Abstract
Neuropeptides play pivotal roles in modulating circadian rhythms. Pigment-dispersing factor (PDF) is critical to the circadian rhythms in Drosophila locomotor activity. Here, we demonstrate that diuretic hormone 31 (DH31) complements PDF function in regulating free-running rhythmicity using male flies. We determined that Dh31 loss-of-function mutants (Dh31#51) showed normal rhythmicity, whereas Dh31#51;Pdf01 double mutants exhibited a severe arrhythmic phenotype compared to Pdf-null mutants (Pdf01). The expression of tethered-PDF or tethered-DH31 in clock cells, posterior dorsal neurons 1 (DN1ps), overcomes the severe arrhythmicity of Dh31#51;Pdf01 double mutants, suggesting that DH31 and PDF may act on DN1ps to regulate free-running rhythmicity in a hierarchical manner. Unexpectedly, the molecular oscillations in Dh31#51;Pdf01 mutants were similar to those in Pdf01 mutants in DN1ps, indicating that DH31 does not contribute to molecular oscillations. Furthermore, a reduction in Dh31 receptor (Dh31r) expression resulted in normal locomotor activity and did not enhance the arrhythmic phenotype caused by the Pdf receptor (Pdfr) mutation, suggesting that PDFR, but not DH31R, in DN1ps mainly regulates free-running rhythmicity. Taken together, we identify a novel role of DH31, in which DH31 and PDF hierarchically regulate free-running rhythmicity through DN1ps.
Introduction
In Drosophila, pigment-dispersing factor (PDF) is required for robust locomotor behavioral output1. PDF coordinates circadian networks and controls the timing of morning and evening peaks in locomotor activity2–6. In the absence of PDF (Pdf01), flies show a loss of morning anticipation, an advanced evening activity peak, shorter free-running periods, and dampened molecular oscillations in clock cells7–11, suggesting that PDF is a main neuropeptide responsible for orchestrating the activity of each pacemaker neuron. However, the lack of PDF does not completely dampen free-running rhythm; approximately half of Pdf01 mutants still maintain weak rhythmic locomotor activity under constant dark (DD) conditions9,10. The data suggest that PDF may not be the only molecule responsible for regulating free-running rhythmicity. Therefore, we sought to identify another neuropeptide that complements the role of PDF in free-running rhythmicity.
In addition to PDF, diuretic hormone 31 (DH31) activates the PDF receptor (PDFR), which regulates locomotor activity12. DH31 is expressed in clock neurons in the brain. An RNA-seq analysis using sorted clock cells in the brain suggested that DH31 is expressed in lateral neurons (LNvs) and dorsal neurons 1 (DN1s)13, and DH31 antibody staining shows that DH31 is expressed in posterior dorsal neurons 1 (DN1ps)14,15. However, we and others have shown that Dh31 mutants exhibit normal locomotor activity rhythms14,15.
Along with locomotor activity rhythms, DH31 plays roles in sleep and temperature preference rhythm (TPR). A recent study showed that PDF signaling is relayed to DN1s, which express DH31, to promote awakening at dawn14. We also recently showed that DH31 acts on dorsal neurons 2 (DN2s) via PDFR to modulate TPR, particularly the decrease in preferred temperature at the transition from day to night15. Therefore, we hypothesized that normal locomotor activity rhythms in Dh31 single mutants might be a result of normal PDF signaling. To this end, we examined locomotor activity rhythms using Dh31-Pdf double mutants.
Here, we identify a novel role for DH31 in regulating the circadian rhythms of locomotor activity. We determined that PDF and DH31 hierarchically function to regulate free-running rhythmicity by acting on the same clock cells (DN1ps). These neuropeptides appear to play important roles in modulating the clock networks involved in free-running rhythmicity. Therefore, the identification of this novel DH31 function deepens our mechanistic understanding of the circadian rhythms of locomotor activity.
Results
DH31 is involved in regulating free-running rhythmicity
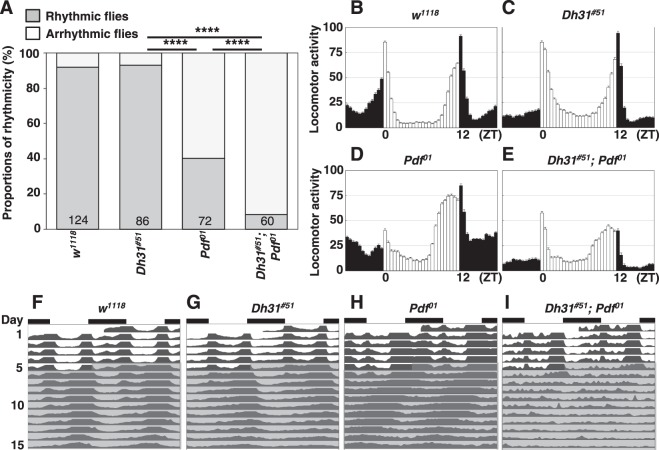
To re-evaluate the function of DH31 in regulating the circadian rhythms of locomotor activity, we focused on a double mutant of Dh31#51 (a loss-of-function mutant)16 and Pdf01 (a null mutant)7 and examined the phenotypes for rhythmicity, free-running period, morning anticipation and evening activity peaks.
We determined that both w1118 (WT) and Dh31#51 mutant flies maintained a robust free-running rhythmicity (WT: 92% rhythmic, power = 1371.7, Dh31#51: 93% rhythmic, power = 678.2) (Fig. 1A,F,G and Table 1). These data are consistent with previous reports using Dh31#51 mutants15 and another Dh31 mutant14. In contrast, the Pdf01 mutants exhibited a weak free-running rhythmicity (40% rhythmic, power = 243.0) (Fig. 1A,H and Table 1), which is also consistent with previous reports9,10. However, we determined that the free-running rhythmicity of Dh31#51;Pdf01 double mutants was strongly disrupted: 92% of the flies exhibited an arrhythmic phenotype, and only 8% showed weak amplitudes (power = 226.7) (Fig. 1A,I and Table 1). These data indicate that the Dh31#51;Pdf01 double-mutant phenotype is more severely arrhythmic than either single mutant, suggesting that DH31 is involved in modulating free-running rhythmicity.
Figure 1.
Dh31-Pdf double mutants exhibited severely disrupted free-running rhythmicity. (A) Comparison of free-running rhythms for different genotypes. The proportions of rhythmic (gray bar) and arrhythmic (white bar) flies over 10 days in DD were compared via χ2 analysis. ****P < 0.0001. Numbers in the bar graphs represent the number of flies. (B–E) Average daily actogram over 4 days in LD for each genotype: w1118 (B), Dh31#51 (C), Pdf01 (D), and Dh31#51;Pdf01 (E). (F–I) Double-plotted averaged actogram of rhythmic flies over 5 days in LD and 10 days in DD for each genotype: w1118 (F), Dh31#51 (G), Pdf01 (H), and Dh31#51;Pdf01 (I).
Table 1.
