Abstract
Background
The prognosis of patients with hepatic metastases from neuroendocrine tumors (NET) is generally good, and radioembolization with Yttrium-90 microspheres is a locoregional therapy that is used in efforts to improve hepatic disease control and survival. This study aims to describe the survival outcomes and toxicities associated with radioembolization for hepatic-predominant metastatic NET in a large single-institution cohort.
Methods
A total of 59 patients underwent radioembolization for metastatic NET with hepatic predominant disease at a single academic center. Patient outcomes were analyzed by Kaplan-Meier survival analysis and toxicities were detailed and described. Ten patients within the cohort underwent post-treatment dosimetric analysis using PET-MRI and normal liver dosimetry was correlated with hepatic fibrosis and toxicity.
Results
Median overall survival from time of radioembolization in the patient cohort was 31 months, and the 1- and 2-year overall survival was 80.4% and 65.6% respectively. Median hepatic progression-free survival and overall progression-free survival were 18 and 13 months, respectively. Three patients died of hepatic failure that was possibly therapy-related. Ten patients underwent evaluation of post-treatment dosimetry following radioembolization. In patients who did not develop hepatotoxicity or hepatic fibrosis, mean dose to normal liver was 25.4 Gy, while the mean liver dose in patients who experienced toxicity (hepatic fibrosis in n=2 and death from hepatic failure in n=1) was 59.1 Gy.
Conclusions
Overall survival following radioembolization for hepatic metastases from NET is excellent; however, deaths that are potentially treatment-related have been observed. Preliminary data regarding dose to normal liver is suggestive of a relation between dosimetry and toxicity, however further work is required to further elucidate the mechanism, correlation with dosimetry, as well as additional patient and tumor factors that may predispose these patients to toxicity.
Keywords: Liver, neuroendocrine tumors (NET), radioembolization
Introduction
Neuroendocrine tumors (NET) are neoplasms originating from enterochromaffin cells which typically arise from the mid-gut, islet cells in the pancreas, and less frequently, the lung or other sites. Although these neoplasms can have an indolent course, distant metastases can occur and the liver is the most common site (1). Over the past several decades, the diagnosis of this tumor has been increasing in incidence (2), and many patients have hepatic metastases at the time of diagnosis (3). Patients with metastatic NET have good long-term prognosis, with median overall survival (OS) of 24 months for pancreatic NET and 56 months for NET arising from the small bowel (2,4). Systemic treatment options include cytotoxic chemotherapy, somatostatin receptor antagonists such as octreotide, biologic agents including everolimus, sunitinib and others, and more recently radionuclides such as lutetium dotatate. Loco-regional therapies, however, are often attractive options in the setting of progressive or symptomatic hepatic metastases.
Radioembolization using Yttrium-90 (Y90) microspheres has been well-described and is now a standard technique to provide a targeted loco-regional approach to the treatment of liver metastases (5-7). This therapy has been used to improve quality of life in patients with refractory symptoms related to carcinoid syndrome as well as for definitive control of progressive metastases (8). While this treatment is generally well-tolerated, there have been reports of hepatic toxicity following radioembolization in this patient population. This work represents a large single center experience in treating patients with metastatic NET using Y90, with a focus on survival outcomes and toxicities.
Methods
Patients with liver-predominant metastatic NET treated with radioembolization at a single institution from 2009 through 2015 were included in this analysis. This retrospective study was approved by the institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Inclusion criteria included patients with unresectable liver-predominant metastatic NET refractory to systemic therapy, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, ability to undergo angiography, adequate renal function (creatinine ≤2.0 mg/dL), and adequate hepatic function (bilirubin ≤2.0 mg/dL). Before radioembolization, patient treatment options were discussed at a weekly gastrointestinal tumor board meeting attended by radiation oncologists, medical oncologists, interventional radiologists and hepatobiliary surgeons. Exclusion criteria for radioembolization included significant extrahepatic disease, gastrointestinal arterial flow that might result in extrahepatic deposition, excess lung shunting that would result in lung dose >30 Gy, and concurrent chemotherapy or radiotherapy. Patients on octreotide continued on the medication while receiving radioembolization.
