Abstract
The large volume of municipal sludge causes environmental problems in cities worldwide. In this study, municipal sludge, mixed with construction waste residue, was used as substrate to plant Ailanthus altissima. The growth of A. altissima, the substrate characteristics, and substrate microbial diversity were measured to investigate potential recycling and reusing pathways of municipal sludge. The obtained results showed that compared to garden soil, the mixed substrate was weakly alkaline, and had higher nutrient contents, which is beneficial for A. altissima, and results in better growth in mixed substrate. Although the contents of the main heavy metals in the mixed substrate were significantly higher than in garden soil, the values met the criterion of Class II soil in the Environmental Quality Standard for the Soils of China (GB15618-1995). Different substrates showed a variety of microbial diversities. Proteobacteria was the top microbial phylum in all samples, and higher relative abundances were found in samples containing municipal sludge. After growing A. altissima, the relative abundances of Acidobacteria and Gemmatimonadetes increased in the mixed substrate. Therefore, addition of construction waste residue and growth of A. altissima caused a difference. The microbial communities in the mixed substrate with A. altissima are both plant friendly and environmentally friendly. These results suggest this mixed substrate as a potentially feasible and effective pathway for the reuse and recycling of municipal sludge for vegetation restoration.
Introduction
Excessive amounts of municipal sludge with high pollutant content have become a worldwide environmental problem. Especially in cities, the treatment and disposal of municipal sludge poses severe challenges due to land shortages, strict legal regulations, increasing costs, and public concern1. For example, China generates a large volume of sewage per year. In 2015 about 40 million tons of municipal sludge were generated, and the yield has been predicted to reach almost 80 million tons. Reducing the volume of municipal sludge is the dominant disposal goal of municipal sludge treatment. In China, landfill, composting, natural drying, and burning are the four main treatment modes of municipal sludge. The disposal amount by landfill accounts for 65.00% of the total municipal sludge, which may result in secondary pollution. Ecological and environmentally friendly recycling and reusing modes are urgently required for the treatment of municipal sludge.
Municipal sludge contains an abundance of nutrients. Dewatered municipal sludge contains 50.00–70.00% organic matter, 30.00–50.00% mineral components, 3.40–4.00% N, 0.50–2.50% P, and a large amount of micronutrients2–4. Therefore, municipal sludge has good potential for use as soil conditioner and fertilizer in land application. Due to relatively low contents of lignin or cellulose, the organic matter in municipal sludge mineralizes and degrades rapidly, thus generating and increasing abundant nitrate in soils5. The addition of municipal sludge composts to the soil improves soil properties, such as bulk density, porosity, and water holding capacity6. A significant enrichment in mineral nitrate and available phosphorus is detected in soil after municipal sludge application and both the pH value and electrical conductivity also increase7. However, municipal sludge also contains different contaminants, including heavy metals, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), adsorbable organohalogens (AOX), pesticides, surfactants, hormones, pharmaceuticals, and nanoparticles8. These can create potential pollution to the soil and are hazardous to the health of humans9,10. Therefore, the land application of municipal sludge in agriculture and horticulture has security risks, while the application of municipal sludge in forests is a relatively ideal disposal pathway. The advantages are that forests are often deficient in major nutrients that can be found in abundance in municipal sludge, and that forests are not the major contributors to the human food chain.
Municipal sludge not only improves forestry soil fertility by increasing soil organic matter and available nitrogen and phosphorus, but also promotes the growth and reproduction of trees, including increases in the height and diameter of trees, which is advantageous for the growth of shrubs and herbs of trees11. The application of municipal sludge containing low heavy metal concentrations also improves the structure and the organic matter of the clay loam brown forest soil12. Moreover, municipal sludge has also been used as substrate for vegetation restoration and reconstruction13,14, which is a disposal route with broad prospect. All relevant previous studies have focused on the effect of municipal sludge on soil properties and plant growth; however, the influence on affected microbes received little attention. On the one hand, the many microbes in municipal sludge are pathogenic species, which may result in the pollution of soil. On the other hand, many microbes also can improve the physical and chemical features of soil to promote the growth of plants. So during the application of municipal sludge, the change of microbe diversity need to be investigated.
The construction waste is a main solid waste in city, which causes huge consumption of limited resources and leads to serious environment problem. In some areas all or part of construction waste is unlawfully deposited on land, or in natural drainages including water, contrary to regulations to protect human health, commerce and the environment. At present, landfill is the main disposal pathway of the construction waste, which increases the burden on landfill loading and operation, and the waste from sources such as solvents or chemicals can result in soil and water pollution. China is the largest producer of construction waste in the world. In 2015, totally 2,400 million tons of construction waste was produced in China, which accounts for 40% of the total amount of municipal waste. It is predicted that the amount of construction waste will arrived at 50 and 75 tons in 2020 and 2030, respectively15. But at present, the recycling utilization rate of construction waste is only about 5% in China, so the big city besieged by plenty of waste, which harms the urban safety and ecological development. Thus the effective and harmless method for the disposal of construction waste is in serious demand. Basis on the laboratory study of construction waste in the substrate mix for extensive green roofs16, we think that using construction waste as substrate in vegetation restoration is feasible.
In this study, the application of construction waste combined with municipal sludge in vegetation restoration was considered. The seeds of Ailanthus altissima were sowed on the substrate composed of municipal sludge mixed with construction waste residue. Then the growth of A. altissima, the substrate characteristics, and substrate microbial diversity were measured to investigate the potential recycling and reusing pathway of municipal sludge.
Results
The pH values of different substrates were tested (Fig. 1). The pH value of pure sludge (W) was about 7.00, while the pH value of garden soil (TYC) was about 8.00. When the sludge was mixed with construction waste residue (WJ), the pH value significantly increased by 6.90% compared with W (P <0.05). And after planting A. altissima, the pH value of mixed substrate (WJC) rose to 7.80.
Figure 1.
