Abstract
Pulmonary hypertension (PH) frequently complicates the course of patients with various forms of chronic lung disease (CLD). CLD-associated PH (CLD-PH) is invariably associated with reduced functional ability, impaired quality of life, greater oxygen requirements and an increased risk of mortality. The aetiology of CLD-PH is complex and multifactorial, with differences in the pathogenic sequelae between the diverse forms of CLD. Haemodynamic evaluation of PH severity should be contextualised within the extent of the underlying lung disease, which is best gauged through a combination of physiological and imaging assessment. Who, when, if and how to screen for PH will be addressed in this article, as will the current state of knowledge with regard to the role of treatment with pulmonary vasoactive agents. Although such therapy cannot be endorsed given the current state of findings, future studies in this area are strongly encouraged.
Short abstract
State of the art and research perspectives in pulmonary hypertension in chronic lung disease and hypoxia http://ow.ly/XcW730meWxy
Introduction
This article provides an update on pulmonary hypertension (PH) associated with chronic lung disease (CLD), with the main focus being on chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) [1]. There is evidence that PH is associated with other CLDs such as cystic fibrosis and bronchopulmonary dysplasia [2, 3]. CLD-associated PH (CLD-PH) is clearly linked with reduced functional status and worse outcomes [4, 5]. Even in patients who fulfil diagnostic criteria for group 1 pulmonary arterial hypertension (PAH), the presence of minor lung disease affects survival [6]. Moreover, there is data suggesting that mean pulmonary arterial pressure (mPAP) ≤25 mmHg is associated with worse outcome in CLD-PH [7, 8]. Whether the presence of PH is causative or a surrogate of other factors affecting outcomes remains largely uncertain.
PH in the context of acute exacerbations of the various CLDs will not be discussed. However, it is important that defining PH should not be undertaken during an acute exacerbation, but under stable conditions. For purposes of consistent nomenclature, the lung condition will be mentioned first, followed by “-PH” since mostly it is the lung condition which initially manifests clinically.
Epidemiology and clinical relevance of PH in lung disease
Chronic obstructive lung disease
The prevalence of PH in COPD (COPD-PH) is in general dependent on the severity of the disease, but also on the definition of PH and the method of diagnostic assessment. Specific genetic signatures are also linked with the development of PH in COPD [9]. Several studies in patients with spirometric Global Initiative for Chronic Obstructive Lung Disease stage IV showed that up to 90% have mPAP >20 mmHg, with most ranging between 20 and 35 mmHg. Approximately 1–5% of COPD patients have mPAP >35–40 mmHg at rest [10]. Even under moderate exercise conditions, COPD patients may show a rapid rise in mPAP, indicating loss of lung vasculature, vascular distensibility and/or vessel recruitment capability. In addition, exercise PH in COPD may be due to comorbid left heart disease. There is a cluster of patients representing a “pulmonary vascular COPD phenotype”, characterised by less severe airflow limitation, hypoxaemia, very low diffusing capacity of the lung for carbon monoxide (DLCO), normo- or hypocapnia and a cardiovascular exercise limitation profile [11]. Interestingly, the vascular lesions in COPD-PH patients are morphologically similar to those in idiopathic PAH (IPAH). It has previously been established that the presence of PH has a stronger association with mortality in COPD than forced expiratory volume in 1 s (FEV1) or gas exchange variables [1]. In addition, an enlarged pulmonary artery diameter, as detected by computed tomography (CT) scan, predicts hospitalisation due to acute COPD exacerbation [4, 12].
Idiopathic pulmonary fibrosis and other idiopathic interstitial pneumonias
Most of the data on the prevalence and impact of PH complicating fibrotic lung disorders emanates from the idiopathic pulmonary fibrosis (IPF) literature. In IPF, mPAP ≥25 mmHg has been reported in 8–15% of patients upon initial work-up, with greater prevalence in advanced (30–50%) and end-stage (>60%) disease [13–15]. Additionally, echocardiography studies have suggested a high prevalence of PH [16]. However, echocardiography and other non-invasive measures, including an enlarged main pulmonary artery on CT scan, are limited in their accuracy to detect PH in lung diseases, thus serving as screening tools only [16, 17]. Nonetheless, both of these modalities have been shown to provide independent prognostic information in patients with fibrotic lung disease. In most patients, PH is mild to moderate, but may also be severe [14]. One longitudinal study suggested that mPAP increases by around 1.8 mmHg per year, but rapid progression of PH has also been reported in late-stage IPF patients [18]. Intriguingly, there is limited correlation between PH severity and lung function impairment or high-resolution CT fibrosis score [14–16, 19, 20], whereas distinct gene signatures have been observed in IPF-PH lungs [21]. PH may also be associated with an increased risk for acute exacerbation in advanced IPF [22]. Adverse outcomes with mPAP thresholds ≥25 mmHg have been reported in IPF. Indeed, the prognosis of fibrotic idiopathic interstitial pneumonia (IIP) with PH is worse than IPAH [23].
Combined pulmonary fibrosis and emphysema, and other lung diseases
Combined pulmonary fibrosis and emphysema (CPFE) is currently defined by the simultaneous presence of emphysema in the upper lobes and fibrosis in the lower lobes on chest CT. Patients with CPFE are particularly prone to develop PH, with estimates suggesting a prevalence of 30–50% [24]. Typically, normal or mildly abnormal lung volumes and the absence of airflow obstruction are accompanied by a markedly impaired diffusion capacity, significant hypoxaemia and PH. The PH appears to contribute to the functional limitation in CPFE and is associated with poor survival [24, 25].
Sarcoidosis
The prevalence of PH in sarcoidosis ranges from 5.7% to 74% [26]. Sarcoidosis-PH has a reported 5-year survival of 50–60% [27, 28]. While the vast majority of patients with sarcoidosis-PH have extensive parenchymal disease, it may also occur in patients without pulmonary fibrosis [27–29]. The mechanisms underlying PH in sarcoidosis are complex, and include fibrosis-associated remodelling and obliteration of pulmonary vessels, extrinsic compression of central pulmonary vessels by lymphadenopathy or mediastinal fibrosis, pulmonary veno-occlusive-like lesions, granulomatous involvement of pulmonary vessels, left ventricular dysfunction, and portopulmonary hypertension [27].
Other CLDs
The prevalence of PH in patients with pulmonary Langerhans cell histiocytosis is high, with haemodynamic features frequently resembling PAH, while PH complicating lymphangioleiomyomatosis tends to be mild to moderate and mostly related to the extent of parenchymal involvement [30, 31]. PH may complicate the course of adults with a history of bronchopulmonary dysplasia and cystic fibrosis [2, 3]. PH may also develop in patients with chronic hypersensitivity pneumonitis and, possibly, lung cancer [32, 33].
