Abstract
Background
Self-rated health (SRH), which is frequently used in epidemiological research, has consistently been shown to be a strong predictor of morbidity and mortality, even after controlling for demographic, social and medical risk factors. However, less is known about the relationship between SRH and all-cause and cause-specific mortality in young adulthood.
Objective
To investigate SRH in young people (13–35 years-old) as a predictor of all-cause mortality in young adulthood (deaths before age 54) and examine the associated causes of death.
Methods
We used data from two large population-based cohort studies (N = 23,679): Young-HUNT1 (1995–1997, persons 13 to 20 years old, participation rate = 90%) and HUNT2 (1995–1997, persons 20 to 35 years old, participation rate = 70%). These data were linked to the Norwegian Cause of Death Registry up to 2014, and 247 deaths were identified. Other predictors we examined included age, gender, baseline smoking, physical activity and physical and mental disability.
Results
Participants reporting ‘not so good’/‘poor’ SRH had approximately twice the risk of death compared to those reporting ‘good’ or ‘very good’ SRH at baseline. The association between low SRH and risk of death was attenuated when the models were adjusted for other predictors, but remained statistically significant. The causes of death differed somewhat between SRH levels. Most of the deaths for people reporting ‘very good’ SRH at baseline were mostly due to neoplasms (34%) and other external causes (30%). The causes of death were more varied for people reporting ‘not so good’/‘poor’ SRH, with suicide (23%), other external causes (21%) and other/unknown causes of death (17%) being the most frequent causes.
Conclusion
SRH predicts all-cause mortality in young adulthood, with poor SRH being associated with death in young adulthood. The findings also indicate different causes of death for different SRH. This knowledge is important for identifying groups at risk for later disease, which can potentially be used to prevent morbidity in the adult population.
Keywords: self-rated health, subjective health, epidemiology, mortality, registry study, young adulthood
Highlights
-
•
Self-rated health (SRH) has shown to be a predictor of mortality.
-
•
We found that SRH in young people (13–35 years-old) predicted all-cause mortality in young adulthood (deaths before age 54).
-
•
The findings also indicate different causes of death for different SRH.
1. Introduction
Self-rated health (SRH) is a simple, subjective assessment of health status, which is extensively used in epidemiological research (Møller et al., 1996, Idler and Benyamini, 1997, Jylhä, 2009, Schnittker and Bacak, 2014, Doiron et al., 2015) under various names, e.g. self-assessed health (Doiron et al., 2015), self-perceived health (Shields & Shooshtari, 2001) and self-reported health (McGee, Liao, Cao, & Cooper, 1999). SRH is commonly measured with a single question (‘How is your health at the moment?’) in which people are asked to rate their health on a four- or five-point scale, from poor to excellent, or to compare their health with that of age peers (Lundberg and Manderbacka, 1996, Idler and Benyamini, 1997, White et al., 2009). SRH has been shown to be high reliability and valid, and it has predictive power over a range of health outcomes. Indeed, it has frequently been found to be an independent predictor of mortality, even after controlling for demographic, social and medical risk factors (Idler and Benyamini, 1997, Heistaro et al., 2001, Breidablik et al., 2010, Jylhä, 2009, Ganna and Ingelsson, 2015).
The association between SRH and mortality was initially shown in the 1982 Manitoba Longitudinal Study on Aging (MLSA) (Mossey & Shapiro, 1982). A review by Idler and Benyamini (1997) of twenty-seven community studies demonstrated impressively consistent findings that SRH was an independent predictor of mortality, despite the studies’ inclusion of numerous specific health-status indicators and other relevant covariates known to predict mortality (Idler & Benyamini, 1997).
The cross-sectional General Social Survey, which was conducted repeatedly between 1980 and 2002, showed the validity of SRH increased over time (individuals were apparently better at assessing their health in 2002 than they were in 1980) and the relationship between SRH and mortality became stronger during that period of time (Schnittker & Bacak, 2014). Moreover, a publication from the Norwegian HUNT study by Schou, Krokstad, and Westin (2006) found that adults who reported poor SRH had higher mortality as a group than adults who reported good SRH (adjusting for age, education and established illness) — even higher than adults who reported cardiac infarction or diabetes (Schou et al., 2006).