All patients underwent pretreatment evaluation including history and physical, laboratory testing, and liver imaging. Baseline laboratory tests included complete blood count, liver function tests, INR, and chromogranin A. Patients were treated with resin (SIR-Spheres, Sirtex Medical, Lane Cove, Australia) or glass microspheres loaded with Y90 (TheraSpheres, MDS Nordion, Kanata, ON, Canada). The decision to use resin or glass microspheres was made by the treating physician. Pretreatment angiography and 99mTC-MAA were performed before radioembolization to examine the hepatic artery anatomy and assess for gastrointestinal arterial flow and lung shunting. Radioembolization was performed as previously described (9). All procedures were performed on an outpatient basis.
Ten patients included in analysis underwent PET-MRI within 24 hours after radioembolization for evaluation of post-implant dosimetry as previously described (10). For these patients, the whole liver and hepatic tumors were individually segmented in MimVista (MIM Software, Cleveland, OH) to generate a treated normal liver volume (whole liver minus tumor). The Y90 PET images were used to generate dose maps and to create dose volume histograms (DVH) as previously described by Fowler et al. (10) and the minimum delivered dose (Gy) to a given volume of the treated normal liver was assessed.
Following radioembolization, patients returned to routine clinical follow which typically involved physical examination, laboratory testing and imaging at 3 month intervals. Dates of expiration were recorded and patients were otherwise censored at last clinic follow-up.
Tumor response was assessed using contrast CT or MRI images using both mRECIST (11) and EASL (12) criteria. Hepatic progression was defined as the appearance of new hepatic lesions or enlargement of existing disease. Progression-free survival (PFS) was defined as time to any progression (hepatic or distant) or death. OS was based on all-cause mortality. Clinical and biochemical toxicities following radioembolization were assessed using Common Terminology Criteria for Adverse Events of the National Cancer Institute (version 4.0).
All analyses were calculated from the date of radioembolization using the Kaplan-Meier method. For PFS and hepatic PFS, patients were censored at date of last imaging in the absence of progression. All statistical analyses were performed using the R survival package available on CRAN at http://cran.r-project.org.
Results
Baseline characteristics
A total of 59 patients were included in analysis. Baseline patient characteristics are summarized in Table 1. Median patient age was 62 years (range, 26–79 years). Most patients were Caucasian and had an ECOG PS of 0. All patients had multifocal hepatic disease and bilobar involvement. The majority of patients had primary tumors that originated in the pancreas, midgut or an unknown site, and most tumors were grade 1 or 2 histologically. Half of patients had undergone resection of the primary tumor and most patients had liver-only disease. Most patients had prior treatment with octreotide. Fourteen patients had undergone prior liver-directed therapy, including surgery, 13 had received prior cytotoxic chemotherapy, and 21 had received prior biologic therapy.
Table 1. Baseline patient characteristics.
| Clinical variable | Value, n (%) |
|---|---|
| Age (years) | |
| ≤60 | 31 (52.5) |
| >60 | 28 (47.5) |
| Gender | |
| Male | 32 (54.2) |
| Female | 27 (45.7) |
| Race | |
| Caucasian | 47 (79.7) |
| African-American | 11 (18.6) |
| Other | 1 (1.7) |
| Eastern Cooperative Oncology Group (ECOG) | |
| 0 | 34 (57.6) |
| 1 | 21 (35.6) |
| 2 | 1 (1.7) |
| Unknown | 3 (5.1) |
| Site of primary | |
| Pancreas | 18 (30.5) |
| Foregut | 3 (5.1) |
| Midgut | 16 (27.1) |
| Hindgut | 3 (5.1) |
| Lung | 7 (11.9) |
| Kidney | 1 (1.7) |
| Thymus | 1 (1.7) |
| Unknown | 10 (16.9) |
| Tumor grade | |
| 1 | 23 (39.0) |
| 2 | 22 (37.3) |
| 3 | 8 (13.6) |
| Unknown | 6 (5.1) |
| Carcinoid syndrome | |
| Yes | 22 (37.3) |
| No | 37 (62.6) |
| Extrahepatic metastases | |
| Yes | 21 (35.6) |
| No | 38 (64.4) |
| Resection of primary tumor | |
| Yes | 30 (50.1) |
| No | 29 (49.2) |
| Prior liver-directed therapy | |
| Yes | 16 (27.1) |
| No | 43 (72.9) |
| Type of prior therapy | |
| Surgery | 7 (11.9) |
| Chemoembolization | 5 (8.5) |
| Radiofrequency ablation | 1 (1.7) |
| Bland embolization | 3 (5.1) |
| Prior octreotide | |
| Yes | 45 (76.3) |
| No | 14 (23.7) |
| Prior biologic therapy | |
| Yes | 25 (42.4) |
| No | 34 (57.6) |
| Biologic agent | |
| Bevacizumab | 6 (5.1) |
| Everolimus | 12 (10.2) |
| Sunitinib | 6 (5.1) |
| Pazopanib | 1 (1.7) |
| Prior chemotherapy | |
| Yes | 13 (22.0) |
| No | 46 (78.0) |
| Pre-treatment chromogranin A | |
| ≤500 | 19 (52.8) |
| >500 | 17 (47.2) |
| Tumor volume | |
| ≤200 cc | 25 (46.3) |
| <200 cc | 29 (53.7) |
Data missing in pretreatment Chromogranin A (n=36) and tumor volume (n=54).