Detection of PH in CLD
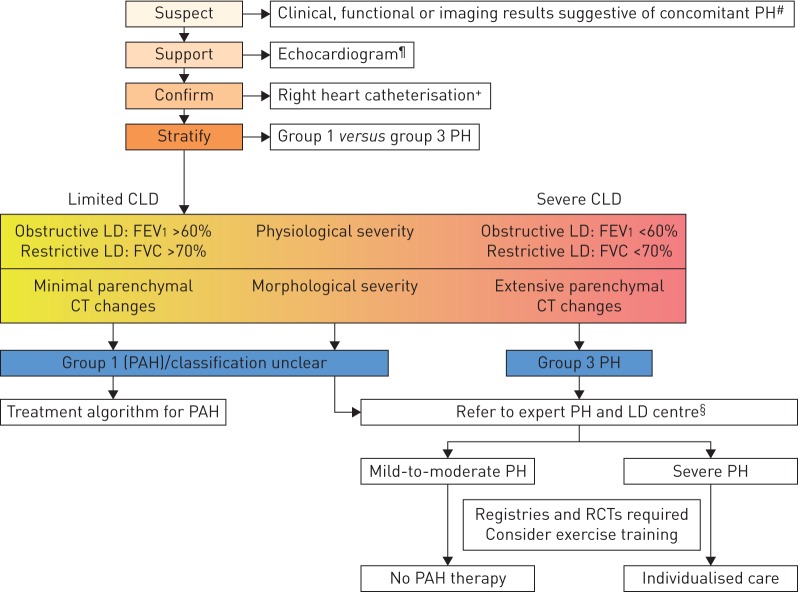
Non-invasive modalities that might raise suspicion for the presence of PH in CLD include circulating biomarkers, pulmonary function testing, echocardiography and imaging (figure 1). Plasma levels of brain natriuretic peptide (BNP) or N-terminal pro-BNP are elevated in severe CLD-PH, but have less sensitivity and specificity for moderate PH and may be confounded by left heart abnormalities [34, 35]. In both ILD and COPD, PH is generally associated with a lower DLCO, diminished exercise capacity and more impaired gas exchange at rest or during exercise than expected based on ventilatory impairments [14, 16, 19, 36, 37].
FIGURE 1.
Evaluation of pulmonary hypertension (PH) in chronic lung disease (CLD). FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; CT: computed tomography; PAH: pulmonary arterial hypertension; RCT: randomised controlled trial; DLCO: diffusing capacity of the lung for carbon monoxide; KCO: transfer coefficient of the lung for carbon monoxide. #: suggestive findings include: 1) symptoms and signs (dyspnoea out of proportion, loud P2, signs of right heart failure, right axis deviation on ECG, elevated natriuretic peptide levels); 2) pulmonary function test abnormalities (low DLCO (e.g. <40% of predicted), elevated %FVC/%DLCO ratio (low KCO)); 3) exercise test findings (including decreased distance, decreased arterial oxygen saturation or increased Borg rating on 6-min walk test and decreased circulatory reserve, preserved ventilatory reserve on cardiopulmonary exercise testing); and 4) imaging findings (extent of LD, enlarged pulmonary artery segments, increased pulmonary artery/aorta diameter ratio >1 on CT). ¶: signs supporting the diagnosis of PH include elevated systolic pulmonary arterial pressure and signs of right ventricular dysfunction. However, echocardiography measures are only suggestive and have limited accuracy in patients with CLD. +: strongly consider referring the patient to a PH expert centre. §: expert centres should comprise multidisciplinary teams. Any decision for individualised treatment should follow a goal-orientated approach with predefined treatment targets, to be stopped if these targets are not met after a predefined time period.
Echocardiography is considered the best non-invasive modality to screen for CLD-PH. However, the ability to determine peak tricuspid regurgitation velocity to estimate the right ventricular systolic pressure is limited in these patients [38]. Alternate echocardiographic measures including right ventricular outflow tract diameter, tricuspid annular plane systolic excursion, and qualitative assessment of right chamber structure and function have been advocated in IPF and COPD [39, 40].
The ratio of the main pulmonary artery to ascending aorta diameter on imaging may predict PH in both COPD and IPF, with a ratio >1 (range 0.9–1.1) suggested as a threshold [12, 41, 42]. Combining the pulmonary artery/aorta diameter ratio and other non-invasive measures (including echocardiographic and physiological variables) improves the accuracy of predicting PH [36, 41].
Right heart catheterisation (RHC) remains the gold standard for the diagnosis of CLD-PH. However, suspicion for underlying PH does not always mandate performance of RHC in patients with established lung disease if there is no therapeutic or management consequence.
Recommendations
When to perform RHC
RHC should be performed in patients with CLD when significant PH is suspected and the patient's management will likely be influenced by RHC results, including referral for transplantation, inclusion in clinical trials or registries, treatment of unmasked left heart dysfunction, or compassionate use of therapy.
RHC may be considered when:
1) Clinical worsening, progressive exercise limitation and/or gas exchange abnormalities are not deemed attributable to ventilatory impairment.
2) An accurate prognostic assessment is deemed sufficiently important.
Pressure measurements during RHC
As a result of exaggerated changes in intrathoracic pressures during the breathing cycle in patients with lung disease, a floating average over several breaths (without a breath hold) is suggested for measurement of mean pressures, including the pulmonary capillary wedge pressure.
We suggest adapting the definition for PH in the context of CLD-PH:
1) CLD without PH (mPAP <21 mmHg, or mPAP 21–24 mmHg with pulmonary vascular resistance (PVR) <3 Wood Units (WU)).
2) CLD with PH (mPAP 21–24 mmHg with PVR ≥3 WU, or mPAP 25–34 mmHg) (CLD-PH).
3) CLD with severe PH (mPAP ≥35 mmHg, or mPAP ≥25 mmHg with low cardiac index (<2.0 L·min−1·m−2)) (CLD-severe PH).
The rationale for the choice of mPAP ≥35 mmHg as a cut-off for severe PH follows previously presented evidence [1]. There are currently no valid data to support the routine use of acute vasodilator testing in CLD-PH.
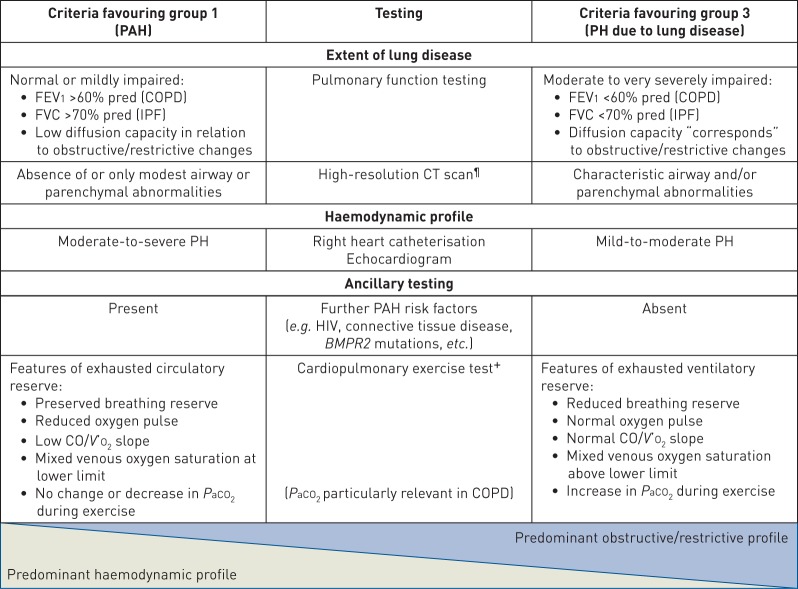
The randomised controlled trials (RCTs) in group 1 for PAH therapies set exclusion criteria using pulmonary function testing in the following ranges: total lung capacity <60–70% of predicted, FEV1 <55–80% of predicted or FEV1/forced vital capacity (FVC) ratio <50–70%. PAH studies have not previously utilised chest imaging to exclude patients with lung disease; indeed, it is possible that a number of patients with lung volumes above these inclusion thresholds might have an underappreciated burden of parenchymal lung disease. However, lung diseases (especially COPD) are common conditions and PAH developing in such patients may not be attributable to these diseases, but may be coincidental. Criteria for discrimination between group 1 and group 3 PH are summarised in table 1. The spectrum of severity of both the pulmonary vascular and parenchymal lung disease is likely a continuum, which often makes the distinction between group 1 and group 3 PH very difficult. When there is uncertainty whether to classify a patient with lung disease and PH into group 1 or group 3, then the patient should be referred to centres with expertise in both PH and CLD.
