Abstract
MiRNAs are naturally occurring, small, non-coding RNA molecules that post-transcriptionally regulate the expression of a large number of genes involved in various biological processes, either through mRNA degradation or through translation inhibition. MiRNAs play important roles in many aspects of physiology and pathology throughout the body, particularly in cancer, which have made miRNAs attractive tools and targets for translational research. The types of non-coding RNAs, biogenesis of miRNAs, circulating miRNAs, and direct delivery of miRNA were briefly reviewed. As a case of point, the role and perspective of miR-302, a family of ES-specific miRNA, on cancer, iPSCs, heart disease were presented.
Keywords: Biomedical engineering, Biochemistry, Biotechnology, Cancer research, Cell biology, Developmental biology, Genetics, Molecular biology
1. Introduction
Human genome contains all the genetic information in our body in the form of DNA. When the complete sequence of all DNA was worked out, only small portion of it are genes, which give the human species traits. The vast majority of human DNA was considered as “junk DNA.” That is, they have no functional purpose. However, some of these “junk” DNAs, called microRNAs (miRNAs), have been shown to be essential for a variety of vital functions, including reprogramming of somatic cells to pluripotent stem cells as well as progression and metastasis of cancer cells. In other words, the miRNA is a small single-stranded non-coding RNA molecule (sRNA or ncRNA) (containing about 19–25 nucleotides) that functions in preventing the formation of target proteins through translational repression. RNAs shorter than 30 nucleotides (nt) and directly affects RNA processing and degradation is considered sRNA. ncRNA is transcribed from a DNA sequence that is also considered a RNA gene; these types of RNA are transcribed directly by type III RNA polymerases or processed indirectly from a large transcript of type II RNA polymerases. Examples of ncRNAs include transfer RNA, nucleolar RNA, nuclear RNA, phage RNA and viral RNA [1]. A type of small, single-stranded RNA that possesses the reverse complement of the mRNA transcript of another protein coding gene is called microRNA (miRNA).
2. Main text
2.1. Small interfering RNAs (siRNAs), microRNAs (miRNAs), and intronic miRNAs
Small interfering RNAs (siRNAs) are small double-stranded RNA (dsRNA) mostly observed in triggering post-transcriptional gene silencing in plants, fungi and low-end animals [2], while miRNAs are single-stranded RNAs that are approximately 18- to 25-nucleotides each and are transcribed from DNA to suppress protein-coding gene expression in high-end plants and animals [3]. Consisting of the reverse complement of the mRNA transcripts of its targeted genes, miRNAs function to either degrade target mRNAs or inhibit protein synthesis of the target protein-coding genes. Long RNA transcripts consisting of at least a hairpin-like miRNA precursor (pre-miRNA) make up the primary transcripts (pri-miRNA) of the miRNA genes. Assisted by microprocessor and exported from the nucleus by Exportin-5, ribonuclease Drosha processes pri-miRNAs in the nucleus to pre-miRNAs. Dicer, the cytoplasmic RNase III enzyme, removes the miRNA from the pre-miRNA stem region. Based on the structures of the double-stranded RNA (dsRNA) and the hairpin resemblance, the miRNA and siRNAs are closely related.
Gene suppression by miRNA is dependent on the complementarity between one or multiple target sequences in the gene transcript (mRNA) and the miRNA. The binding of miRNA to mRNA forms dsRNA with the aid of RNA-induced silencing complex (RISC), causing the gene transcripts to degrade through processes such as RNA interference (RNAi) [3, 4]. In other cases, miRNAs form an RISC to block protein translation and therefore mediates posttranscriptional regulation without degrading mRNAs. A majority of miRNAs can target more than one mRNA and more than one miRNA can target the same mRNA. Through simultaneous cross-interactions between multiple miRNAs and mRNAs, miRNAs often exhibit many functions in perfecting the protein-coding gene expression and fine-tuning the process of gene regulation.
The underlying mechanisms of siRNA- and miRNA-mediated gene silencing, called RNA interference (RNAi), demonstrate the cellular ability to simultaneously suppress multiple genes containing homologous sequences, including transgenes and endogenous genes, and sometimes may further involve RNA-mediated DNA methylation [2]. RNAi, in other words, occurs when small regulatory RNAs bind with a complementary sequence of the target gene transcripts to form dsRNA regions, so as to interfere with the gene expression. Dicer, in the cytoplasm, digests the dsRNAs into siRNAs. Dicer, consisting of an amino-terminal helicase and PAZ domain and two RNA III and one dsRNA-binding motifs, binds to the dsRNA, excising it into siRNAs which find other completely complementary single-stranded RNA molecules. RNAs complementary to the siRNAs are then destroyed by RNases. Also known as transgene quelling or PTGS, post-transcriptional gene silencing, the introduction of dsRNA sequences containing high complementarity to the target gene transcripts can further stimulate RNA-dependent RNA polymerase activities to amplify the resulting siRNAs and so as to enhance the targeted gene silencing. Yet, in mammals dsRNAs longer than 30 nucleotides (nt) can activate an antiviral response, leading to nonspecific degradation of RNA transcripts, the overall shutdown of host cell protein synthesis, and the production of interferon. Because of this phenomenon, gene-specific RNAi activity is not produced by the long dsRNA in mammalian cells. Similar or same phenomena in the different biological systems of other species have different names.
The miRNA produced from intronic RNA fragments, called intronic miRNAs, is significantly different from intergenic miRNAs. Intronic miRNAs require Pol-II and spliceosomal components for its biogenesis while intergenic miRNAs do not. Intronic miRNAs exhibit the following four characteristics: i) they and their encoding gene transcripts must share the same promoter, ii) their location must be in the non-protein-coding region of a primary gene transcript, iii) they and the gene transcripts are coexpressed, and iv) in order to form mature miRNAs, the nuclear RNA splicing and excision processes must remove them from the transcripts of their coding genes. Many of the miRNAs that are to be discussed later are oriented oppositely to the protein-coding gene transcript meaning that they are not intronic miRNAs given that they don't share a promoter with the gene; with these miRNAs, their promoters are situated in the antisense orientation to the gene [5, 6, 7].
In eukaryotes, intronic and other ncRNAs have possibly evolved to give a second level gene expression. Group II introns in bacteria and organellar genomes consist of both retrotransposable elements, which make this intron mobile, and catalytic RNAs. These introns, due to their mobility, are reversely spliced directly into the DNA target site and then reverse transcribed by the intron encoded gene. To minimize the damage to the host, the introns are spliced out of the gene transcript after it has been inserted into the DNA. Group II introns, eukaryotic spliceosomal introns, and non-LTR-retrotransposons have a possible evolutionary relationship. Some of these new introns can stem from other introns in the same gene.
Transposons are ancient DNAs that exist in the common ancestor genome, enter the host many times, like retroviruses, for selfish reasons [8]. A genome can be destroyed if there is too much transposon activity; to prevent this destruction, certain organisms form a mechanism to halt or silence transposon and virus activities. As an example, retroviruses and transposons incorporated in a bacteria's genome are removed when the bacteria frequently removes its genes. In eukaryotes, having miRNAs is a possible way of reducing transposon activity. Like the variety of antibody production, miRNA could be involved in virus resistance. Class II transposons have the function to cut and paste; transposase, an enzyme, binds to transposons at the ends, which are the same, and to the target site on the genome, cut to leave sticky ends. These two, the ends and the target site, are linked by ligases. By leaving multiple copies of themselves, transposons increase the size of the genome. Transposons, for the genome to modulate gene regulation through miRNAs, are advantageous. The insertion of transposons to the introns of the protein-coding gene could allow for the transposons themselves, their secondary structures, or a part of them to become intronic miRNAs.
