeding, which could not be rescued by the enhanced antioxidant reaction through Nrf2 pathway. This may partly explain that Nrf2 overexpression had no significant effect on liver steatosis in adropin-KO mice. Future studies investigate deeply into the physiological function of adropin and its role in metabolic diseases may shed light on this issue.
CBP has been reported to bind to the Nrf2 transcription region to promote its transcriptional activity [31], [37]. In addition, CBP can promote the localization of Nrf2 in the nucleus via acetylation of Nrf2 and thus increase Nrf2 transcriptional activity of its downstream genes [38], [39]. Liu et al. [40] have also indicated NF-κB p65 can inhibit the ability of the Nrf2-induced antioxidant response by dissociating the binding of Nrf2 with CBP. In our study, we observe that adropin can increase the expression of CBP, promote its interaction with Nrf2 and inhibit its binding with NF-κB. And adropin failed to induce an increase in Nrf2 transcriptional activity when CBP was knocked down, suggesting that the adropin-induced Nrf2 activation is CBP dependent. It is worth noting that except for the CBP, there are other cofactors that can associate with the Nrf2 and regulate its activity. We also roughly screened the expression of some factors such as Maff, Mafg, Mafk, c-jun and p300. And adropin treatment did not affect their expression like CBP (data not shown). Therefore, in this study we mainly investigated the CBP-Nrf2 interaction in the adropin protective effects. However, we cannot totally exclude other cofactors in this process because they can also modulate Nrf2 activity by binding to Nrf2 or modifying Nrf2 without altering the expression themselves. Further more detailed studies of adropin's regulation of Nrf2 activity may clarify this matter. On the other hand, we also found that adropin-KO mice had lower expression of Nrf2, while adropin treatment upregulated Nrf2 expression. However, this could not be explained by upregulated expression of CBP. In recent years, it has been reported that Nrf2 expression is modulated by many ways of epigenetic modification such as DNA methylation [41], histone modifications [42] and microRNA [43]. Whether epigenetic modification is involved in the upregulation of Nrf2 by adropin is a direction for future research and needs to be further clarified.
Although the prevalence of NAFLD has gradually increased, there are still insufficient effective measures for the clinical treatment of NASH [44]. Due to the critical pathological features of NASH, such as oxidative stress, hepatocyte damage and apoptosis in the progression of NASH, few compounds such as vitamin E [45], CCR2/CCR5 inhibitors and GS-4997 (an inhibitor of apoptosis signal-regulating kinase 1) [46] have been tested for alleviating the progress of NASH. And we attempted to explore whether adropin can be used as a promising peptide for the treatment of NASH. We observed that the injection of adropin peptide can significantly alleviate the progression of MCD-induced NASH in mice. Furthermore, the injection of adropin activated the Nrf2 pathway and increased the antioxidant responses of MCD mice, thus attenuating liver injury.
5. Conclusion
The present study demonstrated that adropin plays a protective role in liver injury of NASH by regulating the Nrf2-ROS pathway and provided a novel viewpoint that adropin could be a potential peptide for use in the treatment of NASH.
Financial support
This work was supported by funding from the Major Projects of Guangzhou Health Collaborative Innovation [Grant number 201604020002 (No. 20, Topic 4, Phase III)], the State Key Program of National Natural Science Foundation of China [Grant number 81730090] and the Guangdong Science and Technology Project [Grant number 2016A050502013].
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.101068.
Contributor Information
Xu Chen, Email: cxu1024@gmail.com.
Hongliang Xue, Email: xuehongliang490@foxmail.com.
Wanjun Fang, Email: fangwj5@mail2.sysu.edu.cn.
Ke Chen, Email: chenk67@mail2.sysu.edu.cn.
Shen Chen, Email: 834035345@qq.com.
Wenqi Yang, Email: yangwenqigw@126.com.
Tianran Shen, Email: tianranshen@gmail.com.
Xuechen Chen, Email: chxuech@mail2.sysu.edu.cn.
Peiwen Zhang, Email: 313743920@qq.com.
Wenhua Ling, Email: lingwh@mail.sysu.edu.cn.