One of the few studies to examine the association between SRH and cause-specific mortality found that the relationship between SRH and mortality differed by cause of death (Benjamins, Hummer, Eberstein, & Nam, 2004). Deaths due to diabetes and infectious and respiratory diseases were most strongly associated with SRH, whereas deaths due to heart disease, stroke and cancer showed a moderate association with SRH. In contrast, SRH had only a weak association or no association with deaths due to accidents, homicides and suicides. The differences found by cause of death indicate that SRH does not predict all causes of mortality equally well (Benjamins et al., 2004). Furthermore, that study showed the relationship between SRH and mortality risk was stronger among men for several causes.
Others predictors, including socioeconomic status, functional status, physical activity and smoking, have been found to explain variations in the associations between SRH and mortality, but the results are not consistent (Idler & Benyamini, 1997). One study examining the ability of SRH to predict mortality among older men and women found that: (a) the relationship between SRH and short-term and long-term mortality was explained by age and health among men; (b) the relationship between SRH and short-term mortality was explained by age, physical and mental health and physical activity among women; and (c) the relationship between SRH and long-term mortality was explained by age, physical health and physical activity among women (Bath, 2003).
There is still much research to be done on the relationship between SRH and mortality. Most of the current studies have examined SRH as a predictor of mortality among older people (Idler & Benyamini, 1997), and since the leading causes of death occur in old age, these will dominate the causes of death examined. Few studies have examined the link between SRH and subsequent mortality in younger adults, and very few have examined the association of SRH with mortality in younger adults over an extended period of time. Because of the marked predictive power of SRH, it is important to gain a better understanding of the relationship between SRH and premature mortality from a long-term perspective, as SRH may be a potential indicator and target for early interventions (Jylhä, 2009). Research involving more specific causes of death may also be useful for understanding the mechanisms underlying the relationship between SRH and mortality risk (Idler & Benyamini, 1997). The aim of the present study was to broaden this knowledge by studying younger individuals and all-cause and cause-specific mortality in young adulthood. The main aim of the study, which used a large Norwegian sample and national register data, was to examine SRH in young people as a predictor for mortality in young adulthood.
The health status of the population of Norway is generally good. In 2017, life expectancy was 84.3 years for women and 80.9 years for men, with the two main causes of death being cardiovascular disease and cancer (Norwegian Institute of Public Health, 2018). According to the Norwegian Institute of Public Health (2018) the mortality rate for cardiovascular disease has fallen over the last 50 years and deaths have largely shifted to the over-80 age groups. In younger age groups, the number of deaths is low. Compared to other countries, Norway has a relatively high number of drug-induced deaths (Norwegian Institute of Public Health, 2018). The main causes of disability and reduced health are musculoskeletal disorders, mental disorders, cardiovascular disease and cancer. Many people are still insufficiently physically active, whereas smoking has decreased, but more than 10 per cent of the adult population still smoke on a daily basis (Norwegian Institute of Public Health, 2018).
On this background the following research questions were examined:
-
–
Is SRH at a young age (13–35 years) a predictor for mortality in young adulthood (deaths before age 54)?
-
–
To what degree is the power of SRH to predict mortality attenuated by adjusting for age, gender, mental and physical disability, smoking and physical activity?
-
–
What are the causes of death for premature mortality, and do participants reporting different levels of SRH show a different distribution of causes of death?
2. Methods
The Nord-Trøndelag Health Study (HUNT) is the largest set of health data about the Norwegian population, which has been collected through several population studies. We used the data from two of these studies: (1) The Young-HUNT1 study (1995–1997) in which 9131 students aged 13–20 years (mean age = 16.1) participated, representing 90% of the adolescent population in the country; and (2) The HUNT2 study (1995–1997) study of persons aged 19–35 years (mean age = 28.0 years), which had a participation rate of 70%. Although the HUNT2 study had participants up to 48 years old, as we are interested in SRH in early periods of life, we only included participants 35 years or younger. Data from the two studies were combined, giving us a total of 23,679 participants; all the results reported herein are based on the combined data from the Young-HUNT1 and HUNT2 studies. The HUNT data were linked to the Norwegian Cause of Death Registry (‘Dødsårsaksregisteret’).
