Abstract
Gastrointestinal stromal tumors (GISTs) are rare neoplasms of the gastrointestinal tract associated with high rates of malignant transformation. Most GISTs present asymptomatically. They are best identified by computed tomography (CT) scan and most stain positive for CD117 (C-Kit), CD34, and/or DOG-1. There have been many risk stratification classifications systems which are calculated based on tumor size, mitotic rate, location, and perforation. The approaches to treating GISTs are to resect primary low-risk tumors, resect high-risk primary or metastatic tumors with imatinib 400 mg daily for 12 months, or if the tumor is unresectable, neoadjuvant imatinib 400 mg daily followed by surgical resection is recommended. Sunitinib is required for KIT exon 9, 13, and 14 mutations, while ponatinib is used for exon 17 mutations and regorafenib for highly refractory tumors. High-risk tumors should be monitored for recurrence with serial abdominal CT scans. Radiofrequency ablation has shown to be effective when surgery is not suitable. Newer therapies of ipilimumab, nivolumab, and endoscopic ultrasound alcohol ablation have shown promising results. This report addresses the epidemiology, clinical presentation, diagnostic imaging, histologic diagnosis, classification and risk stratification, staging and grading, surgical treatment, adjuvant treatment, and metastasis of GISTs.
Keywords: Gastrointestinal stromal tumor (GIST), tyrosine kinase receptor inhibitor (TKI), risk classification, prognosis, outcome
Introduction
Gastrointestinal stromal tumors (GISTs) were originally believed to have originated from the mesenchymal cells of the gastrointestinal tract (GIT) (1,2). Kindblom and associates in 1998 found that these tumors actually originate from the interstitial cells of Cajal (3). Hirota and colleagues discovered that these tumors express CD117 antigen (C-Kit), a gain of function mutation responsible for activating the growth of these tumors (4). Although GISTs are considered rare tumors, most GISTs are discovered incidentally so the true prevalence is unknown. Traditional chemotherapy and radiation are not effective on GISTs, therefore surgical resection has always been the mainstay of treatment (5,6). With the discovery of mutations associated with these tumors, the treatment has changed dramatically. Imatinib mesylate, a selective tyrosine kinase receptor inhibitor (TKI), is used as an adjuvant or neoadjuvant therapy to improve the morbidity and mortality associated with GISTs. Due to growing resistance, sunitinib and regorafenib are effective second-line TKIs (7-13).
Epidemiology
GISTs are rare, accounting for 1% to 2% of gastrointestinal neoplasms (14). Soreide and colleagues reviewed 29 studies consisting of 13,550 patients from 19 different countries with GISTs between January 2000 and December 2014 (15). The median age was 65 (range, 10–100) with a 1:1 male to female ratio. The highest incidence rates (19–22 per million per year) were noted in Hong Kong, Shanghai, Taiwan, and Norway. The lowest incidence was noted in the Shanxi province of China with 4.3 per million per year. Eighteen percent (range, 5–40%) of GISTs were discovered incidentally. GISTs were found in the stomach (56%) (Figure 1), small bowel (32%) (Figure 2), colon and rectum (6%) (Figure 3), esophagus (0.7%), and other locations (5.5%) (15). About 10% to 30% of GISTs progress to malignancy. GISTs occurring outside of the stomach are associated with a higher malignant potential (Table 1) (16). Exophytic growth is noted in 79% of GISTs while intraluminal (Figure 4) or mixed growth occurs less frequently (17).
Figure 1.
Gross photo of a fresh total gastrectomy specimen showing a large polypoid mass near the GE junction at lesser curvature and very close to the proximal resection margin. The specimen is opened along the greater curvature. The tumor is about 7 cm, in size with surface ulceration, hemorrhage, deep fissuring, and tumor fragmentation.
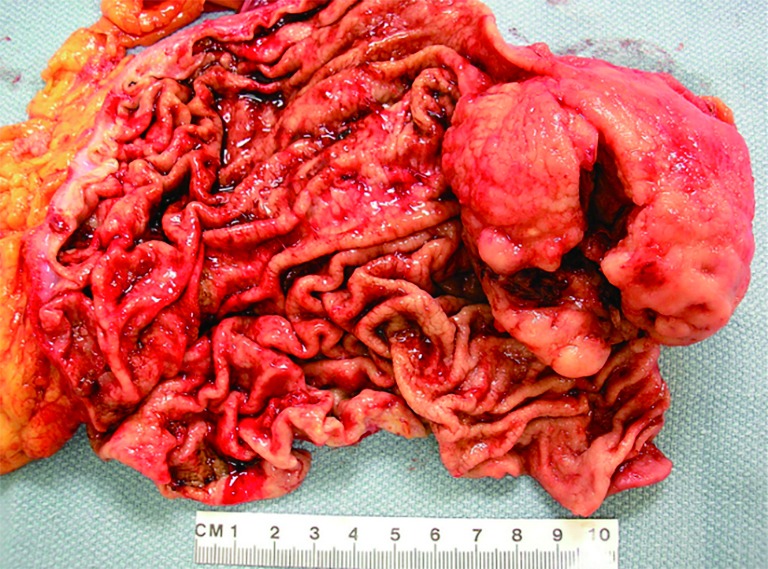
Figure 2.
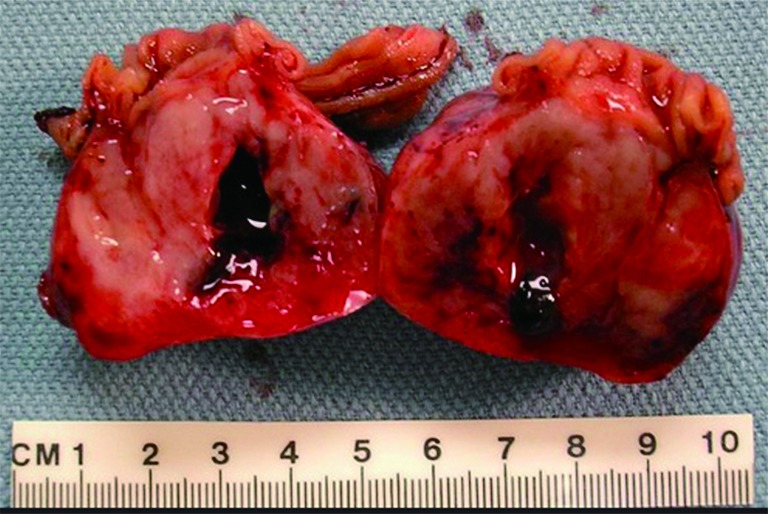
Gross photo. A large, bulky, intraluminal mass in resected small bowel. The cut surface of the tumor has a fish-flesh appearance with hemorrhage, necrosis, and cystic softening.