2.2. Other non-coding RNAs
Other types of non-coding RNAs also play an important role in gene regulation. For instance, PIWI-interacting RNAs (piRNA) control transposon activity [9]. Predominantly expressed in the germlines or different species and a part of the Argonaute family of proteins, they can form RNA-induced silencing complexes by binding to the miRNA or siRNA [9]. A subcategory of piRNAs, called repeated-associated siRNA (rasiRNA), also interacts with the PIWI-Argonaute protein family. Longer than all other ncRNAs, rasiRNA doesn't require the Dicer enzyme and instead utilizes the Argonaute proteins to manage the cleavage process.
RasiRNAs are essential in regulating cell structure and transcriptional silencing. Also, there are trans-acting siRNAs (tasiRNA), a type of siRNAs in plants, that are conserved miRNA pathways in tasiRNA production in both monocot and dicot plants [10]. The signaling pathways of tasiRNAs are thought to be comparable to other siRNAs and the function of tasiRNA is like other siRNA in the RNAi mechanism. Small activating RNAs (saRNA) prompt epigenetic changes on the target promoter, altering the gene expression [11]. Long noncoding RNAs (lncRNAs) are longer than 200 nt long that act at the interface of chromatin-modifying machinery and the genome [12]. In addition, some foreign RNAs, such as phage and viral RNAs, are 30 ribonucleotides long, rendering it a form of small RNA, functioning as a priming initiator for the bacteriophage F1 DNA replication.
2.3. Biogenesis of miRNAs
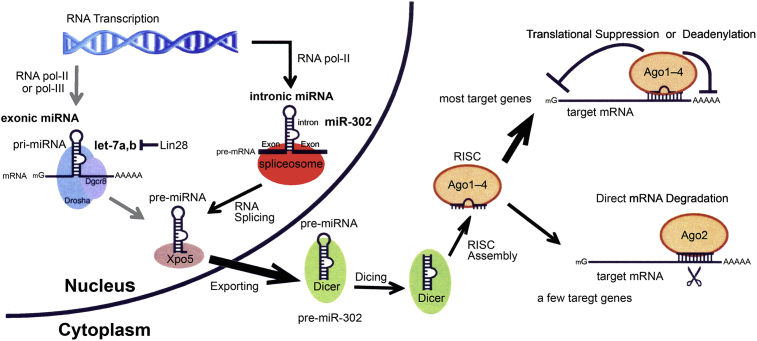
Distributed throughout the entire genome, miRNA genes can be localized in intergenic locations [13] or intronic and exonic regions of the gene. In vertebrates, the biogenesis of miRNA consists of five steps. First, the transcription of miRNAs from DNA facilitated by Type-II RNA polymerases [14, 15] into pre-miRNAs. Second, the pri-miRNAs are processed by the microprocessor complex which comprised of Drosha, DGCR8, and/or spliceosomal components. DGCR8 function to recognize the pri-miRNA while Drosha possesses the endonucleolytic function conferred by the RNase III domines. The structure of pre-miRNAs is comprised of a special hairpin secondary structure that holds area of imperfectly paired dsRNA, which are cleaved to one or multiple miRNAs. This step relies on the origin of the pre-miRNA, located in either an exon or an intron [3, 15]. Since the base of the stem-loop hairpin structure is identified by the Drosha microprocessor complex, it gets cleaved and released in order to form miRNA precursors or pre-miRNAs, 70–90 nt, in the nucleus. Third, the pre-miRNAis exported out of the nucleus by Ran-GTP and Exportin-5 [4, 16]. Fourth, the pre-miRNA is further cleaved by RNase III enzyme Dicer and TRBP or TARBP2, a cofactor transactivation-responsive RNA-binding protein in the cytoplasm to form a miRNA duplex, (about 22 nt long). Within the two strands, one is the miRNA, the leading or guide strand, and the other is known as the miRNA* or the passenger strand, specifically through base pairing of the 3′ UTR and the targeted mRNA based on a partial complementarity between miRNA and the target mRNA, the mature miRNA blocks mRNA translation. The guide strand contains the weakest binding and a U-bias at the 5′-end and an excess of purines. The strand with perfect complementarity between the target mRNA and the miRNA, 5′-end with a C-bias, and an excess of pyrimidines is the passenger strand that gets degraded [17]. Lastly, the RISC is incorporated into a RNP, which executes the RNAi-related gene silencing [18, 19], RNP assimilates the mature miRNA. Certain genes are regulated by the autoregulatory negative feedback from the miRNAs [20, 21]. There are alternative pathways for miRNA biogenesis in animals which are Drosha/DGCR8 independent but Dicer dependent and vice versa.
The intronic is a new class of miRNA derived from the processing of gene introns, which requires type-II RNA polymerases (Pol-II) and spliceosomal components for their biogenesis. For example, the miR-302 family, located in the gene La-related protein 7 (LARP7), was initially identified to be specifically expressed in undifferentiated human embryonic stem cells (hESCs). The biogenesis of the intronic miRNA is however slightly more complicated than that of intergenic and exonic miRNAs, as shown in Fig. 1. Intronic miRNA maturation requires the involvement of intracellular RNA splicing machineries [5, 15]. Intronic miRNA biogenesis consists of six major steps: (i) Transcription of primary miRNA precursors (pri-miRNA, 300–1000 nt) encoded in the intron region of a gene by Type-II RNA polymerases, (ii) Splicing of the long pri-miRNAs out of the encoding gene transcripts by intracellular spliceosomes, (iii) Further processing of the pri-miRNAs by Drosha-like endoribonucleases and DGCR8 in the nucleus to form 60–90nt long single hairpin-like miRNA precursors (pre-miRNAs), (iv) Exporting of pre-miRNAs out of nucleus by Ran-GTP and Exportin-5, (v) Processing of pre-miRNAs into 21–23 nt long mature intronic miRNAs by Dicer, and (vi) Formation of RISC in combination with other proteins to induce gene silencing.
Fig. 1.
The biogenesis of intronic miRNA miR-302. Encoded in the intronic region of the La Ribonucleoprotein domain family member 7 gene (LARP7 or PIP7S), miR-302 is a native intronic miRNA. First, transcribed within the intron of LARP7 gene transcripts by type-II RNA polymerases, and then cellular spliceosomes splice the intron out of LARP7 gene transcripts to form primary miRNA precursors, pri-miRNAs (pri-miR-302). After that, Drosha-like endoribonucleases process the pri-miRNAs into miRNA precursors (pre-miRNAs) with a single hairpin like structure. Then, Exportin-5 exports the pre-miRNAs out of the nucleus into the cytoplasm allowing Dicer-like RnaseIII endoribonucleases to further process and form 21–23nt long mature miRNAs, such as miR-302s. Lastly, following assembly into RNA-induced silencing complexes (RISC) with Argonaute proteins, the mature miR-302s carry out their specific gene silencing functions.
Of the intronic miRNA, 5′UTR and 3′ UTR can be assumed as an intron extension, but their mRNA translation processing is different from the process of the intron found between the two protein-coding exons, or, the in-frame intron. Before the discovery of the intronic miRNAs, in-frame introns were thought to be a large genetic wasteland in gene transcripts. Intronic miRNAs, excised through splicing, linearized from lariat debranching, and resected by nucleases, and other pre-miRNA-like hairpins, can be generated through Drosha and DGCR8-independent pathways. Ago1-4 incorporates both non-canonical miRNAs and canonical miRNAs.
2.4. Assembly of RISC
RNA-induced silencing complex (RISC) contains many associated proteins, which contain RNA so they are ribonucleoproteins. These ribonucleoproteins combine an RNA and an RNA-binding protein, incorporating one strand of a single-stranded RNA (ssRNA) fragment of miRNA, or double-stranded of small interfering RNA (siRNA). The single strand acts as the template for RISC to recognize the transcript of the complementary messenger RNA. Once identified, Argonautes choose the strand with the less stable 5′ end to integrate into RISC and then activate and cleave the mRNA, these actions facilitated by RNase III Dicer, culminating RNA interference, RNAi, and gene silencing [22].