TABLE 1.
Criteria favouring group 1 versus group 3 pulmonary hypertension (PH)#
PAH: pulmonary arterial hypertension; FEV1: forced expiratory volume in 1 s; COPD: chronic obstructive pulmonary disease; FVC: forced vital capacity; IPF: idiopathic pulmonary fibrosis; CT: computed tomography; BMPR2: bone morphogenetic protein receptor type 2; CO: cardiac output; V′O2: oxygen uptake; PaCO2: arterial carbon dioxide tension. #: group 2 and 4 patients are excluded based on the diagnostic criteria of these groups; ¶: parenchymal changes linked to pulmonary veno-occlusive disease may be discriminated from those associated with diffuse parenchymal lung diseases; +: features of a limited circulatory reserve may be noted in severe COPD-PH and severe IPF-PH.
Treatment of PH due in CLD: evidence for appropriate risk–benefit balance of PAH-targeted therapy
The underlying lung disease should be optimally treated according to current guidelines. Long-term oxygen treatment (LTOT) makes intuitive sense in patients with lung disease who are hypoxaemic. However, it has only been prospectively evaluated in COPD. In stabilised hypoxaemic COPD patients, LTOT for 15 h per day prevented the progressive increase of mPAP and when used for >18 h per day produced a slight decrease of mPAP [43, 44]. However, in COPD patients with moderate resting oxygen desaturation (SpO2 89–93%) or exercise-induced oxygen desaturation (SpO2 <90% for ≥10 s), LTOT does not provide benefit in terms of survival or hospitalisations [45]. Evidence for the beneficial effect of LTOT in ILD is less clear than in COPD and there are no studies addressing the impact of LTOT on PH associated with this group of diseases [46].
Safety and efficacy of PAH-targeted therapy in CLD-PH has been evaluated in recent years; however, there have only been a few RCTs in ILD, COPD and sarcoidosis. Pertinent studies which included more than 20 participants are shown in table 2.
TABLE 2.
Randomised controlled trials (RCTs) with pulmonary arterial hypertension (PAH)-targeted therapy in lung disease
| First author (year) [ref.] | Subjects n | Inclusion criteria | Study design | Diagnosis of PH | Baseline haemodynamics# | Baseline PFTs# | Therapy | Duration | Primary end-point result | Other outcomes |
| COPD | ||||||||||
| Vonbank (2003) [86] | 40 | COPD on supplemental oxygen with PH by RHC | RCT (open label) | RHC: mPAP ≥25 mmHg | mPAP 27.6±4.4 mmHg, CI 2.7±0.6 L·min−1·m−2 | FEV1 1.09±0.4 L, FEV1/FVC 44.5% | “Pulsed” nitric oxide with oxygen vs oxygen | 3 months | PVRI, improved | Improved mPAP, CO and PVR; no worsened hypoxaemia |
| Stolz (2008) [53] | 30 | GOLD III–IV; no haemodynamic requirement | RCT (2:1) | Echo | sPAP 32 (29–38) mmHg | Not reported | Bosentan 125 mg 2 times daily | 12 weeks | 6MWD, no change | Worsened hypoxaemia and health-related QoL |
| Valerio (2009) [50] | 32 | COPD with PH by RHC | RCT (open label) | RHC | mPAP 37±5 mmHg | FEV1 37±18% | Bosentan 125 mg 2 times daily | 18 months | No defined primary | mPAP, PVR, BODE index and 6MWD improved |
| Rao (2011) [87] | 33 | GOLD III–IV | RCT | Echo: sPAP >40 mmHg | sPAP 52.7±11.9 mmHg | FEV1 32.5±11.1% | Sildenafil 20 mg 3 times daily | 12 weeks | 6MWD, increased 190 m | Decrease in sPAP |
| Blanco (2013) [88] | 60 | COPD with PH by RHC or echo | RCT | RHC: mPAP ≥25 mmHg; echo: sPAP ≥35 mmHg | sPAP 42±10 mmHg, mPAP 31±5 mmHg | FEV1 32±11% | Sildenafil 20 mg or placebo 3 times daily and PR | 3 months | Exercise endurance time, no change | No change in 6MWD, peak V′O2, QoL or oxygenation |
| Goudie (2014) [89] | 120 | COPD with PH by echo | RCT | Echo: pulmonary acceleration time <120 ms or sPAP >30 mmHg | Echo: sPAP 42±10 mmHg | FEV1 41±16% | Tadalafil 10 mg daily | 12 weeks | 6MWD, no change | Decreased sPAP compared with placebo; no difference in QoL, BNP or SaO2 |
| Vitulo (2016) [49] | 28 | COPD with PH by RHC | RCT (2:1) | RHC: mPAP >35 mmHg (if FEV1 <30%), mPAP ≥30 mmHg (if FEV1 ≥30%) | mPAP 39±8 mmHg, CI 2.4±0.5 L·min−1·m−2, PVR 7±2.6 WU | FEV1 54±22%, DLCO 33±12% | Sildenafil 20 mg 3 times daily | 16 weeks | PVR, decreased 1.4 WU | Improved CI, BODE scores and QoL; no effect on gas exchange |
| ILD | ||||||||||
| Han (2013) [90] | 119 | IPF with echo available (66% of the whole cohort) | RCT | Echo: RVSD | Not available | FVC 57%, DLCO 26% | Sildenafil 20 mg 3 times daily | 12 weeks | 6MWD, less decline in patients with RVSD on sildenafil | Improvement in QoL in patients with RVSD |
| Corte (2014) [60] | 60 | IPF or idiopathic fibrotic NSIP | RCT (2:1) | RHC: mPAP ≥25 mmHg | mPAP 37±9.9 mmHg, CI 2.2±0.5 L·min−1·m−2 | FVC 55.7±20%, KCO 45±22% | Bosentan | 16 weeks | PVRI decrease of 20%, negative | Secondary end-points all negative; no change in functional capacity or symptoms |
| Raghu (2015) [14]¶ | 68 | IPF with group 2 PH (14% of whole cohort) | RCT (2:1) | RHC | mPAP 30±8 mmHg | FVC 67±12%, DLCO 39±15% | Ambrisentan 10 mg·day−1 | Event-driven study terminated early | Disease progression, unfavourable trend | More hospitalised ambrisentan arm |
| Nathan (2017) [57] | 147 | IIP, FVC >45%, mPAP >25 mmHg | RCT | RHC | mPAP 33.2±8.2 mmHg, CI 2.6±0.7 L·min−1·m−2 | FVC 76.3±19%, DLCO 32±12% | Riociguat 2.5 mg 3 times daily | 26 weeks | 6MWD, no difference at study halt | Study stopped early for increased harm to riociguat arm (death and hospitalisation) |
| Sarcoidosis | ||||||||||
| Barnett (2009) [65] | 22 | Any SAPH and treatment with PAH therapy | Retrospective case series | RHC | mPAP 46.1±2.7 mmHg, CO 4.2±0.4 L·min−1 | FVC 53.6±3.3%, FEV1 51.2±3.7% | Bosentan, sildenafil | Median (range) 11 (5.2–46.6) months | 6MWD improved by 59 m | NYHA FC improvement in nine patients |
| Baughman (2009) [66] | 22 | Any SAPH | Prospective open label | RHC | mPAP 33 (20–62) mmHg, CO 5.9 (3.1–9.5) L·min−1, PVR 5.1 (1.96–16.3) WU | FVC 50% (41–101%), FEV1/FVC 73% (53–91%) | Inhaled iloprost | 4 months | 6MWD unchanged 0.6±40 m | 7 patients withdrew; 6 patients with ≥20% decrease in PVR and 3 patients with ≥30 m increase in 6MWD |
| Judson (2011) [91] | 25 | mPAP >25 mmHg, PVR >3 WU, FVC >40%, WHO FC II or III, 6MWD 150–450 m | Prospective open label | RHC | mPAP 32.7±7 mmHg, CO 4.45±0.94 L·min−1·m−2, PVR 5.86±2.3 WU | FEV1 59±21%, FVC 61.5±16.5% | Ambrisentan 10 mg daily | 24 weeks | No change in 6MWD 9.8±55 m | 11 patients discontinued drug at 12 weeks; 10 out of 21 patients who completed had improvements in WHO FC and QoL |
| Baughman (2014) [67] | 39 | mPAP ≥25 mmHg, NYHA FC II or III | RCT (2:1) | RHC | mPAP 36±7 mmHg, CI 2.6±0.7 L·min−1·m−2 | FVC 60±16.6% | Bosentan | 16 weeks | Decrease in mPAP (to 32 mmHg) | No change in 6MWD; PVR decreased from 6.1 to 4.4 WU |