Figure 3.
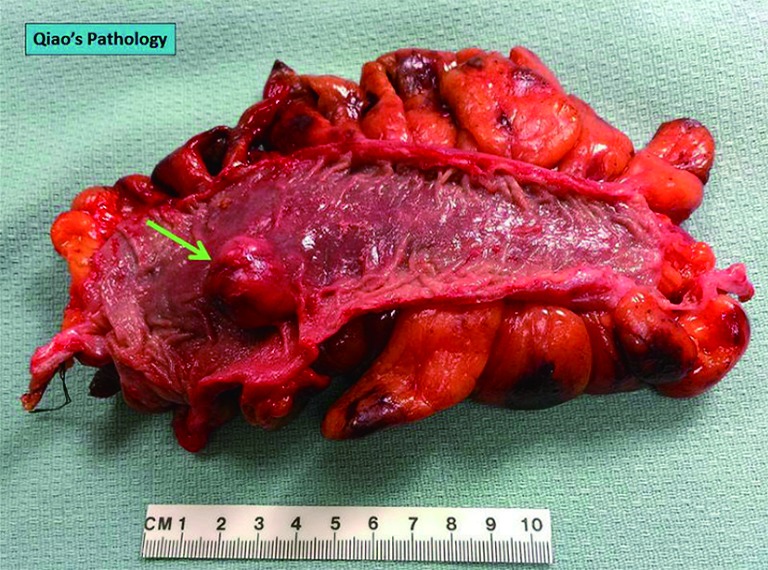
Gross photo showing a firm polypoid mass (green arrow) on the colon mucosal surface.
Table 1. GIST group classification system determined by tumor size, mitotic rate, and probability of metastasis based on location of primary tumor (7).
| Tumor size (cm) | Mitotic rate per HPF* | Gastric | Duodenal | Jejunal and ileal | Rectal |
|---|---|---|---|---|---|
| ≤2 | ≤5/50 | 0% | 0% | 0% | 0% |
| ≤2 | >5/50 | 0% | 50% (high) | – | 54% (high) |
| 3–5 | ≤5/50 | 1.9% (very low) | 4.3% (low) | 8.3% (low) | 8.5% (low) |
| 3–5 | >5/50 | 16% (moderate) | 73% (high) | 50% (high) | 52% (high) |
| 6–10 | ≤5/50 | 3.6% (low) | 24% (moderate) | 34% (high) | 57% (high) |
| 6–10 | >5/50 | 55% (high) | 85% (high) | 86% (high) | 71% (high) |
| >11 | ≤5/50 | 12% (moderate) | 52% (high) | 34% (high) | 57% (high) |
| >11 | >5/50 | 86% (high) | 90% (high) | 86% (high) | 71% (high) |
*, mitotic rate/HPF defined as (number of actively dividing cells/size of microscope field). GIST, gastrointestinal stromal tumor; HPF, high power field.
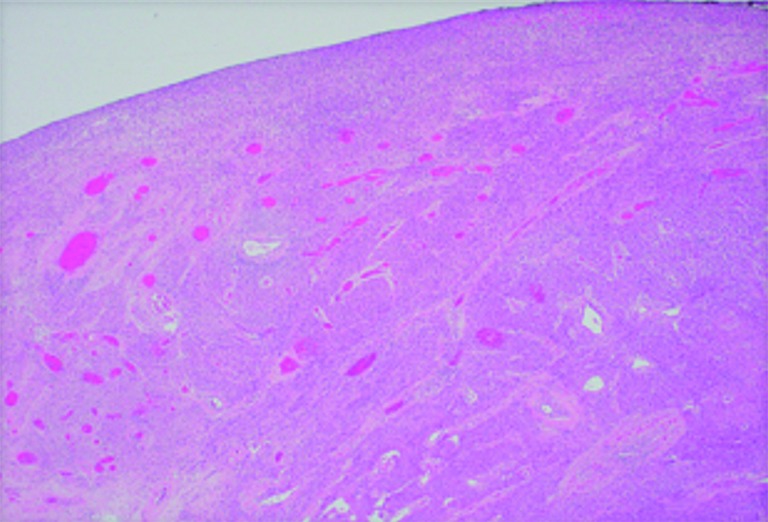
Figure 4.
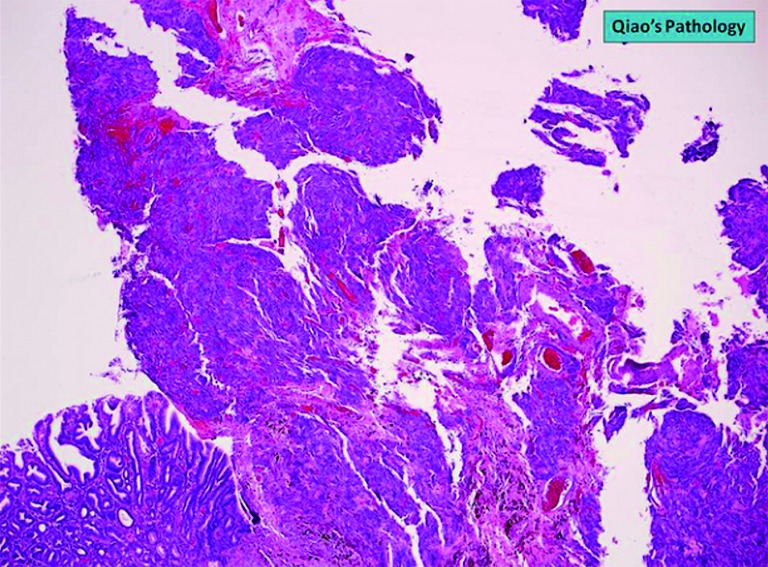
Microscopic photo. H&E stain. 4× objective magnification showing normal gastric mucosa on the left with intraluminal growth of the tumor on the right.