Auxiliary factors and ATP hydrolysis allow for small RNAs to be loaded onto Argonaute proteins. An essential structure to load dsRNA fragments into RISC, RISC-loading complex (RLC), consists of TRBP (the HIV trans-activation response RNA binding protein), Dicer, and Argonaute 2 (Ago2) to assist in the targeting of mRNA. Dicer, a RNase III endonuclease, generates the dsRNA fragments that direct RNAi. Required for the recruitment of Ago2 to the siRNA bound by Dicer, TRBP holds three double-stranded RNA-binding domains. Ago2, an RNase, acts as the catalytic center for RISC. Argonaute proteins, into which the duplex of miRNA is loaded, cling to the mature miRNA and release the star strand. Ago proteins correlate with the cofactors of the GW182/TNRC6 family target transcripts and mediate their destabilization and/or translational suppression [23] with the guide of miRNAs. miRNA and Ago complexes recognize targets by complements to their 5′ ends, preferably nts 2–8 [24, 25, 26, 27]. Prolyl-hydroxylation, ubiquitination, phosphorylation, and poly-ADP-ribosylation, and other posttranslational modifications of Argonaute proteins modifies miRNA activity at large and specific levels.
2.5. Other molecules in RISC assembly
The biogenesis and mechanisms of miRNAs are modified by many different factors. Hsc70/Hsp90, heat-shock organizing protein chaperone machinery [28], facilitates the many steps of the RISC assembly. GW182 family proteins, with a presence of glycine and tryptophan repeats and its molecular weight included in its name, cooperates with the Argonaute proteins and are essential for miRNA-mediated gene silencing in animal cells [29]. Similar to TRBP and the junction among Dicer and TRBP, PACT, a kinase R-activating protein, creates Dicer-PACT complexes, contributing to regulating the proper miRNA length and strand selection in a subset of mammalian miRNAs [30]. RNA-specific adenosine deaminase 1, or ADARI, is involved in A-to-I RNA editing where the adenosine in double-stranded RNA is hydrolytically deaminated into inosine. With this function, ADARI has the ability to alter miRNAs and influence RNA stability, splicing, and miRNA-target interactions [31]. In contrast, certain miRNAs are able to regulate ADARI. A reciprocal feedback loop with miRNA is caused by the overexpression of ADARI [32]. PARN, or poly(A) specific ribonuclease, holds an important role in miRNA-dependent control of mRNA decay and regulation of p53 expression, meaning the facilitation of the biogenesis of many important noncoding RNAs [33, 34]. The N-terminal helicase, a dynamically evolving Dicer domain, can be dimerized by itself and mediated by ATPase activity as a mechanism for RNA length discrimination by a Dicer family protein, which results in the recognition of miRNA targets [35, 36]. A novel component of the Ago2-centered RISCs, eIFIA also enhances Ago2-dependent RNAi and miRNA biogenesis [37].
2.6. Silencing of gene expression
RISC, guided by miRNAs, can explicitly recognize mRNAs. Once RISC binds to target mRNAs, a high degree of miRNA-mRNA complementarity of approximately 6–8 nt long forms, producing translational repression and mRNA cleavage [38]. Central mismatches prevent degradation and facilitate translational repression by the possible mechanisms of RISCs bind to target mRNAs and represses initiation as the cap recognition stage, or at the 60s-ribosomal recruitment stage. RISC can also prevent mRNA to circularize or RISC attachment to target mRNAs, facilitating premature separation from ribosomes. As a result, translation at the post-initiation stage is repressed [39]. Certain miRNAs can bind as ligands to receptors of the Toll-like receptor, TLR, acting as paracrine agonists of TLRs [40]. The miRNAs can also selectively activate innate immune effector cells through the TLR1-NF-ҡB signaling pathway [41].
2.7. Circulating miRNAs
Previously miRNAs and their precursors were thought to be unstable and easily degradable, but were observed to be highly stable in biological fluids, including the serum, saliva, and urine. Circulating miRNAs are stable due to their association with Ago2 protein, multivesicular body, and incorporation into exosomes, microvesicles, apoptotic bodies, or high-density lipoprotein particles [42]. For a variety of diseases, the presence of blood miRNA at different levels acts as biomarkers can be potentially used for early detection and diagnosis [42, 43, 44].
Tiny endosomal membrane vesicles, known as exosomes, about 40–150 nm in diameter, are formed from the inward budding of endosomal members of the late endosomal compartment, resulting in intercellular multi-vesicular endosomes, MVEs [45, 46]. Packed in the MVEs, many exosomes are fused with the plasma membrane, facilitating their release into the extracellular space. Circulating miRNAs either are bound to serum, proteins, or lipoproteins or to be encased in extracellular vesicles, i.e. exosomes, microvesicles, or apoptotic bodies [47, 48].
Despite the obvious potential as biomarkers, there are problems for using circulating miRNAs as routine clinical diagnostic tools. There is no consensus on optimal protocols for the standardization of sample collection, data normalization, and analysis has been achieved. The qPCR and microarray, used to measure circulating miRNAs, depends on the design of the miRNA-specific primers or microarray probes. With miRNAs that have similarities, there could be more difficulties analyzing data obtaining from different laboratories. The limited knowledge on positive and negative miRNAs in human serum samples continues to contribute to the inability to compare different studies. The steps to eventually implement circulating miRNAs in the estimation of the clinical fate of patients with various diseases begins with the validation of miRNA profiles through specific and selective methodologies to achieve accurate measurements, continuing to collecting overall numbers of a broad spectrum of different miRNAs [49].
2.8. Direct delivery of miRNA in vivo
The use of circulating miRNAs may improve the delivery of miRNAs in vivo. Given the high stability under storage and handling conditions of miRNAs and their presence in urine, blood, and other bodily fluids, it is possible that miRNA mimics can be delivered directly in vivo to treat/manage diseases.
Conventional delivery systems for miRNAs include liposomal transfection, liposomes modified by adding long carbon chains and/or positively charged chemical groups such as polyethylene glycol (PEG), glycerol esters, glycerol monooleate, glycolipids, aminated/amide polys, and sugar-encapsulation. These reagents typically fuse with the phospholipid bilayer of cell membranes through passive diffusion. However, there are two major difficulties: high degradation and low penetration of miRNAs. Liposomes, small spherical sacs of phospholipid molecules encompassing a water molecule with the function of carrying miRNAs into the tissues, are normally composed of phosphatidycholine-enriched phospholipid and can also contain various lipid chains with surfactant properties like egg phosphatidylethanolamine. Polyethylenimines, PEIs (positively charged, linear or branched polymers), form nanoscale complexes with small RNAs, resulting in certain RNA protection, cellular delivery, and intracellular release.
Given that the miR-302 family, as intronic and ES-specific miRNAs that were well-studied to date, was demonstrated to play an important role in diverse biological processes, including the pluripotency of human embryonic stem cells (hESCs), self-renewal and reprogramming, we selected and focused on it to illustrate the delivery of miRNAs. We are aware that there are other types of ES-specific miRNAs [50, 51, 52]. The reasons we selected the miR-302 family was due to the fact this was the most well-studied one on iPSCs and anti-tumor activities. In addition, the authors and their co-workers have more experience on miR-302 than other types of ES-specific miRNAs. Liposomes have been frequently used in vitro for miRNAs′ delivery, including miR-302 [53]. PEI mixed with miR-302 was used for intratumoral injections based on the jetPEI Delivery Reagent (Polyplus-transfection, Inc.) [54]. Both delivery methods resulted in efficient silencing of target genes. Neutral Lipid Emulsion, NLE, has been successfully delivered miR-302-367 via tail vein injections [55]. High-fat diet-induced neointima formation and atherosclerotic lesions can be attenuated by intravenous delivery of miR-let-7g mediated through the downregulation of LOX-1 [56]. These findings showed that miRNA mimics delivery in vivo is effective. It is possible that miRNA precursors can also be delivered in vivo.