Free-running rhythms.
| Genotype (DD1–10) | Total | Rhythmic | Tau (hr) | Power | Fig. # | ||
|---|---|---|---|---|---|---|---|
| n | n (%) | Ave | SEM | Ave | SEM | ||
| w 1118 | 124 | 114 (92) | 24 | 0.02 | 1371.7 | 75.1 | 1,2,3,5,S4 |
| Dh31 #51 | 86 | 80 (93) | 24.4 | 0.06 | 678.2 | 46 | 1,2,3,S4 |
| Pdf 01 | 72 | 29 (40) | 22.5 | 0.12 | 243 | 32.5 | 1,2,3,S4 |
| Dh31#51; Pdf01 | 60 | 5 (8) | 23.1 | 0.9 | 226.7 | 37.3 | 1,2,3,S4 |
| tim-Gal4/+, Dh31#51; Pdf01 | 102 | 20 (20) | 23.2 | 0.12 | 406.6 | 42.3 | 2,3 |
| Pdf-Gal4/+, Dh31#51; Pdf01 | 58 | 25 (43) | 22.3 | 0.09 | 371.6 | 35.7 | 2,S4 |
| R18H11-Gal4/+, Dh31#51; Pdf01 | 48 | 9 (19) | 22.5 | 0.13 | 336.8 | 50.3 | 3,S4 |
| +/UAS-Dh31, Dh31#51; Pdf01 | 110 | 21 (19) | 22.6 | 0.11 | 409.8 | 49.5 | 2,S4 |
| +/UAS-Pdf, Dh31#51; Pdf01 | 42 | 9 (21) | 22.4 | 0.34 | 255.8 | 20.8 | 2 |
| +/UAS-t-Dh31, Dh31#51; Pdf01 | 46 | 8 (17) | 23.6 | 0.27 | 247.9 | 40.9 | 3,S4 |
| +/UAS-t-Pdf, Dh31#51; Pdf01 | 47 | 4 (9) | 22 | 0.44 | 277.2 | 43.8 | 3,S4 |
| tim-Gal4 > UAS-Dh31, Dh31#51; Pdf01 | 61 | 31 (51) | 22.6 | 0.06 | 817.9 | 63.2 | 2 |
| tim-Gal4 > UAS-t-Dh31, Dh31#51; Pdf01 | 60 | 22 (37) | 23 | 0.15 | 252.1 | 19.2 | 3 |
| tim-Gal4 > UAS-t-Pdf, Dh31#51; Pdf01 | 48 | 34 (71) | 22.9 | 0.17 | 542.6 | 48.3 | 3 |
| Pdf-Gal4 > UAS-Pdf, Dh31#51; Pdf01 | 31 | 29 (94) | 23.6 | 0.17 | 1043.6 | 109.5 | 2 |
| Pdf-Gal4 > UAS-t-Dh31, Dh31#51; Pdf01 | 59 | 25 (42) | 22.9 | 0.15 | 307.5 | 34.8 | S4 |
| Pdf-Gal4 > UAS-t-Pdf, Dh31#51; Pdf01 | 54 | 22 (41) | 22.5 | 0.16 | 302.7 | 32.4 | S4 |
| R18H11-Gal4 > UAS-Dh31, Dh31#51; Pdf01 | 73 | 20 (27) | 22.5 | 0.11 | 487.4 | 63.7 | S4 |
| R18H11-Gal4 > UAS-t-Dh31, Dh31#51; Pdf01 | 45 | 17 (38) | 23.1 | 0.14 | 492.7 | 79.9 | 3 |
| R18H11-Gal4 > UAS-t-Pdf, Dh31#51; Pdf01 | 41 | 34 (83) | 22.2 | 0.16 | 506.1 | 46.3 | 3 |
| Pdfr 5304 | 55 | 28 (51) | 23.3 | 0.08 | 323.6 | 29.4 | 5 |
| Dh31r 1/Df | 61 | 57 (93) | 24 | 0.05 | 1033 | 81.22 | 5 |
| Pdfr5304; Dh31r1/Df | 84 | 50 (60) | 22.5 | 0.09 | 349.8 | 22.9 | 5 |
| Pdfr5304; Dh31#51 | 88 | 17 (19) | 23.4 | 0.15 | 320.2 | 29.9 | 5 |
Free-running rhythms were calculated from locomotor activity data sets from DD1 to DD10 for each genotype. Figures associated with the free-running rhythm data in each genotype are shown.
We also determined that the free-running period of the Dh31#51;Pdf01 double mutants was 23.1 h (Fig. 1I and Table 1), which was slightly longer than that of the Pdf01 mutants (22.5 h; Fig. 1H and Table 1) and shorter than that of the Dh31#51 mutants (24.4 h; Fig. 1G and Table 1). These data indicate that the Dh31 mutation did not enhance the shorter period caused by the Pdf mutation.
In terms of morning anticipation, compared to WT flies, both the Dh31#51;Pdf01 double mutants and Dh31#51 mutants exhibited abnormal morning anticipation (Fig. S1 and Table S1). However, the anticipation indexes of the Dh31#51;Pdf01 double mutants and Dh31#51 mutants were not significantly different (Table S2). Furthermore, both the Dh31#51;Pdf01 double mutants and Pdf 01 mutants similarly exhibited approximately one-hour advanced peaks in evening activity, which occurred at ZT 10.5–11 (Figs 1D,E and S1 and Tables S3, S4). Thus, the Dh31 mutation did not enhance the abnormal patterns of morning and evening anticipation caused by the Pdf mutation.
In summary, we determined that the lack of DH31 strongly enhanced the arrhythmic phenotype induced by the Pdf mutation but did not affect the free-running period, morning anticipation or timing of the evening activity peak in Dh31#51;Pdf01 double mutants. Therefore, we examined the functions of DH31 in regulating free-running rhythmicity, with a particular focused on the relationship between DH31 and PDF.
DH31 in tim-Gal4-expressing neurons contributes to free-running rhythmicity
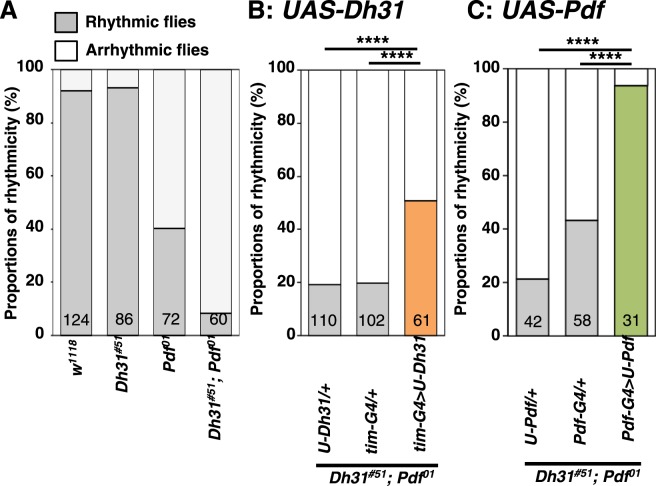
Given that Dh31#51;Pdf01 double-mutant flies exhibited disrupted free-running rhythmicity and that Dh31#51 mutants still showed normal free-running rhythmicity (Fig. 1), it is likely that an abnormal Dh31#51 phenotype might be invisible in the presence of normal PDF signaling. To examine this possibility, we asked whether DH31 expression in clock neurons could overcome the changes in rhythmicity identified in Dh31#51;Pdf01 double mutants.