Appendix A. Supplementary material
Supplementary material
References
- 1.Tiniakos D.G., Vos M.B., Brunt E.M. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu. Rev. Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 2.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 3.Brunt E.M. Pathology of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010;7:195–203. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 5.Malhi H., Gores G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spahis S., Delvin E., Borys J.M., Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid. Redox Signal. 2017;26 doi: 10.1089/ars.2016.6776. (519-+) [DOI] [PubMed] [Google Scholar]
- 7.Khedmat H., Taheri S. Non-alcoholic steatohepatitis: an update in pathophysiology, diagnosis and therapy. Hepat. Mon. 2011;11:74–85. [Google Scholar]
- 8.Kumar K.G., Trevaskis J.L., Lam D.D., Sutton G.M., Koza R.A., Chouljenko V.N. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao S., McMillan R.P., Jacas J., Zhu Q., Li X., Kumar G.K. Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes. 2014;63:3242–3252. doi: 10.2337/db14-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhla A., Hahn S., Butschkau A., Lange S., Wree A., Vollmar B. Lifelong caloric restriction reprograms hepatic fat metabolism in mice. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 2014;69:915–922. doi: 10.1093/gerona/glt160. [DOI] [PubMed] [Google Scholar]
- 11.Partridge C.G., Fawcett G.L., Wang B., Semenkovich C.F., Cheverud J.M. The effect of dietary fat intake on hepatic gene expression in LG/J AND SM/J mice. BMC Genom. 2014;15:99. doi: 10.1186/1471-2164-15-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar K.G., Zhang J.Y., Gao S., Rossi J., McGuinness O.P., Halem H.H. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity. 2012;20:1394–1402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thapa D., Stoner M.W., Zhang M., Xie B., Manning J.R., Guimaraes D. Adropin regulates pyruvate dehydrogenase in cardiac cells via a novel GPCR-MAPK-PDK4 signaling pathway. Redox Biol. 2018;18:25–32. doi: 10.1016/j.redox.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yosaee S., Khodadost M., Esteghamati A., Speakman J.R., Shidfar F., Nazari M.N. Metabolic syndrome patients have lower levels of adropin when compared with healthy overweight/obese and lean subjects. Am. J. Mens. Health. 2017;11:426–434. doi: 10.1177/1557988316664074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oruc C.U., Akpinar Y.E., Dervisoglu E., Amikishiyev S., Salmaslioglu A., Gurdol F. Low concentrations of adropin are associated with endothelial dysfunction as assessed by flow-mediated dilatation in patients with metabolic syndrome. Clin. Chem. Lab. Med. 2017;55:139–144. doi: 10.1515/cclm-2016-0329. [DOI] [PubMed] [Google Scholar]
- 16.Wu L.Z., Fang J., Chen L.L., Zhao Z.W., Luo Y.K., Lin C.G. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 2014;52:751–758. doi: 10.1515/cclm-2013-0844. [DOI] [PubMed] [Google Scholar]
- 17.Day C.P., James O.F. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 18.Egnatchik R.A., Leamy A.K., Noguchi Y., Shiota M., Young J.D. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metab.-Clin. Exp. 2014;63:283–295. doi: 10.1016/j.metabol.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihalas B.P., De Iuliis G.N., Redgrove K.A., McLaughlin E.A., Nixon B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-06372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damgaard D., Bjorn M.E., Jensen P.O., Nielsen C.H. Reactive oxygen species inhibit catalytic activity of peptidylarginine deiminase. J. Enzym. Inhib. Med. Chem. 2017;32:1203–1208. doi: 10.1080/14756366.2017.1368505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalashnikova I., Mazar J., Neal C.J., Rosado A.L., Das S., Westmoreland T.J. Nanoparticle delivery of curcumin induces cellular hypoxia and ROS-mediated apoptosis via modulation of Bcl-2/Bax in human neuroblastoma. Nanoscale. 2017;9:10375–10387. doi: 10.1039/c7nr02770b. [DOI] [PubMed] [Google Scholar]
- 22.Irie M., Sohda T., Iwata K., Kunimoto H., Fukunaga A., Kuno S. Levels of the oxidative stress marker gamma-Glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J. Int. Med. Res. 2012;40:924–933. doi: 10.1177/147323001204000311. [DOI] [PubMed] [Google Scholar]