Clinical presentation
GISTs present asymptomatically in 18% of cases, especially in cases of smaller tumors of the intestinal tract (15,18). These tumors are usually found incidentally on abdominal CT scans, during endoscopy, or during surgical procedures for other manifestations. Symptomatic patients may present with nonspecific symptoms of nausea, vomiting, abdominal distension, early satiety, abdominal pain, and rarely as a palpable abdominal mass (18). Larger tumors may cause obstruction of the gastrointestinal lumen by endophytic growth or compression of the GIT from exophytic growth leading to dysphagia, obstructive jaundice, or constipation, depending on the location of the mass. Perforated neoplasms will present with signs of peritonitis or gastrointestinal bleeding. Indolent or massive intraperitoneal bleeding is secondary to pressure necrosis and ulceration (17,19).
Diagnostic imaging
GISTs can be identified on abdominal ultrasound, CT scan, magnetic resonance imaging (MRI), and positron emission transverse tomography (PET). CT enterography is the best modality to use to identify location of these tumors, any perforation, invasion of these tumors into nearby structures, or metastasis (Figure 5) (20-22). CT-guided biopsy also aids in the definitive diagnosis of GISTs. Some tumors are discovered incidentally or in emergent cases where preoperative biopsy does not play a role.
Figure 5.
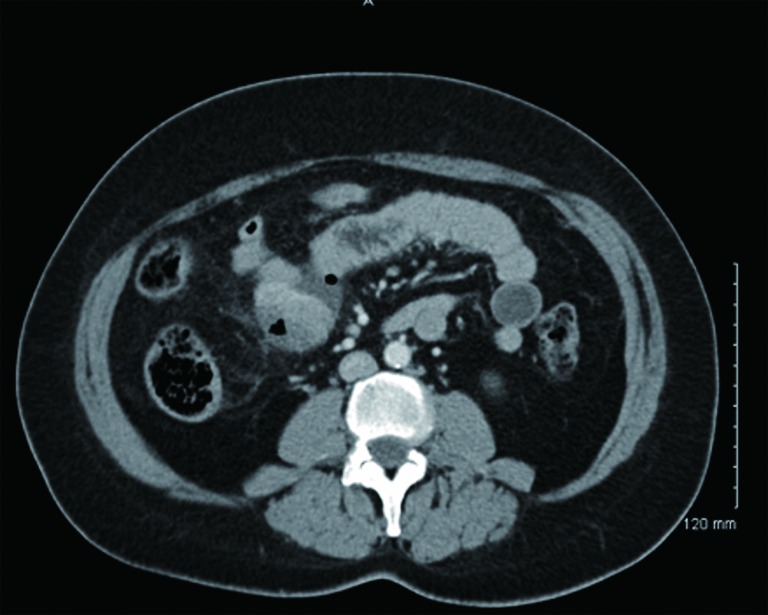
CT with oral contrast showing a mass compressing the mid-jejunal lumen with perforation.
While abdominal ultrasound is not the primary method used to visualize GISTs, ultrasound is useful if the tumor is larger than 5 cm (22,23). However, many factors affect the reliability of ultrasound as a modality, such as the presence of necrosis, ulceration, air in the bowel, and operator expertise (23,24).
Ghanem and Colleagues studied the findings of CT imaging on patients with either primary (n=20) or recurrent (n=16) GISTs by dividing the patients into groups based on tumor size. Tumors were classified as small (<5 cm), intermediate (5–10 cm), or large (>10 cm) and visualized with CT imaging. On CT, small GISTs had symmetric masses that were well demarcated with sharp borders and exhibited intraluminal growth patterns. Intermediate-sized GISTs had less symmetry, exhibited intraluminal and extraluminal growth patterns, and showed signs of infiltration to other organs in nine patients with primary tumors (45%) and two patients with recurrent tumors (12.5%). Lastly, large GISTs exhibited aggressive behavior with peritoneal or distant metastasis (20).
Tateishi and colleagues studied histologic tumor grade and mortality rate in patients with low-grade (n=44) and high-grade (n=25) GISTs by visualizing the tumors using CT imaging. They found that tumors larger than 11.1 cm on CT were associated with increased mortality or high grade histology. These tumors were found to have irregular borders and margins with invasion to adjacent organs and hepatic or peritoneal metastasis (23). They concluded that CT scanning can assess tumor size and metastasis, therefore determining treatment response to adjuvant therapies.
MRI is similar to CT imaging of GISTs in that MRI’s also provide information about size, tumor perforation, metastasis, and tumor invasion into adjacent structures, but MRI is preferred when identifying rectal GISTs, liver metastasis, hemorrhage, and necrosis of tumors (21). Small GISTs on MRI appear symmetrically round while large GISTs appear nonsymmetrical and lobulated (21,22). However, when compared to MRI, CT has the advantage of displaying the thickness of the entire small bowel, leading to better visualization of deep ileal loops and mesentery (23).
PET scans with 2-[F-18]-fluoro-2-deoxy-D-glucose, in conjunction with CT, provides useful information in tumor staging. PET/CT imaging can be used to identify areas of necrosis in lesions and differentiate benign versus malignant tumors (22). Similar to CT scanning, PET/CT scanning is sensitive to determining the effectiveness of adjuvant therapies. Additionally, PET/CT imaging is more accurate in the imaging of liver metastasis than CT imaging alone (22,24).
Invasive imaging
The use of endoscopy was studied by Park et al. in a retrospective study of 174 patients who underwent surgical excision of GISTs between 2008 and 2014. Intraluminal growth was found in 109 patients (62.4%) while the remaining 65 (37.4%) showed extraluminal growth (25). Patients with intraluminal GISTs showed smaller tumor sizes and ulceration on endoscopy three months prior to diagnosis while extraluminal GISTs were undetected. As a result, the researchers concluded that endoscopy has a limited role in the detection of GISTs due to the high prevalence of extraluminal tumors.
