Abstract
Urogenital schistosomiasis is a neglected tropical disease caused by the parasite Schistosoma haematobium, which resides in the vasculature surrounding the urogenital system. Previous work has suggested that helminthic infections can affect the intestinal microbiome, and we hypothesized that S. haematobium infection could result in an alteration of immune system-microbiota homeostasis and impact the composition of the gut microbiota. To address this question, we compared the fecal microbiomes of infected and uninfected schoolchildren from the Argungu Local Government Area of Kebbi State, Nigeria, detecting significant differences in community composition between the two groups. Most remarkably, we observed a decreased abundance of Firmicutes and increased abundance of Proteobacteria – a shift in community structure which has been previously associated with dysbiosis. More specifically, we detected a number of changes in lower taxa reminiscent of inflammation-associated dysbiosis, including decreases in Clostridiales and increases in Moraxellaceae, Veillonellaceae, Pasteurellaceae, and Desulfovibrionaceae. Functional potential analysis also revealed an enrichment in orthologs of urease, which has been linked to dysbiosis and inflammation. Overall, our analysis indicates that S. haematobium infection is associated with perturbations in the gut microbiota and may point to microbiome disruption as an additional consequence of schistosome infection.
Introduction
Schistosomiasis, or bilharzia, is a parasitic disease that infects hundreds of millions of people each year and is endemic to various tropical regions, notably in Africa1. The disease is caused by infection with trematode helminths of the genus Schistosoma, which live and sexually reproduce in the circulatory system of human hosts. Specifically, the species S. mansoni and S. japonicum live in venules surrounding the gut, while S. haematobium lives in the vessels around the urogenital system. There, adult worm pairs produce eggs that migrate through the surrounding tissue to be excreted primarily in the feces or urine, depending on the species, with the ultimate goal of reaching freshwater sources. They then reproduce asexually in their intermediate host – freshwater snails – before infecting humans present in contaminated water, entering through the skin before migrating to the vasculature2–7. The disease is typically diagnosed by microscopic examination of feces or urine for the presence of schistosome eggs3, although some more sensitive techniques have been developed8. Treatment of schistosomiasis by administration of the anti-helminth drug praziquantel is the main control strategy employed in endemic areas9.
The pathology of the disease generally arises from immunological reactions to eggs that become lodged in the tissue surrounding the gastrointestinal or urogenital system while attempting to migrate to the gut or bladder lumen. The eggs generally provoke a TH2 immune response, which is characteristic of extracellular insults including helminths and their eggs, leading to granuloma formation and fibrotic lesions that can have severe long-term consequences4,10–16. Eventually, the immune response is down-regulated, helping to preserve host health and integrity but allowing the parasite to persist for years4,17,18. This altered immune state may interplay with other immune insults, reducing the effectiveness of certain vaccines and altering the course of viral, bacterial, and parasitic co-infections4,7,19–31. On the other hand, it may also help to reduce the prevalence or severity of autoimmune disorders, and there is research interest in the therapeutic potential of helminths or their antigens to treat inflammatory conditions32–38.
There is evidence that both systemic immunological changes and helminth infection specifically are associated with changes in the gut microbiota. A number of previous studies have indicated that infection with a range of helminths – including gastrointestinal nematodes, tapeworms, tissue flukes, and schistosomes – can have impacts on the composition and function of the gut microbiome, suggesting that alterations to the gut microflora may be an under-recognized side effect of helminth infection39–50. However, most of this work has been done in animal models or humans infected with intestinal parasites, making it difficult to separate systemic immunological changes from effects local to the intestinal niche. In contrast, while it can occasionally localize to the enteric system (particularly during heavy infection or co-infection with S. mansoni)51,52, S. haematobium primarily lives within the vasculature surrounding the bladder and thus provides an opportunity to study whether helminth infection can impact the microbiome indirectly via systemic immunological or other changes that may disrupt gut homeostasis. Such a link between systemic immunity and the microbiota has been recently proposed in a Ugandan cohort, in which low CD4+ T-cell counts in HIV patients were associated with significant changes in the gut microbiome53; additionally, several studies suggest that immunosuppression can alter the composition and function of the gut microbiota54–57. Therefore, we hypothesized that urogenital schistosomiasis may disturb immune-microbial homeostasis and allow for changes in the resident taxa.