- 23.Seki S., Kitada T., Yamada T., Sakaguchi H., Nakatani K., Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 24.Liu S.L., Shi W., Li G.L., Jin B., Chen Y.Q., Hu H. Plasma reactive carbonyl species levels and risk of non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2011;26:1010–1015. doi: 10.1111/j.1440-1746.2011.06672.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal A.J., Mofrad P.S., Contos M.J., Sargeant C., Luketic V.A., Sterling R.K. A pilot study of Vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Redondo A.B., Aguado A., Briones A.M., Salaices M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 2016;114:110–120. [Google Scholar]
- 27.Chao T.I., Jiang X.S., Fukada H., Haj F., Bettaieb A., Torok N. Hepatocyte NOX4 plays an important role in modulating stress response-mediated fibrogenic injury during NASH. Hepatology. 2013;58 (221a-a) [Google Scholar]
- 28.Fulle S., Protasi F., Di Tano G., Pietrangelo T., Beltramin A., Boncompagni S. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Brookes P.S. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic. Biol. Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Gupte A.A., Lyon C.J., Hsueh W.A. Nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2), a key regulator of the antioxidant response to protect against atherosclerosis and nonalcoholic steatohepatitis. Curr. Diabetes Rep. 2013;13:362–371. doi: 10.1007/s11892-013-0372-1. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T., Sherratt P.J., Pickett C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 32.Keleku-Lukwete N., Suzuki M., Yamamoto M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid. Redox Signal. 2017 doi: 10.1089/ars.2017.7358. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhry S., Nazmy M.H., Meakin P.J., Dinkova-Kostova A.T., Walsh S.V., Tsujita T. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2010;48:357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Ramadori P., Drescher H., Erschfeld S., Schumacher F., Berger C., Fragoulis A. Hepatocyte-specific Keap1 deletion reduces liver steatosis but not inflammation during non-alcoholic steatohepatitis development. Free Radic. Biol. Med. 2016;91:114–126. doi: 10.1016/j.freeradbiomed.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Sharma R.S., Harrison D.J., Kisielewski D., Cassidy D.M., McNeilly A.D., Gallagher J.R. Experimental nonalcoholic steatohepatitis and liver fibrosis are ameliorated by pharmacologic activation of Nrf2 (NF-E2 p45-related factor 2) Cell. Mol. Gastroenterol. Hepatol. 2018;5:367–398. doi: 10.1016/j.jcmgh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganesh Kumar K., Zhang J., Gao S., Rossi J., McGuinness O.P., Halem H.H. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity. 2012;20:1394–1402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katoh Y., Itoh K., Yoshida E., Miyagishi M., Fukamizu A., Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 38.Sun Z., Chin Y.E., Zhang D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai Y., Garduno L., Theodore M., Yang J., Arinze I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G.H., Qu J., Shen X. NF-kappab/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Khor T.O., Huang Y., Wu T.Y., Shu L.M., Lee J., Kong A.N.T. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82:1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 42.Correa F., Mallard C., Nilsson M., Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3 beta. Neurobiol. Dis. 2011;44:142–151. doi: 10.1016/j.nbd.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sangokoya C., Telen M.J., Chi J.T. MicroRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oseini A.M., Sanyal A.J. Therapies in non-alcoholic steatohepatitis (NASH) Liver Int. 2017;37(Suppl 1):97–103. doi: 10.1111/liv.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowdley K.V., Wilson L.A., Van Natta M.L., Pai R.K., Sanyal A.J. Efficacy and safety of vitamin E in nonalcoholic steatohepatitis patients With and Without diabetes: pooled analysis from the PIVENS and FLINT NIDDK NASH CRN trials. Hepatology. 2015;62 (264a-a) [Google Scholar]
- 46.Rotman Y., Sanyal A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–190. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material