The Cause of Death Registry covers all deaths in Norway, regardless of whether the deceased was registered as a Norwegian citizen. Deaths of Norwegians who die abroad are also registered. All deaths (about 40,000 each year) are reported by doctors, who are required to complete a death certificate (Norwegian Institute of Public Health, 2015; https://www.fhi.no/en/hn/health-registries/cause-of-death-registry/cause-of-death-registry-/).
2.1. Measures
2.1.1. Self-rated health (SRH)
SRH in the HUNT surveys was measured on a four-point ordinal scale in response to the question ‘How is your health at the moment?’; the response options were ‘very good’, ‘good’, ‘not so good’ and ‘poor’. Since very few respondents reported their health as ‘poor’, we have grouped them together with the respondents reporting ‘not so good’ in the statistical analyses.
2.1.2. Additional predictors: Mental and physical disability, smoking and physical activity
There were minor wording differences between the questions in the Young-HUNT1 and HUNT2 questionnaires, but the questions and response options were fairly similar for our predictors. Briefly, we used the following definitions: Smoking was defined as ‘Yes, I smoke (cigarettes) daily’ for the question ‘Do you smoke?’; Physical and mental disability was defined as a response of ‘a little’ or higher on the sub-items ‘Impairment due to physical illness’ and ‘Impairment due to mental health problems’ for the question ‘Are you disabled in any of these ways (functional impairment)?’; Being physically active was defined as the response ‘Yes’ for the question ‘Are you actively involved in sports?’ (Young-HUNT1), and an average of ‘3 or more hours weekly’, or ‘vigorous’ physical activity in the previous year (HUNT2).
2.2. Ethics
All the participants and the parents or guardians of participants under the age of 16 years gave their written consent to participate in the HUNT study and to the use of data for research. It was explained that participation was voluntary. The study was approved by the Norwegian Data Inspectorate, the Regional and National Committees for Medical and Health Research Ethics and the Norwegian Directorate of Health. Register owners approved the use of data from the HUNT, the Cause of Death Registry and the linkage of the data.
2.3. Data analysis / statistical methods
The cause of death was registered using ICD codes for the underlying cause of death. As the deaths had been recorded over a long time-span, ICD-8, ICD-9 and ICD-10 codes were used, and we reclassified them according to the European Shortlist for Causes of Death, 2012 (COD SL-2012). We report the following level 1 categories of the COD SL-2012: ‘Neoplasms’ (2.x), ‘Diseases of the circulatory system’ (7.x), ‘Diseases of the nervous system and the sense organs’ (6.x), ‘External causes of mortality’ (17.x, further subdivided into ‘Suicide and intentional self-harm,’ 17.2; and other causes, i.e. all 17.x codes except 17.2), and other or unknown causes.
First, we report the absolute mortality (number and proportion of deaths) stratified by SRH at baseline and by cause of death. We also report the relative proportion of each cause of death, stratified by SRH at baseline.
Time from inclusion to death (or censoring) was analysed using Kaplan–Meier estimates and proportional hazard models. The age at inclusion differed between the participants; hence, we used the variants of these methods that take ‘delayed entry’ into account. For privacy reasons, we only had access to birthdates with two-month’s precision (e.g. ‘January/February 1970’), and therefore, we used the date in the middle of each two-month interval in all the analyses.
To examine SRH as a predictor of death, either alone or along with other predictors, we fitted several proportional hazard models. We included age (at HUNT study inclusion), gender, study group (Young-HUNT1 or HUNT2) and SRH as predictors in the base model. To assess the degree to which the predictive ability of SRH was attenuated by adjusting for other predictors (potential confounders), we fitted two additional models: one used mental and physical disability as additional predictors, and one used mental and physical disability, smoking and physical activity as additional predictors. The proportional hazard assumption was tested using a global test on the complete-case data.
To examine if the net effects differed between genders, we also fitted similar models with an interaction between gender and the other predictors. To preserve statistical power, we only analysed the overall interaction between gender and the other predictors taken as a group, not the individual interaction between gender and each predictor.
As there was a number of missing responses to the questions, especially the smoking question, we used multiple imputations to handle this for the regression analyses. The imputation was done by predictive mean matching, using the ‘aregImpute()’ function from the ‘rms’ R package with default options, except that we used 500 imputations. This uses a very flexible imputation model, e.g. with splines for continuous variables. The variables used in the imputation models were the ones included in the regression models. We also examined other variables for potential inclusion in the imputation model, but they had relatively little predictive power/effect on the imputations.