Radiologic-histopathologic correlation
The diagnosis of GISTs are made with histopathology and immunochemistry. GISTs have three different histologic findings, including spindle (70%) (Figure 6A), epithelioid (20%) (Figure 6B), or mixed type (10%). They are often misdiagnosed as leiomyoma or leiomyosarcoma prior to immunohistochemical analysis (3). Additionally, if tumors perforate, microabscesses can be seen on microscopy (Figure 7) (26). Approximately 88% of GISTs stain positive for both CD117 (Figure 8A) and DOG-1 (Figure 8B). A recent analysis of 70 cases of GIST showed positive expression of CD117 and DOG-1 in 95.71% and 88.57% of cases respectively (27). Literature suggests DOG-1 appears to be more sensitive and specific than CD117. However, in GISTs with a PDGFRA mutation, their sensitivities decrease to 9% and 79% respectively (28). Imaging reveals homogeneous densities in small tumors and larger tumors reveal irregular lobulated margins, mucosal ulceration, central and coagulative necrosis (Figure 9), hemorrhage cavitation, and heterogeneous enhancement (17). Necrosis of GISTs can also be seen on histological images and can progress to calcifications (Figure 10), which can be viewed with CT or MRI imaging.
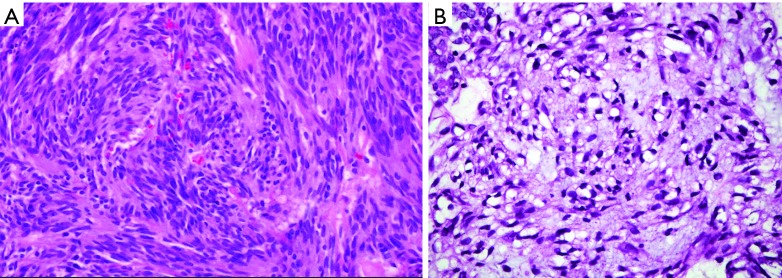
Figure 6.
Microscopic photo, H&E stain. (A) 40× objective magnification image of a GIST spindle cell type; (B) 100× objective magnification image of a GIST epithelioid type. GIST, gastrointestinal stromal tumor.
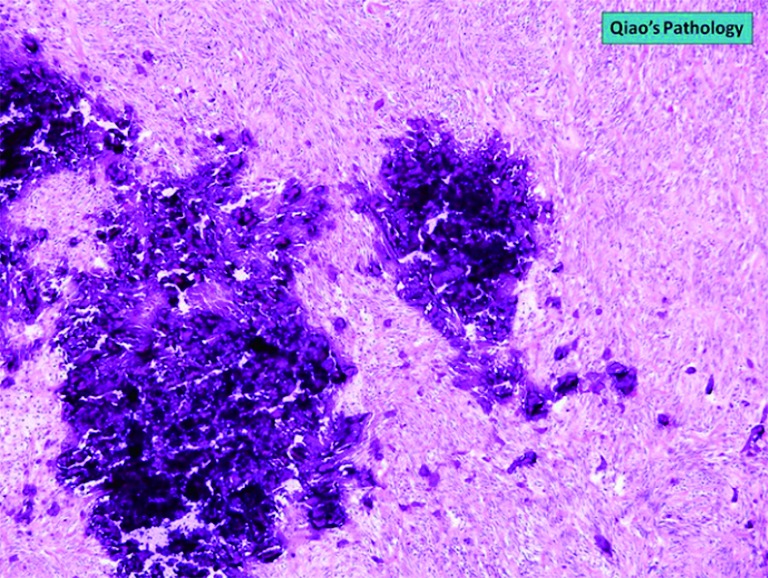
Figure 7.
Microscopic photo, H&E stain. 4× objective magnification image of a GIST showing microabscesses. GIST, gastrointestinal stromal tumor.
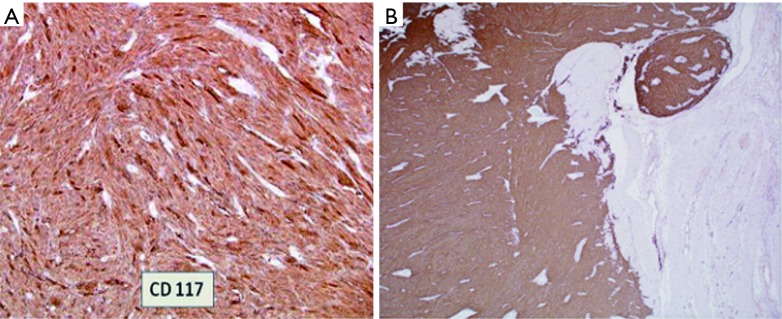
Figure 8.
Microscopic photo. (A) 40× objective magnification of GIST that stains CD117 positive; (B) 40× objective magnification of GIST that stains DOG-1 positive. GIST, gastrointestinal stromal tumor.
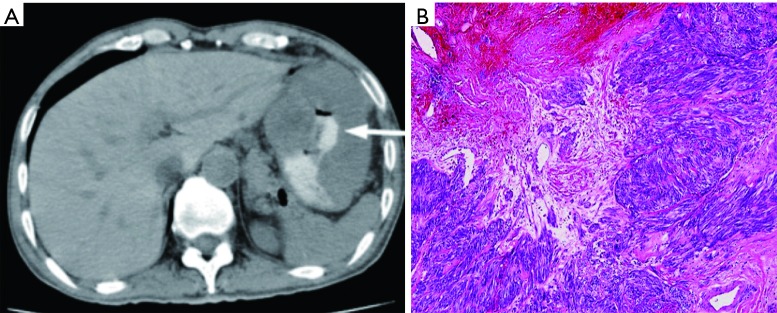
Figure 9.
CT Imaging correlating with histology. (A) Abdominal CT transverse cut showing extraluminal mass with contrast filling area of necrosis (arrow) (9); (B) microscopic photo, H&E stain. 4× objective magnification showing a GIST with ischemic and coagulative necrosis. GIST, gastrointestinal stromal tumor.
Figure 10.
Microscopic photo, H&E stain. 10× objective magnification of calcifications.