Treatment characteristics
Details regarding the radioembolization treatments are described in Table 2. Mean lung shunting at time of pretreatment mesenteric angiography was 3%, and only 4 patients had lung shunting greater than 10%. Thirty-seven patients required prophylactic embolization of selected visceral arteries, such as the gastroduodenal or right gastric. Forty-two patients (71%) underwent sequential lobar treatments, while 17 patients (29%) received a single lobar administration. No patients in the study received a single whole liver administration of microspheres. Thirty-eight patients (64%) underwent radioembolization with resin microspheres, while 21 patients (46%) were treated with glass microspheres. Mean administered activity was 1.71 GBq with resin microspheres and 5.43 GBq with glass microspheres.
Table 2. Radioembolization treatment characteristics.
| Technical variable | Value |
|---|---|
| Lung shunting | 3% (1–19.6%) |
| Prophylactic embolization | |
| Yes | 37 (63%) |
| No | 22 (37%) |
| Treatment volume | |
| Sequential lobar | 42 (71%) |
| Single (lobar) | 17 (29%) |
| Single (whole liver) | 0 (0%) |
| Glass microspheres | 21 (46%) |
| Activity delivered (GBq) | 5.43 (1.83–16.95) |
| Resin microspheres | 38 (64%) |
| Activity delivered (GBq) | 1.71 (0.47–2.25) |
| Stasis | 2 (5%) |
Symptomatic response
Twenty-two patients had symptoms of carcinoid syndrome (flushing and/or diarrhea) that was refractory to medical therapy prior to radioembolization. Five of these patients had documented improvement in symptoms following treatment.
Survival
Mean and median follow-up for all patients was 25 and 26 months, respectively. Mean and median follow-up for patients alive at time of analysis was 27 and 29 months. Twenty-eight patients had died at time of analysis. Kaplan-Meier curves for OS, hepatic progression-free survival (PFS) and overall PFS are shown in Figure 1. The median OS was 31 months (95% CI, 27 months to unreached), as shown in Table 3. The 1- and 2-year OS was 80.4% and 65.6% respectively. Median hepatic PFS was 18 months (95% CI, 13–27), and 1- and 2-year hepatic PFS were 63.2% and 37.7%. Median PFS was 13 months (95% CI, 8–19) and 1- and 2-year PFS were 50.6% and 27.8%.
Figure 1.
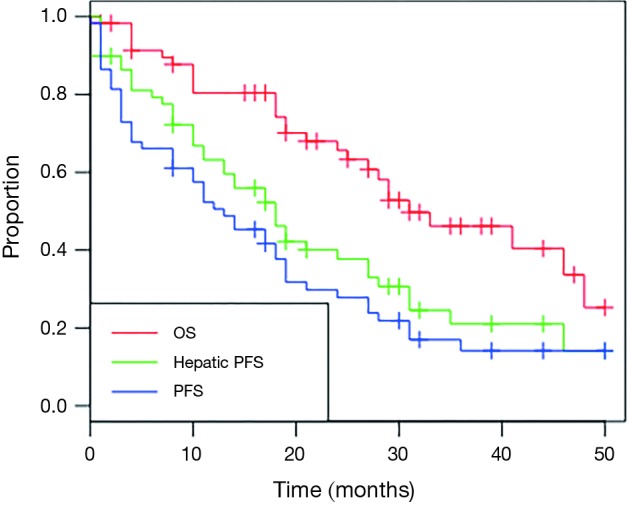
Kaplan-Meier survival curves depicting overall survival, hepatic progression-free survival and progression-free survival for patients with metastatic neuroendocrine tumor treated with radioembolization.
