Abstract
Complex craniofacial surgeries of damaged tissues have several limitations, which present complications and challenges when trying to replicate facial function and structure. Traditional treatment techniques have shown suitable nerve function regeneration with various drawbacks. As technology continues to advance, new methods have been explored in order to regenerate damaged nerves in an effort to more efficiently and effectively regain original function and structure. This article will summarize recent bioengineering strategies involving biodegradable composite scaffolds, bioactive factors, and external stimuli alone or in combination to support peripheral nerve regeneration. Particular emphasis is made on the contributions of growth factors and electrical stimulation on the regenerative process.
Keywords: Peripheral nerve regeneration, Composite materials, Growth factor, Electrical stimulation
Graphical abstract
Highlights
-
•
Craniofacial nerve repair surgeries have limitations and often result in insufficient restoration of facial function.
-
•
Nerve repair strategies for critical defects have often resulted in failure to re-establish sufficient nerve function.
-
•
Biochemical molecules promote tissue regeneration by differentiation of recruited cells to mature neuronal fates.
-
•
Electrical stimulation promotes regeneration of axons and provide signals for native cells to differentiate.
1. Introduction
The craniofacial skeleton and related tissues, including nerve and bone, are involved in several major functions ranging from protecting the brain to speaking, hearing, and breathing [1]. Specially, craniofacial nerves are involved in mechanisms to detect and react to changes in one's internal and external environment and are integral in maintaining proper overall function [2]. Due to their complex structure and networking, craniofacial repair surgeries have limitations and often result in insufficient restoration of facial function and structure. As a consequence, there are limited strategies for nerve repair and regeneration thereby over 20 million Americans experience nerve impairment exceeding a cost of $150 billion [3]. This creates a critical need for nerve regeneration, especially for craniofacial nerves and their peripheral extensions.
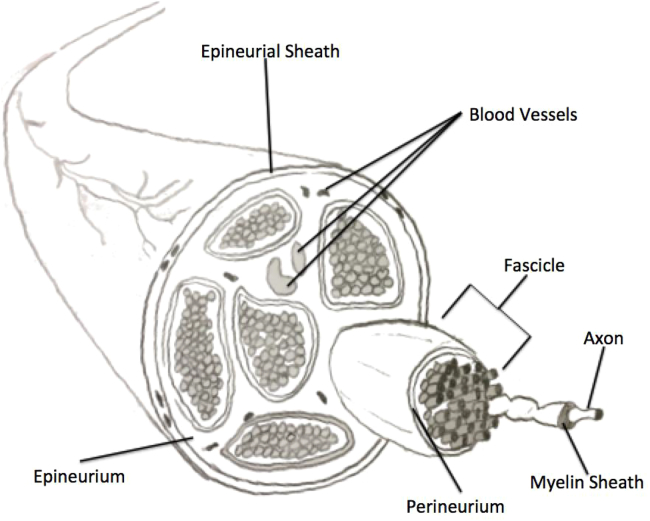
The nervous system is divided into two sections: the central nervous system (CNS) and the peripheral nervous system (PNS). The PNS is comprised of both sensory and motor neurons which carry information from all the parts of the body to and from the CNS. Cranial nerves are also categorized under peripheral nervous system and emerge from the brain or brain stem to innervate areas of head and neck. The facial cranial nerves innervate facial muscles that are responsible for expressions. A peripheral nerve consists of a cell body which gives out extensions, called axons that are crucial for targeting distant tissues and organs [3]. These axons are coated with myelin sheath membranes, formed by Schwann cells, and are arranged together in bundles called fascicles [3] (Fig. 1). Due to the complex anatomical structure of nerve bundles and its mechanism of degeneration, nerve repair and regeneration strategies for critical defects have often resulted in failure to re-establish sufficient nerve function.
Fig. 1.
Structural representation of a nerve in the peripheral nervous system. Highlighted are the fascicle structures all encased in the Epineurial Sheath.
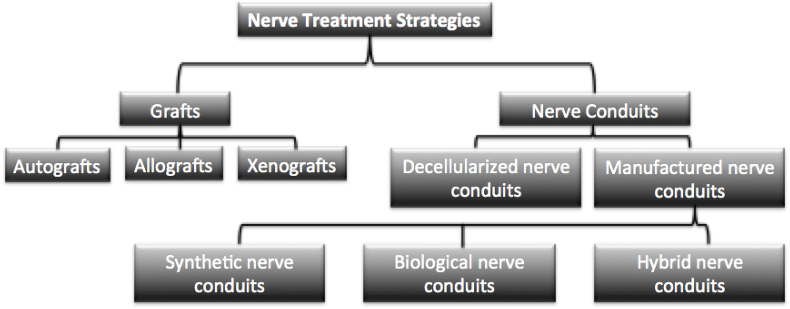
Various strategies for repair have been implemented depending on the type of trauma experienced [4,5]. In the event of peripheral nerve injury (PNI), a regulated sequence of events involving cellular bodies in the spinal cord and ganglia occur in order to regenerate damaged nerves. Nerve injuries are classified into three broad categories depending on the severity of the injury: neuropraxia, axonotmesis, and neurotmesis [4]. Neurapraxia is the least severe type of injury and is not associated with long term loss of function [6]. In contrast, axonotmesis occurs when there is a disruption of the nerve axon and the surrounding myelin sheath. The most severe type of nerve injury is neurotmesis, which involves a complete disconnection of the nerve and will be the focus for this review. This type of nerve injury results in a complete loss of function and axonal regrowth is limited due to potential formation of scar tissue, known as neuroma [4]. For neurotmesis repair, current methods include direct end to end repair, grafts and synthetic nerve conduits (Fig. 2). These nerve replacement strategies are used clinically with direct end to end suturing, considered the current preferred standard [6]. Direct end to end suturing is the easiest method but cannot be used with critically sized injuries as the tension induced from stretching the nerve can cause functional failure. On the other hand, the use of grafts for nerve repair is dependent on availability of the tissue, location from which the tissue is obtained and immune response from the host. These factors can limit the success of grafts for nerve regeneration. Nerve grafts can be autografts or allografts. Autografts are derived from the healthy part of patient's body thereby it reduces the chances of immunological rejection. However, this causes tissue damage at the donor site, termed donor site morbidity, and therefore limits their applicability [6]. For allografts, the tissue is harvested from another donor of the same species. While this increases availability, it introduces a minute risk of disease transmission and immunological response. Due to the limitations of the above two methods, nerve conduits made from biocompatible polymers such as polyglycolic acid (PGA), collagen, and polycaprolactone (PCL) are currently being used [7,8]. However, current conduits can only be used for defects of a few centimeters and primarily function as structural support and concentrators of clotting factors between the distal and proximal stumps [6]. Alternate methods from the gold standards of current clinical practices involve tissue engineering strategies using composite biomaterials.
