Abstract
Background
The US Preventive Services Task Force of Colorectal Cancer (USPSTF) recommends against continuing screening for colorectal cancer (CRC) past 75 years in adequately screened individuals. Survival and staging data for CRC that compares elderly vs. younger populations has not been published. This study aims to compare staging (0–4) of CRC in groups of 60–69, 70–79 and 80–89-year-old; also, to compare surgical and no treatment (i.e., no surgery) survival outcomes (5–10 years) in these age groups.
Methods
Male veterans within groups 60–69, 70–79 and 80–89 years of age who were diagnosed with CRC between 2000 and 2015 were selected from Veterans affairs national cancer cube registry.
Results
Their staging, surgery or no treatment, and 5–10 years survival data was obtained from the cancer cube. Surgical and survival data was obtained only for stage 0-2 as surgery is currently the standard of treatment for these stages.
Conclusions
Highest number of CRC cases diagnosed across each age group was stage 1 with stage 2 being second. In surgical treatment group the survival was statistical different for 80–89 age group as compared to 60–69 (34.4%) and 70–79 (30.86%) although octogenarians did have a surprisingly high mean of 25.45%. The 5–10-year survival data for no treatment group (i.e., no surgery) was very poor.
Keywords: Colorectal cancer (CRC), octogenarians, survival, staging, surgery
Introduction
Colorectal cancer (CRC) is very debilitating and common disease. According to Surveillance, Epidemiology and End Results (SEER) (1) database CRC is the 4th most common type of cancer and 2nd highest in cancer related death after lung in the United States. Around 140,250 new cases of CRC are diagnosed annually in the USA out of which 97,220 are colon and 43,030 are rectal (2). Almost 50,630 Americans are expected to die from colon cancer each year. CRC incidence is very low before age 40 and rises progressively thereafter to almost 3.7/1,000 per year by age 80 (SEER). CRC originates in adenomas or flat dysplasia and evolve into cancer with slow progression over a decade. Colorectal screening is the process of detecting early-stage CRCs and precancerous lesions in asymptomatic people. Since the implementation of CRC screening the death rate from CRC has been declining at the rate of 2.5 to 3 percent each year between 2004 and 2013. We have seen 53% reduction in CRC mortality (3). The US Preventive Services Task Force of Colorectal Cancer (USPSTF) is a panel of gastroenterologist recommends screening asymptomatic average risks persons beginning at 50 years of age and stopping at age 75 in adequately screened individuals (4).
Screening can be done via either direct visualization method such as colonoscopy, CT colonography, flexible sigmoidoscopy, flexible sigmoidoscopy with fecal immunochemical test (FIT) or via stool-based tests such as guaiac-based fecal occult blood test, FIT, or multi-targeted stool DNA test or via serology test such ad SEPT9 DNA test (4). Though according to USPSTF there is no difference in the effectiveness of these above studies, colonoscopy is better in detecting precancerous lesions and can be therapeutic at the time of the procedure by removing the adenomatous polyp prior to malignant transformation (5).
Once the diagnosis of CRC is made, staging is an important factor as it guides our treatment. Tumor, node, metastasis (TNM) staging from the combined American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) is the preferred staging system (6). Treatment for stage 1 is surgical resection alone. For stage 3 which includes lymph node positive disease, curative surgery is followed with adjuvant chemotherapy as standard of care. For stage 2 disease which includes node negative disease, adjuvant chemotherapy has not shown any significant benefits (7). Approximately 80 percent of colon cancers are usually localized to either the wall (39%) or regional lymph node (37%) (8). Laparoscopic assisted colectomy is an acceptable option for localized cancer (9).
Five-year survival shown by SEER database is around 65% for CRC (10). The survival drops from stage 1 being 93%, stage 2 being 72–85%, stage 3 being 44 to 83% and stage 4 being 8% (11). Survival is higher for lower stages. The SEER database is geographically limited and is not nationwide. The SEER registry consists of 10–12 states. Though it gives a good approximation about the overall survival of colon cancer, we need more nationwide studies to compare numbers.
Octogenarians fall outside the age bracket for screening colonoscopy but usually tend to have colonoscopies for diagnostic reasons. USPSTF recommends against screening these patients as they are poor candidates for surgery which is the mainstay treatment for majority of CRC cases. Furthermore, CRC staging and survival rates in the Veterans Affair (VA) geriatric population have not been studied.
