Abstract
Ferrites samples Mg1-xLixFe2O4 for x = 0.0, 0.1, 0.2, 0.3, were synthesized by solid-state sintering method. Detailed investigations were made on the structural, morphological, magnetic and electrical proprieties of these samples. A detailed investigation was performed on power generation of these samples and role of Li-doping has been discussed. The X-ray Diffraction (XRD) pattern confirms the spinel phase formation in samples without any impurity. It is observed from Scanning Electron Microscopy that average grain size of samples decreases with lithium doping in MgFe2O4. The saturation magnetization of MgFe2O4 (15.4 emu/g) is found to increase with Lithium percentage and maximum 39.3 emu/g for Mg0.7Li0.3Fe2O4 sample. Ferrites play a crucial role in magnetic recording, microwave magnetic devices and many applications in medical sciences. Recently, it was observed that ferrites can be an alternate source of green energy by inventing hydroelectric cell (HEC). The processes of water adsorption and dissociation on the metal-oxide surface, plays an important role in production of electricity in ferrites. When, water is sprayed on hydroelectric cell the thermodynamic driving force is responsible for the formation of stable metal-oxygen or metal-hydroxyl bonds. The reactivity of ferrite surface towards water is based on the interaction of these ions and the d orbital of the Fe atom. Due to this interaction, water dissociated in H3O+ and OH− ions and migrates toward silver and zinc electrodes respectively. A typical hydroelectric cell of 2 inch diameter produces 17.1 mA of peak current and 949 mV voltage with a maximum output power of 15.85 mW for Li = 0.2 doped MgFe2O4 sample.
Keyword: Materials science
1. Introduction
Ferrites belong to a group of magnetic materials extensively used in many applications such as microwave devices, computer memory chip, magnetic recording media, radio frequency coil fabrication, transformer cores, rod antennas and many branches of telecommunication and electronic engineering [1, 2, 3, 4, 5]. It is due to its attractive features such as high Curie temperature, high saturation magnetization and square hysteresis loop. Most of the soft ferrites have spinel structure which consists of the 8 formula units [6, 7] of M2+Fe23+O4. Normally ‘M’ is a divalent atom of radius between 0.80 Å to 1.0 Å, such as Mn, Mg, Cd, Fe, Zn, Cu etc and Fe23+ is a trivalent atom. The structure of spinel ferrite is complex, which consists of a cubic closed-packed array of 32 oxide ions, which forms 64 tetrahedral and 32 octahedral site in one unit cell [containing eight formula units (M2+Fe23+O4)8]. These unit cells contain two kinds of sites. One is called octahedral or B-site, in which the oxygen ions around it occupy the corner of an octahedron. The other is called as tetrahedral or A site, in which M2+ is located at the centre of a tetrahedron whose corners are occupied by oxygen ions [8]. The cation distribution on A and B sites, in general is represented by: [M2+δ Fe3+1-δ ]A [M2+1-δ Fe3+1+δ]B O2-4, where δ = 1 for normal spinel structure and δ = 0 for inverse spinel structure. The distribution of cations among the tetrahedral and octahedral site play an important role in deciding the electric, dielectric, magnetic and structural properties of spinel ferrites [9, 10].
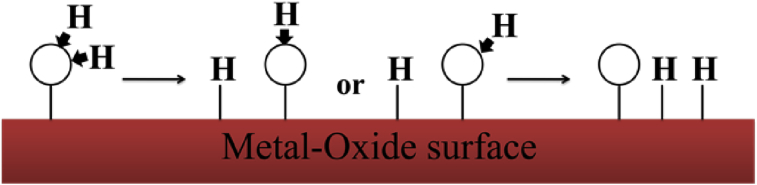
Metal-oxide surfaces offer great potential for a wide variety of applications like magnetic recording media, water-gas shift reaction, photocatalytic splitting etc. [11, 12, 13]. Generation of electric current and voltage by a hydroelectric cell using dissociation of water has unfolded new green electrical energy source [14]. The adsorption of water on oxide surface can be in different manner. First, well-ordered ionic single-crystal samples are often nonreactive for H2O dissociation. It may be due to that the dissociation of water on the surface is by reaction at the defect sites and facet edges in the oxide polycrystalline samples, and defect sites are very less on the single crystals [15]. The influence of defect lattice sites and oxygen deficit surfaces are important for dissociation of water. The second distinctive feature of H2O adsorption is the formation of relatively strong chemisorption bonds on some ionic surfaces, so that molecular adsorbed H2O can be stable even at room temperature [16]. Water adsorption at metal-oxide surfaces is governed by a subtle balance between water-water hydrogen bonding and water-metal oxide interactions, which together determine the stability of the water structures formed. Adsorbed water can dissociate in hydroxyl, atomic oxygen and atomic hydrogen as experimentally observed by many researchers [17, 18]. The possible dissociation pathways of water are illustrated schematically in Fig. 1.