Fig. 2.
Flow Chart of the various nerve treatment strategies.
The basic paradigm of tissue engineering is based on design and fabrication of novel biomaterial scaffolds that can house cells and deliver biomolecular and physical signals to cells for successful tissue regeneration. A variety of biomaterials are available from synthetic to natural sources for tissue engineering applications. Main categories of biomaterials include metals, ceramics, and polymers. Among these, polymers have ideal properties such as high ductility, superior tensile strength, and ease of suturing for soft tissues such as nerve. Polymers are also able to affect the physicochemical and mechanical properties of a biomimetic matrix. Generally, polymers can be characterized as synthetic or natural. Currently, most common biodegradable synthetic polymers used in medicine are polylactic acid (PLA), polyglycolic acid (PGA), copolymers of PLA and PGA (PLGA), polycaprolactone (PCL), polyanhydrides, polyorthoesters, polycarbonates, and polyfumarates [9,10]. These polymers are synthesized from monomer units in a laboratory through various form of polymerization such as free radical polymerization. On the other hand, natural polymers, such as collagen, chitosan and silk fibroin [6,11,12], are derived from natural sources such as rat tail, crab shells and silk worm, respectively.
The success of any scaffold used in surgery is dictated by its biocompatibility, porosity, bioresorbability, and mechanical strength [1]. First, a scaffold with adequate biocompatibility will sucessfully integrate with the native tissue without producing extreme foregin body response. This ensures successful long and short term implantation of these materials. Tissue integration is also dependent on porosity of the scaffold. Porous scaffolds with interconnected pores allow cellular infiltration and proliferation to enbale tissue regeneration and integration [13,14]. A scaffold used for soft tissue must reabsorb to be slowly replaced by regenerated tissue. For being bioresorbable, a material must breakbown over time in the body into non-toxic subunits. This material property ensures prevention of necrosis of the native and newly regenerated tissue. Last, the success of a scaffold is largely dependent on its mechanical properties. Mechanical properties such as tissue comparable tensile strength is essential in order to ensure proper load transfer and stability during implantation of the scaffold [1,15,16]. If the scaffold is not mechanically competent it will break under load and if it is too strong then it can lead to phenomena such as stress shielding leading to atrophy of surrounding natural tissue [17]. While this phenomenon is classic to bone, the principle applies to all tissues in the body. Many natural and synthetic materials possess some of these ideal properties, however, none are perfect. Therefore, biomaterial composites of both synthetic and natural materials provides an ideal solution by combining the optimal properties of each component into one system [13,[18], [19], [20]].
While polymeric materials provide effective biomimetic scaffolds for endogenous nerve cell attachment and proliferation, other biomolecular and physical signals are needed to stimulate cells to regenerate damaged tissue. Various biochemical molecules have shown to be effective in promoting regeneration of damaged tissue by supporting differentation of recruited cells to mature neuronal fates. For example, fibroblast growth factor and insulin like growth factor support peripheral nerve regeneration [[21], [22], [23]]. Additionally, growth factors such as neural growth factor (NGF) induce differention of mesenchymal stem cells into mature neural lineages [24]. Aside from chemical cues, electrical stimulaiton is also known to not only promoting regeneration of axons but also provide signals for native cells to differentiate [25,26].
This review will focus on current biomateiral composites being developed for craniofacial nerve tissue engineering in the hopes of providing an improved treatment method to the current standard. Additionally, an indepth look into the complimentary chemical and electrical cues that accompany these strategies will be investigated.
2. Peripheral nerve injuries
Neuronal axons in the PNS require axoplasmic flow from the main cell body for its survival. In an event of trauma, the axon distal to the point of injury suffers from Wallerian degeneration. The distal nerve end below the site of the lesion degenerates, while the proximal end (axon on the same side as the cell body) regrows outwards, eventually re-forming synaptic connections with nearby tissues [27]. Wallerian degeneration is a process unique to the PNS and Schwann cells (SC) play an important role in axonal regeneration.
Upon peripheral nerve injuries, an immune response occurs through activated Schwann cells and macrophages that leads to clearing the neuron and myelin debris at the distal nerve stump [28]. Schwann cells (SCs) are capable of denervation and provide a variety of functions during Wallerian degeneration. They grow and migrate towards the site of injury and serve as physical guides for the regenerating axon form the proximal side, forming the well documented “bands of Bunger,” serving as a scaffold [29]. In addition, SCs secrete a variety of neurotrophic factors and cytokines to assist in the regeneration process, including NGF, cell adhesion molecules, and extracellular matrix components [29].
As mentioned previously, myelin found on neurons in the PNS is formed by Schwann cells whereas CNS myelin is generated by oligodendrocytes. While oligodendrocytes and Schwann cells are often compared to each other in terms of function, a major difference between the two is in their ability to repair neurons after injury. Schwann cells promote nerve regeneration and repair, whereas oligodendrocytes inhibit neuron repair after an injury [27,30,31].
3. Bioinstructive nerve conduits
Recent advances in tissue engineering and regenerative medicine have promoted the use and study of manufactured nerve conduits. Nerve conduits are an alternative to allografts or autografts and are used for treatment of large nerve defects. These nerve guidance conduits (NGCs) have several advantages over donor nerve grafts such as ease of availability, limited scar tissue formation, reduced immune response and no donor site morbidity. Furthermore, chances of adverse immune responses can be significantly decreased with the use of appropriately sterilized biomaterials through techniques such as gamma irradiation and ethylene oxide treatment. The surface of the NGCs can also be altered to support axonal elongation between from the proximal to the distal stump [32]. Current tissue engineering strategies utilize 3D-scaffolds made from biomaterials designed for better biocompatibility, biodegradation and porosity [33,34]. Scaffolds serve as a biomimicking microenvironment and support for cells that eventually results in tissue regeneration. With time, the scaffold degrades which eliminates the need for removal of the implant from the body [35,36].