Classification and risk stratification
Many classification systems have evolved over the years but none have proved to be superior to the other. In 2002, Fletcher and colleagues collaborated to create the NIH classification, the first GIST classification system. It determined the risk of recurrence by categorizing patients into very low, low, intermediate, and high-risk groups by looking at the size and mitotic activity of the tumor (Table 2) (29). They concluded that tumors >5 cm in diameter plus a mitotic count higher than 5/50 high power fields (HPF) and tumors >10 cm with any mitotic rate have a higher risk of recurrence, subsequently requiring adjuvant drug therapy (29).
Table 2. Comparison of GIST imaging modality advantages and disadvantages.
| Imaging modality | Advantages | Disadvantages |
|---|---|---|
| CT enterography | Identifies location of tumors, perforation, local invasion, metastasis, definitive diagnosis (CT-guided biopsy). Displays full thickness of small bowel and mesentery. Determines sensitivity to adjuvant therapy | – |
| Abdominal ultrasound | Useful for visualization of tumors >5 cm in diameter | Inconsistent reliability in presence of necrosis, ulceration, and air in bowel. Variable efficacy due to operator skill |
| Magnetic resonance imaging | Identifies location of tumors, perforation, local invasion, and metastasis | Does not display full thickness of small bowel and mesentery |
| PET/CT combination imaging | GIST tumor staging, identifying areas of necrosis, differentiating benign vs. malignant tumors, determining sensitivity to adjuvant therapy. Better for imaging liver metastasis than CT alone | – |
GIST, gastrointestinal stromal tumor.
In 2006, Miettinen and colleagues evaluated 1,765 stomach GISTs and 906 small intestine GISTs and proposed a new classification system called the Armed Forces Institute of Pathology (AFIP) classification system. They discovered that in addition to tumor size and mitotic rate, anatomical location is an important prognostic factor (16). They were also the first to define the total area for mitotic counting (5 mm2). This classification system shows that the risk of recurrence for a tumor of the same size and mitotic count is greater for non-gastric GISTs than for gastric GISTs (16). Goh and associates revised the AFIP classification by combining the very low and low risk into one group and introducing a very high-risk group. They found that there was no significant difference in recurrence in the very low and low risk groups and only tumors >5 cm with >10 mitoses per HPF had a high risk of recurrence. In 2008, Joensuu and associates created the modified NIH classification, which determined that tumor rupture during surgery had a significant negative prognosis (Table 3). A GIST is considered high risk if the tumor is >10 cm with any mitotic index, the tumor is >5 cm with a mitotic count >5/50 HPF, or if the tumor has ruptured (Table 4) (30). It was determined that in non-ruptured GISTs, the mitotic rate is the most important prognostic factor and determines when adjuvant drug therapy is necessary (30).
Table 3. Modified NIH risk of recurrence (21).
| Risk | Tumor size (cm) | Mitotic count (HPF) | Tumor site |
|---|---|---|---|
| Very low risk | <2 | <5/50 | Any |
| Low risk | 2.1–5 | <5/50 | Any |
| Intermediate risk | <5 | 6–10/50 | Gastric |
| 5.1–10 | <5/50 | Gastric | |
| High risk | Any | Any | Perforated tumor |
| >5 | >5/50 | Any | |
| >10 | Any | Any | |
| Any | >10/50 | Any | |
| 2.1–5 | >5/50 | Non-gastric | |
| 5.1–10 | <5/50 | Non-gastric |
HPF, high power field.
Table 4. NIH risk assessment of GIST (20).
| Risk | Tumor size (cm) | Mitotic count (HPF) |
|---|---|---|
| Very low risk | <2 | <5/50 |
| Low risk | 2.1–5 | <5/50 |
| Intermediate risk | <5 | 6–10/50 |
| 5–10 | <5/50 | |
| High risk | >5 | >5/50 |
| >10 | Any mitotic count |
GIST, gastrointestinal stromal tumor; HPF, high power field.
Staging and grading
Woodall and associates proposed that a GIST staging system can be determined by the tumor-grade-metastasis (TGM) system. They retrospectively reviewed 2,537 GISTs from 1977 to 2004. The median age was 64 years old with 48% of the patients being men. At a 21-month follow-up, 23% of patients had metastasis and 5% had lymph node involvement. According to the TGM system, tumors measuring less than 70 mm are classified as T1 whereas tumors measuring greater than 70 mm are classified as T2. The second criteria defines grade I and II tumors as G1 and grade III and IV tumors as G2. Lastly, the presence of metastasis was defined as M1 and no metastasis was M0. In the TGM staging system, grade and metastasis were the greatest prognostic factors (31).
The French Federation of Cancer Centers Sarcoma Group (FNCLCC) and the National Cancer Institute (NCI) system are the most widely accepted grading scales for soft tissue sarcomas. The FNCLCC grading is based on mitotic activity, necrosis, and differentiation of the tumor. According to this system, these factors have a strong correlation to metastasis and mortality (32). The NCI system concluded that the quantification of cellularity, pleomorphism, and location determine prognosis (32). The grading and staging systems of GISTs can be beneficial in determining the effectiveness of Imatinib as a neoadjuvant or adjuvant therapy.
Surgical treatment
The gold standard of treatment for GISTs is surgical resection through laparoscopy, however if the patient is unstable then an open laparotomy is the preferred method of treatment (33). Laparoscopic surgery (LSG) is recommended for GISTs that are less than 5 cm and located in the stomach and small bowel. Chen and associates retrospectively reviewed 58 cases of GISTs. Sixteen cases (27%) underwent LSG and 42 cases (73%) underwent open surgery (OSG). Recurrence was observed in one LSG patient at a median follow-up of 33 months and 2 OSG patients at a median follow-up of 40 months. The LSG group resumed a normal diet sooner, had shorter postoperative hospital stays, and required less pain management when compared to the OSG group. Post-operative morbidity was 6.3% and 19% in the LSG and OSG groups respectively, thus laparoscopy is the preferred method of resection (33).