Fig. 1.
Schematic representation of possible dissociation of adsorbed water.
In this work, we have reported the effect of Li doping on the structural, magnetic and electrical properties of magnesium ferrite. A detailed investigation has been made on electric current and voltage generated by a series of samples of Mg1-xLixFe2O4.
2. Experimental
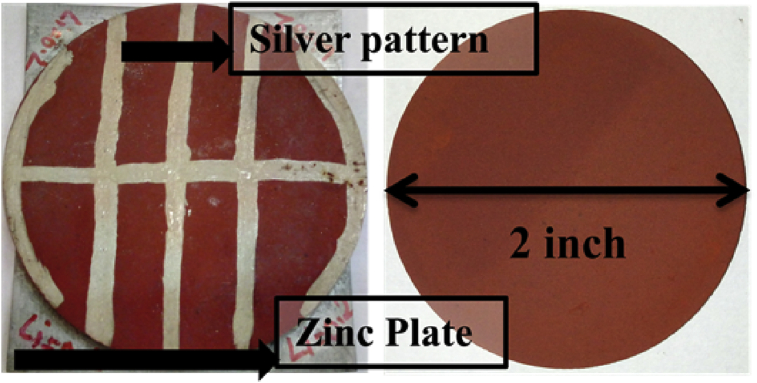
The samples were prepared by the conventional double sintering ceramic technique, in which oxides or carbonates were used as precursors. According to the stoichiometric composition of Mg1-xLixFe2O4 (x = 0.0, 0.1, 0.2, 0.3), specified molar ratio of the magnesium Oxide (MgO), iron oxide (Fe2O3) and lithium carbonate (Li2CO3) were mixed and ground by wet grinding in distilled water. Mixed powders were pre-sintered at 750 °C for 5 h in ambient atmosphere. After pre-sintering the obtained powders were again mixed and grinded followed by pellets formation. Two kinds of pellets were prepared for each sample, one was two inch of diameter for hydroelectric cell and second was 8 mm in diameter for electrical characterization. These obtained pellets of all samples were finally sintered at 1050 °C for 5 h. Heating and cooling rate was controlled at 5 °C/min. Finally, obtained pellets of 8 mm diameter were silver coated on the opposite faces for electrical characterization. And in pellet of two inch diameter a zinc plate was fix on the one face and other face was coated with silver in comb pattern for hydroelectric characterization as shown in Fig. 2. The structural characterization of samples was carried out by the X-ray diffraction (XRD Rigaku Miniflex II, step size = 0.02°) technique using Cu Kα radiation (wavelength λ = 1.5406 Å). A scanning electron microscope (JEOL/JSM-6610LV) was used to observe the microstructure details of the samples. Magnetic measurements were performed using vibrating sample magnetometer (VSM, Lake Shore7304) for all samples at room temperature. Current and voltage generated by each hydroelectric cell was measured by digital multi meter (DMM). A.C. resistivity of samples was measured by using Novocontrol technologies Alpha-A high performance Frequency Analyzer.
Fig. 2.
Image of ferrites pellets with silver comb pattern and zinc plate work as hydroelectric cell.