Based on the biomaterial used, manufactured nerve conduits can be categorized as: synthetic, natural, or composite. In general, synthetic nerve conduits provide higher degree of controllability, better mechanical properties, and poor bioactivity compared to their natural counterpart. Commonly used, U.S. Food and Drug Administration (FDA) approved, synthetic biomaterial such as polylactic acid (PLA) and polylactic-co-glycolic acid (PLGA) are known for low inflammatory response and ease of processing. PLGA has been shown to give control over its rate of degradation by altering the ratio of its monomer components [37]. Additionally, PLGA scaffolds have the unique ability of adhering to Schwann cells and directing their growth [38,39,39]. Recently, rapid prototyping techniques such as 3D bio-printing are being employed for synthetic nerve conduit fabrication. This technology constructs individualized synthetic nerve grafts with an anatomy and physiology similar to natural nerve [40]. Since 3D bio-printing uses non-thermal extrusion method, biomaterials combined with various cells and neural growth factors can be printed which has improved axonal regeneration of long-segmented nerve defects [40]. These scaffolds are utilized for their biocompatibility, tunable physiochemical properties, and ability to promote cell attachment.
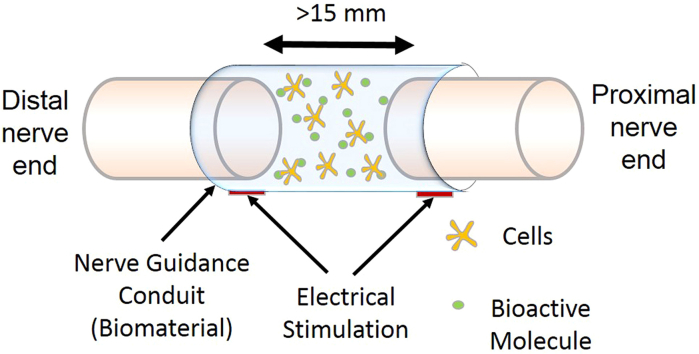
Natural biopolymers used for the fabrication of NGCs typically have regenerative bioactivity along with good mechanical properties. Clinically, FDA approved collagen type I nerve conduits have been implemented for defects smaller than 3 cm [6]. For critically sized injuries other materials such as fibrin and silk have been investigated [41]. A multichannel electrospun silk nerve conduit, mimicking natural tissue ECM structure, was created by Dinis et al., which closely resembled the tubular structure of epineurial tube [42]. This scaffold possessed an ultimate tensile strength extremely close to that of a sciatic nerve [42]. A defect size of 15 mm is considered a limiting factor for nerve regeneration in rats compared to over 3 cm in humans [43]. An in vivo study compared autografts, synthetic and natural biomaterial conduits for critically sized defects [40]. The results showed that even though chitosan tubes were biocompatible and showed better regeneration than synthetic silicone tube, they were inferior to the autograft. Nerve regeneration in rats with autografts was 50% more compared to rats implanted with chitosan nerve conduits [40].
Even though natural and synthetic polymers have been used for tissue engineered scaffolds, research is also being done on composite polymers to enhance material properties. Composite polymers embody the material properties of two or more polymers prepared for a specific application. In nerve tissue engineering, composite nerve conduits can contain a blend of both natural and synthetic polymers. An example of a composite nerve conduit is PCL blended with collagen through electrospinning [44,45]. PCL is a synthetic polymer used for scaffolds for its porous nature, biocompatibility, cell proliferation, and cell adhesion properties [46]. Whereas, collagen is a structural protein present within natural ECM that activates integrin receptors on the ends of the distal axons and glial cells [35,44]. When fabricated together and seeded with cells, this conduit promotes neurite outgrowth, extension and glial migration. Comparing PCL to the PCL/collagen blend (C/PCL), it was seen that C/PCL increased Schwann cell migration, neurite outgrowth and fibroblast sheathing cells, which can be an appropriate material for nerve regeneration [44]. Biomaterial composites can also be formed by mixing two synthetic polymers with distinct properties such as polypyrrole (PPy) and poly(D, l-lactic acid) (PDLLA) composite conduit made and tested by Xu et al. [47]. Since nerve tissue is electrically active, PPy, a conductive polymer, promotes regeneration and differentiation of re-growing nerves. However, PPy is very brittle and non-degradable which makes it a poor biomaterial candidate. Addition of PDLLA, a biodegradable and non-cytotoxic polymer, improved the degradation rate and healing properties of the composite biocompatible conductive matrix [47].
While the properties of biomaterial clearly plays a vital role in peripheral nerve regeneration, one also has to account for the elasticity, geometry, and topology of the final scaffold. Specifically, aligned and grooved topologies on scaffold surfaces has shown enhanced extension compared to non-textured counterparts [48]. Two experiments done by Mobasseri et al. highlight this effect. In both of their studies the group utilized non-grooved, sloped, square, and V-shaped morphologies for aligned PCL/PVA NGC films [32,49]. In the first study, hybrid neuroblastoma glioma rat cells displayed enhanced cellular proliferation and elongation in the alignment direction on both the V-shaped and sloped geometries [32]. The group then tested their conduits in a 10 mm Sprague Dawley rat sciatic nerve defect. In vivo, the grooved samples showed a significantly higher number of regenerated axons at a 3 week time point compared to non-grooved conduits. Whereas the sloped topology conduits were not statistically different from autograft controls in terms of final target innervation and compound motor action potential amplitude [32].
Likewise, the elasticity of a scaffolds is an important aspect to consider when utilizing it for nerve regeneration. As mentioned above, it is critical for biomaterials to exhibit specific properties in order to serve as suitable nerve conduits – namely, to be porous, adhesive, biodegradable, and have the appropriate amount of compliance/flexibility amongst other things [50]. The elastic modulus of the scaffold has been shown to impact neuronal stem cell differentiation and growth, thus it is an important parameter to consider [51]. Blending polymers together can maximize and optimize the properties needed to serve as a successful scaffold. Studies have shown that combining certain polymers together can optimize the elasticity, as demonstrated by the blending of PLGA and Poly ethyleneglycol [52,53].
4. Utilization of growth factors
Growth factors are a potential therapeutic option for supporting neuronal growth and enhancing peripheral nerve regeneration. They can be used synchronously with biomaterial composites to promote cell differentiation and proliferation. Extensive research within the literature reports the positive effects of certain neurotrophins that act on subpopulations of neurons in specific stages of development [54]. The three classic Neurotrophins are nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3). All have affinity to different Receptor Tyrosine Kinases Receptors (RTKs) on target cells. RTKs are trans-membrane proteins that ultimately cause a sequence of cellular responses or a signal transduction cascade following substrate binding.