Wu and colleagues retrospectively reviewed 57 patients from 1995 to 2002 with a median follow-up of 18 months (range, 4–81 months). The median age was 61 years and half the patients were females. Twenty-eight (49%) patients underwent surgical open laparotomy with resection with curative intent and the other 29 (51%) were treated post-surgically with imatinib for metastatic disease (n=26) or adjuvant therapy (n=3). Seventy-nine percent (n=22) of the curative intent group had completely negative margins. Three patients had metastases that were completely resected with the tumor and two patients had successful resection after neoadjuvant imatinib therapy. CD117 staining was positive in 96% of the tumors and metastasis was discovered in 17 patients at the time of surgery. Twenty-six patients with metastatic disease were treated with imatinib and tumor regression was noted in 22 patients at 19 months. In total, 23 patients (40%) were alive without disease, 22 patients (39%) are alive with disease, 7 patients died, and 5 patients were lost to follow-up. They concluded that complete surgical resection with negative margins is the curative treatment for GISTs. However, if there is metastatic disease then imatinib should be recommended as the targeted therapy in conjunction with surgery (6).
Adjuvant therapy
The 3 agents approved for the treatment of GISTs are imatinib (Gleevec), sunitinib (Sutent), and ponatinib. Imatinib is a TKI that works by binding to the ATP binding sites on CD117 and PDGFRA, blocking signal transduction. GISTs that are CD117 and PDGFRA positive are thought to benefit from this therapy. DeMatteo and colleagues randomized 713 patients with resected primary GISTs measuring >3 cm that stained for CD117. They randomized 359 patients with imatinib and 354 with a placebo. The recurrence-free survival (RFS) at 1 year was 98% with imatinib versus 83% in the placebo group (P<0.001) (8). In 2002, Demetri and colleagues studied 147 patients with unresectable or metastatic GISTs that expressed CD117. They were randomly assigned to 400 or 600 mg of imatinib daily. At a follow-up of 24 weeks, both doses were deemed effective and there was not an increased risk of toxicity with 600 mg (9). The SSG XVIII trials determined that high risk GISTs showed better RFS with 3 year treatment versus 1 year treatment with imatinib therapy, with 5-year recurrence-free rates at 66% and 48% respectively (10). However, many GISTs with CD117 mutations in exons 9, 11, 13, 14 and 17 have imatinib resistance (Table 5) (11,35). In 2014, they found that patients with CD117 exon 11 mutations have a better survival rate than CD117 exon 9 mutations. They looked into the 10-year outcomes of the phase III SWOG trial, which focused on the use of imatinib, on the long-term survival in patients with metastatic GISTs. The trial studied two doses of imatinib in 695 patients with inoperable advanced GISTs and concluded that the long-term survival rate (8 or more years) was 27% for those who got the dose of 400 mg/d and 25% for those who got the dose of 800 mg/d.
Table 5. Clinical response of advanced GISTs to imatinib 400 mg correlated with mutational status (34).
| Genotype | Percentage of cases | Imatinib response |
|---|---|---|
| CD117 exon 11 mutation | 70% | 85% |
| CD117 exon 9 mutation | 15% | 45% |
| CD117 exon 13 mutation | <5% | Some (few cases) |
| CD117 exon 17 mutation | <5% | Some (few cases) |
| PDGFRA D842V mutation | 4% | None |
| PDGFRA other mutations | 1% | Some (few cases) |
| No CD117 or PDGFRA mutation | 5–10% | Little |
GIST, gastrointestinal stromal tumor.
Gronchi and colleagues studied the role of high dose imatinib in the setting of resistant GISTs. Of the 395 patients, the GIST genotypes were analyzed and the results showed that 282 (71%) had CD117 exon 11 mutations, 67 (17%) had no CD117 or PDGFRA mutations, 32 (8%) had CD117 exon 9 mutations, and 14 (4%) had both CD117 and PDGFRA mutations (28). Sunitinib treatment is the preferred therapy for exon 9 mutations and wild-type GISTs (no CD117 or PDGFRA mutations) (Table 6) (13). In patients with early detection of exon 11 mutations, a low dose of imatinib (400 mg/day) is adequate (11,13,35). Ramaswamy and colleagues showed that CD117 exon 9 mutations have 38 months of overall survival compared to CD117 exon 11 mutation with a survival rate of 66 months (36). Patients with exon 13 or 14 mutations benefit from sunitinib and exon 17 mutations benefit from ponatinib (Table 6) (11-13).
Table 6. Summary of recommended adjuvant therapy.
| CD117/C-Kit mutation | Imatinib | Sunitinib | Ponatinib |
|---|---|---|---|
| Exon 9 | – | Standard dosing‡ | – |
| Exon 11 | Early: low dose†; late: high dose† | – | – |
| Exon 13 | – | Standard dosing‡ | – |
| Exon 14 | – | Standard dosing‡ | – |
| Exon 17 | – | – | Standard dosing‡ |
| No exon mutation | – | Standard dose‡ | – |
Metastasis
In addition to surgery and targeted therapy, it is important that patients who fall into the high-risk categories for metastasis follow-up with serial CT scans every three months for five years (7,30). Patnaik and associates retrospectively reviewed 42 patients with primary GISTs and the overall metastasis rate was found to be 59.5% (n=25). The median age was 50 years old (range, 24–82 years) and the male to female ratio was 2:1. At the time of presentation, 22 patents (52.3%) had metastatic lesions and three patients (7.1%) developed metastasis after resection of the primary tumor. Common locations for GIST metastasis are to the liver (28%), and the mesentery and omentum (30%). Less frequently, tumors will metastasize to the lung (7%), subcutaneous tissues (4.7%), lymph nodes (4.7%), or bone (2.3%) (37).
If a patient cannot undergo surgical resection, then CT guided radiofrequency ablation (RF) may be an option. Yamanaka and colleagues studied 21 metastatic GIST liver tumors in seven patients. Solitary nodules were noted in two patients and multiple nodules in five patients. The tumor sizes were between 1.2–4.2 cm (median, 2.2 cm). After 12 sessions of RF, all but one patient (4.8%) had tumor regression. One patient died of subarachnoid hemorrhage at 5.9 months and two patients were noted to have new metastatic lesions to the liver and lung. No GIST-associated deaths occurred and the overall survival rate was 85.7% (P<0.05) at follow-up of 30.6 months (38).
Metastatic GISTs are developing resistance to imatinib and sunitinib. In 2013, Demetri and colleagues studied the efficacy of regorafenib, a kinase inhibitor, in patients at 57 hospitals in 17 countries with GISTs that were metastatic or unresectable with resistance to imatinib and sunitinib. The results of the study showed that regorafenib at a standard dosage of 160 mg daily can decrease metastasis and the size of tumors in highly refractory populations (9). Later that year, the FDA approved regorafenib as a standard treatment in drug resistant GISTs.