3. Results and discussion
3.1. Structural analysis
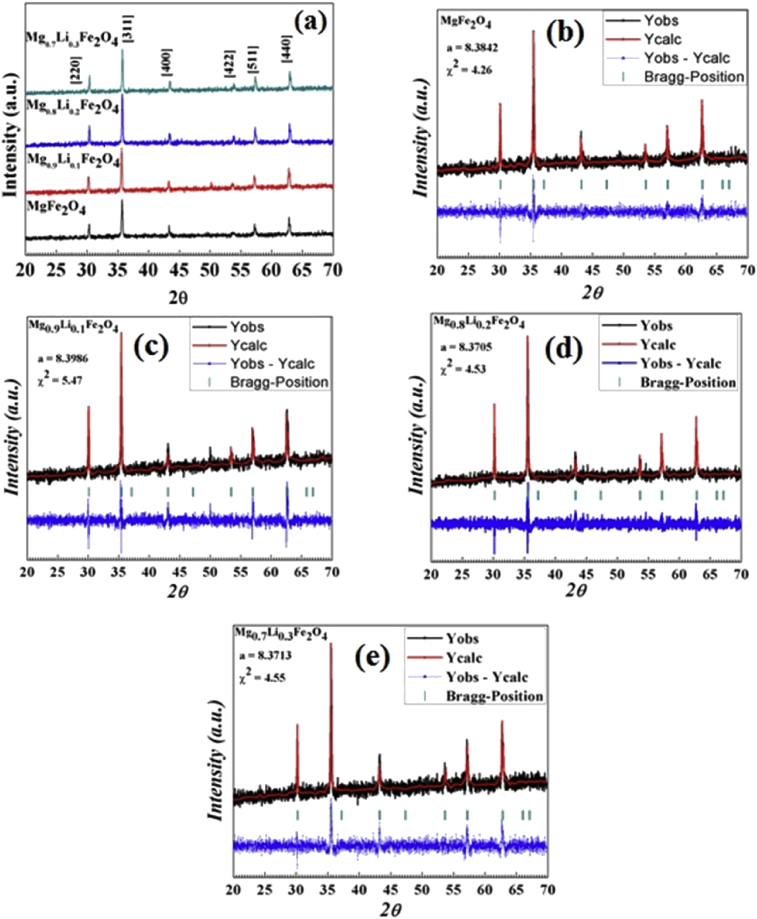
Fig. 3 shows the X-ray powder diffraction pattern of Mg1-xLixFe2O4 (0 ≤ x ≤ 0.3) samples. This can be observed from the XRD pattern of MgFe2O4, the obtained peak positions are same as reported in JCPDS file (73-1720), which exhibits the inverse-spinel phase formation with Fdm space group. FullProf programme have been used for Rietveld refinement technique to analyze the XRD patterns of samples. The structural model Fdm (space group) and initial structural parameters (Wyckoff positions) of metal 8a, iron 16d and oxygen atoms 32e have been taken for all samples. The method employs a least-squares procedure to compare Bragg's intensities and those calculated from a possible structural model. The fitting quality of the experimental data is assessed by computing the parameters such as ‘goodness of fit χ2 and R factors [19]. The typical Rietveld refinement X-ray pattern for all samples is given in Fig. 3. Fitting parameters, goodness of fit (χ2) and lattice constant and X-ray density calculated by the Rietveld analysis are listed in Table 1. The lattice parameter value increases from 8.3842 Å to 8.3713 Å which indicates the incorporation of Li+ ions in the Magnesium ferrite.
Fig. 3.
(a) X-ray powder diffraction pattern of Mg1-xLixFe2O4 samples and Rietveld Refinement patterns of (b) x = 0.0, (c) x = 0.1, (d) x = 0.2, (e) x = 0.3 samples.
Table 1.
Structural parameters of Mg1-xLixFe2O4 samples.
| Samples | Lattice parameter a = b = c (Ǻ) | χ2 | X-ray density (g/cc) |
|---|---|---|---|
| MgFe2O4 | 8.3842 | 4.26 | 4.51 |
| Mg0.9Li0.1Fe2O4 | 8.3986 | 5.47 | 4.45 |
| Mg0.8Li0.2Fe2O4 | 8.3705 | 4.53 | 4.44 |
| Mg0.7Li0.3Fe2O4 | 8.3713 | 4.55 | 4.41 |
3.2. Scanning Electron Microscopy (SEM)
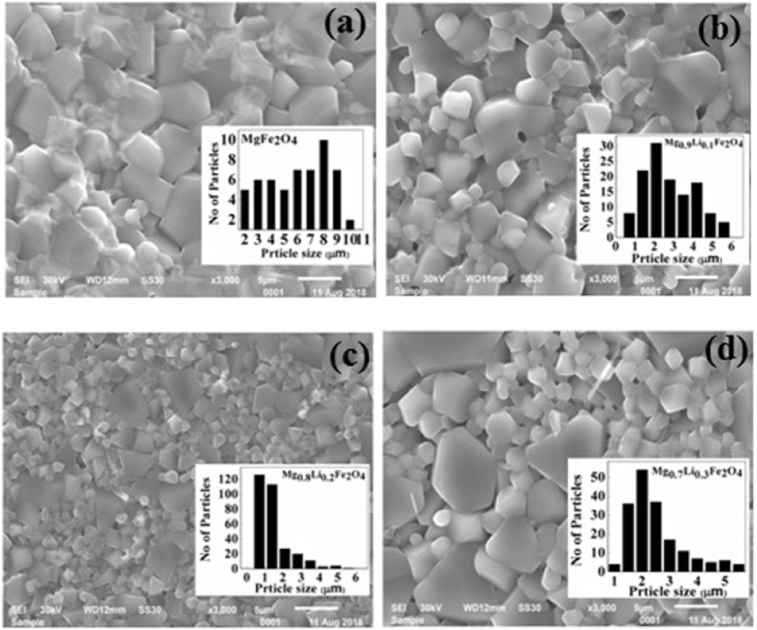
Magnetic and electrical properties of ferrite samples are strongly dependent on their microstructure. Typical SEM images of the sintered pellets for the Mg1-xLixFe2O4 (0 ≤ x ≤ 0.3) samples are shown in Fig. 4. It can be observed that the micrographs of all samples exhibit randomly oriented fine crystalline structure. The grains of Mg1-xLixFe2O4 samples are found to be closely packed. All samples are mostly homogeneous in nature but with different grain size distribution, which may be due to the difference in growth rate of individual phases in the samples.