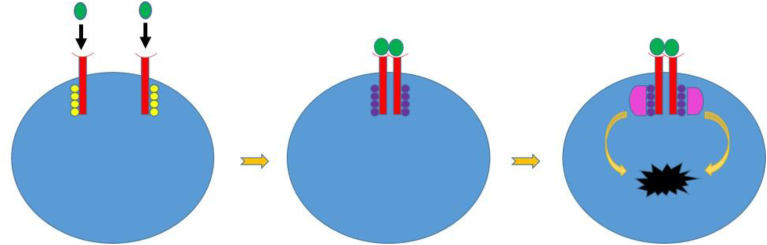
The mechanism of activation of RTK post neurotrophin binding can be based on an already existing model for epithelial growth factor receptor (EGFR) as shown in Fig. 3. Inactive RTK monomers are present across the plasma membrane comprising of three major domains: ligand binding extracellular domain, hydrophobic transmembrane domain and cytosolic domain [27]. As seen in Fig. 3, following substrate binding, the two monomers aggregate and dimerize. The tyrosine kinase domains on the cytosolic domain of the receptor then auto-phosphorylates the other monomer, with the phosphate groups derived from breaking down of ATP into ADP and Phosphate. After auto-phosphorylation, the active tyrosine kinase domains facilitates binding of other proteins to the phosphorylated tyrosine domains [27]. This triggers a conformational change within them, leading to a transduction cascade which ultimately results in cellular response, as depicted in Fig. 3.
Fig. 3.
Receptor Tyrosine Kinase (RTKs) mechanism. It is a transmembrane protein spanning the length of the membrane. Initially, it is inactive, with each monomer (red) having an open substrate binding site, and the tyrosine kinase domains (yellow) are un-phosphorylated and inactive. After substrate (green) binds, the monomers aggregate, dimerize, and the tyrosine kinase domains cross-phosphorylate each other, they are now active (purple). Finally, relay proteins (pink) bind to the activated, phosphorylated tyrosine kinase domains, and undergo conformational changes that ultimately lead to transduction cascades and cellular events (black shape).
Similar to EGRF model, Receptor Tyrosine Kinases serve multiple roles during nerve regeneration. As mentioned above, RTKs play a key role serving as a membrane receptor for major neurotrophic growth factors promoting axonal regeneration, survival, and neurite outgrowth. Additionally, RTKs are transported through retrograde mechanisms from neuronal projections back to the cell body, where they promote transcription and translation of proteins that serve to enhance long-term neuronal growth and survival [[55], [56], [57]].
4.1. Nerve growth factor
NGF in its mature form has high affinity for Tropomyosin Receptor Kinase A (TrKA). It promotes neuronal survival through its anti-apoptotic effects [58]. NGF only supports a limited set of neuronal populations [59] of the central and peripheral nervous system, specifically supporting the growth and survival of peripheral sympathetic and neural crest-derived sensory neurons [60,61]. Additionally, NGF can act on sympathetic (cholinergic) neurons as well as sensory neurons [62]. It promotes differentiation, survival and synaptic connections amongst neurons via transduction pathways including the Ras and PLC (phospholipase C) pathways [27]. NGF has been abundantly characterized which makes it an attractive candidate for in vivo and in vitro studies.
4.2. Brain-derived neurotrophic factor
BDNF supports neuronal growth and differentiation via Tropomyosin Receptor Kinase B (TrKB) activation [63]. Like other neuronal growth factors, BDNF's effects are only on certain subpopulations of neurons. Brain-derived neurotrophic factors have been shown to promote survival of a subpopulation of sensory dorsal root ganglion neurons [60] while its effect on sympathetic neurons are minimal. BDNF's basic functions also includes induction of neurite outgrowth of neurons [27,63] by altering local levels of Ca2+ signaling in growth cones, thus enhancing directional growth and the neurite extension process [27]. In addition to supporting sensory neurons in dorsal root ganglia (DRGs) and neurons found in the inferior vagus ganglion, BDNF also supports the survival and outgrowth of motor neurons and their axons. A study confirmed that continuous administration of BDNF in-vivo within chick embryos supported 40% of motor neurons that typically undergo degeneration and apoptosis during embryonic development [64].
4.3. Neurotrophin-3
Neurotrophin-3 has overlapping neurotrophic activity to NGF and BDNF and has high affinity receptor to Tropomyosin Receptor Kinase C (TrkC) [27], but is able to act on a specific subgroup of neurons. NT-3 promotes neurite outgrowth of both neural placode derived nodose ganglion and paravertebral chain sympathetic ganglia suggesting a broader specificity than either NGF or BDNF [59]. Additionally, NT-3 has been shown to support specifically neurons within the trigeminal ganglion early in development and neurons within the superior cervical sympathetic ganglia early in development [64]. Promotion of growth within the trigeminal ganglion is of particular interest for craniofacial nerve injuries, given the widespread distribution of the trigeminal nerve and its branches. In chick embryos, continuous administration of NT-3 in vivo supported 36% of motor neurons that are typically lost during early embryonic development between day 6 and day 10 [64].
4.4. Vascular endothelial growth factor
Other than the three major neurotrophins responsible for neuronal cell survival, differentiation and apoptosis, there are growth factors that can effectively promote neuronal regeneration. One such growth factor is vascular endothelial growth factor (VEGF). Naturally, VEGF promotes angiogenesis which is a crucial step towards tissue growth and repair [65]. Increased vascularization promoted by VEGF has seen to also promote regeneration of nerve fibers, axonal outgrowth from the DRG and SCG (superior cervical ganglia) and even neuronal survival in vitro [66,67].
Specifically, VEGF secretes a 165 amino acid binding variant that is seen to bind to neuropilin-1, a neuronal cell surface molecule on neurons, which plays a crucial role in chemorepulsive signaling during axonal outgrowth [66]. In studies conducted by Sondell et al., when VEGF was added to nerve grafts, Schwann cell invasion and neovascularization were promoted, both of which are important processes of the nerve regeneration process [65,67]. These treated nerves were seen to have increased tissue organization, vascularization, angiogenesis and myelinated nerve fibers [67]. Additionally, VEGF causes activation of the flk-1 receptor, the MAPK pathway, and a receptor on Schwann cells, which can stimulate axonal outgrowth from the DRG and SCG [67].