| Keir (2014) [92] | 33 | Any SAPH | Retrospective case series | RHC | mPAP 44±8.6 mmHg, PVR 10±5.1 WU, CI 2.1±0.6 L·min−1·m−2, TAPSE 17.5 (8–27) mm | FEV1 51.8±18.3%, FVC 64.8±22.3% | Sildenafil n=29, bosentan n=4 | 6 months | None identified | 6MWD improved 14 m; BNP and TAPSE improved |
| Bonham (2015) [93] | 26 | Any treated SAPH, no left-sided disease | Retrospective case series | RHC | mPAP 46 (38–56) mmHg, CI 2.1 (1.8–2.6) L·min−1·m−2, PVR 8.3 (5.7–11.1) WU | FEV1 48% (38–59%), FVC 48% (44–64%), DLCO 29% (25–44%) | Epoprostenol n=7, treprostinil n=6, ERA n=12, PDE5i n=20 | Variable | None identified | Increased CI/CO, decreased PVR (median 12.7 months in 10 prostacyclin patients) and improved N-terminal pro-BNP |
PH: pulmonary hypertension; PFT: pulmonary function test; COPD: chronic obstructive pulmonary disease; RHC: right heart catheterisation; mPAP: mean pulmonary arterial pressure; CI: cardiac index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PVR(I): pulmonary vascular resistance (index); CO: cardiac output; GOLD: Global Initiative for Chronic Obstructive Lung Disease; sPAP: systolic PAP; 6MWD: 6-min walk distance; QoL: quality of life; BODE: body mass, airflow obstruction, dyspnoea, exercise capacity; echo: echocardiography; PR: pulmonary rehabilitation; V′O2: oxygen uptake; BNP: brain natriuretic peptide; SaO2: arterial oxygen saturation; WU: Wood Units; DLCO: diffusing capacity of the lung for carbon monoxide; ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis; RVSD: right ventricular systolic dysfunction; NSIP: non-specific interstitial pneumonia; KCO: transfer coefficient of the lung for carbon monoxide; IIP: idiopathic interstitial pneumonia; SAPH: sarcoidosis-associated PH; NYHA: New York Heart Association; FC: Functional Class; WHO: World Health Organization; TAPSE: tricuspid annular plane systolic excursion; ERA: endothelin receptor antagonist; PDE5i: phosphodiesterase type 5 inhibitor. #: for RCTs, the data for the treatment arm are reported as mean±sd or median (interquartile range); ¶: subgroup analysis of the ARTEMIS-IPF trial (study performed to evaluate the antifibrotic effects of the study medication, not all patients had PH).
COPD
Effect on pulmonary haemodynamics
Long-term use of PAH-targeted therapy improves pulmonary haemodynamics in COPD patients with PH, as shown in two different meta-analyses [47, 48]. Beneficial haemodynamic effects with long-term PAH therapy, assessed by RHC, have been demonstrated with both sildenafil and bosentan [49, 50].
Effect on exercise tolerance, symptoms and quality of life
The effect of PAH-targeted therapy on exercise capacity in patients with COPD-PH is less apparent. Two meta-analyses failed to show significant improvement in 6-min walk distance (6MWD), whereas a third reported an improvement in 6MWD in COPD patients with demonstrated PH [47, 48, 51].
The effect of PAH-targeted therapy on dyspnoea or quality of life measures in COPD-PH is also generally disappointing when evaluated in RCTs [47, 48]. However, one recent study conducted in COPD patients with severe PH showed that sildenafil significantly improved the BODE (body mass, airflow obstruction, dyspnoea, exercise capacity) index, the modified Medical Research Council scale and the Short Form-36 general health domain [49]. Taken together, the available studies do not provide clear evidence that the effect of PAH-targeted therapy on pulmonary haemodynamics in COPD-PH translates into an improvement in exercise tolerance and symptoms.
Effect on oxygenation
Vasodilator treatment may worsen gas exchange due to the inhibition of hypoxic pulmonary vasoconstriction, thereby increasing ventilation/perfusion mismatching in COPD [52]. Evidence for a long-term benefit of PH therapy in COPD-PH is heterogeneous [47]. While deterioration of gas exchange was shown in some studies with the long-term use of bosentan or sildenafil, no change was observed in others using sildenafil or tadalafil [49, 53, 54] and rarely resulted in treatment withdrawal [55]. It is important to note that any reduction in oxygenation related to pulmonary vasodilation might be compensated for by an increased cardiac output that may maintain or even improve tissue oxygen delivery, especially with exercise.
Conclusion
Although preliminary evidence suggests that currently available vasoactive medications may have a benefit in COPD-PH patients with mPAP ≥35 mmHg, further studies are required before PAH therapies can be recommended. Therefore, these patients should be a target population for larger prospective studies. This does not preclude COPD patients with lower mPAP being enrolled in future studies, especially if the cardiac index is low or PVR is significantly elevated.
Idiopathic interstitial pneumonias
Safety
Treatment with PAH-targeted therapies in patients with IIP has yielded important safety signals in some RCTs. The ARTEMIS study was terminated prematurely because an interim analysis indicated that ambrisentan-treated patients with IPF were more likely to have disease progression, particularly hospitalisations due to respiratory events [56]. Thus, ambrisentan is contraindicated in patients with IPF.
The RISE-IIP trial evaluated the effect of riociguat on 6MWD in patients with IIP. The study was terminated early on the basis of interim results showing increased mortality and risk of serious adverse events in the riociguat group [57]. Accordingly, riociguat is contraindicated in patients with IIP-PH.
Effect on pulmonary haemodynamics
Uncontrolled studies have shown improvement in pulmonary haemodynamics in patients with IIP-PH using riociguat and treprostinil [58, 59]. However, RCTs have failed to substantiate such an improvement in this population. The BPHIT study did not show significant changes in pulmonary haemodynamics in patients with IIP-PH treated with bosentan during 16 weeks [60]. The ARTEMIS-IPF trial also failed to show any significant effect of ambrisentan on pulmonary haemodynamics in the subgroup of patients who underwent a second assessment with RHC [14].
Effect on exercise tolerance
A recent meta-analysis did not show improvement in 6MWD in patients with ILD-PH treated with PAH-targeted therapy [48]. The STEP-IPF study, which was enriched for underlying IPF-PH by the inclusion of patients with DLCO <35% of predicted, failed to meet its primary end-point of a 20% increase in the 6MWT distance [61]. In contrast, open-label studies with sildenafil, riociguat and treprostinil did show significant improvements in 6MWD, with an average increase of 46 m over baseline [48]. The largest observational study to date of severe IIP-PH patients (n=151) found that the improvement in 6MWD at 6 months in response to PAH therapy was equivalent to that seen in IPAH patients [23].