Table 3. Overall survival (OS), hepatic progression-free survival (PFS) and PFS after radioembolization.
| Clinical response | Time from treatment |
|---|---|
| OS | |
| Median (months) | 31 (27–unreached) |
| 1-year (%) | 80.4 (70.6–91.5) |
| 2-year (%) | 65.6 (53.8–80.1) |
| Hepatic PFS | |
| Median (months) | 18 [13–27] |
| 1-year (%) | 63.2 (51.8–77.1) |
| 2-year (%) | 37.7 (26.6–53.5) |
| PFS | |
| Median (months) | 13 [8–19] |
| 1-year (%) | 50.6 (39.2–65.1) |
| 2-year (%) | 27.8 (18.1–42.7) |
Radiographic response
Post-radioembolization imaging was used to assess local response to therapy by both mRECIST and EASL criteria and is described in Table 4. Seven patients had no imaging follow-up and could not be assessed for treatment response. Three patients had a radiographic complete response (CR) by both mRECIST and EASL criteria. A partial response (PR) was noted in 27 patients by mRECIST and 28 patients by EASL. Stable disease (SD) was noted in 18 and 15 patients, respectively and progressive disease (PD) was seen in 4 and 6 patients respectively. The overall response rate was 58% (30 out of 52 evaluable patients).
Table 4. Radiographic response [modified Response Evaluation Criteria in Solid Tumors (mRECIST) and European Association for the Study of Liver (EASL)] after radioembolization.
| Radiographic response | Number (%) |
|---|---|
| mRECIST response | |
| Complete response (CR) | 3 (5.1) |
| Partial response (PR) | 27 (45.8) |
| Stable disease (SD) | 18 (30.5) |
| Progressive disease (PD) | 4 (6.8) |
| Unknown | 7 (11.9) |
| EASL response | |
| CR | 3 (5.1) |
| PR | 28 (47.5) |
| SD | 15 (15.3) |
| PD | 6 (10.2) |
| Unknown | 7 (11.9) |
Toxicities
Grade 3 or higher clinical or biochemical toxicities were noted in a total 9 patients and are detailed in Table 5. Three patients developed gastrointestinal ulceration, 4 patients had reported encephalopathy, and 2 developed ascites. Eighteen patients had grade 3 or higher hematologic toxicity, predominantly lymphopenia. In addition, 4 patients were noted to have radiographically detected radiation-induced hepatic fibrosis without clinical sequelae.
Table 5. Clinical and biochemical toxicities.
| Toxicity | ≤ Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Clinical toxicities | ||||
| Encephalopathy | 4 | |||
| Gastrointestinal ulcer | 2 | 1 | ||
| Abdominal pain | 16 | 4 | ||
| Fever | 2 | |||
| Ascites | 5 | 2 | ||
| Fatigue | 7 | |||
| Anorexia | 5 | 1 | ||
| Nausea | 13 | 1 | ||
| Radiation fibrosis | 6 | |||
| Groin hematoma | 1 | |||
| Hepatic failure | 1 | 3 | ||
| Biochemical toxicities | ||||
| ALT | 22 | 3 | ||
| AST | 26 | 2 | ||
| Albumin | 17 | 2 | ||
| Bilirubin | 3 | 2 | 1 | |
| Alkaline phosphatase | 33 | 2 | ||
| INR | 1 | 2 | ||
| Hematologic toxicities | ||||
| Absolute lymphocyte | 23 | 14 | 1 | |
| Platelets | 11 | 2 | 1 |
There were 3 deaths from hepatic failure that were potentially treatment-related. One patient, a 26-year-old female with a grade 2 tumor, died 3 months after treatment with resin microspheres. She had been treated the prior year with chemoembolization and had also previously undergone a Whipple procedure. Another patient, a 39-year-old male, with grade 1 tumor, died 21 months after sequential bilobar infusion with glass microspheres. Imaging features typical of cirrhosis were found nine months after Y90 infusion. A third subject, a 77-year-old male with grade 3 tumor, who had previously undergone a right hepatic trisegmentectomy, died 5 months following left lobe treatment from what was felt to be radiation-induced liver disease.