Methods
Our aim is to study the staging of CRC in octogenarians and survival after treatment. We want the gastroenterologist panel to have this data for reference if ever need while deciding on screening and treatment guidelines. Furthermore, it will help us to compare CRC survival in the VA versus the general population.
We performed a retrospective data analysis of US male veterans who were diagnosed with CRC from 2000–2015. Our study was approved by the Samuel S. Stratton VA Medical Center Institutional Review Board, FWA #00002073; IRB #00000950. Study was a retrospective review so subject informed consent was not obtained. Data was accessed using National Veterans Affairs Cancer Care Cube Registry (CCCR) (10) that incorporates the data source from the oncology domain tables on the corporate data warehouse (CDW) raw server which is updated every two weeks. The registry was accessed on 4/3/2017, and data input after this date is not included in this study. Each patient with CRC is considered a unique case and is added to the CCCR. We divided patient into three age groups at time of diagnosis: 60–69, 70–79 and 80–89 years old. Younger patients such as 60–69 and 70–79 years old were considered for comparison as most of the patients that undergo treatment are in these age groups. Accession year which refers to the year in which the patient was first seen at the reporting institution for diagnosis and/or treatment of the primary cancer was 2000–2015. The number of CRC patients in each group was further divided into stage 0, 1, 2, 3, 4.
Survival for 5–10 years was studied only for stages 0, 1, 2 as treatment for these patients is surgery. This would help us control confounding in survival from the treatment stand point. Furthermore, 5–10-year survival without any treatment was also measured. Only males were studied, as females have a very low population in the VA.