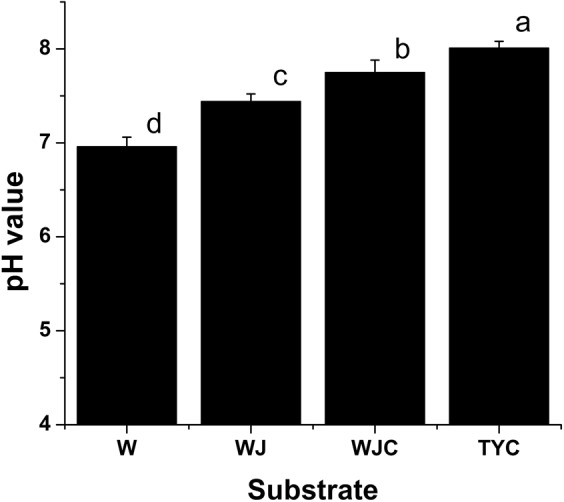
The pH values of substrates. Each bar is the average, and standard errors are shown. W, the pure sludge; WJ, the mixed substrate (sludge + construction waste) without planting A. altissima; WJC, the mixed substrate (sludge + construction waste) after planting A. altissima; TYC, the control substrate (garden soil) after planting A. altissima. Statistical significance [least significant difference (LSD)] of the difference in pH value of substrates is indicated by different small letters (P < 0.05).
Table 1 showed the nutrient contents of substrates. We found that in W, the nutrient contents were significantly higher than in TYC (P < 0.05). In W the contents of organic matter, total nitrogen, total phosphorus, and total potassium were 6.68, 11.23, 23.11, and 3.39 folds of those in TYC, respectively. Moreover, mixture with construction waste residue resulted in the significant decreases of these nutrient contents (P < 0.05), and further reduction was found after planting A. altissima. In WJC, the contents of organic matter, total nitrogen, total phosphorus, and total potassium decreased to 53.71%, 34.12%, 27.35%, and 45.86% of those in W, respectively, which was also lower than those in WJ; however, these values were still far higher than those in TYC (P < 0.05). The contents of available nitrogen, available phosphorus, and available potassium were also found to show similar patterns.
Table 1.
The nutrient contents of substrates.
| Nutrient index | Content (g·kg−1) | |||
|---|---|---|---|---|
| W | WJ | WJC | TYC | |
| Organic matter | 124.70 ± 2.34 a | 77.06 ± 2.04 b | 66.98 ± 0.80 c | 18.68 ± 1.19 d |
| Total nitrogen | 13.92 ± 0.36 a | 5.5 ± 0.23 b | 4.75 ± 0.10 c | 1.24 ± 0.03 d |
| Available nitrogen | 0.54 ± 0.01 a | 0.51 ± 0.01 b | 0.07 ± 0.00 c | 0.07 ± 0.00 c |
| Total phosphorus | 12.25 ± 0.75 a | 4.51 ± 0.10 b | 3.35 ± 0.07 c | 0.53 ± 0.01 d |
| Available phosphorus | 0.40 ± 0.01 b | 0.43 ± 0.00 a | 0.03 ± 0.00 c | 0.03 ± 0.00 c |
| Total potassium | 5.8 ± 0.17 a | 4.23 ± 0.74 b | 2.66 ± 0.04 c | 1.71 ± 0.08 d |
| Available potassium | 0.45 ± 0.01 a | 0.45 ± 0.01 a | 0.08 ± 0.00 b | 0.09 ± 0.00 b |
Note: Each value is the average, and standard errors are shown. Statistical significance [least significant difference (LSD)] of the difference in nutrient contents of substrates is indicated by different small letters (P < 0.05).
The contents of heavy metals in substrates were also examined (Table 2). It was found that the sludge-containing substrates had significantly higher contents of heavy metals than in TYC (P < 0.05), especially in W. The contents of Cu and Zn in W were 4.53 and 5.95-fold of those in TYC. Similar to the nutrient contents, the contents of heavy metals gradually decreased from W to WJ and to WJC. In WJC, the contents of As, Cd, Cr, Cu, Pb, and Zn were also significantly higher than those in TYC by 14.35%, 100.00%, 22.50%, 175.90%, 40.88%, and 317.88%, respectively (P < 0.05).
Table 2.
Contents of heavy metals in substrates.
| Heavy metal | Content (mg·kg−1) | |||
|---|---|---|---|---|
| W | WJ | WJC | TYC | |
| As | 18.14 ± 0.62 a | 9.21 ± 0.67 b | 7.33 ± 0.57 c | 6.41 ± 0.25 d |
| Cd | 0.71 ± 0.07 a | 0.46 ± 0.04 b | 0.38 ± 0.03 b | 0.19 ± 0.01 c |
| Cr | 53.73 ± 2.29 a | 44.88 ± 0.72 b | 32.72 ± 0.52 c | 26.71 ± 0.83 d |
| Cu | 98.56 ± 2.86 a | 64.76 ± 1.74 b | 59.98 ± 1.63 c | 21.74 ± 0.62 d |
| Pb | 26.56 ± 0.71 a | 24.54 ± 0.79 b | 23.06 ± 0.86 b | 16.37 ± 0.41 c |
| Zn | 347.12 ± 6.45 a | 250.78 ± 4.47 b | 243.71 ± 4.04 b | 58.32 ± 2.54 c |
Note: The average and standard errors are shown. Statistical significance [least significant difference (LSD)] of the difference in contents of heavy metals of substrates is indicated by different lower-case letters (P < 0.05).
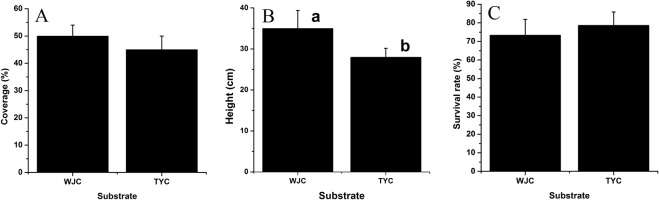
After growth for eighteen months, the survival rate, height, and coverage of A. altissima were investigated (Fig. 2). The survival rate of in WJC was about 6.66% lower than that in TYC (Fig. 2A). However, the height and coverage of in WJC were higher than those in TYC (Fig. 2B,C). The height in WJC almost arrived at 35.00 cm, which was 25.00% higher than that in TYC. Compared to TYC, the coverage in WJC was 11.11% higher.