5. Utilization of electrical stimulation
Cellular differentiation can be guided by either growth factors that trigger cell signaling pathways or by external physical stimulus. Physical stimulus such as stretch, compression routines [68] or nanotopologies [69] have shown effective outcomes for musculoskeletal and nerve tissue engineering, respectively. These physical stimulus are sensed by the mechanosensors of the cell and transduced to initiate cell signaling cascade. Likewise, application of external electrical stimulation using suitable medium such as conducting polymers has been shown to affect cellular behavior due to the changes in ion influx across the cellular membrane, which propagates intracellular transduction pathways [70]. Generally, the cell membrane maintains a steady state potential, called resting potential. Small electrical activity called action potential can alter the transmembrane potential from negative to positive. Information is transferred in neurons along the axons by means of series of action potentials that elicits a growth-controlling transport processes across the plasma membrane [71]. Severed neurons must be able to switch from a transmitting mode to a growth mode in order to express growth-associated proteins such as GAP-43, tubulin, and actin [72]. As shown in Elzinga et al., electrical stimulation promotes axonal outgrowth through promotion of intracellular cAMP, increased expression of neurotrophic factors (including glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF)) [73] along with their receptors (tyrosine kinase B (Trk B)), and an increased expression of genes associated with nerve regeneration such as actin, tubulin, galectin-1, growth associated protein-43 (GAP-43), and neurotrophin-4/5 [[73], [74], [75]].
Electrical stimulation (ES) has shown to have a wide range of positive effects on different tissues. Many of these effects have been observed and studied in vivo as well as in vitro. Utilization of electrical stimulation has shown an increase in nerve regeneration and decreased atrophy of axotomized nerves [70,76]. The effects of ES on neurons and within peripheral nerves following injury has been well documented [77]. ES accelerates both sensory and motor nerve regeneration and healing after an injury [77]. When ES is performed within 30 days following injury, it increases myelin formation at the location of the damaged nerve, prevents Schwan cell apoptosis and promotes their activity –crucial both for myelin production and nerve regeneration [77]. ES also increases the expression of BDNF, its receptor TrKB activation, and Growth Associated Protein 43 (GAP-43) within nervous tissue, promoting regeneration. Additionally, ES promotes muscle growth, regeneration and remodeling following an injury and can assist in preventing disuse atrophy following lower motor neuron (LMN) injury [78].
Interestingly, ES also increases intra-muscular levels of BDNF and glial cell derived neurotrophic factor (GDNF) mRNA [79]. This has been shown to promote neuronal regeneration in surrounding nervous tissue and enhanced synaptic connections at the neuromuscular junction [79]. A proposed mechanism for this phenomenon (intra-muscular neuronal growth factors influencing extra-cellular neurons) is that diffusion of BDNF and GDNF out of the muscle fibers and into the surrounding damaged neurons promotes neuronal regeneration.
ES also increases levels of VEGF mRNA and VEGF protein [80,81]. As discussed previously, VEGF promote angiogenesis in vivo and endothelial cell proliferation in vitro [80]. Angiogenesis is critical for both tissue regeneration and remodeling [81]. It is hypothesized that the metabolic imbalance created by ES results in increased VEGF to promote angiogenesis. ES causes continuous muscle contraction which requires nutrients and oxygen (O2). O2 is perhaps the limiting factor of all nutrients required by muscle and VEGF functions to increase O2 delivery to hypoxic tissues [81]. ES helps prevent disuse atrophy by conserving type-1 twitch muscle fibers [78]. This is perhaps indirectly due to the increased VEGF and angiogenesis allowing for the increased O2 delivery which is utilized by type-1 muscle fibers via the electron transport chain for ATP synthesis and subsequent muscle contraction. ES also increases proliferation and differentiation of myoblasts into myocytes which is due to an increase in expression of genes associated with myogenic differentiation [[82], [83], [84], [85], [86]].
In order for electrical stimulation to elicit electrical surge through the scaffold, the nerve conduit needs to be made of electrically conductive polymers. Electrical stimulation is seen to promote cell proliferation and growth in vitro using minimum amount of electrical stimulation, time, or voltage threshold in order to initiate a cellular cascade [35,87].
For in vitro experiments, one common method to deliver DC electrical stimulation to cells within a culture plate is through the use of salt bridges immersed within the cells culture media [88]. This allows the salt bridges to separate the cells from the electrodes in order to prevent any fluctuations in pH or the formation of chemical byproducts [88,89]. Although this method is useful, there are several drawbacks to using this type of chamber setup: the wells and working area are small which limit the number of cells that can be simultaneously electrically stimulated, the amount of time that the cells can be exposed to the electrical stimulation is limited because of the concentration and heat difference between the salt bridge and the culture media, and there are issue with maintaining sterile conditions [88]. Studies using carbon and metallic electrodes have also been used, however, these studies used single petri dishes, which reduced the amount of cells that could be studied at once, increased the time for the experiments, and reduced replication of the experiments [88,90]. In order to overcome all the described problems, a new plate design was created consisting of platinum electrodes secured to the lid of a 6-well culture plate. This design uses a 1 mm gauge platinum wire, 50 mm in length, and bent into an L shape. By attaching the electrodes to the lid of the culture plate, the ES design allows for easy handling, negligible media evaporation, and easy sterilization. The individual electrode tips are soldered to copper wires coated in silver run in a parallel circuit [88]. The silver-coated wires are then attached to a DC power supplier, which can change the voltage, duration, and frequency. Table 1 provides a list of commercially available machines that are used for electrical stimulation and compares their mode of action.
Table 1.
Chart depicting various commercially available machines for electrical stimulation and their mechanisms of action.
| Machine | Voltage Range | Pulse Length | Electrodes | Reference |
|---|---|---|---|---|
| Transcutaneous Electrical Nerve Stimulation (TENS) | 0–350 V | 1–250 μs | Electrode pads | [93] |
| Variable | Variable | |||
| The Jouan PS10/15 Electropulsator | 0–1500 V | 5 μs- 24 msec | Parallel Plates | [94] |
| Variable | Variable | |||
| BTX ECM 830 | 0–500 V | 0.3 ms- 99 msec | Parallel Plates or electrode needles | |
| 20–3000 V | 5 μs- 99 μs | |||
| Variable | Variable |
ES has shown to work therapeutically in vivo and mechanistically in vitro. Although literature is lacking regarding the precise mechanisms on a cellular level through which ES manipulates tissues, the clinical argument is strong supporting the benefits of ES in a wide variety of tissues [78,91,92]. ES could become the future standard of care but more research is needed to define the parameters necessary to maximize its benefits.