Fig. 4.
SEM images of Mg1-xLixFe2O4 samples (a) x = 0.0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3 and their grain size distribution (in-set).
We can observe that the pure sample MgFe2O4 exhibits dual average sizes, 3–4 μm and 8μm of grain distribution. We can observe that densification increases as we increase the amount of lithium in MgFe2O4. The dual nature and grain size growth is decreasing with lithium doping. Mg0.8Li0.2Fe2O4 sample shows highly dense microstructure with average grain size 1–2 μm. Reduction in grain size due to doping may arise due to different diffusion rates of constituting elements of the compounds [20]. This modified microstructure may enhance the magnetic and electrical properties of ferrite samples.
3.3. Magnetic properties
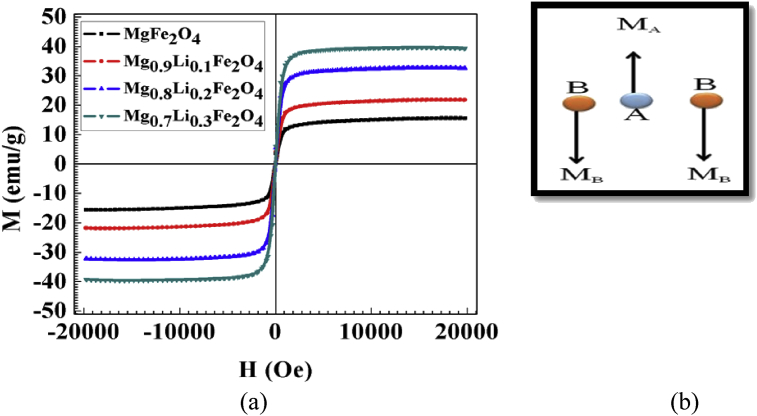
The M-H loops for all samples have been recorded from VSM up to the maximum field of ± 2T at room temperature, shown in Fig. 5. All samples are showing well saturated M-H loop. It is observed that the saturation magnetization increases slowly with increase in Li concentration in Mg1-xLixFe2O4 ferrites as shown in Table 2. It is well known that magnesium ferrite is inverse spinel with cations distribution as [A [B O4, where x represents the degree of inversions, which is defined as the fraction of the (A) sites occupied by Fe3+ cations [21]. The magnetic properties in the ferrites depend on the interaction between the magnetic moments on the tetrahedral (A-site) and octahedral (B-site) sites and their exchange interactions. In a spinel ferrite, each ion at A-site has 12 B-site ions as nearest neighbours while a B-site ion has six A-site and six B-site ions nearest neighbours. According to Neel's two sublattice model, the A-B super exchange interaction pre-dominant to the sublattice A-A and B-B interactions. A pictorial presentation of alignment of A and B-site magnetic moments according to Neel's model is given in Fig. 5(b). Hence the net magnetization of spinel ferrites is given by μ = μB - μA [22]. Lithium doping in the MgFe2O4 may reduce the A-B interaction which increases the magnetic moment in the compounds. An increase in saturation magnetization is observed with Li contain which may be due to replacement of Mg2+ ions with Li+ in A-site and simultaneous migration of Fe3+ ions from A-sites to B-sites as reported by S. Rahman et al. [23].
Fig. 5.
(a) M-H loops of Mg1-xLixFe2O4 samples at room temperature and (b) Neel's collinear model to explain the magnetic behaviour in ferrites.
Table 2.