Figure 2.
Survival rate (A), height (B), and coverage (B) of A. altissima in the WJC and TYC. Average values and standard errors are shown. Statistical significance [least significant difference (LSD)] of the difference in height of seedlings on substrates is indicated by different lower-case letters (P < 0.05).
After high-throughput sequencing using the Illumina HiSeq platform, the data of samples were obtained and analyzed (Supplementary Table S1). The Q30 values of all samples exceeded 98.00%, and the GC values were about 55.00%. Moreover, Venn diagrams of OTUs exhibited the difference of microbial diversity between different samples (Supplementary Fig. 3).
To analyze the diversity among all samples, microbial species and their proportions at phylum levels are shown in Fig. 3. Due to the contained sludge, the dominant microbes showed similar proportions in W, WJ, and WJC, which largely differed from TYC. For these three samples, Proteobacteria was the largest proportion of population, which nearly arrived at 50.00%. In TYC, although Proteobacteria also occupied the highest proportion, it only reached about 35.00%, and Acidobacteria accounted for a further high proportion with about 25.00%. In W, WJ, and WJC, the proportion of Acidobacteria did not exceed 10.00%. Bacteroidetes also accounted for high proportions which was larger than that in TYC. Acidobacteria and Thaumarchaeota were found to gradually increase from W to TYC, while the proportion of Firmicutes presented the opposite pattern. In WJC and TYC Gemmatimonacetes also exhibited higher proportions than in W and WJ, which nearly arrived at 12.00% and 5.50%, respectively.
Figure 3.
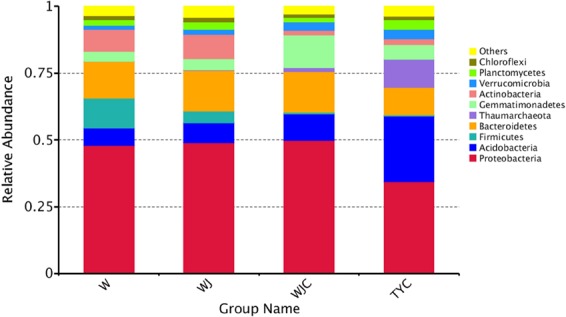
Relative abundance of the dominant microbial phyla observed in 16S rRNA gene sequences in different substrate samples.
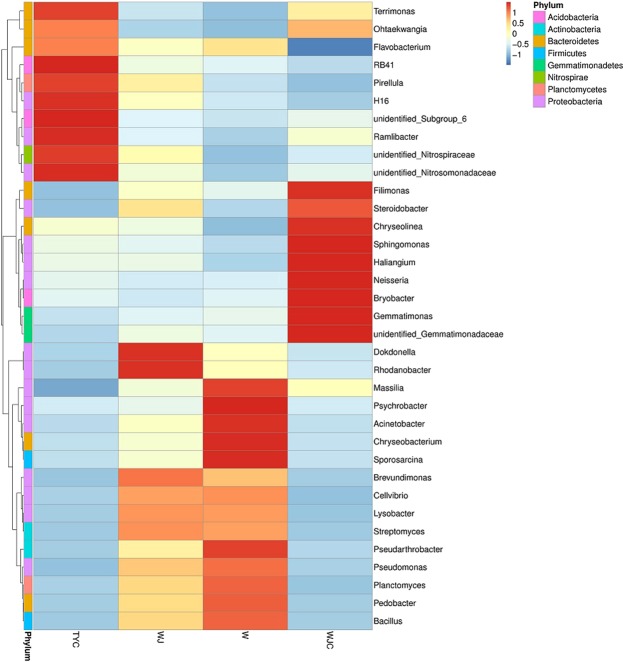
To demonstrate the different compositions of the microbial community structures in four samples, hierarchical cluster analysis of microbial communities at the genus level in shown in Fig. 4. The relative abundances of these 35 genera varied significantly in different soil samples. However, in W and WJ, the relative abundances showed a similar pattern to some extent. Both in W and WJ, Brevundimonas, Cellvibrio, Lysobacter, Streptomyces, Pseudomonas, Planctomyces, Pedobacter, Bacillus, and Pseudarthrobacter presented relatively high abundances. In W, the abundances of Massilia, Psychrobacter, Acinetobacter, Chryseobacterium, Sporosarcina, and Pseudarthrobacter were higher than those of other genera. In WJ, Dokdonella and Rhodanobacter exhibited the highest abundances. In WJC Filimonas, Steroidobacter, Chryseolinea, Sphingomonas, Haliangium, Neisseria, Bryobacter, Gemmatimonas, unidentified_Gemmatimonadaceae, and Ohtaekwangia were found to have high relative abundances. Ten genera (e.g., Terrimonas, Ohtaekwangia, Flavobacterium, Pirellula, and Ramlibacter) showed high abundances in TYC. In these samples, the microbial genera with high abundances belonged to more than two phyla, which indicated high microbial diversities in these samples.
Figure 4.
Hierarchically clustered heatmap analysis of microbial community at the genus level. The scale bar shows the standardized Z-value of the microbial relative percentages in four samples. The larger value in the scale bar shows the larger relative abundance of this genus in the sample.
The beta diversity difference among samples of W, WJ, WJC, and TYC was analyzed in Fig. 5. Significant differences were not observed between W and WJ or between WJC and TYC. However, a significant difference was found between the samples (W and WJ) without plants and the samples (WJC and TYC) with planting A. altissima.
Figure 5.