Statistics
We calculated percentage of patients surviving 5–10 years after getting surgery for stages 0, 1, 2 by dividing number of patients surviving in that stage over the total number of CRC patients in that stage. Similarly, we calculated percentage of patient surviving 5–10 years for stages 0, 1, 2 that didn’t go under treatment, i.e., no surgery. Mean and 95% confidence interval for the percentage of patents surviving 5–10 years with and without surgery were calculated using an excel spread sheet. Statistical significance was accepted at P<0.05.
Results
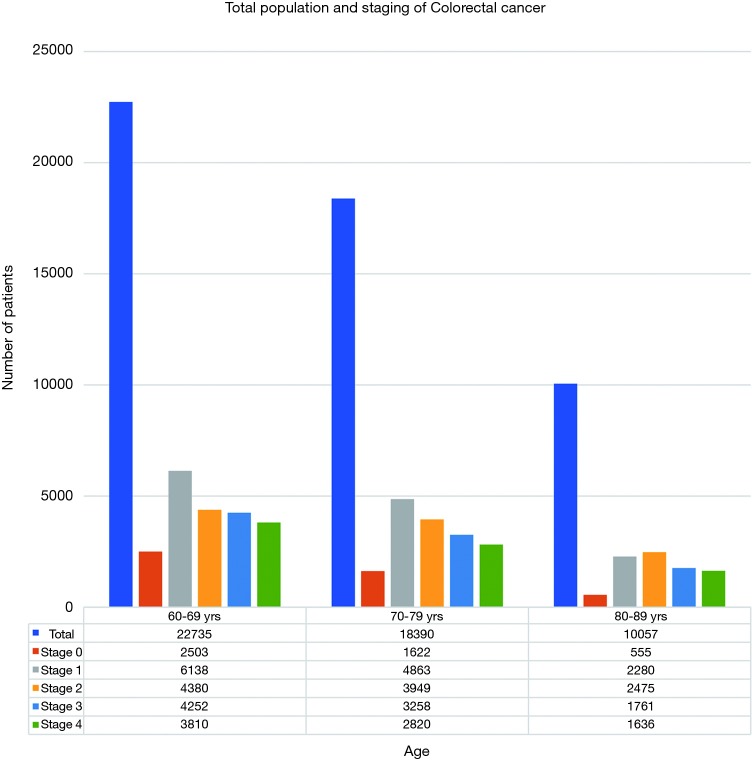
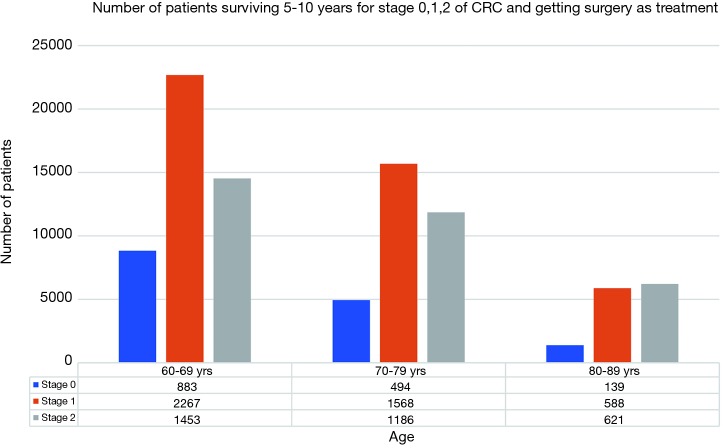
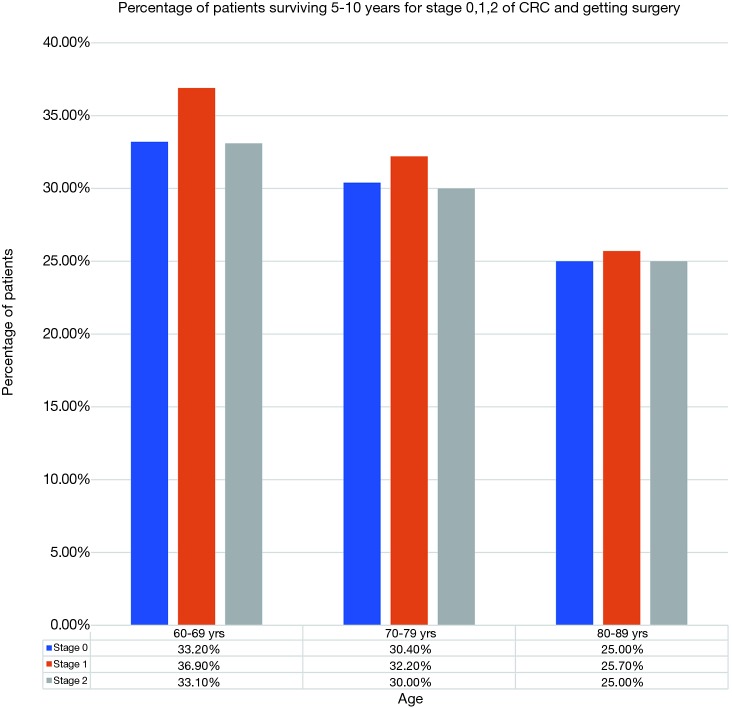
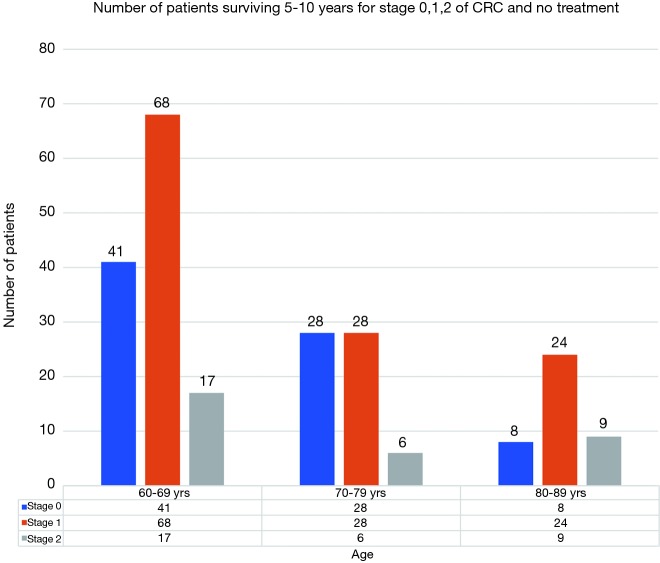
A total of 22,735 patients within age 60–69, 18,390 within age 70–79 and 10,057 within age 80–89 years were diagnosed with CRC in year 2000–2015 with majority being stages 1 and 2 as shown in Figure 1. Stages 0, 1, 2 accounted for 57% of patients in age group 60–69 years, 56% in age group 70–79 years and 52% in age group 80–89 years. Figure 2 shows number of patients undergoing surgery for stages 0, 1, 2 and surviving 5–10 years. Similarly, Figure 3 shows percentage of patients surviving 5–10 years for stages 0, 1, 2 and getting surgery. On the contrary, Figures 4 and 5 shows data for patients in stage 0, 1, 2 who never received any treatment, i.e., surgery and survived 5–10 years.
Figure 1.
Total population and staging of colorectal cancer.
Figure 2.
Number of patients surviving 5–10 years for stage 0, 1, 2 of CRC and getting surgery as treatment. CRC, colorectal cancer.
Figure 3.