Ten patients in this retrospective cohort were enrolled on a prospective institutional study in which they underwent post-radioembolization PET-MRI for evaluation of post-treatment dosimetry. Three of these patients had evidence of radiation-induced fibrosis (one with grade 5 hepatic failure). Mean delivered radiation dose to the normal liver (normal liver defined as volume of treated lobe minus tumor volume within treated lobe) was calculated for each of the ten patients. Patients who developed hepatic fibrosis had higher mean normal liver dose than those without toxicity (59.1 vs. 25.4 Gy, P=0.008), shown in Table 5. This is shown graphically in the DVH (Figure 2), demonstrating minimum delivered dose (Gy) to a given volume of the treated normal liver. The DVH curves for the 3 patients with hepatic fibrosis demonstrate a larger volume of liver receiving 20–80 Gy than the patients who did not have toxicity. The mean lobar dose and presence or absence of subsequent radiotoxicity to the liver in the ten patients who underwent assessment of post-radioembolization dosimetry is shown in Figure 3.
Figure 2.
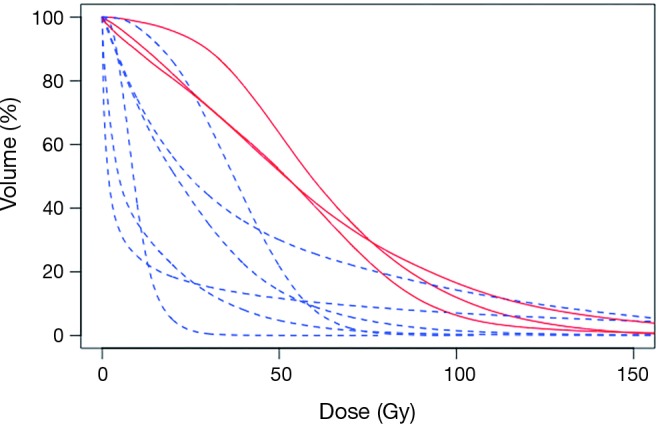
Dose volume histograms for the normal hepatic parenchyma for the treated lobe for 10 patients who underwent assessment of post-radioembolization dosimetry. DVHs for patients with hepatic toxicity are shown in red (solid line) and for patients without toxicity are shown in blue (dashed line). Qualitatively, patients who had greater liver volumes receiving 30–90 Gy were more likely to have toxicity.
Figure 3.
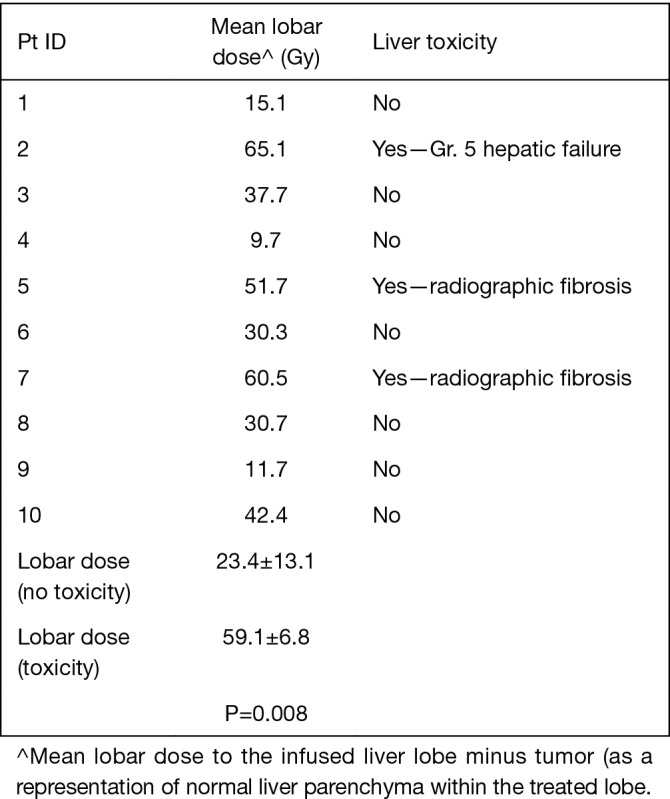
Hepatic toxicity as related to delivered dose administered.