Glycylglycerins are a type of novel sugar alcohols that are tightly bound with negatively charged miR-302 precursor through electro-affinity, forming sugar-like coats as microcapsules [57]. Mixed with miR-302, glycylglycerins protect the miRNA from degradation, even at room temperature [57]. Protecting the siR-302 from degradation by the glycylglycerins which coated the siRNA also silenced the target genes of miR-302. An emulsion vehicle containing only injectable pharmaceutical ingredients such as excipients that have been used in FDA-approved intravenous injectable drug products or intravenous clinical trial materials, with additional special ingredients has been developed. Consisting of oil droplets less than 200 nm in diameter, the emulsion can be sterilized through a 0.2 μm filter. The mixture of glycylglycerin emulsions with miR-302 or miR-302 precursor was used for glycylglycerin and miR-302/miR-302 precursor delivery. Given that miRNA precursors possibly have longer half-lives than a mature miRNA, miRNA precursors were as effective as mature miRNAs [58], the development of direct delivery of miRNA precursor as drugs for treating and/or managing diseases is feasible. Direct delivery of miRNA precursors in vivo can also expedite the assimilation of miRNAs and/or miRNA precursors as drugs.
It is intriguing to notice that miRNAs can also be taken orally. It has been long known that the development of bee queens and workers is due to diet; the former eat royal jelly, a substance secreted by the glands of nurse bees, the latter a combination of pollen and honey called bee bread. The bee bread contains miR-162a that suppresses amTOM, which is a stimulatory gene in caste differentiation. In this way, miR-162a fine-tunes the honeybee caste development [59]. Conceivably, miRNAs and/or miRNA precursors, with proper formulation such as sugar alcohols, glycylglycerins, recently isolated from miR-302-mediated iPSCs that bind with miR-302 precursor via electro-affinity and protect the miRNA from degradation [60], could be developed as drugs for medication to be taken orally in the near future.
2.9. miRNAs and cancer
Development of products and technologies in the miRNA field has shed light on possible application of miRNAs in cancer. The development and metastasis of cancer is closely associated with cell proliferation, apoptosis, cell migration, cell invasion, and the epithelial-to mesenchymal transition [60]. miRNAs act as tumor suppressors or oncogenes depending on their target genes [61]. As a result, miRNAs regulate the expression of different genes that play important roles in cancer cell growth, apoptosis, cell invasion, migration, and metastasis [61, 62, 63, 64, 65, 66, 67, 68, 69]. Further, miRNA mimics and molecules targeting miRNAs (anti-miRs) are showing hope for the development of therapeutics. A few siRNA and miRNA have been approved for clinical trials against numerous diseases including hepatitis, T cell lymphoma, diabetes and fatty liver diseases, mesothelioma, scleroderma, and multiple solid tumors [70].
Given that a single miRNA frequently targets hundreds of mRNAs and miRNA regulatory pathways, the fact that embryonic stem cell-specific miRNAs, ESC-specific miRNAs, have anti-tumor activities in various cancers is intriguing. The ESC-specific miRNAs, miR-302-367 and miR-371-373, are abundant in embryonic cells but suddenly dropped to undetectable levels when differentiated [71]. Also, ESC-specific miR-302 induced somatic cells or cancer cells to change into tumor-free pluripotent embryonic stem cells [54, 72, 73]. miR-302 has also been found in different types of cancers, targeting different gene expressions [74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88]. Conceivably, miR-302 can be used to silence the expression of several key genes in cell cycle, global demethylation, apoptosis, and DNA repair along with BMI-1, CXCR4, AKT1/2, Runx-1, and EGFR genes simultaneously.
Long noncoding RNAs (lncRNAs) also hold multiple important roles for many different biological processes such as transcriptional regulation, cell growth, and tumorigenesis depending on the gain-of-function and loss-of-function studies. For example, overexpression of H19, HOTAIR, LINC00672, HULC, MALATI, PANDAR, BANCR, SPRY4-1T1, and CASC11 promotes tumor growth which allows lncRNAs to act as biomarkers for diagnosis [88, 89, 90, 91, 92, 93, 94]. Certain lncRNAs inhibit the activity of miRNAs [89, 92, 93].
2.10. miRNAs and iPSCs
The classic reprogramming of somatic cells into iPSCs was shown to be enhanced by miRNAs through ectopic expression of four transcription factors: Oct4, Sox2, Klf4, and cMyc [94]. Without any exogenous factors, the embryonic stem cell-specific or ESC-specific miRNAs [95, 96] can generate iPS cells more efficiently than the classical 4 transcription factors. In pluripotent stem cells, four miRNA clusters, two from mice, miR-290 and miR-302, and two from humans, miR-371 and miR-302, are highly expressed [71, 95, 96, 97]. During the early stages of development, ESC-specific miR-302 is expressed at a high level, which then declines [95, 96]. miR-302, in hESCs and hiPSCs, is the most predominant miRNA; ideal levels of miR-302 are vital for cellular reprogramming [98]. For efficient reprogramming of somatic cells and iPSC generation in the absence of canonical reprogramming factors, miR-302 family members are essential [96, 99]. Potentially, miR-302 can convert one type of cells to another in vivo.
Certain animals can regenerate large sections of their bodies such as amphibian limb regenerations. The changing of one type of tissue to another has been experimentally demonstrated, including metaplasia, the transformation of B lymphocytes to macrophages [100] or pancreatic exocrine cells to different types of other endocrine cells [101]. The embryonic-like pluripotent stem cells that miR-302 induced using skin cancer cells formed no teratomas when injected in an undifferentiated form into the muscle of SCID mice, implying that they were tumor-free [72]. iPSCs were also induced by miR-302/367 and other ESC-specific miRNAs [94, 102]. The combinational approach of using mature miRNAs like miR-200c, mi-302, and miR-369 also induced mouse and human iPSCs [53]. The mechanisms underlying miR-302-induced iPSCs are arbitrated by an epigenetic reprogramming similar to the natural zygotic reprogramming process in two- to eight-cell-stage embryos, targeting epigenetic regulator like AOF1/2. MECP1-p66, MECP2, and MBD2 [98]. Cycling D1/D2, CDK2, BMI-1 [103], PTEN [11], and other cell cycle regulators along with TGF-β regulators such as Lefty1/2 [104] and TGFBR2, and BMP inhibitors including DAZAP2, SLAIN1, and TOB2 are targeted genes [54, 72, 96, 105, 106, 107]. Given that the induction of tumor-free iPSCs by miR-302 requires a concentration of approximately twice of that found in iPSCs or hEPSc as a result of demethylation [98], it is possible that a lower concentration of miR-302 would reprogram somatic cells to adult stem cells or even convert cancer cells to normal cells. The ability to target different types of genes allows miR-302 to suppress tumor growth makes the ESC-specific miRNA ideal for regenerative medicine.
Among Oct4/Sox. Lefty, and miR-302, a reciprocal feedback has been detected [96, 108, 109, 110, 111]. miR-302 can be continuously produced by miR-302-mediated iPSCs given the miR-302/Oct 4 reciprocal feed-back mechanism [16,112], providing a model for making miR-302 precursors. From the discovery that lncRNAs and miR-302 precursors have longer half-lives than mature miRNAs [113], the miR-302 precursor with hairpin structure represent a better candidate for therapeutic applications. miRNAs are more adequate at assimilating into the RNA-induced silencing complex [58,114]. Cost-effective miR-302 precursors can be generated for targeted treatment of different diseases if a Pol-II-promoter-driven prokaryotic RNA transcription system can be developed. Another possibility of innovative paradigm in treating and managing tumors in patients can be presented by reprogramming cancer cells to form noncancerous/normal cells.