Percentage of patients surviving 5–10 years for stage 0, 1, 2 of CRC and getting surgery. CRC, colorectal cancer.
Figure 4.
Number of patients surviving 5–10 years for stage 0, 1, 2 of CRC and no treatment. CRC, colorectal cancer.
Figure 5.
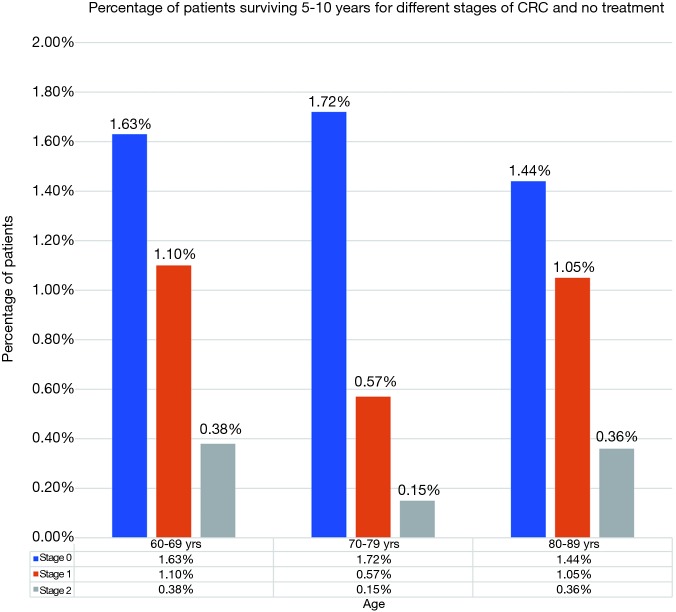
Percentage of patients surviving 5–10 years for different stages of CRC and no treatment. CRC, colorectal cancer.
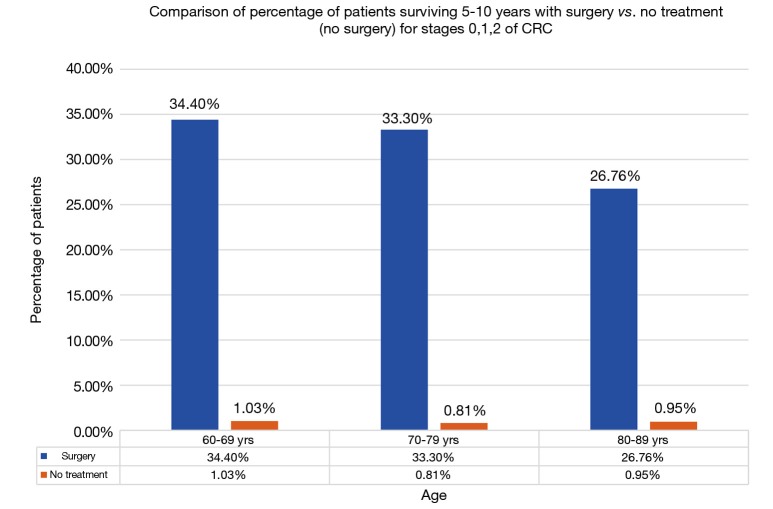
After surgery, 5–10-year survival for 60–69 age group averaged about 34.40% (95% CI: 31.94–36.85%) for stages 0–2. Similarly, for 70–79 and 80–89 age group it was 30.86% (95% CI: 29.54–32.19%) and 25.45% (95% CI: 24.77–25.69%) respectively (Figure 3). There was no statistically difference in survival between age groups 60–69 and 70–79. There was a statistical difference in survival between age group 80–89 years and the other two age groups, though the mean survival percentage of these octogenarians was surprisingly high. The 5–10-year survival data for patients without treatment i.e., not undergoing surgery was 1.03% (95% CI: 0.32–1.74%), 0.81% (95% CI: −0.10% to 1.73%), and 0.95% (95% CI: 0.33–1.56%) for age groups 60–69, 70–79 and 80–89 respectively (Figure 5). Comparison between the surgery and no surgery group is shown in Figure 6 with a significant difference in all age groups regarding survival 5–10 years.
Figure 6.
Comparison of percentage of patients surviving 5–10 years with surgery vs. no treatment (no surgery) for stages 0, 1, 2 of CRC. CRC, colorectal cancer.