Because we and others showed that DH31 is expressed in a subset of circadian clock cells (DN1ps)14,15, we first expressed DH31 in DN1ps using R18H11-Gal4 (a DN1p driver) (Fig. S4B). However, 73% of the rescued flies exhibited arrhythmicity (Figs S3G, S4B, and Table 1: R18H11-Gal4 > UAS-Dh31, Dh31#51;Pdf01), which was similar to the results for the Gal4 and UAS control flies (Fig. S3D,F and Table 1: R18H11-Gal4/+, Dh31#51;Pdf01, +/UAS-Dh31, Dh31#51;Pdf01). These data suggest that DH31 expression in R18H11-Gal4-expressing neurons is not sufficient to restore free-running rhythmicity.
R18H11-Gal4 is expressed in only ~ four to six DN1ps14, and we also found that DH31 is expressed in anterior DN1s (DN1as) (Supplemental Fig. S5). As such, DH31 expression from non-R18H11-Gal4-expressing DN1s might also play a role in regulating free-running rhythmicity. We therefore expressed DH31 using tim-Gal4 (a driver for all clock cells) in the Dh31#51;Pdf01 double mutants and assessed whether DH31 expression alone could prevent the severe arrhythmicity. We determined that 51% of the rescued flies showed restored free-running rhythmicity (Figs 2B and S3P, and Table 1: tim-Gal4 > UAS-Dh31, Dh31#51;Pdf01), while the Gal4 and UAS control flies from the double-mutant background still exhibited severe arrhythmic phenotypes (Figs 2B and S3B,F, and Table 1: tim-Gal4/+, Dh31#51; Pdf01, +/UAS-Dh31, Dh31#51; Pdf01). The data indicated that DH31 expression in tim-Gal4-expressing neurons in Dh31#51;Pdf01 mutants is sufficient to recover a similar level of rhythmicity to that of Pdf01 mutants (Fig. 2A,B) and suggested that DH31 in tim-Gal4-expressing neurons contributes to free-running rhythmicity.
Figure 2.
DH31 expression in tim-Gal4-expressing neurons or PDF expression in LNvs rescued severe arrhythmicity in Dh31-Pdf double mutants. (A-C) Comparison of free-running rhythms in different genotypes: w1118, Dh31#51, Pdf01 and Dh31#51;Pdf01 (A), UAS-Dh31/+, tim-Gal4/+ and tim-Gal4 > UAS-Dh31 from the Dh31#51;Pdf01 double-mutant background (B) and UAS-Pdf /+, Pdf-Gal4/+ and Pdf-Gal4 > UAS-Pdf from the Dh31#51;Pdf01 double-mutant background (C). The data in Fig. 2A are the reproduced from Fig. 1A. The proportions of rhythmic (gray bar) and arrhythmic (white bar) flies over 10 days in DD were compared via χ2 analysis. ****P < 0.0001. Numbers in the bar graphs represent the number of flies.
PDF and DH31 regulate free-running rhythmicity in a hierarchical fashion
Given that Pdf01 single mutants exhibited an arrhythmic phenotype, PDF should be able to regulate a free-running rhythm without DH31. To confirm this hypothesis, PDF was expressed in LNvs using Pdf-Gal4 (a LNv driver) in Dh31#51;Pdf01 double-mutant flies. These flies strongly recovered their free-running rhythm, with a rhythmicity level that was similar to that of Dh31#51 and WT flies (Figs 2A,C and S3O, and Table 1: Pdf-Gal4 > UAS-Pdf, Dh31#51;Pdf01), suggesting that PDF secretion from LNvs is sufficient to restore rhythmicity in these double-mutant flies. Taken together, we concluded that PDF and DH31 regulate free-running rhythmicity in a hierarchical fashion, in which PDF functions in a primary role, and DH31 functions in a secondary role.
Importantly, the disruption of morning anticipation and the advanced peak in evening activity in Dh31#51;Pdf01 double mutants were also recovered in Pdf-Gal4 > UAS-Pdf, Dh31#51;Pdf01 flies (S2I,L,M and Tables S1 and S2). These data also highlight that the overexpression of PDF from LNvs is sufficient to restore normal phenotypes in the double mutants and to prevent the abnormal morning anticipation phenotype in Dh31 mutants.
Both DH31 and PDF act on DN1ps to regulate free-running rhythmicity
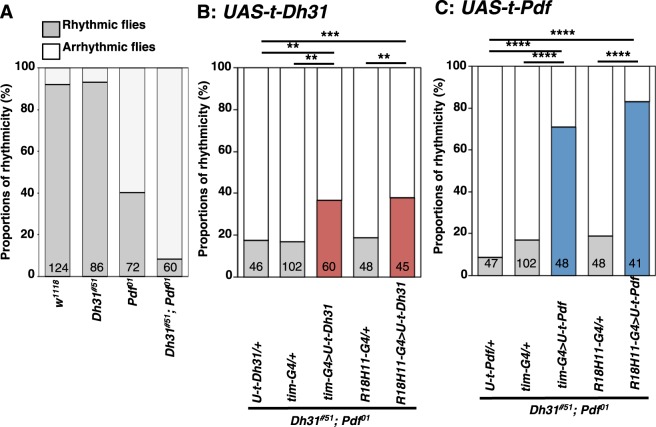
To determine how PDF and DH31 regulate free-running rhythmicity at the cellular level, we first verified that both PDF and DH31 act on clock cells to regulate free-running rhythmicity. Membrane-tethered peptides have both linker and anchor peptides that couple with the cell membrane, which results in cell-autonomous binding and the constant activation of the receptors on specific cells17,18. By using tethered-PDF (t-PDF), we determined that t-PDF expression in all clock cells using tim-Gal4 restored rhythmicity to 71% in the double mutants, showing a rhythmicity close to that of the Dh31#51 flies (Figs 3A,C and S3B,H,I, and Table 1: tim-Gal4 > UAS-t-Pdf, Dh31#51;Pdf01). Similarly, t-DH31 expression in all clock cells using tim-Gal4 also restored rhythmicity to 37%, which was similar to the rhythmicity of the Pdf01 flies (Figs 3A,B and S3A–C, and Table 1: tim-Gal4 > UAS-t-Dh31, Dh31#51;Pdf01). Therefore, we confirmed that both PDF and DH31 act on clock cells to regulate free-running rhythmicity.
Figure 3.
t-DH31 or t-PDF expression in DN1ps prevented severe arrhythmicity in Dh31-Pdf double mutants. (A-C) Comparison of free-running rhythms in different genotypes: w1118, Dh31#51, Pdf01 and Dh31#51;Pdf01 (A), UAS-t-Dh31/+, tim-Gal4/+, tim-Gal4 > UAS-t-Dh31, R18H11-Gal4 (DN1ps driver)/+ and R18H11-Gal4 > UAS-t-Dh31 from the Dh31#51;Pdf01 double-mutant background (B) and UAS-t-Pdf /+, tim-Gal4/+, tim-Gal4 > UAS-t-Pdf, R18H11-Gal4/+ and R18H11-Gal4 > UAS-t-Pdf from the Dh31#51;Pdf01 double-mutant background (C). The data in Fig. 3A are reproduced from Fig. 1A. The proportions of rhythmic (gray bar) and arrhythmic (white bar) flies over 10 days in DD were compared via χ2 analysis. ****P < 0.0001, ***P < 0.001 and **P < 0.01. Numbers in the bar graphs represent the number of flies.