Newer therapies
Heinrich et al. studied the efficacy of ponatinib, particularly in patients with exon 11 mutations when imatinib, sunitinib, and regorafenib therapy had failed. Ponatinib has activity against BCR-ABL, CD117, and PDGFRA. The median survival was seven months for CD117 exon 11 mutations with ponatinib. They determined that ponatinib is effective in treating resistant GISTs but the adverse effects need to be further investigated (12).
Singh and colleagues discussed the use of nivolumab (Opdivo) and ipilimumab (Yervoy) immunotherapy at the 2018 Gastrointestinal Cancers Symposium. Nivolumab can be used alone or in combination with ipilimumab, a CTLA4 blocker. This study divided patients with advanced/metastatic GISTs into either the nivolumab treatment alone (240 mg every 2 weeks) or nivolumab plus ipilimumab treatment (1 mg/kg every 6 weeks), for up to 2 years. The median progression-free survival was 15.3 weeks in nivolumab alone and 18 weeks in the nivolumab plus ipilimumab. The reported side effects of nivolumab were fatigue (26.3%), pruritus (15.8%), and arthralgia (10.5%). Ipilimumab had reported side effects of rash (21.1%), arthralgia (10.5%), and pruritus (10.5%). In a current ongoing study, patients with TKI resistant or unresectable GISTs have benefited from the use of ipilimumab and nivolumab therapy with tumor size regression of 40% (39).
Endoscopic ultrasound-guided (EUS) injection of alcohol can be used for ablation of GISTs or metastatic lesions located in the liver, adrenal glands, or pelvic lymph nodes. Günter et al. reported on a 59-year-old male who was diagnosed with a 40-mm GIST via biopsy. Surgical resection was not feasible due to severe chronic obstructive lung disease. He underwent a EUS-guided tumor ablation with 1.5-mL of 95% ethanol (40). Seven weeks after the injection, endosonography showed a 1.5 cm ulcer at the injection site but no evidence of a tumor. The ulcer at the injection site resolved with proton pump inhibitor therapy. Follow-up therapy at 2 years demonstrated complete remission of the tumor. EUS alcohol ablation for GISTs requires further research, but may be an effective treatment when surgery is contraindicated due to comorbidities.
Conclusions
GISTs are rare tumors that account for a small percentage of gastrointestinal neoplasms. GISTs that occur outside the stomach are associated with a higher malignancy potential. Usually GISTs are an incidental finding and therefore most of the time present asymptomatically. However, if GISTs present symptomatically they can present with nausea, vomiting, abdominal distension, abdominal pain, or peritonitis. GISTs are best identified by CT scan but also can be seen on abdominal ultrasound, MRI, and PET. The pathology of GISTs consist of either spindle cells, epithelioid cells, or mixed cell types. GISTs most commonly stain positive for CD117 and DOG-1. GISTs are staged using the TGM system, which determines that grade and metastasis are the best predictors of prognosis. The grading systems of GISTs are used to determine the effectiveness of imatinib as neoadjuvant or adjuvant therapy. Laparoscopic surgical resection of GISTs with adjuvant imatinib 400 mg daily is the gold standard for the treatment of GISTs. However, if the tumor is unresectable then neoadjuvant imatinib 400 mg daily followed by resection is recommended. Metastasis are very common and can be seen in the liver and mesentery and omentum, but are treated the same as high risk GISTs. There is limited evidence showing the effectiveness of RF for nonoperative liver metastasis.
To determine the risk of malignancy potential and recurrence, researchers follow the NIH, AFIP, and modified NIH classifications that are calculated based on tumor size, mitotic rate, location, and perforation. TKIs are recommended for high risk GISTs. FDA approved treatments are imatinib, sunitinib, and regorafenib. The standard dose for high risk GISTs is imatinib 400 mg daily. Sunitinib is required for CD117 exon 9, 13, or 14 mutations, while ponatinib is used for CD117 exon 17 mutations, and regorafenib in highly refractory tumors. As research continues to grow in this area, newer studies are showing the effectiveness of novel therapies such as ipilimumab, nivolumab, and EUS alcohol ablation.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. 10.1097/00000478-198309000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Rammohan A, Sathyanesan J, Rajendran K, et al. A gist of gastrointestinal stromal tumors: A review. World J Gastrointest Oncol 2013;5:102-12. 10.4251/wjgo.v5.i6.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. 10.1126/science.279.5350.577 [DOI] [PubMed] [Google Scholar]
- 5.Corbin KS, Kindler HL, Liauw SL. Considering the role of radiation therapy for gastrointestinal stromal tumor. Onco Targets Ther 2014;7:713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu PC, Langerman A, Ryan CW, et al. Surgical treatment of gastrointestinal stromal tumors in the imatinib (STI-571) era. Surgery 2003;134:656-65; discussion 665-6. 10.1016/S0039-6060(03)00314-3 [DOI] [PubMed] [Google Scholar]
- 7.Benjamin RS, Casali PG. Adjuvant Imatinib for GI Stromal Tumors: When and For How Long? J Clin Oncol 2016;34:215-8. 10.1200/JCO.2015.64.0102 [DOI] [PubMed] [Google Scholar]
- 8.DeMatteo RP. Nanoneoadjuvant therapy of gastrointestinal stromal tumor (GIST). Ann Surg Oncol 2009;16:799-800. 10.1245/s10434-009-0316-9 [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. 10.1016/S0140-6736(12)61857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg BL. The SSG XVIII/AIO trial: results change the current adjuvant treatment recommendations for gastrointestinal stromal tumors. Am J Clin Oncol 2013;36:89-90. 10.1097/COC.0b013e31827a7f55 [DOI] [PubMed] [Google Scholar]
- 11.Gronchi A, Blay JY, Trent JC. The role of high-dose imatinib in the management of patients with gastrointestinal stromal tumor. Cancer 2010;116:1847-58. 10.1002/cncr.24944 [DOI] [PubMed] [Google Scholar]