Observed magnetic parameters from M-H loops of Mg1-xLixFe2O4 samples at room temperature.
| Sample | Hc (Oe) | Mr (emu/g) | Ms (emu/g) |
|---|---|---|---|
| MgFe2O4 | 96.4 | 1.9 | 15.4 |
| Mg0.9Li0.1Fe2O4 | 81.1 | 2.4 | 21.7 |
| Mg0.8Li0.2Fe2O4 | 61.9 | 2.9 | 32.6 |
| Mg0.7Li0.3Fe2O4 | 52.3 | 3.3 | 39.3 |
3.4. Hydroelectric properties
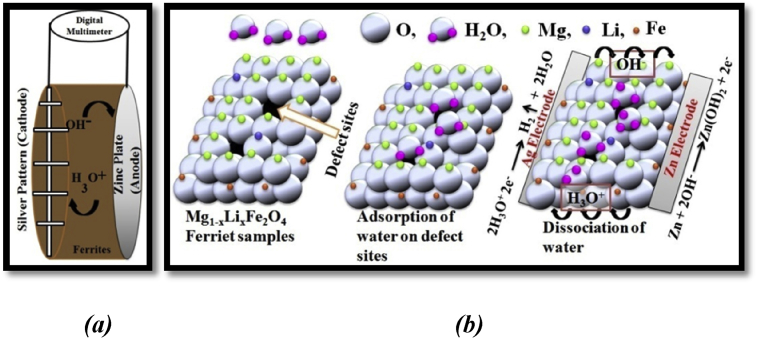
The working of a hydroelectric cell is based on adsorption of water molecule and its dissociation and conduction of OH−, H3O+ ions in the ferrites medium. The mechanism of water molecule adsorption and dissociation on ferrites surface is schematically represented in Fig. 6. As the water molecules come in contact with the ferrite surface, immediately water molecules dissociate into hydronium (H3O+) and hydroxide (OH−) ions [14]. Hyronium ions are reduced at Ag electrode to release hydrogen gas and hydroxide ions are oxidized at Zn electrode as shown by Eqs. i, ii, iii).
| 4H2O → 2H3O+ + 2OH− (dissociation of water molecules) | (i) |
| Zn + 2OH− → Zn(OH)2 + 2e− Eoxd = −0.76 V (at Zn electrode) | (ii) |
| 2e− + 2H3O+ → 2H2O + H2 Ered = 0.22 V (at silver electrode) | (iii) |
Fig. 6.
(a) Schematic diagram of working of a hydroelectric cell and (b) representation of water adsorption and its dissociation on the surface of the Mg1-xLixFe2O4 samples.
As we can observe that the overall voltage generated between the silver and Zn electrodes is Ecell = 0.22 + 0.76 = 0.98 V. This voltage produces an electric field between the electrodes which helps in further motion of ions (H3O+, OH−) in the ferrite medium.
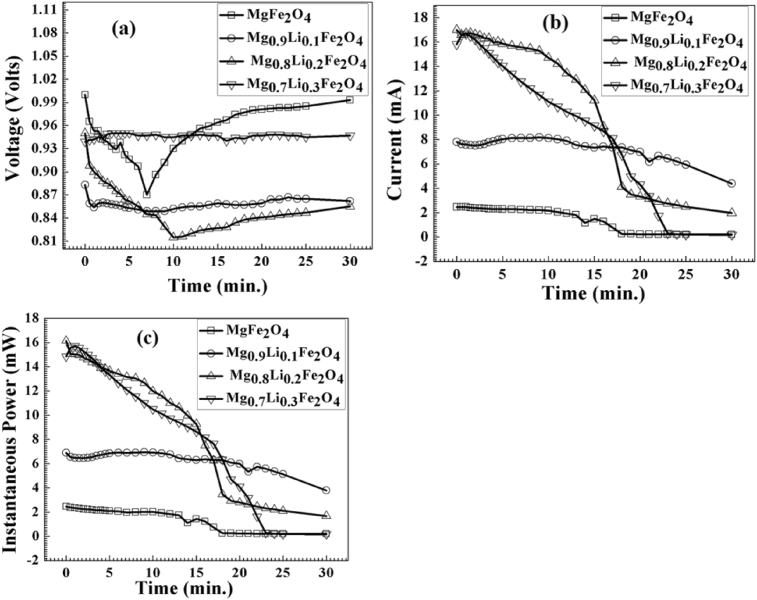
Dissociation of water molecule occurs on the surface of Mg1-xLixFe2O4 samples due to attraction between oxygen lone pair electron of H2O molecule and Fe3+ ions situated at octahedral site, which make strong chemisorption bond in between [24]. Oxygen defects also have been observed play an important role in dissociation of water molecule [25]. Hydroelectric cells of diameter 2 inch fabricated in lab as shown in Fig. 2. We recorded the open circuit voltage and short circuit current for each samples for 30 minutes after dipping in deionized water to analyze the hydroelectric properties of Mg1-xLixFe2O4 cells. We repeated such observations in sets of samples to observe the consistency of results. All the observations have been performed at room temperature and in ambient atmosphere. These hydroelectric cells produced the emf in the range of 859–965 mV and maximum current in the range of 17–2.47 mA as shown in Fig. 7. We observed some fluctuation in open voltage at initial stage (5–10 min.) which becomes almost constant which sustained for more than 24 hours. But the response of current is not up to the values reported by