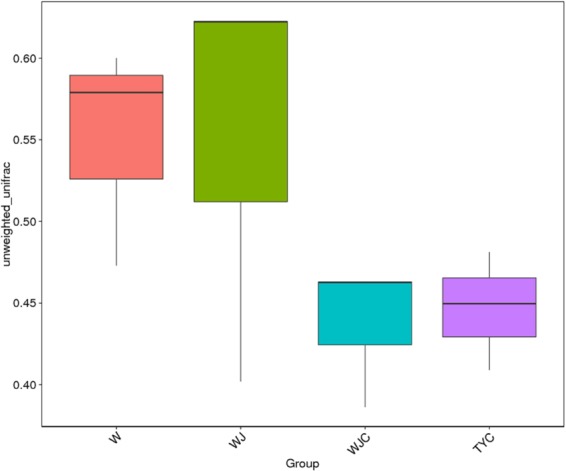
Difference analysis of beta diversity among four sample groups. The average and standard errors are shown. Turkey test was performed on the beta diversity statistical significance [least significant difference (LSD)] of the difference in contents of heavy metals of substrates is indicated by different lower-case letters (P < 0.05).
PCoA clearly showed variations of microbial communities among the four soil samples (Fig. 6). W and WJ were not separated and thus possessed similar microbial community compositions. WJC was separated from W and WJ along the second component (PC2), while TYC was separated from W and WJ along the first component (PC1).
Figure 6.
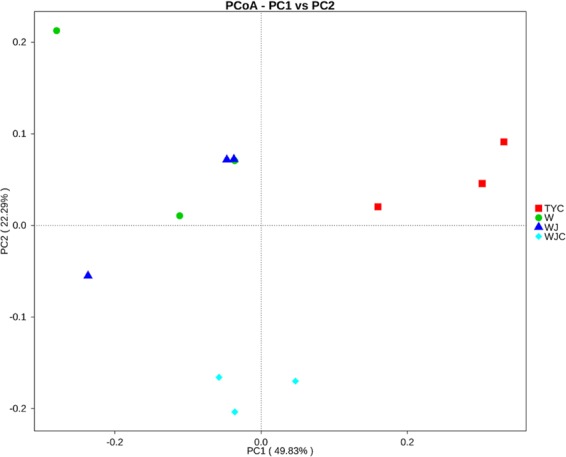
PCoA of microbial communities among different samples.
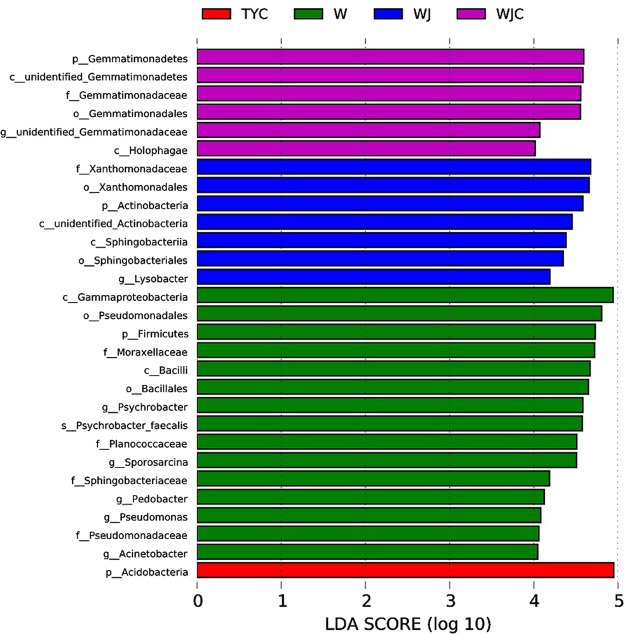
A persistent difference in microbial taxa patterns was found via LEfSe analysis (Fig. 7). In W, the relative abundances of Gammaproteobacteria class, Pseudomonadales order, Firmicutes phylum, Moraxellaceae family, Bacillales order, Psychrobacter genus, Psychrobacter species, Planococcaceae family, Sporosarcina genus, Sphingobacteriaceae family, Pedobacter genus, Pseudomonas genus, Pseudomonadacear family, and Acinetobacter genus were higher than three other substrates, among which the Gammaproteobacteria class showed the highest LDA score. WJ had high relative abundances of Xanthomooadaceae family, Xanthomonadeles order, Actinobacteria pylum, unidentified_Actinobacteria class, Sphingobacteriia class, Sphingobacteriales order, and Lysobacter genus than other substrates. Compared to W, WJ, and TYC, in WJC Gemmatimonadetes phylum, unidentified_Gemmatimonadetes class, Gemmatimonadaceae family, Gemmatimonadales order, unidentified_Gemmatimonadaceae genus, and Holophagae class showed higher relative abundances. Only the phylum Acidobacteria was found to have higher relative abundance in TYC than the other three substrates.
Figure 7.
Liner discriminant analysis coupled with effect size measurements among different substrates. Lineages with LDA values higher than 4 are displayed.
Discussion
Increasing concern is caused by the substantial generation of municipal sewage sludge and the inadequate development of the wastewater treatment industry, which causes environmental problems. Due to its high nutrient content, municipal sludge is used as soil conditioner and fertilizer to recycle its nutrients through the soil-plant system17–19. Sewage sludge has many beneficial effects on soil fertility, enriching it with its nitrogen, phosphorus, and micronutrient contents, and improving the physicochemical, microbiological, and enzymatic properties of soil11,20,21, which is an economic and effective method for disposal. However, a high level of contaminants, especially of heavy metals and pathogenic microbes, leads to a long-term environmental risk in soil18. Application of municipal sludge for vegetation restoration in a degraded ecosystem can avoid the potential risk that toxic contaminants enter the human food chain. This study investigated the potential application of municipal sludge combined with construction waste for vegetation restoration.
The utilized municipal sludge was found to have near neutral in pH, and the mixture with construction waste residue increased the pH value; moreover, after the growth of A. altissima for eighteen months, the pH value further increased. Although the mixed substrate was weakly alkaline, the pH value was evidently lower than that of garden soil. The application of sludge in tree peony-soil ecosystems also decreased the pH value, which was close to neutral soil pH, and was conducive to tree peony20. The municipal sludge used in this study had high nutrient contents (Table 1), which was too high for plants, while the construction waste residue contained few nutrients (Table 3). The addition of construction waste residue not only decreased the nutrient contents to a still high but acceptable level for plants (Table 1), it also improved the air permeability. A. altissima seedlings in the mixed substrate obtained better nutrient conditions than in garden soil; therefore, we can find that the height and coverage of A. altissima in mixed substrate was better than that in garden soil. The plant height, flower diameter, and flower numbers per plant of tree peony significantly increased after sludge compost amendment22. Application of sewage sludge compost as fertilizer in soil with mass ratios of 30.00% also promoted the height, ground diameter, and biomass of Mangifera persiciforma trees23. Compared to the application of sludge to soil, the mixed substrate of municipal sludge and construction waste residue also achieved the same beneficial effect of A. altissima plant growth; moreover, both of them were reused. Although the survival rate of A. altissima in mixed substrate was impacted, and was a little lower than in garden soil, it still achieved about 75.00%.