Discussion
There are many approved systemic and local therapies for metastatic NET, however each approach has limits of efficacy, toxicity and/or feasibility. Somatostatin analogs such as octreotide are useful to treat the symptoms of carcinoid syndrome but metastases can eventually become resistant to therapy (13). Cytotoxic chemotherapy has variable results and can result in significant toxicity (14-16). Newer systemic agents include mTOR inhibitors and tyrosine kinase inhibitors, as well as peptide receptor radionuclide therapy with Lu177-dotatate. Surgical management of hepatic metastases can be effective for both symptomatic palliation and prolonging OS, but is not feasible in the majority of patients due to the diffuse multifocal distribution of the metastases (17). Transplantation remains as an investigational tool, but has had limited success in reported series (18,19). Transcatheter arterial approaches (bland embolization and chemoembolization) have been well-described and are effective at both improving PFS and OS (20). In one report, 92% of patients treated with chemoembolization achieved relief of carcinoid symptoms and PFS was 65% at 1 year (21). However, significant toxicity has been described in up to 10% of patients undergoing bland embolization for treatment of neuroendocrine metastases (22). Radioembolization with Y90 has been well-described in the treatment of metastatic NET and was first reported in five patients by Simon et al. in 1968 (23). The treatment is generally well-tolerated by patients, has a low risk of toxicity, and produces tangible radiographic responses (24-27). Y90 radioembolization is included in the published National Comprehensive Cancer Network guidelines as an effective therapy to treat hepatic metastases from NET (28).
Our current series has demonstrated the benefit of radioembolization as seen by a radiographic response rate (CR and PR) of 58% in a cohort of patients who had generally received one or more types of prior therapy. When including those with SD as seen by cross-sectional imaging using mRECIST criteria, the overall disease control rate was 77%, comparable to the multi-center report by Kennedy et al. which reported CR, PR and SD rates of 2.7%, 60.5%, and 22.7% respectively (27). Their results were based on a three-month follow-up scan using a variety of imaging techniques, similar to our patient population (27). A single center experience of 40 patients demonstrated CR and PR rates of 20.5% and 43.4% and OS rates at 1 and 2 years of 72.5% and 62.5% in a cohort of patients with similar demographics as in the current report (29). This consistent data between studies confirms radioembolization as a useful technique to treat patients who have progressed on other available medical treatments such as systemic chemotherapy, including cytotoxic agents, somatostatin analogs, or biological agents.
Several recent publications have evaluated the incidence of hepatic toxicity in patient with metastatic NET, particularly since this is a patient population with a generally good life expectancy. In one analysis, a cirrhosis-appearing morphology was seen on cross-sectional imaging in 26.7% of patients receiving unilobar infusion of microspheres. This rate doubled in those treated with whole-liver infusion. Those investigators also noted signs of portal hypertension, which was more frequent in patients undergoing whole-liver infusion (30). The incidence of cirrhosis or fibrosis following Y90 for NET has also been reported by others (31-33). Decrease in liver volume, another potential metric of fibrosis, was noted in 12% of patients treated with sequential bilobar Y90 infusions in another study (34). Radiation-induced hepatic fibrosis was noted in only 4 out of 52 patients (7.7%) with available follow-up scans in the current study. However, whole-liver infusion was not used in the current study and the mean follow-up was only 2 years. Hepatic fibrosis and remodeling after radiation therapy is a slow process, and the clinical changes related to the development of portal hypertension may not always be apparent until a longer observation period is present.
Though the timeframe for radioembolization-induced hepatic fibrosis is over the course of many years, three patients in this cohort died of hepatic failure following radioembolization (two within 6 months of treatment). Two of these patients had previously undergone procedures or treatments that may have predisposed them to hepatic injury. There have been few reports of fatal toxicities in patients with metastatic NET following radioembolization. Whitney et al. report an episode of hepatic failure one month following radioembolization (35). In their retrospective review of toxicities in patients treated at a single institution, Su et al. report death from hepatic failure in 2 of patients in the absence of disease progression or subsequent therapies, while 6 additional patients died of liver failure in the setting of disease progression and subsequent exposure to potentially hepatotoxic systemic therapies (30).
Significant efforts have been made to correlate prescribed activity with actual delivered dose, treatment outcomes and toxicities following radioembolization. Ten patients in our cohort had undergone post-radioembolization Y90 PET-MRI to evaluate dose distribution of the delivered microspheres. This technique of using PET to quantify delivered dose after radioembolization is emerging as a strategy to predict response in hepatic tumors (10,36). Srinivas et al have used this technique with PET-CT to calculate delivered dose to normal liver tissue and has shown that a mean dose of 67 Gy to normal liver tissue is associated with increased rates of hepatic complications (36). Similarly, a relationship between dose and toxicity has been described after dosimetric calculations using SPECT-CT (37). In our cohort of ten patients, the mean liver dose for patients who had hepatic toxicity (defined as hepatic fibrosis or hepatic failure) was 59.1 Gy, while the mean liver dose for patients without hepatic toxicity was 25.4 Gy. Due to the multifactorial nature of hepatic failure for patients with metastatic cancer (disease progression, prior and subsequent local and systemic therapies) and the low patient number of patients who underwent analysis of post-radioembolization dosimetry, it is impossible to draw a firm conclusion regarding the correlation of radiation dose to normal liver and incidence of toxicity. However, these data are suggestive that delivered dose to normal hepatic parenchyma may impact long-term hepatic function.