R. K. Kotnala et al. [14]. We observed that current decreases very fast with time. This decrease in current could be a result of saturation of Fe-sites in octahedral due to involvement in transportation of OH− and H3O− ions in the ferrite medium. The decrease in the current of hydroelectric cells is the main barrier for large energy production for longer time. Further investigation is required to rectify this problem. We observed that Li doping improved the voltage and current (power) response in the ferrite samples as shown in Fig. 7. Maximum current is exhibited by Mg0.8Li0.2Fe2O4 sample. Monovalent lithium ions substitute the divalent Magnesium ions which create oxygen vacancy due to charge unbalance in the compounds. This increase in oxygen vacancies with lithium doping may increase chemidissociation which modifies the current in result. But as we discussed, further investigation is required to modify the current in hydroelectric cells for large scale power production.
Fig. 7.
(a) Voltage, (b) current and (c) power generated by hydroelectric cells of the Mg1-xLixFe2O4 samples.
3.5. AC resistivity response
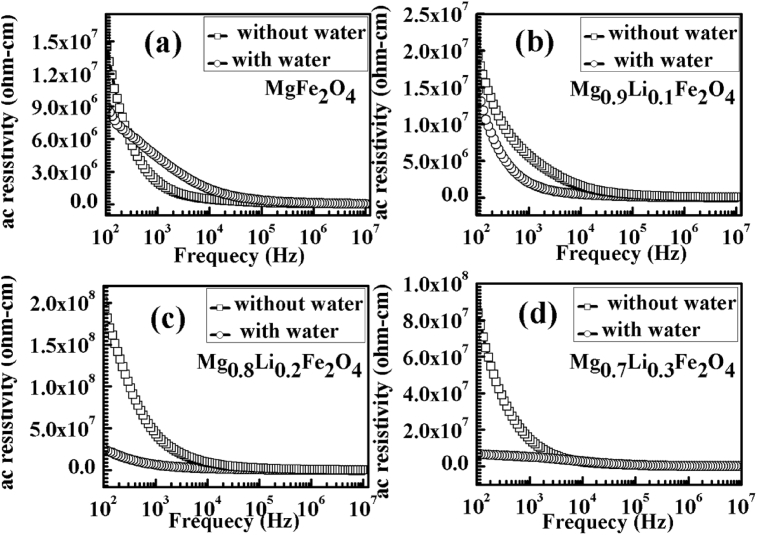
Fig. 8 shows the variation of a.c. resistivity of Mg1-xLixFe2O4 samples with log of frequency at room temperature. Here, we recorded the resistivity of the all dry samples (without water) from 100 Hz to 10 MHz. Then, all samples were dipped in deionized water for some time followed by ac resistivity measurement (with water). From the Fig. 8, we can observe that a.c. resistivity of the samples decrease with the frequency and become almost constant after 10 MHz. This change in the conduction mechanism in the ferrites can be explained on the basis of hopping of charge carriers between Fe3+ and Fe2+ ions on the octahedral sites [25]. At the higher applied frequency up to 10 MHz the hopping phenomenon increases which resulting in a decrease in the resistivity of the ferrite samples. Further increase in the frequency beyond the 10 MHz the resistivity becomes negligible small and remains constant because the hopping frequency no longer follows the applied field [26]. Resistivity of the Mg1-xLixFe2O4 samples decreases after dipping into deionized water (resistivity of deionized water in MΩ). The value of resistivity is minimum for Mg0.8Li0.2Fe2O4 and Mg0.7Li0.3Fe2O4 samples as predicted from Fig. 8. This implies that formation of OH− and H3O+ ions is more in these samples which may help in conduction. These results are also supported by current generation in hydroelectric cells. The maximum current was generated by the Mg0.8Li0.2Fe2O4 and Mg0.7Li0.3Fe2O4 samples. These results, confirm that ferrite Mg1-xLixFe2O4 materials are responsible to dissociate the water molecules in the ions.