Effect on symptoms and quality of life
The effect of PAH-targeted therapy on symptomatic burden in patients with PH-ILD has been assessed in two RCTs and three open-label studies [48]. The STEP-IPF study also demonstrated a positive effect of sildenafil on quality of life compared with the placebo arm, while one study showed significant improvement in shortness of breath using treprostinil [59, 61]. The remaining studies failed to show significant change in quality of life questionnaires or dyspnoea scales [48].
Effect on oxygenation
In ILD, the acute administration of aerosolised iloprost, inhaled nitric oxide or sildenafil does not worsen ventilation/perfusion relationships. In contrast, acute administration of i.v. epoprostenol causes deterioration of gas exchange due to increased perfusion in non-ventilated alveolar units [62–64]. In longer-term studies, treatment with PAH-targeted therapy did not result in worsening of gas exchange in patients with ILD [48].
Conclusion
Riociguat and ambrisentan are both contraindicated in IIP-PH. There is no evidence of benefit for other endothelin receptor antagonists in IIP-PH. Data on the use of sildenafil in IIP-PH is conflicting, while evidence for prostanoid therapy is too limited for any current recommendations. Further RCTs are encouraged.
Combined pulmonary fibrosis and emphysema
Treatment options remain limited with currently little evidence to support PAH therapies in this disease.
Sarcoidosis
There is still a lack of data on the safety and efficacy of drugs approved for PAH in sarcoidosis-PH patients. Case series have suggested beneficial effects of various compounds (table 2) [28, 65, 66]. However, only a single RCT has been performed in this group of patients and the results were inconclusive [67]. At present, no PAH-targeted therapy can routinely be recommended for patients with sarcoidosis-PH.
Other CLDs
Clinical experience and case series suggest beneficial effects of drugs approved for PAH in some patients with pulmonary Langerhans cell histiocytosis and lymphangioleiomyomatosis, but the lack of robust data precludes firm recommendations for any of these other conditions [31, 68].
Retrospective survival analysis in group 3 PH
The impact of PAH-targeted therapy on survival in group 3 PH has been assessed in retrospective studies, which included patients with different CLDs and usually with severe PH. One study found a survival benefit in severe PH-COPD patients with a favourable haemodynamic and functional response after 3 months of therapy, while two studies showed longer survival in patients treated with PAH-targeted therapy (mostly phosphodiesterase type 5 inhibitors) compared with patients who did not receive PAH-targeted treatment [69–71]. In one of these studies, the survival benefit was apparent in patients with severe PH, but not mild-to-moderate PH [70]. These studies need to be interpreted with caution given the nature of their study design with no RCTs as yet attesting to a survival benefit.
Recommendations for treatment of different patient groups with CLD and PH
Further long-term RCTs focusing on, but not limited to, patients with CLD-severe PH and COPD or ILDs are needed. Due to the major differences in underlying pathophysiology, obstructive and restrictive lung diseases should be investigated separately. Combining different groups with lung fibrosis is one potential clinical trial approach, with the advantage of increased patient numbers and abrogation of the diagnostic dilemma that often accompanies fibrotic lung disorders. This might be disadvantageous in view of the differing aetiologies, but there are precedents for this approach in PAH where IPAH has typically been combined with other group 1 subgroups in RCTs. This problem may be addressed by detailed phenotyping of the patients and predefined subgroups to be analysed separately in addition to the overall analysis. Based on physiological testing (lung function, haemodynamics and exercise) as well as CT morphology, the following groups of PH patients may be distinguished with respect to classification and management recommendations (summarised in table 1 and figure 1):
1) Patients with mild obstructive or restrictive lung disease, in whom CT analysis shows no gross parenchymal or airway abnormalities and who present with clinically relevant PH. Whether such patients have PAH (group 1) with concomitant lung disease or PH due to lung disease (group 3) remains a diagnostic dilemma (see earlier). Therefore, these patients should be referred to an expert centre.
2) Patients with more severe obstructive and/or restrictive lung disease (IPF with FVC <70% of predicted, COPD with FEV1 <60% of predicted) and accompanying less severe PH (mPAP 20–24 mmHg with PVR ≥3 WU, or mPAP 25–34 mmHg). These groups represent the majority of patients presenting with CLD-PH. Current data do not support therapy with PAH-approved drugs in these patients. Moreover, as the limitation in exercise capacity in these patients is largely due to ventilatory and not circulatory impairment, any functional benefit from PAH treatment is questionable. Vascular changes may, however, contribute to disease progression and future studies addressing this aspect of the disease may be a worthy endeavour.
3) Patients with more severe obstructive and/or restrictive lung disease and severe PH as defined earlier (mPAP ≥35 mmHg; severe COPD-PH, severe ILD-PH, severe CPFE-PH). These patients have a poor prognosis and should be referred to a centre with expertise in both PH and CLD for individualised patient care. These patients should preferably be included in RCTs if available.
4) Patients with “end-stage” obstructive and/or restrictive lung diseases and associated PH. In these advanced cases, life-preserving measures, such as mechanical ventilatory or extracorporeal membrane oxygenation support, should only be considered as a bridge to transplantation. Patients in any of these groups may be candidates for lung transplantation, an option that should be part of the management algorithm if all else fails and they are otherwise appropriate candidates. RCTs should address whether PAH-approved drugs may improve functional ability, quality of life, prolong time to clinical worsening, improve survival or provide a bridge to transplantation. In the absence of such trials, decisions on individualised patient care should be made in the context of expert centres.
Specific aspects of PH in systemic sclerosis
PH in patients with systemic sclerosis (SSc) can be multifactorial. These patients are at high risk of developing isolated PAH, but they may also develop significant parenchymal lung disease and/or a component of left heart disease. There is often difficulty in discriminating group 1 PAH from group 3 PH in SSc patients, since quite commonly these patients have evidence of parenchymal lung disease on high-resolution CT, which may or may not be accompanied by restrictive physiology. SSc patients with combined pulmonary fibrosis and PH have a particularly high mortality risk [72]. Both PH severity and the extent of pulmonary fibrosis can vary widely. Patients with pre-capillary PH and mild fibrosis are usually classified as having PAH, and have been included in most of the RCTs of PAH medications. However, assessment of the degree of fibrosis was usually based on pulmonary function testing and not scrutiny of the extent of fibrosis on high-resolution CT. Patients with preserved lung volumes can be safely treated with PAH drugs, but there is no evidence for treatment of PH-SSc with more advanced ILD.
Recommendations
While there are clearly SSc patients who are “pure” group 1, there are others who are group 2 or 3. There is likely a significant grey zone of these patients with features of more than one group. To best evaluate the extent of lung disease in relation to the patient's haemodynamic profile requires lung imaging rather than reliance on pulmonary function test criteria only. In the absence of RCTs, SSc patients with PH and more than minimal fibrosis on high-resolution CT should be referred to expert centres for individualised treatment.
Hypoventilation and hypoxia-associated PH
Chronic PH in obstructive sleep apnoea is rare and most commonly mild. In marked contrast, a significant proportion of patients with obesity–hypoventilation syndrome (OHS) or overlap syndrome (combination of obstructive sleep apnoea and COPD) present with PH [73, 74]. This represents an important group of patients given that current estimates suggest that around 0.4% of the US population suffers from OHS, reaching up to 31% among hospitalised patients with a body mass index >35 kg·m−2 [75]. Although variable, PH is frequently severe in these patients, being commonly associated with right ventricular failure and poor outcomes [76]. In addition to the improvements in gas exchange and sleepiness, one RCT and two series documented near normalisation of pulmonary haemodynamics, right ventricular function and exercise capacity after 3–6 months of non-invasive ventilation [73, 74, 76]. Similar findings were reported in a series of patients with PH associated with restrictive thoracic diseases treated with non-invasive ventilation [77].