LNvs are the main clock cells that regulate locomotor activity rhythms2,3,5, and bath application of PDF or DH31 can activate LNvs19. Therefore, we assessed whether DH31 and PDF act on LNvs to regulate rhythmicity. However, neither t-DH31 nor t-PDF expression in LNvs using Pdf-Gal4 was able to restore rhythmicity compared to the phenotypes of the Gal4 control flies (Figs S3K,L,M and S4C,D and Table 1: Pdf-Gal4 > UAS-t-Dh31 and Dh31#51;Pdf01, Pdf-Gal4 > UAS-t-Pdf, Dh31#51;Pdf01). Therefore, our data suggest that both PDF and DH31 are less likely to act on LNvs to modulate rhythmicity.
We subsequently focused on DN1s because small LNvs (sLNvs) project to DN1s20–22, PDF acts on DN1ps to regulate locomotor activity22,23, and Pdfr expression in DN1ps restores the dampened free-running rhythm in Pdfr mutant flies23. We determined that flies with t-PDF expression in DN1ps using R18H11-Gal4 showed 83% rhythmicity, which was close to the rhythmicity of the Dh31#51 flies (Figs 3A,C and S3J, and Table 1: R18H11-Gal4 > UAS-t-Pdf, Dh31#51;Pdf01). Therefore, we confirmed that t-PDF expression in DN1ps rescues the severe disruption of free-running rhythm identified in Dh31#51;Pdf01 double mutants.
To determine whether DH31 also acts on the same group of clock cells, we expressed t-DH31 in DN1ps and assessed the effect on free-running rhythmicity. When t-DH31 was expressed in the DN1ps of Dh31#51;Pdf01 double-mutant flies using the R18H11-Gal4 driver, the free-running rhythmicity was restored to the same level as that of the Pdf01 flies (38%, Figs 3A,B and S3E, and Table 1: R18H11-Gal4 > UAS-t-Dh31, Dh31#51;Pdf01). The rhythmicity of these flies was significantly different from that of the Gal4 or UAS control flies from the double-mutant background (Fig. 3B and Table 1). These results suggest that DH31 also acts on DN1ps to modulate free-running rhythmicity. Thus, our data suggest that both PDF and DH31 can act on DN1ps to regulate free-running rhythmicity.
Dh31 mutation did not enhance the abnormal molecular oscillations caused by the Pdf mutation
To further examine the role of DH31 in the arrhythmic phenotype, we focused on the molecular oscillations in each clock cell. Given that the Pdf mutation causes abnormal molecular oscillations in clock cells8,9 and that the Dh31 mutation enhanced the abnormal free-running rhythms caused by the Pdf mutation (Fig. 1A), we suspected that molecular oscillations in the clock cells of Dh31#51;Pdf01 double mutants would be severely dampened compared to those of Pdf01 mutants.
To test this possibility, we examined the molecular oscillations in each group of clock cells (LNv, LNd, and DN1) by measuring the expression of Vrille (VRI), which is a component of the second clock feedback loop in the core molecular clock system24. In most cases, severe arrhythmicity in the Dh31#51;Pdf01 double mutants was identified by three days after the shift from LD to DD; thus, we compared the expression levels of VRI in each mutant maintained at DD3 (Fig. S3Q). We determined that the intensities of VRI expression in LNvs and LNds in all mutant flies (Dh31#51, Pdf01 and Dh31#51;Pdf01) were less than those in WT flies at ZT 13 and 19 (Figs 4A,B and S6 and Tables S5, S6). Notably, the peak of VRI expression in LNds in Dh31#51 was delayed compared to that in WT and the other mutants (Figs 4B and S6), suggesting that DH31 is involved in regulating molecular rhythms in LNds. Furthermore, the Pdf01 and Dh31#51;Pdf01 mutants exhibited a severe disruption in the molecular rhythm in their DN1s (Figs 4C and S6 and Tables S5, S6). However, there was no significant difference between the Pdf01 and Dh31#51;Pdf01 mutants in LNvs, LNds or DN1s (Figs 4 and S6 and Tables S5, S6). Therefore, the data did not show a significant difference in VRI expression between Pdf01 and Dh31#51;Pdf01 double mutants, suggesting that the Dh31 mutation does not enhance the abnormal molecular oscillations caused by the Pdf mutation. Thus, the role of Dh31 in regulating free-running rhythmicity may differ from that of Pdf.
Figure 4.
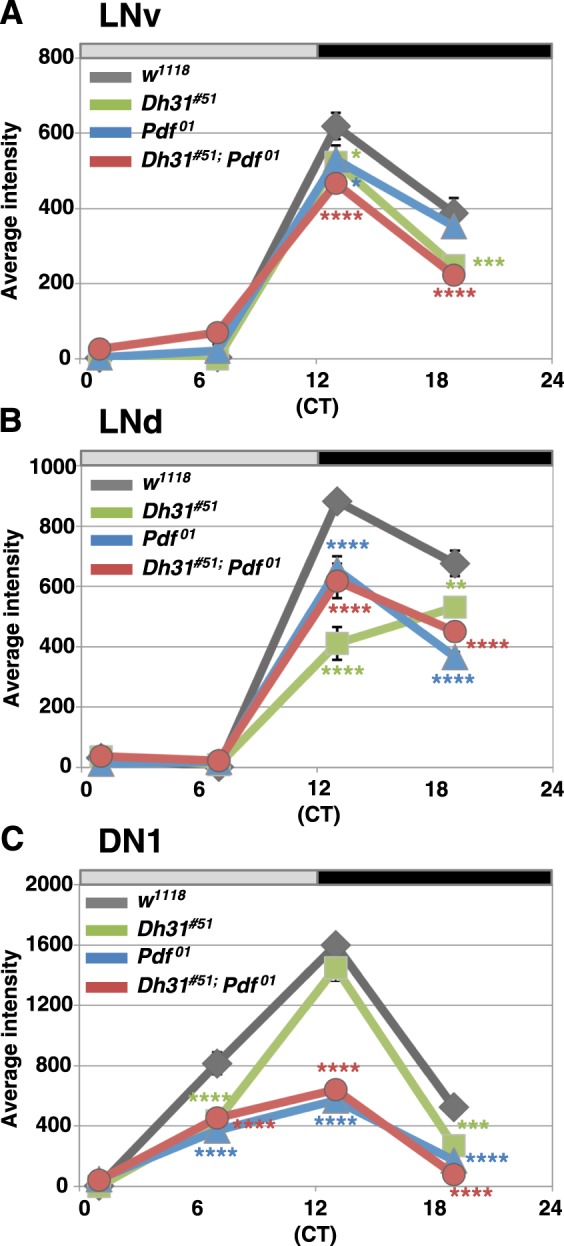
Dh31 mutation did not enhance the abnormal molecular oscillations caused by Pdf mutation. The average levels of VRI expression in 10 brain hemispheres in each subgroup of clock cells (LNv, LNd and DN1) among WT, Dh31#51, Pdf01 and Dh31#51;Pdf01 double mutants at the indicated time points ((circadian time (CT) 1, 7, 13 and 19) in DD3. LNv (A), LNd (B) and DN1 (C). The detailed data of the average intensity are shown in Table S5 The variation of the average intensity in a day in each genotype was compared using two-way ANOVA and Sidak’s multiple-comparison test. The results for comparisons with WT flies at each time point are shown: ****P < 0.0001, ***P < 0.001, **P < 0.01 or *P < 0.05. The remaining comparisons are shown in Table S4.