- 12.Heinrich MC, von Mehren M, Demetri GD, et al. A phase 2 study of ponatinib in patients (pts) with advanced gastrointestinal stromal tumors (GIST) after failure of tyrosine kinase inhibitor (TKI) therapy: Initial report. J Clin Oncol 2014;32:10506 10.1200/jco.2014.32.15_suppl.10506 [DOI] [Google Scholar]
- 13.Mulet-Margalef N, Garcia-Del-Muro X. Sunitinib in the treatment of gastrointestinal stromal tumor: patient selection and perspectives. Onco Targets Ther 2016;9:7573-82. 10.2147/OTT.S101385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltran MA, Cruces KS. Primary tumors of jejunum and ileum as a cause of intestinal obstruction: a case control study. Int J Surg 2007;5:183-91. 10.1016/j.ijsu.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Soreide K, Sandvik OM, Soreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. 10.1016/j.canep.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 16.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [DOI] [PubMed] [Google Scholar]
- 17.Gong J, Kang W, Zhu J, et al. CT and MR imaging of gastrointestinal stromal tumor of stomach: a pictorial review. Quant Imaging Med Surg 2012;2:274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherubl H, Faiss S, Knoefel WT, et al. Management of early asymptomatic gastrointestinal stromal tumors of the stomach. World J Gastrointest Endosc 2014;6:266-71. 10.4253/wjge.v6.i7.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scola D, Bahoura L, Copelan A, et al. Getting the GIST: a pictorial review of the various patterns of presentation of gastrointestinal stromal tumors on imaging. Abdom Radiol (NY) 2017;42:1350-64. 10.1007/s00261-016-1025-z [DOI] [PubMed] [Google Scholar]
- 20.Ghanem N, Altehoefer C, Furtwangler A, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol 2003;13:1669-78. 10.1007/s00330-002-1803-6 [DOI] [PubMed] [Google Scholar]
- 21.Lanke G, Lee JH. How best to manage gastrointestinal stromal tumor. World J Clin Oncol 2017;8:135-44. 10.5306/wjco.v8.i2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernuccio F, Taibbi A, Picone D, et al. Imaging of Gastrointestinal Stromal Tumors: From Diagnosis to Evaluation of Therapeutic Response. Anticancer Res 2016;36:2639-48. [PubMed] [Google Scholar]
- 23.Tateishi U, Hasegawa T, Satake M, et al. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr 2003;27:792-8. 10.1097/00004728-200309000-00018 [DOI] [PubMed] [Google Scholar]
- 24.Belloni M, De Fiori E, Mazzarol G, et al. Endoscopic ultrasound and Computed Tomography in gastric stromal tumours. Radiol Med 2002;103:65-73. [PubMed] [Google Scholar]
- 25.Park CH, Kim EH, Jung DH, et al. Impact of periodic endoscopy on incidentally diagnosed gastric gastrointestinal stromal tumors: findings in surgically resected and confirmed lesions. Ann Surg Oncol 2015;22:2933-9. 10.1245/s10434-015-4517-0 [DOI] [PubMed] [Google Scholar]
- 26.Owens SR. Atlas of esophagus and stomach pathology. New York: Springer-Verlag; 2016. [Google Scholar]
- 27.Kisluk J, Zinczuk J, Kemona A, et al. Expression of CD117, DOG-1, and IGF-1R in gastrointestinal stromal tumours - an analysis of 70 cases from 2004 to 2010. Prz Gastroenterol 2016;11:115-22. 10.5114/pg.2015.52587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol 2008;32:210-8. 10.1097/PAS.0b013e3181238cec [DOI] [PubMed] [Google Scholar]
- 29.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. 10.1053/hupa.2002.123545 [DOI] [PubMed] [Google Scholar]
- 30.Joensuu H, Martin-Broto J, Nishida T, et al. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer 2015;51:1611-7. 10.1016/j.ejca.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 31.Woodall CE, 3rd, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg 2009;144:670-8. 10.1001/archsurg.2009.108 [DOI] [PubMed] [Google Scholar]
- 32.Hou YY, Lu SH, Zhou Y, et al. Stage and histological grade of gastrointestinal stromal tumors based on a new approach are strongly associated with clinical behaviors. Mod Pathol 2009;22:556-69. 10.1038/modpathol.2009.11 [DOI] [PubMed] [Google Scholar]
- 33.Chen YH, Liu KH, Yeh CN, et al. Laparoscopic resection of gastrointestinal stromal tumors: safe, efficient, and comparable oncologic outcomes. J Laparoendosc Adv Surg Tech A 2012;22:758-63. 10.1089/lap.2012.0115 [DOI] [PubMed] [Google Scholar]
- 34.Zhang WY, Li ZS, Jin ZD. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J Gastroenterol 2013;19:3397-403. 10.3748/wjg.v19.i22.3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oppelt PJ, Hirbe AC, Van Tine BA. Gastrointestinal stromal tumors (GISTs): point mutations matter in management, a review. J Gastrointest Oncol 2017;8:466-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramaswamy A, Bal M, Swami R, et al. Early outcomes of exon 11 mutants in GIST treated with standard dose Imatinib. Ann Transl Med 2017;5:134. 10.21037/atm.2017.03.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patnaik S, Jyotsnarani Y, Rammurti S. Radiological features of metastatic gastrointestinal stromal tumors. J Clin Imaging Sci 2012;2:43. 10.4103/2156-7514.99177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka T, Takaki H, Nakatsuka A, et al. Radiofrequency ablation for liver metastasis from gastrointestinal stromal tumor. J Vasc Interv Radiol 2013;24:341-6. 10.1016/j.jvir.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 39.Singh AS, Chmielowski BC, Hecht JR. A randomized phase 2 study of nivolumab monotherapy versus nivolumab combined with ipilimumab in patients with metastatic or unresectable gastrointestinal stromal tumor (GIST). J Clin Oncol 2018;36:55 10.1200/JCO.2018.36.4_suppl.55 [DOI] [Google Scholar]
- 40.Günter E, Lingenfelser T, Eitelbach F, et al. EUS-guided ethanol injection for treatment of a GI stromal tumor. Gastrointest Endosc 2003;57:113-5 10.1067/mge.2003.39 [DOI] [PubMed] [Google Scholar]