Fig. 8.
Variation of resistivity of the Mg1-xLixFe2O4 samples, (a) x = 0.0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3, with and without water.
4. Conclusions
Polycrystalline Mg1-xLixFe2O4 (0.0 ≤ x ≤ 0.3) ferrite samples have been synthesized by solid state method. Phase purity has been confirmed by XRD analysis. The lattice parameters are found to decrease with Li substitution which was calculated by using Rietveld refinement technique. Surface morphology was observed by using SEM images and shows a decrease in grain size with Li substitution. An increase is also observed in saturation magnetization (Ms) with Li substitution in Mg1-xLixFe2O4 samples, which is explained on the basis of Neel's two sub-lattices Model. In hydroelectric cells, voltage, current and power generation has been investigated in detail. Maximum power was observed in Mg0.8Li0.2Fe2O4 sample. Resistivity response with frequency of samples without water and with water confirms that ferrite Mg1-xLixFe2O4 materials are responsible to dissociate the water molecules in ions. But further investigation is required to modify the current in hydroelectric cells for large scale power production. Hence, Ferrites: Magnetic materials may be an alternate source of green and safe electrical energy.
Declarations
Author contribution statement
Pranati Kharbanda, Tushar Madaan: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Isha Sharma, Shruti Vashishtha, Arti Chauhan, Sumit Mittal: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Jarnail S. Bangruwa: Analyzed and interpreted the data; Wrote the paper.
Parveen Kumar: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Vivek Verma: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to Principal, Hindu College, Head, Department of Physics & Astrophysics and USIC, University of Delhi, Delhi for constant encouragement and measurement facilities.
References
- 1.Abbas T., Khan Y., Ahmed M., Anwar S. X-ray diffraction study of the cation distribution in the Mn-Zn-ferrites. Solid State Commun. 1992;82:701. [Google Scholar]
- 2.Schaefer M., Dietzmann G., Wirth H. Magnetic losses in ferrites and nanocrystalline ribbons for power applications. J. Magn. Magn. Mater. 1991;101:95. [Google Scholar]
- 3.Ahmed M.A., Okasha N., El-Dek S.I. Preparation and characterization of nanometric Mn ferrite via different methods. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/6/065603. [DOI] [PubMed] [Google Scholar]
- 4.Rozman M., Drofenik M. Sintering of nano sized Mn Zn ferrite powders. J. Am. Ceram. Soc. 1998;81:1757. [Google Scholar]
- 5.Chen S.H., Chang S.C., Lin L.N. The influence of grain boundary internal stress on permeability: temperature curve for Mn–Zn ferrites. J. Magn. Magn. Mater. 2000;209:193. [Google Scholar]
- 6.Gaudon M., Pailhe N., Wattiaux A., Demourgues A. Structural defects in AFe2O4 (A = Zn, Mg) spinels. Mater. Res. Bull. 2009;44:479–484. [Google Scholar]
- 7.Pradeep A., Priyadharsini P., Chandrasekaran G. Sol–gel route of synthesis of nanoparticles of MgFe2O4 and XRD, FTIR and VSM study. J. Magn. Magn. Mater. 2008;320:2774–2779. [Google Scholar]
- 8.Ahmad I.S., Ahmed M.A., Hammad S., Moustafa A.M. Application of Rietveld method to the structural characteristics of substituted copper ferrite compounds. Cryst. Res. Technol. 2001;36:85–92. [Google Scholar]
- 9.Tahir Farid Hafiz Muhammad, Ahmad Ishtiaq, Ali Irshad, Mahmood Asif, Ramay Shahid M. Structural and dielectric properties of copper-based spinel ferrites. Eur. Phys. J. Plus. 2018;41:133–145. [Google Scholar]
- 10.Li Mingling, Liu Xiansong, Xu Taotao, Nie Yu, Li Honglin, Zhang Cong. Synthesis and characterization of nanosized MnZn ferrites via a modified hydrothermal method. J. Magn. Magn. Mater. 2017;439:228–235. [Google Scholar]