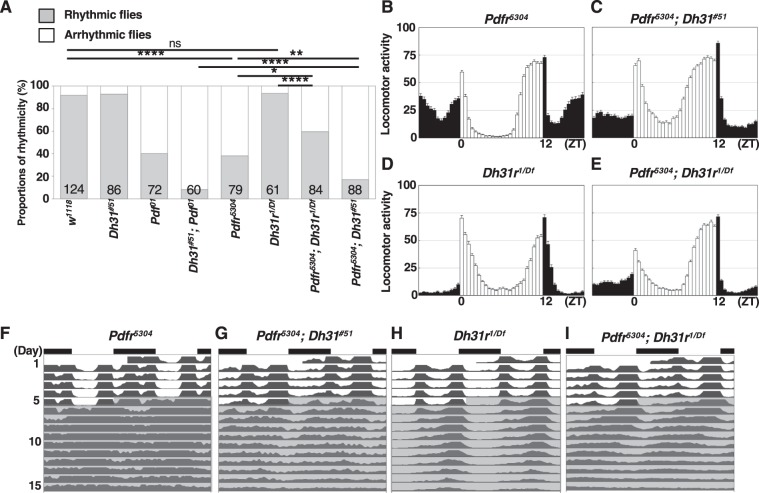
Dh31r mutation did not enhance the severe disruption of locomotor activity rhythms in Pdfr mutant flies
We subsequently focused on the functions of PDF and DH31 receptors in locomotor activity rhythms. In Dh31 receptor (Dh31r) loss-of-function mutant flies (Dh31rf05546/Df(2 R)BSC273, referred to here as Dh31r1/Df), Dh31r mRNA levels in the head were 38% of those observed in w1118 flies25. We recently found that these mutant flies showed abnormal TPRs; however, they exhibited normal locomotor activity rhythms (Fig. 5A,D,H and Table 1: Dh31r1/Df)25. Given the phenotype of Dh31#51;Pdf01 double mutants in locomotor activity rhythms, we expected the Dh31r mutation to also enhance the Pdfr mutant phenotype. To this end, we created Pdfr5304;Dh31r1/Df double mutants. First, we confirmed that Pdfr5304 mutants exhibited a weak rhythmicity (51% rhythmic and power = 323.6), shorter period (23.3 h), loss of morning anticipation and advanced phases in the evening activity peak (Fig. 5A,B,F and Tables 1 and S1), which were very similar to the phenotype of Pdf01 mutants. These findings suggest that Pdfr5304 mutants represent a phenocopy of Pdf01 mutants12,26,27. However, the Pdfr5304;Dh31r1/Df double-mutant phenotype still exhibited 60% rhythmicity (Fig. 5A,I and Table 1), which was similar to that of the Pdfr5304 single mutants (51% rhythmic, Table 1). The morning anticipation and phase-advanced evening activity peak phenotypes were also not influenced by the double mutation of Pdfr and Dh31r (Fig. 5E and Tables S1 and S2). Thus, these data indicate that the Dh31r mutation did not enhance the arrhythmic phenotype caused by the Pdfr mutation.
Figure 5.
DH31R is not involved in locomotor activity. (A) Comparison of free-running rhythms in different genotypes. The proportions of rhythmic (gray bar) and arrhythmic (white bar) flies over 10 days in DD were compared via χ2 analysis. ****P < 0.0001, **P < 0.01 or *P < 0.05. Numbers in the bar graph represent the number of flies. (B-E) Average daily actogram over 4 days in LD for each genotype: Pdfr5304 (B), Pdfr5304; Dh31#51 (C), Dh31r1/Df (D) and Pdfr5304;Dh311/Df (E). (F-I) Double-plotted averaged actogram of rhythmic flies over 5 days in LD and 10 days in DD for each genotype: Pdfr5304 (F), Pdfr5304;Dh31#51 (G), Dh31r1/Df (H) and Pdfr5304;Dh311/Df (I).
Furthermore, as a control, we generated Pdfr5304;Dh31#51 double mutant flies and tested their locomotor activity rhythms. Pdfr5304;Dh31#51 double mutant flies showed only 23% rhythmicity in free running (Fig. 5A,G and Table 1), which was significantly lower than that of Pdfr5304 or Pdfr5304;Dh31r1/Df double-mutant flies (51% or 60%, respectively) (Fig. 5A,F,I and Table 1). The results suggested that both PDF and DH31 signals regulate robust locomotor activity rhythms but that DH31R is unlikely to regulate locomotor activity rhythms.
Discussion
PDF and DH31 regulate free-running rhythmicity in a hierarchical fashion in DN1ps
We demonstrated a novel function of DH31 in regulating Drosophila locomotor activity rhythms. We showed that Dh31#51 mutants maintained a robust free-running rhythm (Fig. 1 and Table 1), whereas Dh31#51;Pdf01 double-mutant flies exhibited a severe disruption of their free-running rhythm compared to Pdf01 mutants (Fig. 1 and Table 1). These findings suggest that Dh31#51 mutants maintain a robust free-running rhythm because the primary factor, PDF, can sustain a strong rhythm (Figs 1 and 6). We showed that ~40% of Pdf01 single-mutant flies exhibited a preserved rhythmic state, which is because DH31 can partially support free-running rhythmicity. Thus, the severe disruptions of free-running rhythm in Pdf01 and Dh31#51 double-mutant flies is likely caused by the loss of both pathways.
Figure 6.