2.11. miRNAs and heart disease
Many miRNAs play an important role in different aspects of the progression of cardiovascular diseases such as cardiac hypertrophy, fibrosis, and myocardial infarction [115,116]. Even with full capacity to regenerate the human embryonic heart and the heart of lower vertebrates, adult human hearts have limited capacity to regenerate lost or damaged myocytes after a cardiac insult due to the low proliferative rate of cardiomyocytes [117]. MiRNA, including the miR-15 family, miR-590, and miR-199a [118], are effective in stimulating cardiomyocyte proliferation and promoting mammalian cardiac repair in vivo [117, 119, 120, 121, 122]. Expressed in abundance in the myocardium, cardiac miRNAs miR-1, miR133a, miR-208a/b, and miR-499 play pivotal roles in cardiomyogenesis, heart function, and pathology [123,124]. Other miRNAs regulate cardiovascular differentiation of cardiomyocyte progenitor cells and stem cells [125], differentiation of vascular smooth muscle cells, or SMCs [126, 127], and endothelial cells, or ECs [128]. Even though miR-21 expression increases in a failing myocardium (a characteristic associated with fibrosis) a recent study claimed that miRNA-21 is not essential for pathological cardiac remodeling [129].
The miR302-367 cluster is important for cardiomyocyte proliferation during development and is sufficient to induce cardiomyocyte proliferation and promote cardiac regeneration in the adult [52]. An increase in cardiomyocyte proliferation induced by miR302-367 was mediated partly through the repression of the Hippo signal transduction pathway. Reactivating the cell cycle in cardiomyocytes, post-natal reexpression of miR302-367 and transient systemic application of mature miR302-367 resulted in reduced scar formation after an experimental myocardial infarction. On the other hand, long-term expression of miR302-367 induced cardiomyocyte dedifferentiation and dysfunction, implying that the optimal concentration of the miRNA is essential for cardiomyocyte regeneration and repair, reduction of fibrosis, and restoration of cardiac function after experimental cardiac infraction. Consistent with the finding that optimal levels of miR-302 are critical to the miR-302 induced iPSCs [94], these observations could lead to a single miRNA-mediated cardiomyocyte regeneration and repair for managing and treating failing hearts in adults.
3. Conclusion
In this review, we have discussed various types of miRNAs, the biogenesis and mechanism of miRNAs. Although numerous miRNAs mimics and inhibitors are commercially available, it is impossible to provide an in-depth analysis of the various aspects of explosive miRNA research. Given that the primary advantage of miRNA is their direct and immediate altering the adult transcriptome and proteome, we have presented the circulating miRNAs and effective methods of delivering miRNAs in vivo, which not only provide tools for biomarkers and therapeutics for various diseases but also may lead to a basis for improved translational research and potentially a better clinical application for treating or managing some diseases.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ying S., Chang D.C., Lin S.L. The microRNA. Meth. Mol. Biol. 2018;177:1–25. doi: 10.1007/978-1-4939-7601-0_1. [DOI] [PubMed] [Google Scholar]
- 2.Fagard M., Boutet S., Morel J.B., Bellini C., Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11650–11654. doi: 10.1073/pnas.200217597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., Kim V.N. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 4.Lund E., Guttinger S., Calado A., Dahlberg J.E., Kutay U. Nuclear export of microRNA precursors. Science. 2003;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 5.Ying S.Y., Lin S.L. Intronic microRNAs (miRNAs) Biochem. Biophys. Res. Commun. 2005;326:515–520. doi: 10.1016/j.bbrc.2004.10.215. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y.S., Nakahara K., Pham J.W., Kim K., He Z., Sontheimer E.J., Carthew R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. PMID:15066283. [DOI] [PubMed] [Google Scholar]
- 7.Tang G. siRNA and miRNA: an insight into RISCs. Trends Biochem. Sci. 2005;30:106–114. doi: 10.1016/j.tibs.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Aravin A.A., Sachidanadam R., Girard A., Fejes-Toth K., Hannon G.J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–747. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 9.Shpiz S., Kwon D., Rozovsky Y., Kalmykova A. rasiRNA pathway controls antisense expression of Drosophila telomeric transposons in the nucleus. Nucleic Acids Res. 2009;37:267–278. doi: 10.1093/nar/gkn960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasciolli V., Mallory A.C., Bartel D.P., Vaucheret H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr. Biol. 2005;15:1–7. doi: 10.1016/j.cub.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Li L.C., Okino S.T., Zhao H., Pookot D., Place R.F., Urakami S., Enokida H., Dahiya R. Small dsRNAs induce transcriptional activation in human cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17337–17342. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Zhu J.K. Emerging roles of RNA processing factors in regulating long non-coding RNAs. RNA Biol. 2014;11:793–797. doi: 10.4161/rna.29731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altuvia Y., Landgraf P., Lithwick G., Elefant N., Pfeffer S., Aravin A., Brownstein M.J., Tuschl T., Margalit H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–2706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y., Kim M., Han J., Yeom K.H., Lee S., Baek S.H., Kim V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin S.L., Chang D., DY W., Ying S.Y. A novel RNA splicing-mediated gene silencing mechanism potential for genome evolution. Biochem. Biophys. Res. Commun. 2003;310:754–760. doi: 10.1016/j.bbrc.2003.09.070. PMID:14550267. [DOI] [PubMed] [Google Scholar]
- 16.Yi R., Qin Y., Macara I.G., Cullen B.R. Exportin-5 mediates the nuclear export of pre- microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijer H.A., Smith E.M., Bushell M. Regulation of miRNA strand selection: follow the leader? Biochem. Soc. Trans. 2014;42:1135–1140. doi: 10.1042/BST20140142. [DOI] [PubMed] [Google Scholar]
- 18.Chen L.L., Carmichael G.G. Decoding the function of nuclear long non-coding RNAs. Curr. Opin. Cell Biol. 2010;22:357–364. doi: 10.1016/j.ceb.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khvorova A., Reynolds A., Jayasena S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. PMID:14567918. [DOI] [PubMed] [Google Scholar]
- 20.Hammond S.M. An overview of microRNAs. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 21.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 22.Watson J.D. Cold Spring Harbor Laboratory Press; San Francisco, CA: 2008. Molecular Biology of the Gene; pp. 641–648. [Google Scholar]