Table 3.
Nutrient contents of construction waste residue.
| Nutrient index | Content (g·kg−1) |
|---|---|
| Organic matter | 0.68 ± 0.01 |
| Total nitrogen | 6.46 ± 0.03 |
| Total phosphorus | 0.22 ± 0.00 |
| Total potassium | 0.96 ± 0.01 |
Note: Average values and standard errors are shown.
Moreover, after mixing with construction waste residue, the contents of main heavy metals in the municipal sludge decreased significantly. In the mixed substrate with A. altissima (with reference to Environmental Quality Standard for Soils of China, GB15618-1995), the contents of As, Cr, and Pb reached the level of first-degree soil, and the contents of Cd, Zn, and Cu met the criterion of second-degree soil. Many previous studies focused on the heavy metals during the land application of municipal sludge, and found that as long as the application amount was controlled within reasonable levels, the pollution and hazard of heavy metals could be avoided7,23–26. Based on our data, with regard to heavy metal contents, the mixed substrate belonged to Class II soil, which could be used to grow crops, vegetables, tea trees, fruit trees, and forage grasses, and would not cause pollution or be hazardous for plants and the environment. In addition, the moisture content (Fig. 8) and the activities of soil enzymes including urease, catalase, invertase, and alkaline phosphatase (Table 4) in the mixed substrate were significantly higher than those in garden soil, which was also beneficial for A. altissima growth.
Figure 8.
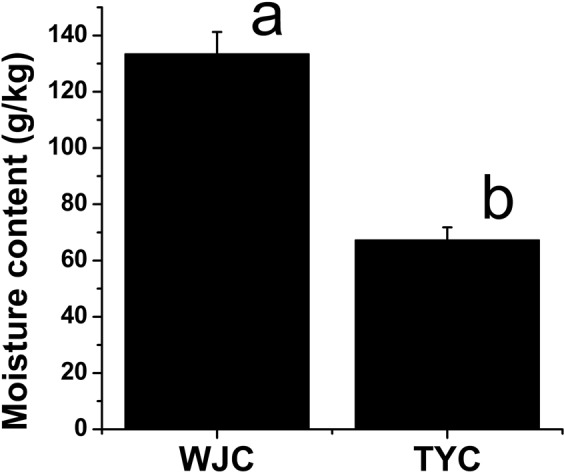
Moisture contents of WJC and TYC. The averages and standard errors are shown. Statistical significance [least significant difference (LSD)] of the difference in moisture contents of substrates is indicated by different lower-case letters (P < 0.05).
Table 4.
Activities of soil enzymes in WJC and TYC.
| Soil enzymes | Activities | |
|---|---|---|
| WJC | TYC | |
| Urease (μg·g−1·2 h−1) | 22.51 ± 1.72 a | 4.76 ± 0.57 b |
| Catalase (mL·g−1·h−1) | 1.43 ± 0.02 a | 0.73 ± 0.02 b |
| Invertase (mg·g−1·24 h−1) | 22.49 ± 0.39 a | 9.96 ± 0.14 b |
| Alkaline phosphatase (μg·g−1·h−1) | 448.34 ± 9.02 a | 171.38 ± 6.77 a |
Note: Average values and standard errors are shown. Statistical significance [least significant difference (LSD)] of the difference in soil enzyme activities of substrates is indicated by different lower-case letters (P < 0.05).
The microbes play an important role for the physicochemical properties of soil and plant growth. Large numbers of microbes can be found in municipal sludge, many of which are species that decompose organic matter, while some of them are pathogenic species that may be environmentally hazardous. Therefore, in the application of municipal sludge, the diversity of microbes is a focal point. In this study, using high-throughput sequencing on the Illumina HiSeq platform, the microbial diversity of mixed substrate was investigated. Our experimental results demonstrated that the microbial community structure observed in the substrate samples containing municipal sludge significantly differed from that of garden soil. Phylum abundance analysis showed that although Proteobacteria was the top microbial phylum in all samples, compared to garden soil, higher values were found in samples that contained municipal sludge. Furthermore, the relative abundance of Proteobacteria in activated sludge of a municipal wastewater treatment plant of Northern China in summer arrived at about 50.50%27. Proteobacteria included many important ammonia oxidizing bacteria involving nitrogen fixation, such as Betaproteobacteria and Gammaproteobacteria28. Therefore, municipal sludge can provide more nitrogen than garden soil. Bacteroidetes, Fimicutes, and Actinobacteria were the other main phyla in samples containing municipal sludge with higher relative abundance than garden soil. However, in a recent study, the proportions of Fimicutes and Actinobacteria in activated sludge were low27. This may result from differences in sludge sources. In garden soil, Acidobacteria and Thaumarchaeota were found to have a higher relative abundance compared to samples containing municipal sludge. Acidobacteria has been reported to be a main bacterial phylum in agricultural or other types of soils, with some variation in their relative abundance29,30. Thaumarchaeota was named by Brochier-Armanet et al.31, and plays an important role in the ammonia oxidization and carbon fixation in soil. No reports exist about Thaumarchaeota in municipal sludge. Therefore, a large difference was found between the substrate samples containing municipal sludge and garden soil.
In addition, after growing A. altissima, the relative abundances of Acidobacteria and Gemmatimonadetes increased in the mixed substrate, especially for Gemmatimonadetes whose relative abundance in WJC was also far higher than that in TYC. Moreover, the relative abundance of Fimicutes decreased after growing A. altissima in the mixed substrate. Therefore, it was demonstrated that the growth of A. altissima resulted in these microbial changes. The planting of apple seedlings was reported to exert a large impact on the bacterial community in the soils of replanted new planting sites32.