One of the drawbacks of our study is that it is retrospective and observational. There is no comparison group who did not undergo radioembolization and there are a relatively small number of total patients, limiting statistical analysis. In addition, it can be difficult ascertain the role of radioembolization with regards to subsequent morbidities because of confounding variables including the use of systemic chemotherapy or biologic agents and progression of tumor causing clinical decline independent of prior Y90 infusion. The analysis of dosimetry by PET-MRI in efforts to correlate dosimetry with toxicity and outcomes is still investigational, and very few patients in this study underwent this analysis. Furthermore, some of our patients had a portion of their treatment done at outside medical facilities, rather than our tertiary care center, making data capture incomplete.
In conclusion, Y90 radioembolization for hepatic predominant metastatic NET results in excellent radiographic response rate and good OS. A small proportion of patients may develop hepatic toxicity ranging from asymptomatic fibrosis to death from liver failure. More work is needed to determine which patients are most likely to benefit from radioembolization and which patient, tumor and dosimetric factors are associated with toxicities.
Acknowledgements
None.
Ethical Statement: This study was approved by the Washington University Institutional Review Board (No. IRB00009237) and informed consent was not required to obtain from the patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol 1987;5:1502-22. 10.1200/JCO.1987.5.10.1502 [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45. 10.1016/S1072-7515(00)00222-2 [DOI] [PubMed] [Google Scholar]
- 4.Fraenkel M, Kim M, Faggiano A, et al. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocrine-Related Cancer 2014;21:R153-63. 10.1530/ERC-13-0125 [DOI] [PubMed] [Google Scholar]
- 5.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. Journal of vascular and interventional radiology 2006;17:1251-78. 10.1097/01.RVI.0000233785.75257.9A [DOI] [PubMed] [Google Scholar]
- 6.Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. Journal of vascular and interventional radiology 2006;17:1571-93. 10.1097/01.RVI.0000236744.34720.73 [DOI] [PubMed] [Google Scholar]
- 7.Salem R, Thurston KG. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 2: special topics. J Vasc Interv Radiol 2006;17:1425-39. 10.1097/01.RVI.0000235779.88652.53 [DOI] [PubMed] [Google Scholar]
- 8.Kennedy A, Coldwell D, Sangro B, et al. Integrating radioembolization into the treatment paradigm for metastatic neuroendocrine tumors in the liver. Am J Clin Oncol 2012;35:393-8. 10.1097/COC.0b013e3182005768 [DOI] [PubMed] [Google Scholar]
- 9.Gui B, Weiner AA, Nosher J, et al. Assessment of the Albumin-Bilirubin (ALBI) Grade as a Prognostic Indicator for Hepatocellular Carcinoma Patients Treated With Radioembolization. Am J Clin Oncol 2018;41:861-6. 10.1097/COC.0000000000000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler KJ, Maughan NM, Laforest R, et al. PET/MRI of Hepatic 90Y Microsphere Deposition Determines Individual Tumor Response. Cardiovasc Intervent Radiol 2016;39:855-64. 10.1007/s00270-015-1285-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. 10.1016/S0168-8278(01)00130-1 [DOI] [PubMed] [Google Scholar]
- 13.Schnirer II, Yao JC, Ajani JA. Carcinoid--a comprehensive review. Acta Oncologica 2003;42:672-92. 10.1080/02841860310010547 [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Lipsitz S, Catalano P, et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005;23:4897-904. 10.1200/JCO.2005.03.616 [DOI] [PubMed] [Google Scholar]
- 15.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71. 10.1200/JCO.2004.04.024 [DOI] [PubMed] [Google Scholar]
- 16.Mayo SC, Herman JM, Cosgrove D, et al. Emerging approaches in the management of patients with neuroendocrine liver metastasis: role of liver-directed and systemic therapies. J Am Coll Surg 2013;216:123-34. 10.1016/j.jamcollsurg.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modlin IM, Latich I, Kidd M, et al. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol 2006;4:526-47. 10.1016/j.cgh.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 18.Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation 1998;66:1307-12. 10.1097/00007890-199811270-00007 [DOI] [PubMed] [Google Scholar]