- 23.Meister G. Argonaute proteins: functional insights and emerging roles. Nat. Rev. Genet. 2013;14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- 24.Brennecke J., Stark A., Russell R.B., Cohen S.M. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doench J.G., Sharp P.A. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai E.C. microRNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 27.Jee D., Lai E.C. Alteration of miRNA activity via context-specific modifications of Argonaute proteins. Trends Cell Biol. 2014;24:546–553. doi: 10.1016/j.tcb.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakanishi K. Anatomy of RISC: how do small RNAs and chaperones activate Argonaute proteins? Wiley Interdiscip. Rev. RNA. 2016;7:637–660. doi: 10.1002/wrna.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eulalio A., Tritschler F., Büttner R., Weichenrieder O., Izaurralde E., Truffault V. The RRM domain in GW182 proteins contributes to miRNA-mediated gene silencing. Nucl. Acids Res. 2009;37:2974–2983. doi: 10.1093/nar/gkp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson R.C., Tambe A., Kidwell M.A., Noland C.L., Schneider C.P., Doudna J.A. Dicer- TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol. Cell. 2015;57:397–407. doi: 10.1016/j.molcel.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grosshans H. Springer Science & Business Media; New York, NY: 2010. Regulation of MicroRNAs. [Google Scholar]
- 32.Cho C.J., Myung S.J., Chang S. ADAR1 and microRNA; a hidden crosstalk in cancer. Int. J. Mol. Sci. 2017;18(4) doi: 10.3390/ijms18040799. pii: E799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skeparnias I., Αnastasakis D., Shaukat A.N., Grafanaki K., Stathopoulos C. Expanding the repertoire of deadenylases. RNA Biol. 2015;7:1–6. doi: 10.1080/15476286.2017.1300222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X., Devany E., Murphy M.R., Glazman G., Persaud M., Kleiman F.E. PARN deadenylase is involved in miRNA-dependent degradation of TP53 mRNA in mammalian cells. Nucl. Acids Res. 2015;43:10925–10938. doi: 10.1093/nar/gkv959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svobodova E., Kubikova J., Svoboda P. Production of small RNAs by mammalian Dicer. Pflugers Arch. 2016;468:1089–1102. doi: 10.1007/s00424-016-1817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzgerald M.E., Vela A., Pyle A.M. Dicer-related helicase 3 forms an obligate dimer for recognizing 22G-RNA. Nucl. Acids Res. 2014;42:3919–3930. doi: 10.1093/nar/gkt1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi T., Arthanari H., Akabayov B., Song H., Papadopoulos E., Qi H.H., Jedrychowski M., Güttler T., Guo C., Luna R.E., Gygi S.P., Huang S.A., Wagner G. eIF1A augments Ago2- mediated Dicer-independent miRNA biogenesis and RNA interference. Nat. Commun. 2015;6:7194. doi: 10.1038/ncomms8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillai R.S., Artus C.G., Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., Zanesi N., Crawford M., Ozer G.H., Wernicke D., Alder H., Caligiuri M.A., Nana-Sinkam P., Perrotti D., Croce C.M. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He S., Chu J., Wu L.C., Mao H., Peng Y., Alvarez- Breckenridge C.A., Hughes T., Wei M., Zhang J., Yuan S., Sandhu S., Vasu S., Benson D.M., Jr., Hofmeister C.C., He X., Ghoshal K., Devine S.M., Caligiuri M.A., Yu J. MicroRNAs activate natural killer cells through toll-like receptor signaling. Blood. 2013;121:4663–4671. doi: 10.1182/blood-2012-07-441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hessvik N.P., Sandvig K., Llorente A. Exosomal miRNAs as biomarkers for prostate cancer. Front. Genet. 2013;1819:1154–1163. doi: 10.3389/fgene.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phuyal S., Hessvik N.P., Skotland T., Sandvig K., Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J. 2014;281:2214–2227. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 44.Marfella R., Di Filippo C., Potenza N., Sardu C., Rizzo M.R., Siniscalchi M., Musacchio E., Barbieri M., Mauro C., Mosca N., Solimene F., Mottola M.T., Russo A., Rossi F., Paolisso G., D’Amico M. Circulating microRNA changes in heart failure patients treated with cardiac resynchro- nization therapy: responders vs. non-responders. Eur. J. Heart Fail. 2013;15:1277–1288. doi: 10.1093/eurjhf/hft088. [DOI] [PubMed] [Google Scholar]
- 45.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harding C.V., Heuser J.E., Stahl P.D. Exosomes: looking back three decades and into the future. J. Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortez M.A., Bueso-Ramos C., Ferdin J., Lopez- Berestein G., Sood A.K., Calin G.A. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Creemers E.E., Tijsen A.J., Pinto Y.M. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 49.Etheridge A., Lee I., Hood L., Galas D., Wang K. Extracellular microRNA: a new source of biomarkers. Mutat. Res. 2017;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuo C.H., Deng J.H., Deng Q., Ying S.Y. A novel role of miR-302/367 in reprogramming. Biochem. Biophys. Res. Commun. 2012;417:11–16. doi: 10.1016/j.bbrc.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 51.Balzano F., Cruciani S., Basoli V., Santaniello S., Facchin F., Ventura C., Maioli M. MiR200 and miR302: two big families influencing stem cell behavior. Molecules. 2018;23(2) doi: 10.3390/molecules23020282. pii: E282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaur S., Abu-Shahba A.G., Paananen R.O., Hongisto H., Hiidenmaa H., Skottman H., Seppänen-Kaijansinkko R., Mannerström B. Small non-coding RNA landscape of extracellular vesicles from human stem cells. Sci. Rep. 2018;8(1):15503. doi: 10.1038/s41598-018-33899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyoshi N., Ishii H., Nagano H., Haraguchi N., Dewi D.L., Kano Y., Nishikawa S., Tanemura M., Mimori K., Tanaka F., Saito T., Nishimura J., Takemasa I., Mizushima T., Ikeda M., Yamamoto H., Sekimoto M., Doki Y., Mori M. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Lin S.L., Chang D.C., Ying S.Y., Leu D., Wu D.T. MicroRNA miR-302 inhibits the tumorigenecity of human pluripotent stem cells by coordinate suppression of the CDK2 and CDK4/6 cell cycle pathways. Cancer Res. 2010;70:9473–9482. doi: 10.1158/0008-5472.CAN-10-2746. [DOI] [PubMed] [Google Scholar]
- 55.Tian Y., Liu Y., Wang T., Zhou N., Kong J., Chen L., Snitow M., Morley M., Li D., Petrenko N., Zhou S., Lu M., Gao E., Koch W.J., Stewart K.M., Morrisey E.E. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 2015;7(279) doi: 10.1126/scitranslmed.3010841. 279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu M., Tao G., Liu Q., Liu K., Yang X. MicroRNA let-7g alleviates atherosclero- sis via the targeting of LOX-1 in vitro and in vivo. Int. J. Mol. Med. 2017;40:57. doi: 10.3892/ijmm.2017.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang-Lin S., Hung A., Chang D.C., Lin Y.W., Ying S.Y., Lin S.L. Novel glycylated sugar alcohols protect ESC-specific microRNAs from degradation in iPS cells. Nucl. Acids Res. 2016;44:4894–4906. doi: 10.1093/nar/gkw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang X., Jia Z. Construction of HCC-targeting artificial miRNAs using natural miRNA precursors. Exp. Ther. Med. 2013;6:209–215. doi: 10.3892/etm.2013.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu K., Liu M., Fu Z., Zhou Z., Kong Y., Liang H., Lin Z., Luo Jun, Zheng H., Wan P., Zhang J., Zen K., Chen J., Hu F., Zhang C.Y., Ren J., Chen X. Plant microRNAs in larval food regulate honeybee caste development. PLoS Genet. 2017;13(8) doi: 10.1371/journal.pgen.1006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. PMID:14744438. [DOI] [PubMed] [Google Scholar]
- 61.Zhang B., Pan X., Cobb G.P., Anderson T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 62.Baranwal S., Alahari S.K. miRNA control of tumor cell invasion and metastasis. Int. J. Canc. 2010;126 doi: 10.1002/ijc.25014. 1283–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palma Flores C., García-Vázquez R., Gallardo Rincón D., Ruiz-García E., Astudillo de la Vega H., Marchat L.A., Salinas Vera Y.M., López-Camarillo C. MicroRNAs driv- ing invasion and metastasis in ovarian cancer: opportunities for translational medicine. Int. J. Oncol. 2017;50:1461–1476. doi: 10.3892/ijo.2017.3948. [DOI] [PubMed] [Google Scholar]