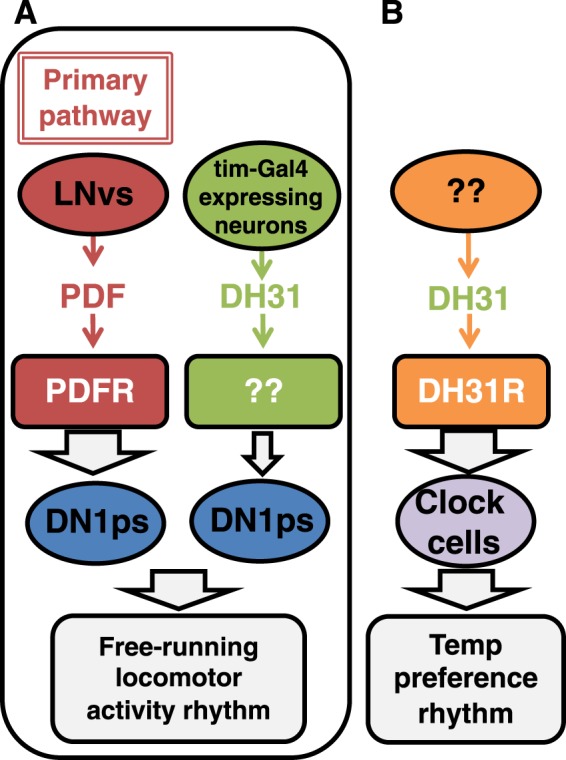
Schematic diagram of the relationship between DH31 and PDF in regulating free-running rhythmicity. (A) A model of the relationship between DH31 and PDF in regulating free-running rhythms. In the primary pathway, PDF acts on DN1ps via the PDFR. In the secondary pathway, DH31 acts on DN1ps via unknown receptor(s). PDF and DH31 independently regulate free-running rhythmicity; however, the effect of the primary pathway on rhythmicity is dominant to that of the secondary pathway. (B) We have previously shown that DH31R regulates TPR25.
PDF is secreted from the main circadian neurons, LNvs, and acts on other clock cells through PDFR to synchronize and maintain robust molecular rhythms11,19. We showed that PDF expression from LNvs in Dh31#51;Pdf01 mutants restored rhythmicity (Fig. 2C), in contrast to t-PDF expression in LNvs (Fig. S4D), indicating that an autoreceptor of PDF signals in LNvs is not sufficient to maintain rhythmicity. Instead, we showed that t-PDF expression in DN1ps restored rhythmicity, which suggests that PDF signaling in DN1ps is sufficient to maintain robust free-running rhythmicity (Fig. 3C). Recently, the responsiveness to PDF was shown to be strongly altered for 24 h via RalA GTPase in sLNvs28. Therefore, we expect that the continuous activation of PDFR by t-PDF generates rhythmic downstream signaling in PDFR-expressing neurons.
We also showed that molecular oscillations in DN1s were strongly dampened in Pdf01 mutants compared with WT flies (Fig. 4C). These data are consistent with previous studies in which the molecular oscillations of PER in Pdf01 mutants held under DD conditions were dampened in DN1s10 and the genetic manipulation of the circadian clocks in PDF-positive cells altered the molecular rhythms in DN1ps23,29. Furthermore, Pdfr expression in DN1ps has been reported to prevent the arrhythmic phenotype in Pdfr 5304 mutants23. These findings support the idea that PDF is secreted from LNvs and acts on DN1ps to regulate free-running rhythmicity.
Furthermore, we showed that t-DH31 expression in DN1ps rescued the Pdf01 and Dh31#51 double-mutant phenotypes, which suggests that DH31 acts on DN1ps to regulate rhythmicity (Fig. 3B). Although it has been suggested that DH31 release might increase at dawn14 and that DH31-mRNA expression levels oscillate for 24 h13, how t-DH31 expression causes rhythmic behavioral output remains unclear. Because DH31 can modestly activate PDFR in vitro12, we cannot exclude the possibility that t-DH31 overexpression might simply activate PDFR in DN1ps instead of the intrinsic PDF signals, thereby restoring locomotor activity rhythms in the flies. However, the rhythmicity of Dh31#51;Pdf01 mutants overexpressing t-DH31 in tim-Gal4-expressing neurons or R18H11-Gal4-expressing DN1ps only reached levels similar to that of the Pdf01 single-mutant flies (Fig. 3A,B). Therefore, DH31 likely acts on DN1ps separately from the PDF pathway.
Although we and others have shown that DH31 is expressed in a subset of DN1ps14,15, DH31 expression using R18H11-Gal4 did not rescue the Pdf01 and Dh31#51 double-mutant phenotypes (Fig. S4B), suggesting that DH31 expression in R18H11-Gal4-expressing neurons is insufficient to maintain rhythmicity. Instead, we showed that DH31 is expressed in DN1as (Fig. S5) and that DH31 expression in tim-Gal4-expressing neurons rescued the phenotype (Fig. 2B), which suggests that DH31 expression in clock neurons maintains rhythmicity. That said, given that DH31 is expressed in nonclock neurons14,15 and that tim-Gal4 is expressed in nonclock cells30, we cannot exclude the possibility that DH31 expression in nonclock neurons might play a role in rescuing the severe phenotype of Dh31#51;Pdf01 mutants. Alternatively, although DH31 expression in LNvs was not detectable via anti-DH31 antibody staining15, a recent RNA-seq analysis detected Dh31 gene expression in both LNvs and DN1s13. Therefore, DH31 expression from LNvs may potentially act on DN1s to support locomotor activity rhythms.
In summary, we propose that PDF and DH31 regulate free-running rhythms in a hierarchical fashion in DN1ps (Fig. 6). As t-DH31 or t-PDF expression in DN1ps resulted in a similar level of rhythmicity as that observed in flies expressing t-DH31 or t-PDF, respectively, in tim-Gal4-expressing neurons (Fig. 3), DN1ps are at least one of the important clock cells that regulate free-running rhythmicity.
The roles of DH31 in locomotor activity rhythms
Given that Dh31#51;Pdf01 mutants exhibited severe arrhythmicity in free-running rhythm, we speculated that the severe arrhythmic phenotype might be a result of abnormal molecular oscillations. However, the molecular oscillations of Dh31#51;Pdf01 mutants were similar to those of Pdf01 mutants (Fig. 4). Therefore, the molecular mechanisms by which DH31 regulates free-running rhythms still remain unclear. Importantly, the peak of VRI expression in LNds in Dh31#5 was at ZT 19, which was delayed compared with those of WT flies and the other mutants (Fig. 4B). The data suggested that DH31 is involved in the regulation of molecular oscillations in LNds. Because LNds are the evening pacemaker2,3, the delayed VRI oscillations in LNds might be associated with the longer period of free-running rhythm in Dh31#51 (24.4 h, Table 1).
Recently, the intracellular calcium rhythms in each clock cell were reported to be nonsynchronous and associated with morning and evening peaks in locomotor activity4. DH31 signaling may possibly contribute to the downstream output that controls molecular rhythms in pacemaker processes, such as intracellular calcium rhythms. Given that PDF from sLNvs regulates strong molecular rhythms in DN1ps and generates robust free-running rhythms under constant conditions10,23,29, DH31 may help maintain vigorous output signals downstream of the molecular clocks in DN1ps.
DH31 receptor is unlikely to regulate locomotor activity rhythms
We recently showed that both Dh31r1/Df mutants and flies undergoing Dh31r knockdown in their neurons showed normal rhythmicity in the locomotor activity rhythm25. In contrast to Dh31#51;Pdf01 double mutants, Pdfr5304;Dh31r1/Df double mutants did not enhance the arrhythmicity observed in Pdfr single mutants (Fig. 5A,F,I and Table 1), which suggests that DH31R does not complement PDFR function; thus, DH31R does not function as a receptor for DH31 in this context. Given that Dh31r1/Df flies showed a strong abnormality in the TPR phenotype25, it is more likely that DH31R does not play an important role in locomotor activity rhythms. However, Dh31r1/Df mutants are not null25, and we cannot exclude the possibility that a small amount of residual DH31R might drive robust locomotor activity rhythms with the PDF pathway.