The parameters used in ES studies in the literature vary greatly both from tissue to tissue, and also within each group. The parameters vary in terms of voltage, frequency, time of stimulation, and delay after injury before ES is used [78,80,91]. More studies and clinical trials are needed to standardize parameters for voltage, frequency, and time of stimulation to achieve maximum benefits of ES.
6. Conclusion
The complex anatomy and physiology of the craniofacial region make incidents that damage nervous tissue very difficult to repair and regenerate. Surgeries utilizing suturing, grafts, and conduits are present, but pose several limitations when trying to recuperate facial function and structure. New strategies and methods focused on tissue engineering approaches for regenerative medicine has become increasingly more prominent with rapidly growing research interest. Studies utilizing electrically conductive nerve conduits seeded with stems cells have proven advantageous for nerve regeneration and outgrowth. Additionally, the utilization of electrical stimulation, nerve growth factors, and even vascular endothelial growth factor have proven beneficial for increased neurite migration and axonal regeneration.
Acknowledgements
The authors acknowledge funding support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (R01EB020640), the Connecticut Regenerative Medicine Research Fund (15-RMBUCHC-08), and the Department of Defense (OR120140).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Tevlin R., McArdle A., Atashroo D. Biomaterials for craniofacial bone engineering. J. Dent. Res. 2014;93:1187–1195. doi: 10.1177/0022034514547271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birch R. 2010. The Peripheral Nervous System: Gross Anatomy; pp. 1–41. [Google Scholar]
- 3.Grinsell D., Keating C.P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res. Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett M.G., Zager E.L. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg. Focus. 2004;16:1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 5.Huebner E.A., Strittmatter S.M. 2009. Axon Regeneration in the Peripheral and central Nervous Systems; pp. 305–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs J. Treatment of acute peripheral nerve injuries: current concepts. J. Hand Surg. 2010;35:491–497. doi: 10.1016/j.jhsa.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Meek M.F., Coert J.H. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann. Plast. Surg. 2008;60:110–116. doi: 10.1097/SAP.0b013e31804d441c. [DOI] [PubMed] [Google Scholar]
- 8.Reid A.J., de Luca A.C., Faroni A., Downes S., Sun M., Terenghi G., Kingham P.J. Long term peripheral nerve regeneration using a novel PCL nerve conduit. Neurosci. Lett. 2013;544:125–130. doi: 10.1016/j.neulet.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal C.M., Ray R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res.: Official J. Soc. Biomateri. 2001;55:141–150. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. [DOI] [PubMed] [Google Scholar]
- 10.James R., Manoukian O.S., Kumbar S.G. Poly (lactic acid) for delivery of bioactive macromolecules. Adv. Drug Deliv. Rev. 2016;107:277–288. doi: 10.1016/j.addr.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y., Zhu J., Xue C., Li Z., Ding F., Yang Y., Gu X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials. 2014;35:2253–2263. doi: 10.1016/j.biomaterials.2013.11.087. [DOI] [PubMed] [Google Scholar]
- 12.Farokhi M., Mottaghitalab F., Shokrgozar M.A., Kaplan D.L., Kim H., Kundu S.C. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. Int. Mater. Rev. 2017;62:367–391. [Google Scholar]
- 13.Gloria A., De Santis R., Ambrosio L. Polymer-based composite scaffolds for tissue engineering. J. Appl. Biomater. Biomech. 2010;8:57–67. [PubMed] [Google Scholar]
- 14.Jeong S.I., Burns N.A., Bonino C.A., Kwon I.K., Khan S.A., Alsberg E. Improved cell infiltration of highly porous 3D nanofibrous scaffolds formed by combined fiber–fiber charge repulsions and ultra-sonication. J. Mater. Chem. B. 2014;2:8116–8122. doi: 10.1039/C4TB01487A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose S., Roy M., Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–554. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olszta M.J., Cheng X., Jee S.S., Kumar R., Kim Y., Kaufman M.J., Douglas E.P., Gower L.B. Bone structure and formation: a new perspective. Mater. Sci. Eng. R Rep. 2007;58:77–116. [Google Scholar]
- 17.Arabnejad S., Johnston B., Tanzer M., Pasini D. Fully porous 3D printed titanium femoral stem to reduce stress‐shielding following total hip arthroplasty. J. Orthop. Res. 2017;35:1774–1783. doi: 10.1002/jor.23445. [DOI] [PubMed] [Google Scholar]
- 18.Daly W., Yao L., Zeugolis D., Windebank A., Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nectow A.R., Marra K.G., Kaplan D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. B Rev. 2011;18:40–50. doi: 10.1089/ten.teb.2011.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G., Kong Y., Zhao Y., Zhao Y., Zhang L., Yang Y. Fabrication and characterization of polyacrylamide/silk fibroin hydrogels for peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2015;26:899–916. doi: 10.1080/09205063.2015.1066109. [DOI] [PubMed] [Google Scholar]
- 21.Hobson M.I., Green C.J., Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anat. 2000;197:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aebischer P., Salessiotis A., Winn S. Basic fibroblast growth factor released from synthetic guidance channels facilitates peripheral nerve regeneration across long nerve gaps. J. Neurosci. Res. 1989;23:282–289. doi: 10.1002/jnr.490230306. [DOI] [PubMed] [Google Scholar]
- 23.Kanje M., Skottner A., Sjo J. Insulin-like growth factor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 1989;486:396–398. doi: 10.1016/0006-8993(89)90531-3. [DOI] [PubMed] [Google Scholar]
- 24.Hamada N., Fujita Y., Kojima T., Kitamoto A., Akao Y., Nozawa Y., Ito M. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem. Int. 2012;60:743–750. doi: 10.1016/j.neuint.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Pires F., Ferreira Q., Rodrigues C.A., Morgado J., Ferreira F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta Gen. Subj. 2015;1850:1158–1168. doi: 10.1016/j.bbagen.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 26.English A.W., Schwartz G., Meador W., Sabatier M.J., Mulligan A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev. Neurobiol. 2007;67:158–172. doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purves D., Cabeza R., Huettel S.A., LaBar K.S., Platt M.L., Woldorff M.G., Brannon E.M. 2008. Cognitive Neuroscience. [Google Scholar]