All the analyses were done in R 3.4.0. Basic survival analyses were done using the ‘prodlim’ package (Gerds, 2018), and survival regression models and imputation models using the ‘rms’ package (Frank & Harrell, 2018).
3. Results
Table 1 shows that out of the 23,679 participants, 264 (1.1%) had died by the end of follow-up (up to year 2014); including 1.5% of the males and 0.8% of the females. The participants reporting ‘not so good’/‘poor’ SRH at baseline had a mortality rate that was approximately twice that of those reporting ‘good’ or ‘very good’ SRH (Table 2). There were 252 participants who did not report their SRH level, of which 7 (2.8%) died by the end of follow-up (suicide = 2, other external causes = 2, neoplasms = 1, nervous system = 1, other unknown = 1). Their mean age was 20.9, and 46% were female.
Table 1.
Baseline characteristics of the study population, stratified by mortality (N = 23,679).
| Total |
Not dead |
Dead |
|||||
|---|---|---|---|---|---|---|---|
| Missing | Count | SD/prop. | Count | SD/prop. | Count | SD/prop. | |
| Participants | 0 | 23,679 | – | 23,415 | – | 264 | – |
| Age (mean, SD) | 0 | 23.5 | 6.8 | 23.5 | 6.8 | 25.3 | 6.9 |
| Male gender | 0 | 11,283 | 48% | 11,118 | 47% | 165 | 62% |
| Study: Young-HUNT 1 | 0 | 8981 | 38% | 8904 | 38% | 77 | 29% |
| Self-reported health | 252 | ||||||
| Very good | – | 7011 | 30% | 6947 | 30% | 64 | 25% |
| Good | – | 13,878 | 59% | 13,738 | 59% | 140 | 54% |
| Not so good/poor | – | 2538 | 11% | 2485 | 11% | 53 | 21% |
| Physical disability | 2748 | 1456 | 7% | 1,413 | 7% | 43 | 19% |
| Mental disability | 3096 | 976 | 5% | 953 | 5% | 23 | 11% |
| Daily smoker | 4916 | 5,121 | 27% | 5041 | 27% | 80 | 36% |
| Physically active | 2002 | 10,895 | 50% | 10,784 | 50% | 111 | 50% |
Abbreviations: SD: Standard deviation; prop.: proportion.
Table 2.
Absolute mortality, stratified by baseline SRH. The mortality figures (proportions) for each cause show the absolute mortality within each SRH level (i.e. they sum to the total mortality within each SRH level) (N = 23,427).
| Total |
Dead |
Cause of death |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| External causes |
|||||||||||||||
| Neoplasms | Circulatory | Nervous system | Suicide | Other ext. | Other/unknown | ||||||||||
| Not so good/poor | 2538 | 53 | (20.9‰) | 9 | (3.5‰) | 6 | (2.4‰) | 6 | (2.4‰) | 12 | (4.7‰) | 11 | (4.3‰) | 9 | (3.5‰) |
| Good | 13,878 | 140 | (10.1‰) | 39 | (2.8‰) | 16 | (1.1‰) | 10 | (0.7‰) | 26 | (1.9‰) | 31 | (2.2‰) | 19 | (1.4‰) |
| Very good | 7011 | 64 | (9.1‰) | 22 | (3.1‰) | 4 | (0.6‰) | 2 | (0.3‰) | 15 | (2.1‰) | 19 | (2.7‰) | 2 | (0.3‰) |
The two study groups (Young-HUNT1 and HUNT2) had a very similar distribution of SRH levels (28%/31% ‘very good’, 61%/58% ‘good’ and 11%/11% ‘not so good’/‘poor’ for Young-HUNT1 and HUNT2, respectively). Thus, study group was not a confounder for the association between SRH and other variables (e.g. mortality).
3.1. Cause of death
There were some differences in the causes of death between the various SRH levels (Table 3). Most of the deaths for the people reporting ‘very good’ SRH at baseline occurred due to neoplasms (34%) or ‘other external causes’ (30%) (e.g. traffic accidents). The causes of deaths for the people reporting ‘not so good’ or ‘poor’ SRH were more uniformly distributed, with suicide (23%), other external causes (21%) and other/unknown causes of death (17%) being the most frequent causes.