Which receptors might function with DH31 to regulate free-running rhythmicity? Given that DH31 can activate PDFR in vitro12, bath applications of DH31 can activate LNvs via PDFR19 and DH31 can function as a ligand of PDFR in TPR at the onset of night15, PDFR may function as a receptors for both DH31 and PDF in the regulation of free-running rhythmicity. However, because the arrhythmicity of Pdfr5304 mutants was not as severe as that of Dh31#51;Pdf01 mutants (Fig. 5), PDFR does not appear to act as a receptor for DH31 in this context (Fig. 6).
Both DH31R and PDFR are class II G-protein coupled receptors (GPCRs), which also include Hector and Diuretic hormone 44 receptors 1 and 2 (DH44R1 and DH44R2, respectively)31,32. Interestingly, the DH44R1 and DH44R2 ligand DH44 has been implicated in circadian output circuits21,33. Therefore, although there is no evidence from in vitro or in vivo experiments, these receptors might nevertheless function as receptors for DH31 to regulate free-running rhythmicity.
Orchestration of neuropeptides regulates locomotor activity rhythms in species ranging from flies to mammals
The orchestration of neuropeptides is critical for regulating circadian clock functions in species that range from flies to mammals. In mammals, several neuropeptides, including vasoactive intestinal polypeptide (VIP), arginine vasopressin (AVP) and neuromedin S (NMS), are expressed in the SCN, which is the center for circadian clock control34,35. The hierarchy of neuropeptide signaling contributes to circadian function in the SCN36. Several recent studies in Drosophila have identified the neuropeptides, including ion transport peptide (ITP)37, neuropeptide F (NPF)38, allatostatin A39, short neuropeptide F40, leucokinin33 and DH4421, that regulate locomotor activity and sleep. However, given that DH31 complements the function of PDF in regulating free-running rhythmicity in the same clock cells, DH31 not only serves as one of the neuropeptides that regulates circadian rhythms but also might selectively influence PDF function in the regulation of free-running rhythms. Thus, our findings shed new light on the next steps required to improve our understanding of the core neuropeptide regulatory mechanisms involved in the circadian rhythm.
Materials and Methods
Fly lines and the generation of transgenic flies
All flies were raised in 12 h light/dark cycles at 25 °C; Zeitgeber Time (ZT) 0 was lights-on, ZT12 was lights-off. w1118 (RRID:BDSC_3605) flies were used as WT flies. UAS-Dh31 was a kind gift of Dr. Paul Taghert. Membrane-tethered DH31 (UAS-t-DH31-ML:B4) and membrane-tethered PDF (UAS-t-PDF-ML:M2a) were used17. yw; Pdf01(RRID:BDSC_26654) flies, yw;Dh31#51 (FBal0304655) flies16 and Pdfr5304 (RRID:BDSC_33068) flies were backcrossed with w1118 flies. tim-Gal4 (RRID:BDSC_7126) (expressed in all clock neurons), Pdf-Gal4 (RRID:BDSC_6900) (expressed in LNvs), and R18H11-Gal4 (RRID:BDSC_48832) (expressed in ~4–6 DN1s)14,20 were obtained from the Bloomington Drosophila Stock Center (stock #7126, # 6900 and #48832, respectively). All Gal4 driver and UAS reporter flies from the Dh31#51;Pdf01double-mutant background were generated via chromosome recombination with w;Dh31#51;Pdf01 double-mutant flies. Dh31r1 is a P-element insertion mutant (PBac{WH}Dh31-Rf05546) and was obtained from the Exelixis Collection at the Harvard Medical School. Dh31rDf is a deletion mutant (Df(2R)BSC273) (RRID:BDSC_23169) and was obtained from the Bloomington Drosophila Stock Center (stock # 23169).
Behavioral analysis of locomotor activity
Locomotor activity assays and data analysis were performed as previously described15,41,42. Flies were reared under 12 h light/dark (LD) cycles at 25 °C. Male flies (1 to 5 days old) were used in the locomotor activity experiments. A Drosophila Activity Monitoring (DAM) system (http://www.trikinetics.com/) was placed in an incubator (MIR-154, Sanyo Scientific, Japan). Lights in the incubator (15-W cool white fluorescent lamps (FL15D, TOSHIBA, Japan)) were connected to an electric timer; the light intensity was approximately 800 lux. Locomotor activity was monitored in 12 h LD cycles for five days and in a constant dark condition for more than ten days at 25 °C. The data were analyzed using Actogram J software43. Free-running periods and power values were calculated using a chi-square periodogram42,44, and flies with a power value < 100 were defined as arrhythmic. Only data from rhythmic flies were averaged to generate a double-plotted actogram. Morning anticipation index (AI) values were calculated as previously described22,45,46. Briefly, AI = (total activity 3 h before lights-on)/(total activity 6 h before lights-on). The AIs of all flies over days 2–5 of the LD cycles were averaged in each genotype. The AIs for different genotypes were compared using Tukey’s multiple-comparison test. The time of evening peaks in all flies over days 2–5 of the LD cycles were averaged in each genotype. The averaged time of evening peaks for different genotypes were compared using Tukey’s multiple-comparison test.
Anti-VRI immunohistochemistry and signal intensity quantification
The signals from VRI antibody staining are relatively strong and specific47. Given that VRI is not degraded by light, we think that VRI staining is easier to handle compared with TIM staining. Therefore, we used VRI to examine the molecular oscillations in each group of clock cells (LNv, LNd, and DN1) in Fig. 4. w1118, Dh31#51, Pdf01, and Dh31#51;Pdf01 flies were raised under 12 h LD cycles at 25 °C. Adult male flies were subsequently entrained to LD cycles for 3 to 4 days and then shifted to constant dark conditions for 3 days (DD3). The flies were fixed at CT 1, 7, 11 or 19 with 4% paraformaldehyde in PBST (PBS plus 0.3% Triton X-100) for 2 h at room temperature, following brain dissection. Immunostaining was performed as previously described15. Briefly, 5% normal goat serum in PBST was used for blocking and antibody incubations with guinea pig anti-VRI antibody (1:200)47 and donkey anti-guinea pig Alexa Fluor® 647 (RRID:AB_10895029) (1:200, Jackson ImmunoResearch). Mounted brains were scanned using a Zeiss LSM5 Pascal confocal microscope. Images were digitally projected as Z-stacks for immunohistochemical analysis. ImageJ software was used to quantify the intensity of the immunostaining signal in each single cell (LNv, LNd and DN1). After background subtraction, the total intensity of each type of clock cell in a brain hemisphere was determined, and the average intensity of 10 brain hemispheres was calculated in Excel.
Experimental design and statistical analysis
Free-running rhythmicity
The proportions of rhythmic and arrhythmic flies in each genotype were compared using χ2 analysis in Prism 7 software, GraphPad Software, Inc. The sample number and proportion of rhythmic flies are shown in Table 1.