At the genus level, heat map analyses and PCoA also showed the difference among these four substrates. Similarly, at phylum level, the microbial communities in TYC were significantly different from W, WJ, and WJC, and compared to W and WJ, WJC was somewhat closer to TYC, which was also demonstrated by the analysis of beta diversity. As the dominant microbial communities in WJ, Dokdonella and Rhodanobacter presented low relative abundance in WJC. Rhodanobacter was reported to be likely involved in the denitrifying process, leading to denitrification in low pH soil33. In this study, Rhodanobacter was found to be part of the prominent microbial communities in the alkaline substrate. For common soil, Rhodanobacter had a negative effect on nitrogen nutrition supply due to nitrogen losses, while for the substrate containing municipal sludge with high nitrogen content, its denitrification can decrease the nitrogen nutrition content to a suitable level for the growth of A. altissima, which played a positive role. However, in WJC, nine microbial communities, such as Filimonas, Chryseolinea, Sphingomonas, and Gemmatimonas accounted for high relative percentages. It has been reported that Sphingomonas can effectively degrade dibenzo-p-dioxin34, hexachlorocyclohexane35, and phenazine-1-carboxylic acid36 in soils. Moreover, Sphingomonas was also capable to degrade fenvalerate, permethrin, fenpropathrin, beta-cypermethrin, cyhalothrin, deltamethrin, bifenthrin, and 3-PBA, and showed multi-resistance to heavy metals and antibiotics37. Therefore, Sphingomonas had potential for degrading organic pollutants, especially pesticides in substrate. Furthermore, Gemmatimonas was confirmed to reduce the emission of N2O, which is a potent greenhouse gas and ozone depletion agent originating from soil38. The high relative abundance of Gemmatimonadaceae suggested that WJC may be beneficial for the atmospheric environment.
Conclusions
This study showed that using municipal sludge mixed with construction waste residue as substrate, increased the growth of A. altissima seedlings and the physiochemical characteristics were suitable for plants, suggesting that the substrates showed potential for vegetation restoration. The different substrates showed various microbial diversities. Proteobacteria was the top microbial phylum in all samples and a higher relative abundance were found in the samples containing municipal sludge. After growing A. altissima, the relative abundances of Acidobacteria and Gemmatimonadetes increased in the mixed substrate. Therefore, the addition of construction waste residue and the growth of A. altissima caused the difference. The microbial communities in WJC was plant-friendly and environment-friendly. The results suggested that using as substrate for vegetation restoration was a potentially feasible and effective pathway for the reuse and recycling of municipal sludge. The heavy metal contents in WJC met the criterion of the Class II soil although the values were significantly higher than in TYC. Therefore, using this mixture as substrate in vegetation restoration was a potentially feasible and effective pathway for the reuse and recycling of municipal sludge and construction waste residue.
Materials and Methods
Experimental Design
The experiment was conducted in the Science and Technology Park of the Beijing University of Agriculture. The municipal sludge used in this study was taken from the Xiaotangshan sewage treatment plant of the Changping district in Beijing, and was naturally dried in the air. The construction waste residue originated from a construction site near the Tingzizhuang village of Changping district in Beijing, and big bricks and stones were removed. The municipal sludge was mixed with the construction waste residue at a volume ratio of 1:1 to obtain the treatment substrate. The garden soil in the nursery was used as control substrate. Both types of experiment plots (5 m × 5 m) were paved with treatment and control substrate to 50 cm thickness, respectively. Three plots were prepared for every type, acting as three replicates.
A. altissima was used as plant material. Five hundred A. altissima seeds (China National Tree Seed Corporation, Beijing) were uniformly sowed (100 seeds per square meter) on two experimental plots in April of 2015. The A. altissima seedlings grew under natural condition.
Soil Sampling
Pure sludge and mixed substrate without planting A. altissima were sampled. After planting A. altissima, both mixed and control substrates were collected in October of 2016. In each plot, at a depth of 10–15 cm, the substrates close to the roots of 10 A. altissima seedlings with medium size were collected and were divided into two parts. One part of the substrates was used to determine the characteristics, while the other part was frozen in liquid N2 immediately for subsequent DNA extraction. The samples are listed in Table 5.
Table 5.
Collected samples.
| Samples | Meaning |
|---|---|
| W | Pure sludge |
| WJ | Mixed substrate without planting A. altissima |
| WJC | Mixed substrate after planting A. altissima |
| TJC | Control substrate after planting A. altissima |
Substrate Characteristics Measurement
The pH value was determined with a Delta 320 pH meter (Mettler-Toledo International Inc., Switzerland) at substrate: water = 1:5. The content of organic matter was determined using the potassium dichromate volumetric method. Using the Kjeldahl determination, the content of total nitrogen was investigated in substrate after heating digestion by concentrated sulfuric acid. The contents of total phosphorus, total potassium, and heavy metals were determined by ICP-AES (6,300, Thermo Fisher Scientific Inc., USA) after microwave digestion.
Growth Investigation of A. altissima
In October, the survival rate of A. altissima was calculated. The height of 30 seedlings with medium size were measured with a meter stick. Moreover, the coverage was investigated using a canopy projection method.
DNA Extraction
Total genomic DNA from soil samples was extracted using the CTAB/SDS method. DNA concentration and purity were monitored on 1.00% agarose gels. Each sample extraction was conducted three times. DNA concentration and purity were monitored on 1.00% agarose gels. According to the concentration, DNA was diluted to 1 ng·L−1 using sterile water. Then, the DNA extractions were used as template DNA for high-throughput sequencing (HTS) analysis by Novogene Bioinformatics Technology Co., Ltd., Beijing, China.
Microbial 16S rRNA Gene Amplification
The protocol of Caporaso et al.39 was used to determine the diversity and composition of the microbial communities in soil samples. PCR amplifications were conducted with the 515F-806R primer set amplifying the V4 region of the 16 S rDNA gene. All PCR reactions were conducted in 30 μL reactions with 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs); 0.2 μM of forward and reverse primers, and about 10 ng template DNA. Thermal cycling consisted of an initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s. Finally, 72 °C for 5 min.