Table 3.
Relative frequencies of causes of death, stratified by SRH. The proportions indicate which causes of death occurred most frequently for each SRH level, and thus sum to 100% within each SRH level (N = 23,427).
| Total | Dead |
Cause of death |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| External causes |
||||||||||||||
| Neoplasms | Circulatory | Nervous system | Suicide | Other ext. | Other/unknown | |||||||||
| Not so good/poor | 2538 | 53 | 9 | (17%) | 6 | (11%) | 6 | (11%) | 12 | (23%) | 11 | (21%) | 9 | (17%) |
| Good | 13,878 | 140 | 39 | (28%) | 16 | (11%) | 10 | (7%) | 26 | (19%) | 31 | (22%) | 19 | (14%) |
| Very good | 7011 | 64 | 22 | (34%) | 4 | (6%) | 2 | (3%) | 15 | (23%) | 19 | (30%) | 2 | (3%) |
3.2. Time of death
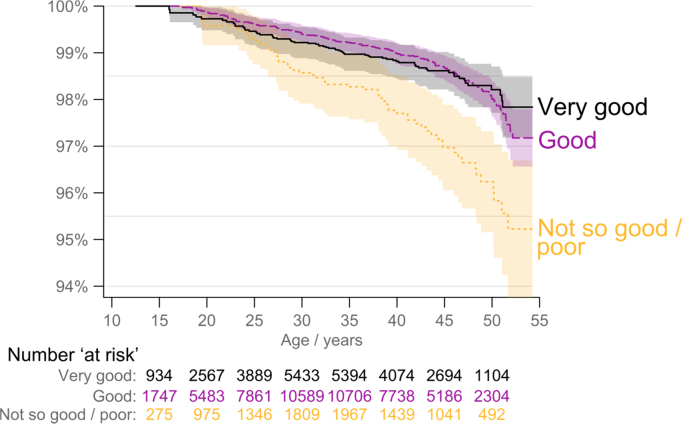
Fig. 1 shows estimated survival/mortality stratified by SRH at baseline, i.e. the estimated probability of surviving to a given age (conditional on being alive at inclusion, which was about 12.3 years for the youngest participant). The mean length of follow-up was 18.3 years for the Young-Hunt 1 group and 18.4 for the Hunt-2 group. There was little difference in the relative number of deaths or time of death for the entire follow-up period for the people in the ‘very good’ and ‘good’ groups. There were few deaths overall up to about 25 years of age, with only minor differences in mortality between the three SRH groups. Mortality in the ‘not so good’/’poor’ SRH group increased after 25 years of age, and remained high compared to the other two groups for rest of the follow-up period. Similar results were observed for analyses stratified by gender (not reported). The differences in mortality were also statistically significant when adjusted for gender, age at inclusion and study group (P < 0.001).
Fig. 1.
Kaplan–Meier estimates of survival (all-cause deaths), stratified by SRH at baseline. The number of people ‘at risk’ increased in the beginning of the time period, since their age at the baseline SRH questionnaire differed between the participants (‘delayed entry’) (n = 23,427).
3.3. Risk of death
Table 4 shows the estimated hazard ratios (HRs) for the various predictors in the three hierarchical models. The SRH status ‘very good’ was used as the reference level. The results for the tests of the proportional hazard assumption had P-values of 0.22, 0.42 and 0.83 for models 1, 2 and 3, respectively. That is, there was no indication that the Cox proportional hazard assumption was violated.
Table 4.
Estimated hazard ratios for various predictors on the risk of death. All models are adjusted for age, gender and study group. The reference level was set to ‘very good’ for SRH and to ‘no’ for all ‘no’/‘yes’ predictors.