- 19.Rosenau J, Bahr MJ, von Wasielewski R, et al. Ki67, E-cadherin, and p53 as prognostic indicators of long-term outcome after liver transplantation for metastatic neuroendocrine tumors. Transplantation 2002;73:386-94. 10.1097/00007890-200202150-00012 [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer 2005;104:1590-602. 10.1002/cncr.21389 [DOI] [PubMed] [Google Scholar]
- 21.Ruutiainen AT, Soulen MC, Tuite CM, et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol 2007;18:847-55. 10.1016/j.jvir.2007.04.018 [DOI] [PubMed] [Google Scholar]
- 22.Brown KT, Koh BY, Brody LA, et al. Particle embolization of hepatic neuroendocrine metastases for control of pain and hormonal symptoms. J Vasc Interv Radiol 1999;10:397-403. 10.1016/S1051-0443(99)70055-2 [DOI] [PubMed] [Google Scholar]
- 23.Simon N, Warner RR, Baron MG, et al. Intra-arterial irradiation of carcinoid tumors of the liver. Am J Roentgenol Radium Ther Nucl Med 1968;102:552-61. 10.2214/ajr.102.3.552 [DOI] [PubMed] [Google Scholar]
- 24.Kalinowski M, Dressler M, König A, et al. Selective internal radiotherapy with Yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: a prospective single center study. Digestion 2009;79:137-42. 10.1159/000209849 [DOI] [PubMed] [Google Scholar]
- 25.Gaba RC, Mendoza-Elias N, Morrison JD, et al. Decision Making for Selection of Transarterial Locoregional Therapy of Metastatic Neuroendocrine Tumors. Semin Intervent Radiol 2017;34:101-8. 10.1055/s-0037-1602590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devcic Z, Rosenberg J, Braat AJA, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med 2014;55:1404-10. 10.2967/jnumed.113.135855 [DOI] [PubMed] [Google Scholar]
- 27.Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008;31:271-9. 10.1097/COC.0b013e31815e4557 [DOI] [PubMed] [Google Scholar]
- 28.Kulke MH, Shah MH, Benson Iii A, et al. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology — Neuroendocrine Tumors. NCCN Clinical Practice Guidelines in Oncology; Version 1 2017:1-116. [Google Scholar]
- 29.Memon K, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys 2012;83:887-94. 10.1016/j.ijrobp.2011.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su YK, Mackey RV, Riaz A, et al. Long-Term Hepatotoxicity of Yttrium-90 Radioembolization as Treatment of Metastatic Neuroendocrine Tumor to the Liver. J Vasc Interv Radiol 2017;28:1520-6. 10.1016/j.jvir.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 31.Saxena A, Chua TC, Bester L, et al. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg 2010;251:910-6. 10.1097/SLA.0b013e3181d3d24a [DOI] [PubMed] [Google Scholar]
- 32.Maker AV, August C, Maker VK, et al. Hepatectomy After Yttrium-90 (Y90) Radioembolization-Induced Liver Fibrosis. J Gastrointest Surg 2016;20:869-70. 10.1007/s11605-016-3077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loree JM, Hiruki T, Kennecke HF. Case Report of Cirrhosis following Yttrium-90 Radioembolization for Pancreatic Neuroendocrine Liver Metastases. Case Rep Oncol 2016;9:76-82. 10.1159/000443985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobs TF, Saleem S, Atassi B, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90yttrium microspheres. Dig Dis Sci 2008;53:2556-63. 10.1007/s10620-007-0148-z [DOI] [PubMed] [Google Scholar]
- 35.Whitney R, Vàlek V, Fages JF, et al. Transarterial chemoembolization and selective internal radiation for the treatment of patients with metastatic neuroendocrine tumors: a comparison of efficacy and cost. Oncologist 2011;16:594-601. 10.1634/theoncologist.2010-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivas SM, Natarajan N, Kuroiwa J, et al. Determination of radiation absorbed dose to primary liver tumors and normal liver tissue using post-radioembolization 90Y PET. Front Oncol 2014;4:255. 10.3389/fonc.2014.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strigari L, Sciuto R, Rea S, et al. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: radiobiologic considerations. J Nucl Med 2010;51:1377-85. 10.2967/jnumed.110.075861 [DOI] [PubMed] [Google Scholar]