- 28.Gaudet A.D., Popovich P.G., Ramer M.S. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jessen K.R., Mirsky R., Lloyd A.C. Vol. 7. 2015. Schwann cells: development and role in nerve repair; p. a020487. (Cold Spring Harb. Perspect. Biol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caroni P., Schwab M.E. Antibody against myelin associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- 31.Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J. Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobasseri A., Faroni A., Minogue B.M., Downes S., Terenghi G., Reid A.J. Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng. 2015;21:1152–1162. doi: 10.1089/ten.tea.2014.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arslantunali D., Dursun T., Yucel D., Hasirci N., Hasirci V. Peripheral nerve conduits: technology update. Med.Devices (Auckl) 2014;7:405–424. doi: 10.2147/MDER.S59124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelke N.B., Lee P., Anderson M., Mistry N., Nagarale R.K., Ma X., Yu X., Kumbar S.G. Neural tissue engineering: nanofiber‐hydrogel based composite scaffolds. Polym. Adv. Technol. 2016;27:42–51. [Google Scholar]
- 35.Mistry N., Moskow J., Shelke N., Yadav S., Berg-Foels W., Kumbar S. 2017. Bio-instructive Scaffolds for Cartilage Regeneration; pp. 115–135. [Google Scholar]
- 36.Kinner B., Capito R., Spector M. 2005. Regeneration of Articular Cartilage; pp. 91–123. [DOI] [PubMed] [Google Scholar]
- 37.Park T.G. Degradation of poly (lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 38.Hadlock T., Sundback C., Hunter D., Cheney M., Vacanti J.P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6:119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 39.Hsu S., Chen C., Lu P.S., Lai C., Chen C. Oriented Schwann cell growth on microgrooved surfaces. Biotechnol. Bioeng. 2005;92:579–588. doi: 10.1002/bit.20634. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez‐Perez F., Cobianchi S., Geuna S., Barwig C., Freier T., Udina E., Navarro X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery. 2015;35:300–308. doi: 10.1002/micr.22362. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., Ding F., Wu J., Hu W., Liu W., Liu J., Gu X. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials. 2007;28:5526–5535. doi: 10.1016/j.biomaterials.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Dinis T., Elia R., Vidal G., Dermigny Q., Denoeud C., Kaplan D., Egles C., Marin F. 3D multi-channel bi-functionalized silk electrospun conduits for peripheral nerve regeneration. J. Mech. Behav. Biomed. Mater. 2015;41:43–55. doi: 10.1016/j.jmbbm.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 43.Lundborg G., Dahlin L.B., Danielsen N., Gelberman R.H., Longo F.M., Powell H.C., Varon S. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp. Neurol. 1982;76:361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- 44.Schnell E., Klinkhammer K., Balzer S., Brook G., Klee D., Dalton P., Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials. 2007;28:3012–3025. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Wang S., Cai L. Polymers for fabricating nerve conduits. Int. J. Polym. Sci. 2010:2010. [Google Scholar]
- 46.Izquierdo R., Garcia‐Giralt N., Rodriguez M. Biodegradable PCL scaffolds with an interconnected spherical pore network for tissue engineering. J. Biomed. Mater. Res. 2008;85:25–35. doi: 10.1002/jbm.a.31396. [DOI] [PubMed] [Google Scholar]
- 47.Xu H., Holzwarth J.M., Yan Y., Xu P., Zheng H., Yin Y., Li S., Ma P.X. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials. 2014;35:225–235. doi: 10.1016/j.biomaterials.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spearman B.S., Desai V.H., Mobini S., McDermott M.D., Graham J.B., Otto K.J., Judy J.W., Schmidt C.E. Tissue‐engineered peripheral nerve interfaces. Adv. Funct. Mater. 2018;28:1701713. doi: 10.1002/adfm.201701713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mobasseri S., Terenghi G., Downes S. Micro-structural geometry of thin films intended for the inner lumen of nerve conduits affects nerve repair. J. Mater. Sci. Mater. Med. 2013;24:1639–1647. doi: 10.1007/s10856-013-4922-5. [DOI] [PubMed] [Google Scholar]
- 50.Verreck G., Chun I., Li Y. Preparation and physicochemical characterization of biodegradable nerve guides containing the nerve growth agent sabeluzole. Biomaterials. 2005;26:1307–1315. doi: 10.1016/j.biomaterials.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X.F., Yang K., Yang X.Q., Liu Y.F., Cheng Y.C., Chen X.Y., Tu Y. Elastic modulus affects the growth and differentiation of neural stem cells. Neural Regen.Res. 2015;10:1523–1527. doi: 10.4103/1673-5374.165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramanian A., Krishnan U.M., Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: biomaterial mediated neural regeneration. J. Biomed. Sci. 2009;16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowlands A., Lim S.A., Martin D., Cooper-White J.J. Polyurethane/poly (lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials. 2007;28:2109–2121. doi: 10.1016/j.biomaterials.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 54.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999;194:1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hausott B., Klimaschewski L. Membrane turnover and receptor trafficking in regenerating axons. Eur. J. Neurosci. 2016;43:309–317. doi: 10.1111/ejn.13025. [DOI] [PubMed] [Google Scholar]
- 56.Ascano M., Bodmer D., Kuruvilla R. Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol. 2012;22:266–273. doi: 10.1016/j.tcb.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zweifel L.S., Kuruvilla R., Ginty D.D. Functions and mechanisms of retrograde neurotrophin signalling. Nat. Rev. Neurosci. 2005;6:615. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 58.Noureddini M., Verdi J., Mortazavi‐Tabatabaei S.A., Sharif S., Azimi A., Keyhanvar P., Shoae‐Hassani A. Human endometrial stem cell neurogenesis in response to NGF and bFGF. Cell Biol. Int. 2012;36:961–966. doi: 10.1042/CBI20110610. [DOI] [PubMed] [Google Scholar]
- 59.Maisonpierre P.C., Belluscio L., Squinto S., Ip N.Y., Furth M.E., Lindsay R.M., Yancopoulos G.D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- 60.Barde Y., Davies A., Johnson J., Lindsay R., Thoenen H. Vol. 71. 1987. pp. 185–189. (Brain Derived Neurotrophic Factor). [DOI] [PubMed] [Google Scholar]
- 61.Ide C. Peripheral nerve regeneration. Neurosci. Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- 62.Meakin S.O., Shooter E.M. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- 63.Verderio C., Bianco F., Blanchard M.P., Bergami M., Canossa M., Scarfone E., Matteoli M. Cross talk between vestibular neurons and Schwann cells mediates BDNF release and neuronal regeneration. Brain Cell Biology. 2006;35:187–201. doi: 10.1007/s11068-007-9011-6. [DOI] [PubMed] [Google Scholar]