|
Model 1 |
Model 2 |
Model 3 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |||||||
| Self-reported health | <.001 | 0.05 | 0.07 | ||||||||||||
| Very good | Ref. | – | – | Ref. | – | – | Ref. | – | – | ||||||
| Good | 1.15 | 0.85 to 1.54 | 0.36 | 1.08 | 0.8 to 1.45 | 0.63 | 1.05 | 0.77 to 1.42 | 0.77 | ||||||
| Not so good/poor | 2.42 | 1.68 to 3.49 | <.001 | 1.63 | 1.08 to 2.46 | 0.02 | 1.56 | 1.02 to 2.37 | 0.04 | ||||||
| Physical disability | – | – | – | 2.06 | 1.14 to 3.71 | 0.02 | 2.06 | 1.15 to 3.70 | 0.02 | ||||||
| Mental disability | – | – | – | 1.32 | 0.68 to 2.55 | 0.41 | 1.28 | 0.67 to 2.47 | 0.46 | ||||||
| Daily smoker | – | – | – | – | – | – | 1.35 | 1.02 to 1.80 | 0.03 | ||||||
| Physically active | – | – | – | – | – | – | 1.07 | 0.82 to 1.39 | 0.64 | ||||||
Abbreviations: HR: hazard ratio; CI: confidence interval.
Having a SRH status of ‘not so good/poor’ in the base model yielded an estimated 142% increase in the (instantaneous) risk of death (HR = 2.42). The risk was somewhat attenuated (HR= 1.56) after adjusting for physical and mental disability, or physical and mental disability, smoking and physical activity, but remained statistically significant (P < 0.04). The group with ‘good’ SRH also had an estimated increase in mortality compared to the ‘very good’ SRH group, but this difference was not statistically significant.
While gender was a statistically significant predictor in all the models (HR = 1.92 for being male in the base model, P < 0.001; other results not shown), there was no significant interaction between gender and the other predictors in any of the models.
4. Discussion
The main aim of this study, which used a large population-based sample and national register data, was to examine SRH in young people as a predictor of premature mortality. In sum, we found that participants reporting ‘not so good’/‘poor’ SRH had approximately twice the risk of death of those reporting ‘good’ or ‘very good’ SRH at baseline. The relationship between SRH and mortality differed somewhat by cause of death. The main causes of death for participants reporting ‘very good’ SRH at baseline were neoplasms (34%) or other external causes (30%) (e.g. traffic accidents). The main causes of death for participants reporting ‘not so good’/‘poor’ SRH were more uniformly distributed, with suicide (23%), other external causes (21%) and other/unknown causes of death (17%) being the most frequent causes. Furthermore, SRH was a predictor of all-cause mortality, after adjusting for age, gender, smoking, physical activity, and mental and physical disability.
The finding that SRH predicted mortality is in line with a substantial body of research showing associations between self-reported health status and subsequent risk of mortality (Idler and Benyamini, 1997, Benjamins et al., 2004). This finding is also in line with one of the few other Norwegian studies examining the relationship between SRH and mortality (Schou et al., 2006).
The current study expands on this finding by demonstrating that the relationship between SRH and mortality differs by cause of death; most of the deaths occurred due to neoplasms or other external causes for people reporting ‘very good’ SRH at baseline, whereas the causes of deaths were more uniformly distributed for the people reporting ‘not so good’/‘poor’ SRH, with suicide, other external causes and other/unknown causes of death being the most frequent causes. A study by Benjamins et al. (2004), which examined the relationship between SRH and cause-specific mortality, showed that the relationship between SRH and mortality differs by cause of death, with deaths due to diabetes and infectious and respiratory diseases being most strongly associated with SRH. SRH in the present study also exhibited a moderately strong association with deaths due to heart disease, stroke, and cancer. In contrast, SRH was only weakly associated or not associated with deaths due to accidents, homicide and suicide (Benjamins et al., 2003). One plausible explanation for these differences may be that individuals with good SRH died of cancer (which either affects people randomly, or does not necessarily affect those who have a pre-existing mental disease or other diseases). In addition, given the relatively few deaths, the differences in mortality may be due to chance, making interpretations problematic.
Interestingly, the current study showed only a small, non-significant difference between ‘very good’ and ‘good’ SRH, in contrast to a large difference documented by other research (Breidablik, Meland, & Lydersen, 2008). Another recent study showed that the predictors of all-cause mortality were not universal, but depended on the level of SRH. The higher mortality of respondents with poor SRH could largely be attributed to health problems, whereas factors other than the presence of illness may explain mortality in cases of average or good SRH (Reile, Stickley, & Leinsalu, 2017). We adjusted for both physical and mental disability in our study, which are proxies for general health problems, and smoking and (lack of) physical activity, that one would expect to have major influences on the risk of death. However, the participants with the lowest SRH still had higher mortality.