AI and time of evening peak
The AI and time of evening peak in each genotype was compared using one-way ANOVA and the Tukey-Kramer test in Prism 7 software, GraphPad Software, Inc. The value of each AI or time of evening peak, sample number, SEM and detailed statistical analysis results are shown in Tables S1–S4.
Signal intensity quantification for immunohistochemistry
The variation of the average intensity in a day in each genotype was compared using two-way ANOVA and Sidak’s multiple-comparison test in Prism 7 software, GraphPad Software, Inc. The results of comparisons with WT flies at each time point are shown in Fig. 4. The remaining comparisons are shown in Table S6. The detailed data of the average intensity, SEM, and sample number are shown in Table S5.
Supplementary information
Acknowledgements
We are grateful to Dr. Paul Taghert for the Pdf01, Pdfr5304, UAS-Pdf, and UAS-Dh31 flies, Dr. Michael Nitabach for the UAS-t-Dh31 and UAS-t-Pdf flies and anti-DH31 antibody, Dr. Paul Hardin for the anti-VRI antibody, and the Bloomington Drosophila fly stock center for the fly lines. We thank Dr. Christian Hong and the Hamada lab members for their comments and advice on the manuscript. This research was supported by RIP funding from the Cincinnati Children’s Hospital, JST (Japan Science and Technology)/Precursory Research for Embryonic Science and Technology (PRESTO), the March of Dimes, and an NIH R01 grant GM107582 to F.N.H.
Author Contributions
F.N.H. and T.G. designed the research. Y.U., H.W.S. and T.G. performed the behavioral experiments. F.A. and T.G. performed the immunostaining. F.N.H., T.G. and Y.U. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37107-3.
References
- 1.Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 3.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 4.Liang X, Holy TE, Taghert PH. Synchronous Drosophila circadian pacemakers display nonsynchronous Ca(2)(+) rhythms in vivo. Science. 2016;351:976–981. doi: 10.1126/science.aad3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Z, Shafer OT. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science. 2014;343:1516–1520. doi: 10.1126/science.1251285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS biology. 2009;7:e1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/S0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshii T, et al. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila’s clock. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klarsfeld A, et al. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS biology. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertens I, et al. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Abruzzi KC, et al. RNA-seq analysis of Drosophila clock and non-clock neurons reveals neuron-specific cycling and novel candidate neuropeptides. PLoS genetics. 2017;13:e1006613. doi: 10.1371/journal.pgen.1006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunst M, et al. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Current biology: CB. 2014;24:2652–2664. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goda T, et al. Drosophila DH31 Neuropeptide and PDF Receptor Regulate Night-Onset Temperature Preference. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:11739–11754. doi: 10.1523/JNEUROSCI.0964-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Head LM, et al. The influence of light on temperature preference in Drosophila. Current biology: CB. 2015;25:1063–1068. doi: 10.1016/j.cub.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi C, et al. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Current biology: CB. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi C, et al. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer OT, et al. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo F, et al. Circadian neuron feedback controls the Drosophila sleep–activity profile. Nature. 2016;536:292–297. doi: 10.1038/nature19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanaugh DJ, et al. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seluzicki A, et al. Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior. PLoS biology. 2014;12:e1001810. doi: 10.1371/journal.pbio.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, et al. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Current biology: CB. 2010;20:591–599. doi: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blau J, Young MW. Cycling vrille expression is required for a functional Drosophila clock. Cell. 1999;99:661–671. doi: 10.1016/S0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- 25.Goda T, et al. Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals. Genes Dev. 2018;32:140–155. doi: 10.1101/gad.307884.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyun S, et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Lear BC, et al. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Klose M, et al. Functional PDF Signaling in the Drosophila Circadian Neural Circuit Is Gated by Ral A-Dependent Modulation. Neuron. 2016;90:781–794. doi: 10.1016/j.neuron.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Z, Bennett AJ, Clem JL, Shafer OT. The Drosophila Clock Neuron Network Features Diverse Coupling Modes and Requires Network-wide Coherence for Robust Circadian Rhythms. Cell Rep. 2016;17:2873–2881. doi: 10.1016/j.celrep.2016.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko M, Park JH, Cheng Y, Hardin PE, Hall JC. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol. 2000;43:207–233. doi: 10.1002/(SICI)1097-4695(20000605)43:3<207::AID-NEU1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Hector CE, Bretz CA, Zhao Y, Johnson EC. Functional differences between two CRF-related diuretic hormone receptors in Drosophila. The Journal of experimental biology. 2009;212:3142–3147. doi: 10.1242/jeb.033175. [DOI] [PubMed] [Google Scholar]
- 32.Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11:1126–1142. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavey M, Collins B, Bertet C, Blau J. Circadian rhythms in neuronal activity propagate through output circuits. Nat Neurosci. 2016;19:587–595. doi: 10.1038/nn.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nature reviews. Neuroscience. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermann-Luibl C, Yoshii T, Senthilan PR, Dircksen H, Helfrich-Forster C. The ion transport peptide is a new functional clock neuropeptide in the fruit fly Drosophila melanogaster. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:9522–9536. doi: 10.1523/JNEUROSCI.0111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He C, et al. Regulation of circadian locomotor rhythm by neuropeptide Y-like system in Drosophila melanogaster. Insect Mol Biol. 2013;22:376–388. doi: 10.1111/imb.12027. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, et al. Allatostatin A Signalling in Drosophila Regulates Feeding and Sleep and Is Modulated by PDF. PLoS genetics. 2016;12:e1006346. doi: 10.1371/journal.pgen.1006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shang Y, et al. Short neuropeptide F is a sleep-promoting inhibitory modulator. Neuron. 2013;80:171–183. doi: 10.1016/j.neuron.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaneko H, et al. Circadian Rhythm of Temperature Preference and Its Neural Control in Drosophila. Current biology: CB. 2012;22:1851–1857. doi: 10.1016/j.cub.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umezaki Y, Yoshii T, Kawaguchi T, Helfrich-Forster C, Tomioka K. Pigment-dispersing factor is involved in age-dependent rhythm changes in Drosophila melanogaster. Journal of biological rhythms. 2012;27:423–432. doi: 10.1177/0748730412462206. [DOI] [PubMed] [Google Scholar]
- 43.Schmid B, Helfrich-Forster C, Yoshii T. A new ImageJ plug-in “ActogramJ” for chronobiological analyses. Journal of biological rhythms. 2011;26:464–467. doi: 10.1177/0748730411414264. [DOI] [PubMed] [Google Scholar]
- 44.Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. Journal of theoretical biology. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-X. [DOI] [PubMed] [Google Scholar]
- 45.Harrisingh MC, Wu Y, Lnenicka GA, Nitabach MN. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:12489–12499. doi: 10.1523/JNEUROSCI.3680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheeba V, Fogle KJ, Holmes TC. Persistence of morning anticipation behavior and high amplitude morning startle response following functional loss of small ventral lateral neurons in Drosophila. PloS one. 2010;5:e11628. doi: 10.1371/journal.pone.0011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glossop NR, et al. VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron. 2003;37:249–261. doi: 10.1016/S0896-6273(03)00002-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.