Recommendations
The mainstay treatment of OHS is non-invasive ventilation, which does not, however, always correct the PH.
PH at high altitude
High altitude is defined as an elevation of >2500 m above sea level. 140 million people permanently reside at high altitudes and >40 million visitors reach high-altitude levels yearly [78]. Data on prevalence of high-altitude PH, defined as mPAP ≥30 mmHg by the International Society for Mountain Medicine [79], are rare and figures range from 5% to 23%, dependent on the geographic region and sex. Thus, hypoxia-induced PH is a major health problem at high-altitude regions of the world [78, 79]. Low ambient oxygen induces hypoxic pulmonary vasoconstriction, which increases PVR. In addition, pulmonary arterial remodelling occurs during long-term hypoxia. Both hypoxic pulmonary vasoconstriction and vascular remodelling act in concert with erythrocytosis, leading to PH at high altitude [80]. Importantly, high-altitude PH is reversible upon re-exposure to normal inspired oxygen tension (PIO2). Acute treatment studies with sildenafil and the ROCK (RhoA/Rho kinase) inhibitor fasudil reduced PAP and/or increased exercise capacity [81–83]. Only sildenafil and captopril have been investigated in long-term studies over a period of 3 months, with lowered PAP levels being noted [84, 85].
Recommendations
Re-exposure to normal PIO2 is the primary treatment of high-altitude PH. Long-term treatment studies with vasodilators are largely missing.
General conclusions
The association between PH and CLD with impaired functional status and worse outcomes is well established. Whether PH is the driver of outcomes or a surrogate for the severity of the underlying CLD remains uncertain. However, given the worldwide prevalence of CLD, and the associated morbidity and mortality of CLD-PH, this area is one of great unmet medical need where future research should be strongly encouraged and supported (table 3).
TABLE 3.
Recommendations and questions for the future direction of research in chronic lung disease (CLD)-associated pulmonary hypertension (PH)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; iPSC: induced pluripotent stem cell; CT: computed tomography; SPECT: single photon emission CT; MRI: magnetic resonance imaging; 6MWT: 6-min walk test; BNP: brain natriuretic peptide; NYHA: New York Heart Association; QoL: quality of life; IIP: idiopathic interstitial pneumonia; CPFE: combined pulmonary fibrosis and emphysema.
Footnotes
Number 10 in the series “Proceedings of the 6th World Symposium on Pulmonary Hypertension” Edited by N. Galiè, V.V. McLaughlin, L.J. Rubin and G. Simonneau
Conflict of interest: S.D. Nathan is a consultant for and has received research funding from Bellerophon, United Therapeutics and Bayer Pharmaceuticals; is a consultant for Third Pole and Actelion, and is a consultant for, has received research funding from and is on the speakers’ bureau of Roche-Genentech and Boehringer Ingelheim.
Conflict of interest: J.A. Barbera reports grants and personal fees from Actelion and MSD, personal fees from Arena, and grants from Bayer and GSK, outside the submitted work.
Conflict of interest: S.P. Gaine reports personal fees from Actelion, United Therapeutics, MSD and GSK, outside the submitted work.
Conflict of interest: S. Harari has received grants for research and speakers fees from Actelion, Boehringer Ingelheim and Roche.
Conflict of interest: F.J. Martinez has received grants from the NIH (IPF UO1, COPD UO1/RO1); personal fees, honoraria and non-personal travel support from the American College of Chest Physicians, Continuing Education, ConCert, Inova Fairfax Health System, MD Magazine, Miller Communications, National Association for Continuing Education, Novartis, Pearl Pharmaceuticals, PeerView Communications, Prime Communications, Puerto Rican Respiratory Society, Roche, Sunovion, Theravance, Potomac, University of Alabama Birmingham and Zambon; personal fees and non-personal or non-financial travel support from AstraZeneca; personal fees, non-personal travel support, and non-financial support for data and safety monitoring board work from Boehringer Ingelheim; personal fees, honoraria, non-personal travel support, and non-financial support for data and safety monitoring board work from Genentech and GlaxoSmithKline; personal fees and honoraria from Columbia University, Integritas, Methodist Hospital Brooklyn, New York University, Unity, UpToDate, WebMD/MedScape, Western Connecticut Health Network, Academic CME, Patara, PlatformIQ, American Thoracic Society, Rockpointe and Rare Disease Healthcare Communications; non-personal travel support from Nitto; personal fees, honoraria, travel support and non-personal travel support from Chiesi; personal fees, honoraria and travel support from Physicians Education Resource and Teva; honoraria and travel support from Canadian Respiratory Network; personal fees from France Foundation; and has participated on scientific advisory boards (no direct financial compensation) for ProterrixBio and Bridge Biotherapeutics; participated on IPF study steering committees (no direct financial compensation) for Afferent/Merck, Gilead, Veracyte, Prometic, Bayer and ProMedior; and participated on an IPF study steering committee and data safety monitoring board (no direct financial compensation) for Biogen.
Conflict of interest: H. Olschewski reports personal fees and non-financial support from Bayer, MSD, Pfizer and Novartis, grants, personal fees and non-financial support from Actelion, grants from Inventiva, and personal fees from Bellerophon, outside the submitted work; and is part-time employee of the Ludwig Boltzmann Institute for Lung Vascular Research.
Conflict of interest: K.M. Olsson received fees for talks and consulting work from Actelion, Bayer, GSK, Pfizer and United Therapeutics.
Conflict of interest: A.J. Peacock has received research grants and personal fees from Actelion Pharmaceuticals, Bayer, GSK, MSD, Pfizer and United Therapeutics, outside the submitted work.
Conflict of interest: J. Pepke-Zaba is a member of the advisory boards for Actelion, Merck, Bayer and GSK, has received grants, personal fees and non-financial support from Actelion, Merck and Bayer, and personal fees from GSK.
Conflict of interest: S. Provencher has received research grants from Actelion Pharmaceuticals and Boehringer Ingelheim, and has received speaker fees from Actelion Pharmaceuticals.
Conflict of interest: N. Weissmann has nothing to disclose.
Conflict of interest: W. Seeger has received consultancy fees from Bayer AG, United Therapeutics, Liquidia, Vectura and Novartis.