Surprisingly, the only covariates examined beside SRH that seemed to be associated with mortality in the fully adjusted model were physical disability and smoking, not mental disability or (lack of) physical activity. But, it should be noted that mental disability was associated with the other predictors included in the model, which could partly explain its lack of association with mortality. Yet, given the large body of research showing the health benefits of physical activity (Samitz, Egger, & Zwahlen, 2011), our finding that this measure did not significantly predict premature mortality was unexpected. However, a previous study reported lower overall mortality rates were more closely associated with recent physical activity than with distant activity (Sherman, D’agostino, Silbershatz, & Kannel, 1999). One explanation for our findings may, therefore, be that we only had a baseline measure of physical activity. Yet, in line with our findings, Pekkanen et al. (1987) found low physical activity clearly was a weaker predictor of death than smoking in a 20-year follow-up of middle-aged Finnish men.
The current study has several strengths that mainly arise from its population-based and historical prospective design, as well as its large number of participants, reinforcing the validity of the data. Additionally, the use of a comprehensive national mortality register reduces selection or information biases, and minimises the risk of measurement error. Its limitations include lack of data on socioeconomic status (SES), as SES has been shown to be an important predictor of overall health and SRH (Goodman, Huang, Schafer-Kalkhoff, & Adler, 2007). However, education, income, work status and marital status would not be appropriate as predictors of early mortality in a study of SRH in young people. First, the adolescents who participated in the study (Young-HUNT1 study, mean age 16.1) would not have any significant income or be married. Thus, including these predictors would probably bias the results. For example, the age of first marriage in Norway is relatively high (currently 34.9 for men and 32.3 years for women). If marital status was included as a predictor, the people who were married would naturally be alive at least until the time of marriage, i.e. they would have a much lower ‘premature mortality’.
Additionally, despite the high number of participants, there were few deaths, something which is expected for a study of mortality in young adulthood, but which also limits the statistical power of the analyses. This is especially true for examining individual causes of death.
In conclusion, SRH in young people predicts all-cause mortality in young adulthood, even when one adjusts for other predictors. There also seems to be differences in causes of death between various SRH levels, but these results are less certain due to the small number of deaths in each ‘cause of death’ category. The results add to earlier findings on the ability of SRH to predict mortality and morbidity later in life. The results of the present study should encourage promotion of subjective health in adolescence and early adult life. The findings also support the use of SRH as a universal measure of health, and provide important knowledge about identifying risks of later diseases, potentially preventing morbidity in the adult population.
Acknowledgments
The Cause of Death Registry in Norway and the Nord-Trøndelag Health Study (The HUNT Study), Norway, provided access to the data used in this study. The HUNT Study is a collaboration between HUNT Research Centre (Faculty of Medicine and health sciences, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. Helse Førde HF, Norway, funded the study.
Acknowledgments
Conflict of interest
No conflict of interest.
Contributor Information
Tina Løkke Vie, Email: tina.lokke.vie@helse-forde.no.
Karl Ove Hufthammer, Email: karl.ove.hufthammer@helse-bergen.no.
Eivind Meland, Email: Eivind.Meland@igs.uib.no.
Hans Johan Breidablik, Email: hans.johan.breidablik@helse-forde.no.
References
- Bath P.A. Differences between older men and women in the self-rated health–mortality relationship. The Gerontologist. 2003;43(3):387–395. doi: 10.1093/geront/43.3.387. [DOI] [PubMed] [Google Scholar]
- Benjamins M.R., Hummer R.A., Eberstein I.W., Nam C.B. Self-reported health and adult mortality risk: An analysis of cause-specific mortality. Social Science Medicine. 2004;59(6):1297–1306. doi: 10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Breidablik H.J., Meland E., Holmen T.L., Lydersen S. Role of parents in adolescent self-rated health: Norwegian Nord-Trøndelag Health Study. Adolescent Health, Medicine and therapeutics. 2010;1:97. doi: 10.2147/AHMT.S12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidablik H.J., Meland E., Lydersen S. Self-rated health in adolescence: A multifactorial composite. Scandinavian Journal of Public Health. 2008;36(1):12–20. doi: 10.1177/1403494807085306. [DOI] [PubMed] [Google Scholar]
- Doiron D., Fiebig D.G., Johar M., Suziedelyte A. Does self-assessed health measure health? Applied Economics. 2015;47(2):180–194. [Google Scholar]
- Frank, E., Harrell, Jr. (2018). rms: Regression Modeling Strategies. R package version 5.1-2. 〈https://CRAN.R-project.org/package=rms〉.