PCR Product Quantification and Qualification
The same volume of 1X loading buffer (contained SYB green) was mixed with PCR products and electrophoresis was conducted on a 2.00% agarose gel for detection. Samples with bright main strip between 400–450 bp were chosen for further experiments.
PCR Products Mixing and Purification
PCR products were mixed at equidensity ratios. Then, mixture PCR products were purified with the GeneJET Gel Extraction Kit (Thermo Scientific).
Library Preparation and Sequencing
Sequencing libraries were generated using the TruSeq DNA PCR-free Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations and index codes were added. The library quality was assessed on the Qubit@2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina HiSeq platform and 250 bp paired-end reads were generated.
Data Analysis
Paired-end reads from the original DNA fragments were merged using FLASH40, which is a very fast and accurate analysis tool that was designed to merge paired-end reads when there were overlaps between reads1 and reads2. Paired-end reads were assigned to each sample according to unique barcodes. Sequences were analyzed using the QIIME software package (Quantitative Insights Into Microbial Ecology)39, and in-house Perl scripts were used to analyze alpha- (within samples) and beta- (among samples) diversity. First, reads were filtered by QIIME quality filters. Then, pick_de_novo_otus.py was used to pick operational taxonomic units (OTUs) by making a OTU table. Sequences with ≥97.00% similarity were assigned to the same OTUs. A representative sequence was picked for each OTU and the RDP classifier was used to annotate taxonomic information for each representative sequence41. QIIME calculates unweighted unifrac, which is phylogenetic measure of beta diversity. Unweighted unifrac was used for Principal Coordinate Analysis (PCoA). To further mine the data of microbial diversity of the differences between the samples, LEfSe was conducted.
All experimental data were analyzed and using SPSS16.0. Statistical significance [least significant difference (LSD)] was performed on pH value, nutrient content, heavy metal content, survival rate, plant height, coverage, moisture content, and soil enzyme activity. Turkey tests were performed on the beta diversity. Origin 8.0 was used for plotting and the data in the figures are shown as the mean ± standard error.
Supplementary information
Acknowledgements
This work was supported by the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20180509), and the Beijing Municipal Natural Science Foundation (8132005).
Author Contributions
X.Y.L. and L.J.L. carried out the experiments and performed the analyses. P.S.L. and Z.H.H designed the experiments and reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pingsheng Leng, Email: bualengpingsheng@163.com.
Zenghui Hu, Email: buahuzenghui@163.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37338-4.
References
- 1.Wei Y, Van Houten RT, Borger AR, Eikelboom DH, Fan Y. Minimization of excess sludge production for biological wastewater treatment. Water Res. 2003;37:4453–4467. doi: 10.1016/S0043-1354(03)00441-X. [DOI] [PubMed] [Google Scholar]
- 2.Fytili D, Zabaniotou A. Utilization of sewage sludge in EU application of old and new methods-a review. Renew Sustain Energy Rev. 2008;12:116–140. doi: 10.1016/j.rser.2006.05.014. [DOI] [Google Scholar]
- 3.Tyagi VK, Lo SL. Sludge: a waste or renewable source for energy and resources recovery? Renew Sustain Energy Rev. 2013;25:708–728. doi: 10.1016/j.rser.2013.05.029. [DOI] [Google Scholar]
- 4.Samolada M, Zabaniotou A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014;34:411–420. doi: 10.1016/j.wasman.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Directive C. Council directive on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off J Eur Commun. 1986;181:0006–0012. [Google Scholar]
- 6.Singh RP, Agrawal M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008;28:347–358. doi: 10.1016/j.wasman.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed B, et al. Sewage sludge used as organic manure in Moroccan sunflower culture: Effects on certain soil properties, growth and yield components. Sci Total Environ. 2018;627:681–688. doi: 10.1016/j.scitotenv.2018.01.258. [DOI] [PubMed] [Google Scholar]
- 8.Siebielska I. Comparison of changes in selected polycyclic aromatic hydrocarbons concentrations during the composting and anaerobic digestion processes of municipal waste and sewage sludge mixtures. Water Sci Tech. 2014;70:1617–1624. doi: 10.2166/wst.2014.417. [DOI] [PubMed] [Google Scholar]
- 9.Fijalkowski KL, Kacprzak MJ, Rorat A. Occurrence changes of Escherichia coli (including O157: h7 serotype) in wastewater and sewage sludge by quantitation method of (EMA) real time-PCR. Desalin. Water Treat. 2014;52:3965–3972. doi: 10.1080/19443994.2014.887499. [DOI] [Google Scholar]
- 10.Kacprzak M, Stańczyk-Mazanek E. Changes in the structure of fungal communities of soil treated with sewage sludge. Biol Fertil Soils. 2003;38:89–95. doi: 10.1007/s00374-003-0633-2. [DOI] [Google Scholar]
- 11.Li G, Yin C, Lin Y, Li Z. Artificial improvement of soil fertility in a regarded forest ecosystem by using municipal sewage sludge. Chin J Applied Ecol. 2002;13:159–162. [PubMed] [Google Scholar]
- 12.Hamuda HEAFB, Ligetvári. F. Impacts of municipal sewage sludge on dynamics of organic matter and biological activities in clay loam brown forest soil. J Residuals Sci Tech. 2011;8:143–149. [Google Scholar]
- 13.Moreno-Peñaranda R, Francisco L, Alcañiz JM. Effects of sewage sludge on plant community composition in restored limestone quarries. Restor Ecol. 2004;12:290–296. doi: 10.1111/j.1061-2971.2004.00310.x. [DOI] [Google Scholar]
- 14.Liu H, Yuan B, Rao H, WU J. Application of composted urban sewage sludge to dam slope vegetation restoration. Water Resour Prot. 2013;29:73–74. [Google Scholar]