References
- 1.Seeger W, Adir Y, Barbera JA, et al. . Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–D116. [DOI] [PubMed] [Google Scholar]
- 2.Berger RMF, Beghetti M, Humpl T, et al. . Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manika K, Pitsiou GG, Boutou AK, et al. . The impact of pulmonary arterial pressure on exercise capacity in mild-to-moderate cystic fibrosis: a case control study. Pulm Med 2012; 2012: 252345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medrek SK, Sharafkhaneh A, Spiegelman AM, et al. . Admission for COPD exacerbation is associated with the clinical diagnosis of pulmonary hypertension: results from a retrospective longitudinal study of a veteran population. COPD 2017; 14: 484–489. [DOI] [PubMed] [Google Scholar]
- 5.Hayes D Jr, Black SM, Tobias JD, et al. . Influence of pulmonary hypertension on patients with idiopathic pulmonary fibrosis awaiting lung transplantation. Ann Thorac Surg 2016; 101: 246–252. [DOI] [PubMed] [Google Scholar]
- 6.Poms AD, Turner M, Farber HW, et al. . Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144: 169–176. [DOI] [PubMed] [Google Scholar]
- 7.Hamada K, Nagai S, Tanaka S, et al. . Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest 2007; 131: 650–656. [DOI] [PubMed] [Google Scholar]
- 8.Kessler R, Faller M, Fourgaur G, et al. . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 158–164. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann J, Wilhelm J, Olschewski A, et al. . Microarray analysis in pulmonary hypertension. Eur Respir J 2016; 48: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaouat A, Bugnet A-S, Kadaoui N, et al. . Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. [DOI] [PubMed] [Google Scholar]
- 11.Boerrigter BG, Bogaard HJ, Trip P, et al. . Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest 2012; 142: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 12.Wells JM, Washko GR, Han MK, et al. . Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012; 367: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura M, Taniguchi H, Kondoh Y, et al. . Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration 2013; 85: 456–463. [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Nathan SD, Behr J, et al. . Pulmonary hypertension in idiopathic pulmonary fibrosis with mild to moderate restriction. Eur Respir J 2015; 46: 1370–1377. [DOI] [PubMed] [Google Scholar]
- 15.Shorr AF, Wainright JL, Cors CS, et al. . Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J 2007; 30: 715–721. [DOI] [PubMed] [Google Scholar]
- 16.Nadrous HF, Pellikka PA, Krowka MJ, et al. . Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest 2005; 128: 2393–2399. [DOI] [PubMed] [Google Scholar]
- 17.Nathan SD, Shlobin OA, Barnett SD, et al. . Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med 2008; 102: 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teramachi R, Taniguchi H, Kondoh Y, et al. . Progression of mean pulmonary arterial pressure in idiopathic pulmonary fibrosis with mild to moderate restriction. Respirology 2017; 22: 986–990. [DOI] [PubMed] [Google Scholar]
- 19.Lettieri CJ, Nathan SD, Barnett SD, et al. . Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest 2006; 129: 746–752. [DOI] [PubMed] [Google Scholar]
- 20.Alhamad EH, Al-Boukai AA, Al-Kassimi FA, et al. . Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology 2011; 260: 875–883. [DOI] [PubMed] [Google Scholar]
- 21.Mura M, Anraku M, Yun Z, et al. . Gene expression profiling in the lungs of patients with pulmonary hypertension associated with pulmonary fibrosis. Chest 2012; 141: 661–673. [DOI] [PubMed] [Google Scholar]
- 22.Judge EP, Fabre A, Adamali HI, et al. . Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J 2012; 40: 93–100. [DOI] [PubMed] [Google Scholar]
- 23.Hoeper MM, Behr J, Held M, et al. . Pulmonary hypertension in patients with chronic fibrosing idiopathic interstitial pneumonias. PLoS One 2015; 10: e0141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottin V, Le Pavec J, Prévot G, et al. . Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J 2010; 35: 105–111. [DOI] [PubMed] [Google Scholar]
- 25.Mejía M, Carrillo G, Rojas-Serrano J, et al. . Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest 2009; 136: 10–15. [DOI] [PubMed] [Google Scholar]
- 26.Shorr AF, Helman DL, Davies DB, et al. . Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J 2005; 25: 783–788. [DOI] [PubMed] [Google Scholar]
- 27.Nunes H, Humbert M, Capron F, et al. . Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax 2006; 61: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucly A, Cottin V, Nunes H, et al. . Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J 2017; 50: 1700465. [DOI] [PubMed] [Google Scholar]
- 29.Baughman RP, Shlobin OA, Alhamad EH, et al. . Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Res Med 2018; 139: 72–78. [DOI] [PubMed] [Google Scholar]
- 30.Fartoukh M, Humbert M, Capron F, et al. . Severe pulmonary hypertension in histiocytosis X. Am J Respir Crit Care Med 2000; 161: 216–223. [DOI] [PubMed] [Google Scholar]
- 31.Le Pavec J, Lorillon G, Jais X, et al. . Pulmonary Langerhans cell histiocytosis-associated pulmonary hypertension: clinical characteristics and impact of pulmonary arterial hypertension therapies. Chest 2012; 142: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira RK, Pereira CA, Ramos RP, et al. . Chronic hypersensitivity pneumonitis and pulmonary hypertension. Eur Respir J 2014; 44: 415–424. [DOI] [PubMed] [Google Scholar]
- 33.Pullamsetti SS, Kojonazarov B, Storn S, et al. . Lung cancer associated pulmonary hypertension: role of microenvironmental inflammation based on tumor cell-immune cell cross-talk. Sci Transl Med 2017; 9: eaai9048. [DOI] [PubMed] [Google Scholar]
- 34.Andersen KH, Iversen M, Kjaergaard J, et al. . Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012; 31: 373–380. [DOI] [PubMed] [Google Scholar]
- 35.Leuchte HH, Baumgartner RA, Nounou ME, et al. . Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am J Respir Crit Care Med 2006; 173: 744–750. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa T, Kondoh Y, Taniguchi H, et al. . A scoring system to predict the elevation of mean pulmonary arterial pressure in idiopathic pulmonary fibrosis. Eur Respir J 2018; 51: 1701311. [DOI] [PubMed] [Google Scholar]
- 37.Glaser S, Obst A, Koch B, et al. . Pulmonary hypertension in patients with idiopathic pulmonary fibrosis – the predictive value of exercise capacity and gas exchange efficiency. PLoS One 2013; 8: e65643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greiner S, Jud A, Aurich M, et al. . Reliability of noninvasive assessment of systolic pulmonary artery pressure by Doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc 2014; 3: e001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Andrea A, Stanziola A, Di Palma E, et al. . Right ventricular structure and function in idiopathic pulmonary fibrosis with or without pulmonary hypertension. Echocardiography 2016; 33: 57–65. [DOI] [PubMed] [Google Scholar]
- 40.Nowak J, Hudzik B, Jastrze Bski D, et al. . Pulmonary hypertension in advanced lung diseases: echocardiography as an important part of patient evaluation for lung transplantation. Clin Respir J 2018: 12: 930– 938. [DOI] [PubMed] [Google Scholar]
- 41.Alkukhun L, Wang XF, Ahmed MK, et al. . Non-invasive screening for pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med 2016; 117: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagi M, Taniguchi H, Kondoh Y, et al. . CT-determined pulmonary artery to aorta ratio as a predictor of elevated pulmonary artery pressure and survival in idiopathic pulmonary fibrosis. Respirology 2017; 22: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 43.Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med 1985; 102: 29–36. [DOI] [PubMed] [Google Scholar]
- 44.Weitzenblum E, Sautegeau A, Ehrhart M, et al. . Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 131: 493–498. [DOI] [PubMed] [Google Scholar]
- 45.Albert RK, Au DH, Blackford AL, et al. . A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016; 375: 1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell EC, Cox NS, Goh N, et al. . Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev 2017; 26: 160080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Tang S, Liu K, et al. . Therapy in stable chronic obstructive pulmonary disease patients with pulmonary hypertension: a systematic review and meta-analysis. J Thorac Dis 2015; 7: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prins KW, Duval S, Markowitz J, et al. . Chronic use of PAH-specific therapy in World Health Organization Group III Pulmonary Hypertension: a systematic review and meta-analysis. Pulm Circ 2017; 7: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitulo P, Stanziola A, Confalonieri M, et al. . Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36: 166–174. [DOI] [PubMed] [Google Scholar]