Discussion
It is projected that in 2030, the population aged 65 and over in United States will be approximately 72 million, almost double its estimated population of 43.1 million in 2012 (12). Demographic changes in the United States will result in a marked increase in the number of cancer diagnoses over the next 20 years. USPTF recommends cessation of screening at age 75 years as risk outweighs the benefits in this elderly population. Most of the studies in the elder patients are either microsimulation, meta-analysis or studies with few patients above 80 years of age (13-15). Usually these elderly patients are poor surgical candidates which deter them from going down the screening route in the first place.
We showed in our data that these octogenarians in the VA population are being diagnosed with CRC with the majority being stage 1 and 2. More than 50% of patients in any age group were from stages 0–2. Finding early stages of CRC can not only help streamline treatment but also improve quality of life. Stages 0–2 do not require chemotherapy for most cases and only depends on surgery as the treatment of choice.
The 5–10-year survival percentage in patients getting surgery was statistically higher in ages 60–69 and 70–79 when compared to age 80–89 but octogenarians surprisingly had a high mean survival rate of 25.45% as compared to 34.40% and 30.86% respectively. Clinically it seems these octogenarians may have comparable surgical outcomes with their younger counterparts. Furthermore, when we compare survival in patients who didn’t get any treatment to the patients who got surgery, the numbers are devastating. The 5–10-year survival in these patients is next to zero. It is not clear whether these patients had baseline comorbid conditions that led to their demise or CRC was the reason of their poor survival.
Our study has its limitations. It is not in a controlled environment in which each individual patient chart is able to be reviewed for comorbid conditions. Patients who underwent colonoscopies above age 75 may have been healthier at baseline leading to better survival post-surgery. There is very little documentation in the cancer cube registry about functional status of the patients. Furthermore, it was not documented whether the cause of death was due to CRC or other comorbid conditions.
In conclusion, our study has the largest cohort to date of any study published on octogenarians in the VA population regarding CRC staging and survival. We believe our large sample size and integrated national Veterans cancer registry indicates that substantial number of octogenarians undergo surgery for treatment of stages 0–2 of CRC and have comparable 5–10-year survival with their younger counterparts. Highest number of CRC cases diagnosed across each age group was stages 1 and 2 with more than 50% of patients falling within stages 0–2. Survival data from surgery and no-treatment cases showed an immense drop in survival for non-treated patients suggesting that these candidates may have other reasons or comorbid conditions leading to their demise. Overall data showing early stage at cancer diagnosis and comparable surgical survival in octogenarians should be kept in mind while making screening guidelines as patients who may have life expectancy 5–10 years could benefit from continued screening and treatment.
Acknowledgements
None.
Ethical Statement: Our study was approved by the Samuel S. Stratton VA Medical Center Institutional Review Board (IRB #00000950). Study was a retrospective review so subject informed consent was not obtained.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.SEER Stat Fact Sheet; colon and rectum. National Cancer Institute. Available online: http://seer.cancer.gov/statfacts/html/colorect.html. Accessed on June 28, 2016.
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544-73. 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force , Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564-75. 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 5.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287-97. 10.1056/NEJMoa1311194 [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt JA, Mayer RJ. Follow-up strategies after curative resection of colorectal cancer. Semin Oncol 2003;30:349-60. 10.1016/S0093-7754(03)00095-2 [DOI] [PubMed] [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 9.Dalibon N, Moutafis M, Fischler M. Laparoscopically assisted versus open colectomy for colon cancer. N Engl J Med 2004;351:933-4; author reply 933-4. 10.1056/NEJM200408263510919 [DOI] [PubMed] [Google Scholar]
- 10.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420-5. [DOI] [PubMed] [Google Scholar]
- 11.Coke P, Gill T. National Cancer Care Cube. 2014 AVAHO Meeting. Portland, Oregon: Federal Practitioner, 2014. [Google Scholar]
- 12.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758-65. 10.1200/JCO.2008.20.8983 [DOI] [PubMed] [Google Scholar]
- 13.van Hees F, Habbema JD, Meester RG, et al. Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis. Ann Intern Med 2014;160:750-9. 10.7326/M13-2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Albéniz X, Hsu J, Bretthauer M, et al. Effectiveness of Screening Colonoscopy to Prevent Colorectal Cancer Among Medicare Beneficiaries Aged 70 to 79 Years: A Prospective Observational Study. Ann Intern Med 2017;166:18-26. 10.7326/M16-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang V, Boscardin WJ, Stijacic-Cenzer I, et al. Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials. BMJ 2015;350:h1662. 10.1136/bmj.h1662 [DOI] [PMC free article] [PubMed] [Google Scholar]