- 64.Gibbons A., Wreford N., Pankhurst J., Bailey K. Continuous supply of the neurotrophins BDNF and NT-3 improve chick motor neuron survival in vivo. Int. J. Dev. Neurosci. 2005;23:389–396. doi: 10.1016/j.ijdevneu.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Pereira Lopes F., Lisboa B., Frattini F. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol. Appl. Neurobiol. 2011;37:600–612. doi: 10.1111/j.1365-2990.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 66.Sondell M., Sundler F., Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk‐1 receptor. Eur. J. Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 67.Sondell M. 2000. Vascular Endothelial Growth Factor (VEGF) and Peripheral Nerve Regeneration. [Google Scholar]
- 68.Thorpe S.D., Buckley C.T., Vinardell T., O'Brien F.J., Campbell V.A., Kelly D.J. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-β3 induced chondrogenic differentiation. Ann. Biomed. Eng. 2010;38:2896–2909. doi: 10.1007/s10439-010-0059-6. [DOI] [PubMed] [Google Scholar]
- 69.Yang F., Murugan R., Ramakrishna S., Wang X., Ma Y., Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 70.Anderson M., Shelke N.B., Manoukian O.S., Yu X., McCullough L.D., Kumbar S.G. Vol. 43. 2015. Peripheral nerve regeneration strategies: electrically stimulating polymer based nerve growth conduits. (Critical Reviews™ in Biomedical Engineering). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghasemi‐Mobarakeh L., Prabhakaran M.P., Morshed M., Nasr‐Esfahani M.H., Baharvand H., Kiani S., Al‐Deyab S.S., Ramakrishna S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Regen. Med. Tissue Eng. 2011;5 doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 72.Fu S.Y., Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 73.Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13:295–310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elzinga K., Tyreman N., Ladak A., Savaryn B., Olson J., Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp. Neurol. 2015;269:142–153. doi: 10.1016/j.expneurol.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 75.Gall C., Murray K., Isackson P.J. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Mol. Brain Res. 1991;9:113–123. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- 76.Gordon T., Gillespie J., Orozco R., Davis L. Axotomy-induced changes in rabbit hindlimb nerves and the effects of chronic electrical stimulation. J. Neurosci. 1991;11:2157–2169. doi: 10.1523/JNEUROSCI.11-07-02157.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu C., Kou Y., Zhang P., Han N., Yin X., Deng J., Chen B., Jiang B. Electrical stimulation promotes regeneration of defective peripheral nerves after delayed repair intervals lasting under one month. PLoS One. 2014;9:e105045. doi: 10.1371/journal.pone.0105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams H.B. The value of continuous electrical muscle stimulation using a completely implantable system in the preservation of muscle function following motor nerve injury and repair: an experimental study. Microsurgery: Official J. Int. Microsurgical Soc. Eur. Fed. Soc. Microsurgery. 1996;17:589–596. doi: 10.1002/(SICI)1098-2752(1996)17:11<589::AID-MICR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 79.Willand M.P., Rosa E., Michalski B., Zhang J.J., Gordon T., Fahnestock M., Borschel G.H. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience. 2016;334:93–104. doi: 10.1016/j.neuroscience.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 80.Hang J., Kong L., Gu J.W., Adair T.H. VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am. J. Physiol. 1995;269:H1827–H1831. doi: 10.1152/ajpheart.1995.269.5.H1827. [DOI] [PubMed] [Google Scholar]
- 81.Annex B.H., Torgan C.E., Lin P., Taylor D.A., Thompson M.A., Peters K.G., Kraus W.E. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 1998;274:H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- 82.Chang K., Kim J.W., Kim J.A. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quigley A.F., Razal J.M., Kita M. Vol. 1. 2012. Electrical stimulation of myoblast proliferation and differentiation on aligned nanostructured conductive polymer platforms; pp. 801–808. (Advanced Healthcare Materials). [DOI] [PubMed] [Google Scholar]
- 84.Yamada M., Tanemura K., Okada S. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cell. 2007;25:562–570. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- 85.Huang Y., Wu H., Tai N., Wang T. Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small. 2012;8:2869–2877. doi: 10.1002/smll.201200715. [DOI] [PubMed] [Google Scholar]
- 86.Huang J., Hu X., Lu L., Ye Z., Zhang Q., Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. 2010;93:164–174. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 87.Anderson M., Shelke N.B., Manoukian O.S., Yu X., McCullough L.D., Kumbar S.G. vol. 43. 2015. Peripheral nerve regeneration strategies: electrically stimulating polymer based nerve growth conduits. (Critical Reviews™ in Biomedical Engineering). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mobini S., Leppik L., Barker J. Direct current electrical stimulation chamber for treating cells in vitro. Biotechniques. 2016;60:95–98. doi: 10.2144/000114382. [DOI] [PubMed] [Google Scholar]
- 89.Song B., Gu Y., Pu J., Reid B., Zhao Z., Zhao M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat. Protoc. 2007;2:1479. doi: 10.1038/nprot.2007.205. [DOI] [PubMed] [Google Scholar]
- 90.Hronik-Tupaj M., Rice W.L., Cronin-Golomb M., Kaplan D.L., Georgakoudi I. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed. Eng. Online. 2011;10:9. doi: 10.1186/1475-925X-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aaron R.K., Ciombor D.M. Therapeutic effects of electromagnetic fields in the stimulation of connective tissue repair. J. Cell. Biochem. 1993;52:42–46. doi: 10.1002/jcb.240520107. [DOI] [PubMed] [Google Scholar]
- 92.Hampton S., King L. Healing an intractable wound using bio-electrical stimulation therapy. Br. J. Nurs. 2005;14:S30–S32. [PubMed] [Google Scholar]
- 93.Cavalcante Miranda de Assis D., Martins Lima E., Teixeira Goes B., Zugaib Cavalcanti J., Barbosa Paixao A., Vannier-Santos M.A., Martinez A.M., Baptista A.F. The parameters of transcutaneous electrical nerve stimulation are critical to its regenerative effects when applied just after a sciatic crush lesion in mice. BioMed Res. Int. 2014;2014:572949. doi: 10.1155/2014/572949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belehradek M., Domenge C., Luboinski B., Orlowski S., Belehradek J., Jr., Mir L.M. Electrochemotherapy, a new antitumor treatment. First clinical phase I‐II trial. Cancer. 1993;72:3694–3700. doi: 10.1002/1097-0142(19931215)72:12<3694::aid-cncr2820721222>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]