- 64.Yan J., Ma C., Gao Y. MicroRNA-30a-5p suppresses epithelial-mesenchymal transition by targeting profilin-2 in high invasive non-small cell lung cancer cell lines. Oncol. Rep. 2017;37:3146–3154. doi: 10.3892/or.2017.5566. [DOI] [PubMed] [Google Scholar]
- 65.Cheng A.M., Byrom M.W., Jeffrey Shelton J., Lance P., Ford L.P. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucl. Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jansson M.D., Lund A.H. MicroRNA and cancer. Mol. Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farazi T.A., Hoell J.I., Morozov P., Tuschl T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acunzo M., Romano G., Wernicke D., Croce C.M. MicroRNA and cancer–a brief overview. Adv. Biol. Regul. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Iorio M.V., Croce C.M. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2017;4:143. doi: 10.1002/emmm.201100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 71.Suh M.R., Lee Y., Kim J.Y., Kim S.K., Moon S.H., Lee J.Y., Cha K.Y., Chung H.M., Yoon H.S., Moon S.Y., Kim V.N., Kim K.S. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Lin S.L., Chang D.C., Chang-Lin S., Lin C.H., DT W., Chen D.T., Ying S.Y. Mir-302 repro- grams human skin cancer cells into a pluripotent ES-cell-like state. RNA. 2008;14:2115–2124. doi: 10.1261/rna.1162708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li H.L., Wei J.F., Fan L.Y., Wang S.H., Zhu L., Li T.P., Lin G., Sun Y., Sun Z.J., Ding J., Liang X.L., Li J., Han Q., Zhao R.C. miR-302 regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4-dependent manner. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2015.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Zhao L., Xiao Q., Jiang L., He M., Bai X., Ma M., Jiao X., Wei M. miR-302a/b/c/d cooperatively inhibit BCRP expression to increase drug sensitivity in breast cancer cells. Gynecol. Oncol. 2016;141:592–601. doi: 10.1016/j.ygyno.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 75.Zhao L., Wang Y., Jiang L., He M., Bai X., Yu L., Wei M. MiR-302a/b/c/d cooperatively sensitizes breast cancer cells to adriamycin via suppressing P-glycoprotein (P-gp) by targeting MAP/ERK kinase kinase 1 (MEKK1) J. Exp. Clin. Canc. Res. 2016;35:25. doi: 10.1186/s13046-016-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ge T., Yin M., Yang M., Liu T., Lou G. MicroRNA-302b suppresses human epithelial ovarian cancer cell growth by targeting RUNX1. Cell. Physiol. Biochem. 2014;34:2209–2220. doi: 10.1159/000369664. [DOI] [PubMed] [Google Scholar]
- 77.Yan G.J., Yu F., Wang B., Zhou H.J., Ge Q.Y., Su J., YL H., Sun H.X., Ding L.J. MicroRNA miR-302 inhibits the tumorigenicity of endometrial cancer cells by suppression of Cyclin D1 and CDK1. Cancer Lett. 2014;345:39–47. doi: 10.1016/j.canlet.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 78.Cai N., Wang Y.D., Zheng P.S. The microRNA-302-367 cluster suppresses the proliferation of cervical carcinoma cells through the novel target AKT1. RNA. 2013;19:85–95. doi: 10.1261/rna.035295.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maadi H., Moshtaghian A., Taha M.F., Mowla S.J., Kazeroonian A., Haass N.K., Javeri A. Multimodal tumor suppression by miR-302 cluster in melanoma and colon cancer. Int. J. Biochem. Cell Biol. 2016;81(Pt A):121–132. doi: 10.1016/j.biocel.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Wang L., Yao J., Shi X., Hu L., Li Z., Song T., Huang C. MicroRNA-302b suppresses cell proliferation by targeting EGFR in human hepatocellular carcinoma SMMC-7721 cells. BMC Canc. 2013;13:448. doi: 10.1186/1471-2407-13-448. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Koga C., Kobayashi S., Nagano H., Tomimaru Y., Hama N., Wada H., Kawamoto K., Eguchi H., Konno M., Ishii H., Umeshita K., Doki Y., Mori M. Reprogramming using microRNA-302 improves drug sensitivity in hepatocellular carcinoma cells. Ann. Surg. Oncol. Suppl. 2014;4:S591–S600. doi: 10.1245/s10434-014-3705-7. [DOI] [PubMed] [Google Scholar]
- 82.Wang L., Yao J., Zhang X., Guo B., Le X., Cubberly M., Li Z., Nan K., Song T., Huang C. miRNA-302b suppresses human hepatocellular carcinoma by targeting AKT2. Mol. Canc. Res. 2014;12:190–202. doi: 10.1158/1541-7786.MCR-13-0411. [DOI] [PubMed] [Google Scholar]
- 83.Cai D., He K., Chang S., Tong D., Huang C. MicroRNA-302b enhances the sensitivity of hepatocellular carcinoma cell lines to 5-FU via targeting Mcl-1 and DPYD. Int. J. Mol. Sci. 2015;16:23668–23682. doi: 10.3390/ijms161023668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bourguignon L.Y., Wong G., Earle C., Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J. Biol. Chem. 2012;287 doi: 10.1074/jbc.M111.308528. 32800–12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen L., Min L., Wang X., Zhao J., Chen H., Qin J., Chen W., Shen Z., Tang Z., Gan Q., Ruan Y., Sun Y., Qin X., Gu J. Loss of RACK1 promotes metastasis of gastric cancer by inducing a miR-302c/IL8 signaling loop. Cancer Res. 2015;75:3832–3841. doi: 10.1158/0008-5472.CAN-14-3690. [DOI] [PubMed] [Google Scholar]
- 86.Khodayari N., Mohammed K.A., Lee H., Kaye F., Nasreen N. MicroRNA-302b targets Mcl-1 and inhibits cell proliferation and induces apoptosis in malignant pleural mesothelioma cells. Am. J. Cancer Res. 2016;6 1996–2009. PMID:27725905. [PMC free article] [PubMed] [Google Scholar]
- 87.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anti Cancer Agents Med. Chem. 2017;17:152–163. doi: 10.2174/1871520616666160502122724. PMID:27137076. [DOI] [PubMed] [Google Scholar]
- 88.Li A.X., Xin W.Q., Ma C.G. Fentanyl inhibits the invasion and migration of colorectal cancer cells via inhibiting the negative regulation of Ets-1 on BANCR. Biochem. Biophys. Res. Commun. 2015;465:594–600. doi: 10.1016/j.bbrc.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 89.Zhou X., Ji G., Ke X., Gu H., Jin W., Zhang G. MiR-141 inhibits gastric cancer pro-liferation by interacting with long noncoding RNA MEG3 and down-regulating E2F3 expression. Dig. Dis. Sci. 2015;60:3271–3282. doi: 10.1007/s10620-015-3782-x. [DOI] [PubMed] [Google Scholar]
- 90.Guo Q., Cheng Y., Liang T., He Y., Ren C., Sun L., Zhang G. Comprehensive analysis of lncRNA-mRNA co-expression patterns identifies immune-associated lncRNA biomarkers in ovarian cancer malignant progression. Sci. Rep. 2015;5:17683. doi: 10.1038/srep17683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang L., Li C., Lan T., Wu L., Yuan Y., Liu Q., Liu Z. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol. Med. Rep. 2016;13:1541–1550. doi: 10.3892/mmr.2015.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xue Y., Ni T., Jiang Y., Li Y. LncRNA GAS5 inhibits tumorigenesis and enhances radiosensitivity by suppressing miR-135b expression in non-small cell lung cancer. Oncol. Res. 2017;25:1305. doi: 10.3727/096504017X14850182723737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mei Y., Si J., Wang Y., Huang Z., Zhu H., Feng S., Wu X., Wu L. Long noncoding RNA GAS5 suppresses tumorigenesis by inhibiting miR-23a 5 expression in non-small cell lung cancer. Oncol. Res. 2017;25:1027. doi: 10.3727/096504016X14822800040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Judson R.L., Babiarz J.E., Venere M., Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat. Biotechnol. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li N., Long B., Han W., Yuan S., Wang K. microRNAs: important regulators of stem cells. Stem Cell Res. Ther. 2017;8:110. doi: 10.1186/s13287-017-0551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosa A., Brivanlou A.H. Regulatory non-coding RNAs in pluripotent stem cells. Int. J. Mol. Sci. 2013;14:14346–14373. doi: 10.3390/ijms140714346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stadler B., Ivanovska I., Mehta K., Song S., Nelson A., Tan Y., Mathieu J., Darby C., Blau C.A., Ware C., Peters G., Miller D.G., Shen L., Cleary M.A., Ruohola-Baker H. Characterization of microRNAs involved in embryonic stem cell states. Stem Cell. Dev. 2010;19:935–950. doi: 10.1089/scd.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin S.L., Chang D.C., Lin C.H., Ying S.Y., Leu D., Wu D.T. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39:1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuo C.H., Ying S.Y. Advances in microRNA-mediated reprogramming technology. Stem Cell. Int. 2012;2012:823709. doi: 10.1155/2012/823709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Slack J.M. Metaplasia and somatic cell reprogramming. J. Pathol. 2009;217:161–168. doi: 10.1002/path.2442. [DOI] [PubMed] [Google Scholar]