- 50.Valerio G, Bracciale P, Grazia DA. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009; 3: 15–21. [DOI] [PubMed] [Google Scholar]
- 51.Park J, Song JH, Park DA, et al. . Systematic review and meta-analysis of pulmonary hypertension specific therapy for exercise capacity in chronic obstructive pulmonary disease. J Korean Med Sci 2013; 28: 1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbera JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: advances in pathophysiology and management. Drugs 2009; 69: 1153–1171. [DOI] [PubMed] [Google Scholar]
- 53.Stolz D, Rasch H, Linka A, et al. . A randomized, controlled trial of bosentan in severe COPD. Eur Respir J 2008; 32: 619–628. [DOI] [PubMed] [Google Scholar]
- 54.Lederer DJ, Bartels MN, Schluger NW, et al. . Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD 2012; 9: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calcaianu G, Canuet M, Schuller A, et al. . Pulmonary arterial hypertension-specific drug therapy in COPD patients with severe pulmonary hypertension and mild-to-moderate airflow limitation. Respiration 2016; 91: 9–17. [DOI] [PubMed] [Google Scholar]
- 56.Raghu G, Behr J, Brown KK, et al. . Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 2013; 158: 641–649. [DOI] [PubMed] [Google Scholar]
- 57.Nathan SD, Behr J, Collard HR, et al. . RISE-IIP: riociguat for the treatment of pulmonary hypertension associated with idiopathic interstitial pneumonia. Eur Respir J 2017; 50: OA1985. [Google Scholar]
- 58.Hoeper MM, Halank M, Wilkens H, et al. . Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J 2013; 41: 853–860. [DOI] [PubMed] [Google Scholar]
- 59.Saggar R, Khanna D, Vaidya A, et al. . Changes in right heart haemodynamics and echocardiographic function in an advanced phenotype of pulmonary hypertension and right heart dysfunction associated with pulmonary fibrosis. Thorax 2014; 69: 123–129. [DOI] [PubMed] [Google Scholar]
- 60.Corte TJ, Keir GJ, Dimopoulos K, et al. . Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2014; 190: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 2010; 363: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olschewski H, Ghofrani HA, Walmrath D, et al. . Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med 1999; 160: 600–607. [DOI] [PubMed] [Google Scholar]
- 63.Blanco I, Ribas J, Xaubet A, et al. . Effects of inhaled nitric oxide at rest and during exercise in idiopathic pulmonary fibrosis. J Appl Physiol 2011; 110: 638–645. [DOI] [PubMed] [Google Scholar]
- 64.Ghofrani HA, Wiedemann R, Rose F, et al. . Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360: 895–900. [DOI] [PubMed] [Google Scholar]
- 65.Barnett CF, Bonura EJ, Nathan SD, et al. . Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest 2009; 135: 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baughman RP, Judson MA, Lower EE, et al. . Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2009; 26: 110–120. [PubMed] [Google Scholar]
- 67.Baughman RP, Culver DA, Cordova FC, et al. . Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest 2014; 145: 810–817. [DOI] [PubMed] [Google Scholar]
- 68.Cottin V, Harari S, Humbert M, et al. . Pulmonary hypertension in lymphangioleiomyomatosis: characteristics in 20 patients. Eur Respir J 2012; 40: 630–640. [DOI] [PubMed] [Google Scholar]
- 69.Hurdman J, Condliffe R, Elliot CA, et al. . Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013; 41: 1292–1301. [DOI] [PubMed] [Google Scholar]
- 70.Lange TJ, Baron M, Seiler I, et al. . Outcome of patients with severe PH due to lung disease with and without targeted therapy. Cardiovasc Ther 2014; 32: 202–208. [DOI] [PubMed] [Google Scholar]
- 71.Tanabe N, Taniguchi H, Tsujino I, et al. . Multi-institutional retrospective cohort study of patients with severe pulmonary hypertension associated with respiratory diseases. Respirology 2015; 20: 805–812. [DOI] [PubMed] [Google Scholar]
- 72.Launay D, Montani D, Hassoun PM, et al. . Clinical phenotypes and survival of pre-capillary pulmonary hypertension in systemic sclerosis. PLoS One 2018; 13: e0197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Held M, Walthelm J, Baron S, et al. . Functional impact of pulmonary hypertension due to hypoventilation and changes under noninvasive ventilation. Eur Respir J 2014; 43: 156–165. [DOI] [PubMed] [Google Scholar]
- 74.Masa JF, Corral J, Caballero C, et al. . Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea. Thorax 2016; 71: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nowbar S, Burkart KM, Gonzales R, et al. . Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116: 1–7. [DOI] [PubMed] [Google Scholar]
- 76.Castro-Anon O, Golpe R, Perez-de-Llano LA, et al. . Haemodynamic effects of non-invasive ventilation in patients with obesity–hypoventilation syndrome. Respirology 2012; 17: 1269–1274. [DOI] [PubMed] [Google Scholar]
- 77.Schonhofer B, Barchfeld T, Wenzel M, et al. . Long term effects of non-invasive mechanical ventilation on pulmonary haemodynamics in patients with chronic respiratory failure. Thorax 2001; 56: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mirrakhimov AE, Strohl KP. High-altitude pulmonary hypertension: an update on disease pathogenesis and management. Open Cardiovasc Med J 2016; 10: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Léon-Velarde F, Maggiorini M, Reeves JT, et al. . Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 2005; 6: 147–157. [DOI] [PubMed] [Google Scholar]
- 80.Wilkins MR, Ghofrani HA, Weissmann N, et al. . Pathophysiology and treatment of high-altitude pulmonary vascular disease. Circulation 2015; 131: 582–590. [DOI] [PubMed] [Google Scholar]
- 81.Ghofrani HA, Reichenberger F, Kohstall MG, et al. . Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: a randomized, double-blind, placebo-controlled crossover trial. Ann Intern Med 2004; 141: 169–177. [DOI] [PubMed] [Google Scholar]
- 82.Xu Y, Liu J, Qian G. Meta-analysis of clinical efficacy of sildenafil, a phosphodiesterase type-5 inhibitor on high altitude hypoxia and its complications. High Alt Med Biol 2014; 15: 46–51. [DOI] [PubMed] [Google Scholar]
- 83.Kojonazarov B, Myrzaakhmatova A, Sooronbaev T, et al. . Effects of fasudil in patients with high-altitude pulmonary hypertension. Eur Respir J 2012; 39: 496–498. [DOI] [PubMed] [Google Scholar]
- 84.Aldashev AA, Kojonazarov BK, Amatov TA, et al. . Phosphodiesterase type 5 and high altitude pulmonary hypertension. Thorax 2005; 60: 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niazova ZA, Batyraliev TA, Aikimbaev KS, et al. . High-altitude pulmonary hypertension: effects of captopril on pulmonary and systemic arterial pressures. J Hum Hypertens 1996; 10: Suppl. 3, S141–S142. [PubMed] [Google Scholar]
- 86.Vonbank K, Ziesche R, Higenbottam TW, et al. . Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 2003; 58: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao RS, Singh S, Sharma BS, et al. . Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011; 53: 81–85. [PubMed] [Google Scholar]
- 88.Blanco I, Santos S, Gea J, et al. . Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42: 982–992. [DOI] [PubMed] [Google Scholar]
- 89.Goudie AR, Lipworth BJ, Hopkinson PJ, et al. . Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2014; 2: 293–300. [DOI] [PubMed] [Google Scholar]
- 90.Han MK, Bach DS, Hagan PG, et al. . Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest 2013; 143: 1699–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Judson MA, Highland KB, Kwon S, et al. . Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28: 139–145. [PubMed] [Google Scholar]
- 92.Keir GJ, Walsh SLF, Gatzoulis MA, et al. . Treatment of sarcoidosis-associated pulmonary hypertension: a single centre retrospective experience using targeted therapies. Sarcoidosis Vasc Diffuse Lung Dis 2014; 31: 82–90. [PubMed] [Google Scholar]
- 93.Bonham CA, Oldham JM, Gomberg-Maitland M, et al. . Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest 2015; 148: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]