- Ganna A., Ingelsson E. 5 year mortality predictors in 498 103 UK Biobank participants: A prospective population-based study. The Lancet. 2015;386(9993):533–540. doi: 10.1016/S0140-6736(15)60175-1. [DOI] [PubMed] [Google Scholar]
- Gerds, T.A. (2018). prodlim: Product-Limit Estimation for Censored Event History Analysis. R package version 2018.04.18. 〈https://CRAN.R-project.org/package=prodlim〉.
- Goodman E., Huang B., Schafer-Kalkhoff T., Adler N.E. Perceived socioeconomic status: A new type of identity that influences adolescents' self-rated health. Journal of Adolescent Health. 2007;41(5):479–487. doi: 10.1016/j.jadohealth.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistaro S., Jousilahti P., Lahelma E. Self rated health and mortality: A long term prospective study in eastern Finland. Journal of Epidemiology and Community Health. 2001;55:227–232. doi: 10.1136/jech.55.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler E.L., Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997:21–37. [PubMed] [Google Scholar]
- Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science Medicine. 2009;69(3):307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Lundberg O., Manderbacka K. Assessing reliability of a measure of self-rated health. Scandinavian Journal of Social Medicine. 1996;24:218–224. doi: 10.1177/140349489602400314. [DOI] [PubMed] [Google Scholar]
- McGee D.L., Liao Y., Cao G., Cooper R.S. Self-reported health status and mortality in a multiethnic US cohort. American Journal of Epidemiology. 1999;149(1):41–46. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- Mossey J.M., Shapiro E. Self-rated health: A predictor of mortality among the elderly. American journal of public health. 1982;72:800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller L., Kristensen T.S., Hollnagel H. Self rated health as a predictor of coronary heart disease in Copenhagen, Denmark. Journal of Epidemiology Community Health. 1996;50(4):423–428. doi: 10.1136/jech.50.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2018. Public health report: Health status in Norway 2018. [Google Scholar]
- Pekkanen J., Nissinen A., Marti B., Tuomilehto J., Punsar S., Karvonen M. Reduction of premature mortality by high physical activity: A 20-year follow-up of middle-aged Finnish men. The lancet. 1987;329(8548):1473–1477. doi: 10.1016/s0140-6736(87)92218-5. [DOI] [PubMed] [Google Scholar]
- Reile R., Stickley A., Leinsalu M. Large variation in predictors of mortality by levels of self-rated health: Results from an 18-year follow-up study. Public Health. 2017;145:59–66. doi: 10.1016/j.puhe.2016.12.034. [DOI] [PubMed] [Google Scholar]
- Samitz G., Egger M., Zwahlen M. Domains of physical activity and all-cause mortality: Systematic review and dose–response meta-analysis of cohort studies. International Journal of Epidemiology. 2011;40(5):1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- Schnittker J., Bacak V. The increasing predictive validity of self-rated health. PloS One. 2014;9(1):e84933. doi: 10.1371/journal.pone.0084933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou M.B., Krokstad S., Westin S. How is self-rated health associated with mortality? Tidsskrift for den Norske laegeforening. 2006;126(20):2644–2647. [PubMed] [Google Scholar]
- Sherman S.E., D’agostino R.B., Silbershatz H., Kannel W.B. Comparison of past versus recent physical activity in the prevention of premature death and coronary artery disease. American Heart Journal. 1999;138(5):900–907. doi: 10.1016/s0002-8703(99)70015-3. [DOI] [PubMed] [Google Scholar]
- Shields M., Shooshtari S. Determinants of self-perceived health. Health Reports. 2001;13(1):35–52. [PubMed] [Google Scholar]
- White A.M., Philogene G.S., Fine L., Sinha S. Social support and self-reported health status of older adults in the United States. American Journal of Public Health. 2009;99(10):1872–1878. doi: 10.2105/AJPH.2008.146894. [DOI] [PMC free article] [PubMed] [Google Scholar]