- 101.Li W., Nakanishi M., Zumsteg A., Shear M., Wright C., Melton D.A., Zhou Q. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife. 2014;3 doi: 10.7554/eLife.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anokye-Danso F., Trivedi C.M., Juhr D., Gupta M., Cui Z., Tian Y., Zhang Y., Yang W., Gruber P.J., Epstein J.A., Morrisey E.E. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang Z., Ahn J., Guo D., Votaw J.R., Shim H. MicroRNA-302 replacement therapy sensitizes breast cancer cells to ionizing radiation. Pharm. Res. 2013;30:1008–1016. doi: 10.1007/s11095-012-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barroso-del Jesus A., Lucena-Aguilar G., Sanchez L., Ligero G., Gutierrez-Aranda I., Menendez P. The Nodal inhibitor Lefty is negatively modulated by the microRNA miR- 302 in human embryonic stem cells. FASEB J. 2011;25:1497–1508. doi: 10.1096/fj.10-172221. [DOI] [PubMed] [Google Scholar]
- 105.Liao B., Bao X., Liu L., Feng S., Zovoilis A., Liu W., Xue Y., Cai J., Guo X., Qin B., Zhang R., Wu J., Lai L., Teng M., Niu L., Zhang B., Esteban M.A., Pei D. MicroRNA cluster 302– 367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J. Biol. Chem. 2011;286:17359–17364. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Subramanyam D., Lamouille S., Judson R.L., Liu J.Y., Bucay N., Derynck R., Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lipchina I., Elkabetz Y., Hafner M., Sheridan R., Mihailovic A., Tuchil T., Sander C., Studer L., Betel D. Genome-wide identification of microRNA targets human ES cells reveals a role for miR-302 in modulating BMP response. Genes Dev. 2011;25:2173–2186. doi: 10.1101/gad.17221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu H., Deng S., Zhao Z., Zhang H., Xiao J., Song W., Gao F., Guan Y. Oct4 regulates the miR-302 cluster in P19 mouse embryonic carcinoma cells. Mol. Biol. Rep. 2011;38:2155–2160. doi: 10.1007/s11033-010-0343-4. [DOI] [PubMed] [Google Scholar]
- 109.Card D.A., Hebbar P.B., Li L., Trotter K.W., Komatsu Y., Mishina Y., Archer T.K. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol. Cell Biol. 2008;28:6426–6438. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu S., Wilson K.D., Ghosh Z., Han L., Wang Y., Lan F., Ransohoff K.J., Burridge P., Wu J.C. MicroRNA-302 increases reprogramming efficiency via repression of NR2F2. Stem Cells. 2013;31:259–268. doi: 10.1002/stem.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin S.L. Concise review: deciphering the mechanism behind induced pluripotent stem cell generation. Stem Cells. 2011;29:1645–1649. doi: 10.1002/stem.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siomi M.C., Miyoshi T., Siomi H. piRNA-mediated silencing in Drosophila germlines. Semin. Cell Dev. Biol. 2010;21 doi: 10.1016/j.semcdb.2010.01.011. 754–749. [DOI] [PubMed] [Google Scholar]
- 113.Yang J.S., Lai E.C. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Terasawa K., Shimizu K., Tsujimoto G. Synthetic pre-miRNA-based shRNA as potent RNAi triggers. J. Nucl. Acids. 2011;2011:131579. doi: 10.4061/2011/131579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gurha P. MicroRNAs in cardiovascular disease. Curr. Opin. Cardiol. 2016;31:249–254. doi: 10.1097/HCO.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 116.van Rooij E., Olson E.N. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat. Rev. Drug Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Amerongen M.J., Engel F.B. Features of cardiomyocyte proliferation and its potential for cardiac regeneration. J. Cell Mol. Med. 2008;12:2233–2244. doi: 10.1111/j.1582-4934.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eulalio A., Mano M., Dal Ferro M., Zentilin L., Sinagra G., Zacchigna S., Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 119.Wu G., Huang Z.P., Wang D.Z. MicroRNAs in cardiac regeneration and cardio- vascular disease. Sci. China Life Sci. 2013;56:907–913. doi: 10.1007/s11427-013-4534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boon R.A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S., Kaluza D., Tréguer K., Carmona G., Bonauer A., Horrevoets A.J., Didier N., Girmatsion Z., Biliczki P., Ehrlich J.R., Katus H.A., Müller O.J., Potente M., Zeiher A.M., Hermeking H., Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 121.Porrello E.R., Johnson B.A., Aurora A.B., Simpson E., Nam Y.J., Matkovich S.J., Dorn G.W., II, van Rooij E., Olson E.N. MiR15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Porrello E.R., Mahmoud A.I., Simpson E., Johnson B.A., Grinsfelder D., Canseco D., Mammen P.P., Rothermel B.A., Olson E.N., Sadek H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. U. S. A. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ikeda S., He A., Kong S.W., Lu J., Bejar R., Bodyak N., Lee K.H., Ma Q., Kang P.M., Golub T.R., Pu W.T. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol. Cell Biol. 2009;29:2193–2204. doi: 10.1128/MCB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Montgomery R.L., Hullinger T.G., Semus H.M., Dickinson B.A., Seto A.G., Lynch J.M., Stack C., Latimer P.A., Olsen E.N., van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sluijter J.P., van Mil A., van Vliet P., Metz C.H., Liu J., Doevendans P.A., Goumans M.J. MicroRNA-1 and -499 regulate differen- tiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:859–868. doi: 10.1161/ATVBAHA.109.197434. [DOI] [PubMed] [Google Scholar]
- 126.Yoo J.K., Kim J., Choi S.J., Noh H.M., Kwon Y.D., Yoo H., Yi H.S., Chung H.M., Kim J.K. Discovery and characterization of novel microRNAs during endothelial differentiation of human embryonic stem cells. Stem Cell. Dev. 2012;21:2049–2057. doi: 10.1089/scd.2011.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao W., Zhao S.P., Zhao Y.H. MicroRNA-143/-145 in cardiovascular diseases. BioMed Res. Int. 2015;2015:531740. doi: 10.1155/2015/531740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen T., Margariti A., Kelaini S., Cochrane A., Guha S.T., Hu Y., Stitt A.W., Zhang L., Xu Q. MicroRNA-199b modulates vas-cular cell fate during iPS cell differentia-tion by targeting the Notch ligand Jagged1 and enhancing VEGF signaling. Stem Cells. 2015;33:1405–1418. doi: 10.1002/stem.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Montgomery R.L., Yu G., Latimer P.A., Stack C., Robinson K., Dalby C.M., Kaminski N., van Rooij E. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol. Med. 2014;6:1347–1356. doi: 10.15252/emmm.201303604. [DOI] [PMC free article] [PubMed] [Google Scholar]