- 15.Lu H. Analysis of the problem and outlet of China’s construction waste. Environ Sustain Dev. 2018;43(3):45–48. [Google Scholar]
- 16.Mickovskia SB, Bussa K, McKenzieb BM, Sömener B. Laboratory study on the potential use of recycled inert construction waste material in the substrate mix for extensive green roofs. Ecol Eng. 2013;61:706–714. doi: 10.1016/j.ecoleng.2013.02.015. [DOI] [Google Scholar]
- 17.Yilmaz DD, Temizgül A. Asessment of arsenic and selenium concentration with chlorophyll contents of sugar beet (Beta vulgaris var. saccharifera) and wheat (Triticum aestivum) exposed to municipal sewage sludge doses. Water Air Soil Pollut. 2012;223:3057–3066. doi: 10.1007/s11270-012-1088-6. [DOI] [Google Scholar]
- 18.Nogueirol RC, de Melo WJ, Bertoncini EI, Alleoni LRF. Concentrations of Cu, Fe, Mn, and Zn in tropical soils amended with sewage sludge and composted sewage sludge. Environ Monit Assess. 2013;185:2929–2938. doi: 10.1007/s10661-012-2761-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhao S, Shang X, Duo L. Accumulation and spatial distribution of Cd, Cr, and Pb in mulberry from municipal solid waste compost following application of EDTA and (NH4)2SO4. Environ Sci Pollut Res. 2013;20:967–975. doi: 10.1007/s11356-012-0992-z. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Chopra AK. Accumulation and translocation of metals in soil and different parts of French Bean (Phaseolus vulgaris L.) amended with sewage sludge. Bull Environ Contam Toxicol. 2013;10:128–142. doi: 10.1007/s00128-013-1142-0. [DOI] [PubMed] [Google Scholar]
- 21.Bourioug M, et al. Sewage sludge application in a plantation: effects on trace metal transfer in soil-plant-snail continuum. Sci Total Environ. 2015;502:309–314. doi: 10.1016/j.scitotenv.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Xue D, Huang X. The impact of sewage sludge compost on tree peony growth and soil microbiological, and biochemical properties. Chemosphere. 2013;93:583–589. doi: 10.1016/j.chemosphere.2013.05.065. [DOI] [PubMed] [Google Scholar]
- 23.Chu S, et al. Municipal sewage sludge compost promotes Mangifera persiciforma tree growth with no risk of heavy metal contamination of soil. Sci Rep. 2017;7:13408. doi: 10.1038/s41598-017-13895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boswell FC. Municipal sewage sludge and selected element application to soil: effect on soil and fescue. J Environ Qual. 1975;4:267–272. doi: 10.2134/jeq1975.00472425000400020029x. [DOI] [Google Scholar]
- 25.Morera MT, Echeverria J, Garrido J. Bioavailability of heavy metals in soils amended with sewage sludge. Can J Soil Sci. 2002;82:433–438. doi: 10.4141/S01-072. [DOI] [Google Scholar]
- 26.Huang X, Xue D, Xue L. Changes in soil microbial functional diversity and biochemical characteristics of tree peony with amendment of sewage sludge compost. Environ Sci Pollut R. 2015;22:11617–11625. doi: 10.1007/s11356-015-4407-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Miao MS, Kong Q, Ni S. Evaluation of microbial diversity of activated sludge in a municipal wastewater treatment plant of northern China by high-throughput sequencing technology. Desalin Water Treat. 2016;57:1–6. doi: 10.1080/19443994.2016.1186566. [DOI] [Google Scholar]
- 28.Juretschko. S, et al. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol. 1998;64:3042–3051. doi: 10.1128/aem.64.8.3042-3051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caporaso JG, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Z, et al. Effect of biofertilizer for suppressing Fusarium wilt disease of banana as well as enhancing microbial and chemical properties of soil under greenhouse trial. Appl Soil Ecol. 2015;93:111–119. doi: 10.1016/j.apsoil.2015.04.013. [DOI] [Google Scholar]
- 31.Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol. 2008;6:245–252. doi: 10.1038/nrmicro1852. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Zhang Q, Zhou J, Wei Q. Illumina amplicon sequencing of 16s rRNA tag reveals bacterial community development in the rhizosphere of apple nurseries at a replant disease site and a new planting site. PloS one. 2014;9:e111744. doi: 10.1371/journal.pone.0111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green SJ, et al. Denitrifying bacteria from the genus Rhodanobacter dominate bacterial communities in the highly contaminated subsurface of a nuclear legacy waste site. Appl. Environ. Microbiol. 2012;78:1039–1047. doi: 10.1128/AEM.06435-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore ERB, Wittich RM, Fortnagel P, Timmis KN. 16S ribosomal RNA gene sequence characterization and phylogenetic analysis of a dibenzo-p-dioxin-degrading isolate within the new genus Sphingomonas. Lett Appl Microbiol. 1993;17:115–118. doi: 10.1111/j.1472-765X.1993.tb01438.x. [DOI] [Google Scholar]
- 35.Böltner D, Moreno-Morillas S, Ramos JL. 16S rDNA phylogeny and distribution of line genes in novel hexachlorocyclohexane-degrading Sphingomonas strains. Environ Microbiol. 2005;7:1329–1338. doi: 10.1111/j.1462-5822.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, et al. Isolation, identification, and degradation characteristics of phenazine-1-carboxylic acid–degrading strain Sphingomonas sp. DP58. Curr Microbiol. 2007;55:284–287. doi: 10.1007/s00284-006-0522-7. [DOI] [PubMed] [Google Scholar]
- 37.Yu F, Shan S, Luo L, Guan L, Qin H. Isolation and characterization of a Sphingomonas sp. strain F-7 degrading fenvalerate and its use in bioremediation of contaminated soil. J Environ Sci Heal B. 2013;48:198–207. doi: 10.1080/03601234.2013.730299. [DOI] [PubMed] [Google Scholar]
- 38.Park D, Kim H, Yoon S. Nitrous oxide reduction by an obligate aerobic bacterium, Gemmatimonas aurantiaca Strain T-27. Appl Environ Microbiol. 2017;83:e00502–17. doi: 10.1128/AEM.00502-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.