- 11.Suzuki Y. Epitaxial spinel ferrite thin films. Annu. Rev. Mater. Res. 2001;31:265–289. [Google Scholar]
- 12.reddy G.K., Gunasekera K., Boolchand P., Dong J., Smirniotis P.G. Cr- and Ce-doped ferrite catalysts for the high temperature water-gas shift reaction: TPR and Mossbauer spectroscopic study. J. Phys. Chem. C. 2011;115(4):920–930. [Google Scholar]
- 13.Verma Vivek, Dar M. Abdullah, Pandey Vibhav, Singh Anterpreet, Annapoorni S., Kotnala R.K. Magnetic properties of nano-crystalline Li0.35Cd0.3Fe2.35O4 ferrite prepared by modified citrate precursor method. Mater. Chem. Phys. 2010;122:133–137. [Google Scholar]
- 14.Kumar Kotnala Ravinder, Shah Jyoti. Green hydroelectrical energy source based on water dissociation by nanoporous ferrite. Int. J. Energy Res. 2016;40:1652–1661. [Google Scholar]
- 15.Hair M.L. Dekker; New York: 1967. Infrared Spectroscopy in Surface Chemistry. [Google Scholar]
- 16.Kurtz R.L., Henrich V.E. Chemisorption of H2O on the surface of Ti2O3: role of d electrons and ligand geometry. Phys. Rev. B. 1982;26:6682. [Google Scholar]
- 17.Zhou Zijian, Zhao Zhenghuan, Zhang Hui, Wang Zhenyu, Chen Xiaoyuan, Wang Ruifang, Chen Zhong, Gao Jinhao. Interplay between longitudinal and transverse contrasts in Fe3O4 nanoplates with (111) exposed surfaces. ACS NANO. 2014;8:7976–7985. doi: 10.1021/nn5038652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rim Kwang Taeg, Eom Daejin, Chan Siu-Wai, Flytzani-Stephanopoulos Maria, Flynn George W., Wen Xiao-Dong, Batista Enrique R. Scanning tunneling microscopy and theoretical study of water adsorption on Fe3O4: implications for catalysis. J. Am. Chem. Soc. 2012;134:18979–18985. doi: 10.1021/ja305294x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar Lawrence, Kumar Pawan, Narayan Amarendra, Kar Manoranjan. Rietveld analysis of XRD patterns of different sizes of nanocrystalline cobalt ferrite. Int. Nano Lett. 2013;3:8. [Google Scholar]
- 20.KumarVashisth Balesh, Bangruwa Jarnail S., Beniwal Anu, Gairola S.P., Kumar Ashok, Singh Nidhi, Verma Vivek. Modified ferroelectric/magnetic and leakage current density properties of Co and Sm co-doped bismuth ferrites. J. Alloy. Comp. 2017;698:699–705. [Google Scholar]
- 21.Sankaramahalingam Angappan, Lawrence John Berchmans. Structural, optical, and magnetic properties of MgFe2O4 synthesized with addition of copper. Synth. React. Inorg. Metal Org. Nano Metal Chem. 2012;42:121–127. [Google Scholar]
- 22.Abdullah Dar M., Batoo Khalid Mujasam, Verma Vivek, Siddiqui W.A., Kotnala R.K. Synthesis and characterization of nano-sized pure and Al-doped lithium ferrite having high value of dielectric constant. J. Alloy. Comp. 2010;493:553–560. [Google Scholar]
- 23.Rahman S., Nadeem K., Rehman M.A., Mumtaz M., Naeem S., Papst I.L. Structural and magnetic properties of ZnMg-ferrite nanoparticles prepared using the coprecipitation method. Ceram. Int. 2013;39:5235–5239. [Google Scholar]
- 24.Jain Shipra, Shah Jyoti, Dhakate S.R., Gupta Govind, Sharma C., Kotnala R.K. Environment-friendly mesoporous magnetite nanoparticles-based hydroelectric cell. J. Phys. Chem. C. 2018;122:5908–5916. [Google Scholar]
- 25.Verma Vivek, Pandey Vibhav, Shukla V.N., Annapoorni S., Kotnala R.K. Remarkable influence on the dielectric and magnetic properties of lithium ferrite by Ti and Zn substitution. Solid State Commun. 2009;149:1726–1730. [Google Scholar]
- 26.Abdullah Dar M., Verma Vivek, Gairola S.P., Siddiqui W.A., Singh Rakesh Kumar, Kotnala R.K. Low dielectric loss of Mg doped Ni–Cu–Zn nano-ferrites for power applications. Appl. Surf. Sci. 2012;258:5342–5347. [Google Scholar]