In this study, we investigated the impact of S. haematobium infection on the intestinal microbiome of adolescents aged 11–15 years in the Argungu Local Government Area of Kebbi State, Nigeria. As assessed by the Nigerian Federal Ministry of Health, Kebbi State has the highest prevalence of S. haematobium infection in the country but a very low prevalence of S. mansoni, making it an ideal location to study impacts of urogenital schistosomiasis specifically58. Kebbi State also has a low prevalence of soil-transmitted helminths, decreasing the likelihood of coinfections58. We chose to focus on adolescent schoolchildren, as children and adolescents are most likely to be infected with S. haematobium due to exposure and immunological factors38,59–65. Additionally, detection of differences in the human microbiome can be difficult given significant variation between individuals, which can be influenced by age, sex, diet, disease states, and other conditions66; to help minimize some such confounding factors, we selected subjects living in the same region, attending the same school, and falling into a relatively narrow age range.
Results
Study Overview and Participants
In order to examine the differences in the gut microbiomes of young adolescents infected with S. haematobium, we sequenced the fecal microbiomes of 49 adolescent students: 24 individuals infected with S. haematobium and 25 controls (Table S1). A t-test indicated that the ages of the subjects do not significantly differ between the two groups (p = 0.3228), and survey data indicates that important exposure and lifestyle factors are not systematically different (Table S2). In both groups, most samples were from male students, as fewer girls attend school in the area and females are less likely to have schistosomiasis both in Kebbi State and elsewhere in Nigeria58–61,63,67–70. We performed analyses of community composition between male and female subjects and found no significant differences or distinct PCoA clustering (Fig. S1); therefore, males and females were grouped together for overall analyses.
In our analysis, we sequenced the V4 region of the 16S rRNA gene and were able to identify most OTUs down to the genus level using the SILVA 16S database71. We analyzed alpha and beta diversity in the infected and uninfected subjects, in addition to examining differences in specific taxa through computational analysis and qPCR. Finally, we used the 16S sequencing results to predict the functional potential of the infected and control gut communities.
Metrics of Diversity Between Infection Groups
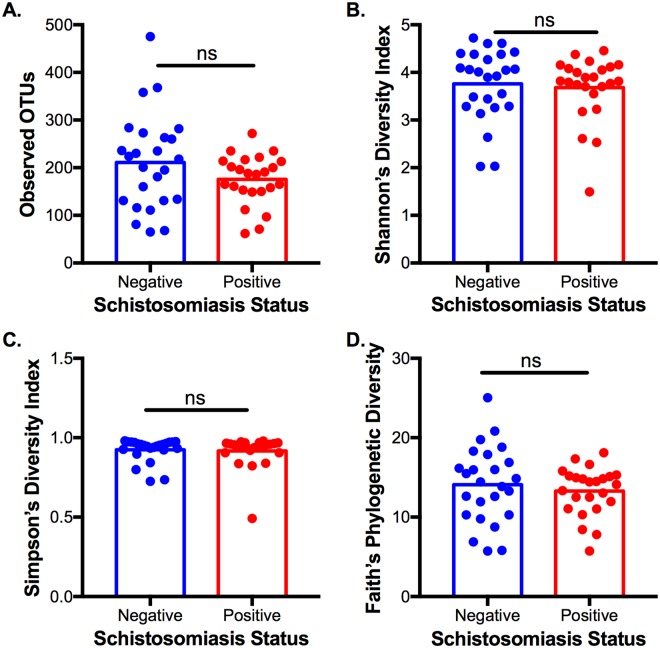
We first examined several metrics of alpha diversity, which measures the diversity of taxa within each individual microbial community, of infected and control adolescents (Fig. 1). Observed OTUs reflects the taxonomic richness of the community (Fig. 1A), the Shannon and Simpson Diversity Indices account for both richness and abundance of taxa (Fig. 1B,C), and Faith’s Phylogenetic Diversity also considers the phylogenetic relatedness of the taxa (Fig. 1D). Using all four metrics, there was no significant difference in alpha diversity between the schistosomiasis-infected and -uninfected subjects, indicating that infection does not systematically impact the diversity of an individual’s gut microbiota.
Figure 1.
Measures of Alpha Diversity in Schistosomiasis-positive and -negative Individuals. (A) Observed OTUs: p = 0.12. (B) Shannon’s Index of diversity: p = 0.77. (C) Simpson’s Index of diversity: p = 0.69. (D) Faith’s Phylogenetic Diversity: p = 0.49. Statistics: two-tailed t-test with Welch’s correction, error bars indicate SEM.
In contrast, we found significant differences between the microbial communities of infected and uninfected subjects when examining beta diversity, which measures the divergence in community composition between different samples. Again, we tested this using multiple metrics: Bray-Curtis dissimilarity reflects differences in the taxa present independent of their relatedness, unweighted UniFrac distance indicates differences in taxa while considering their phylogenetic relatedness, and weighted UniFrac also accounts for the abundances of the differential taxa. Using principal coordinate analyses (PCoA), we noted clustering of infected and control samples (Fig. 2) and a permutational MANOVA indicated that this difference is statistically significant in all cases. We found the greatest difference between the groups using unweighted UniFrac, suggesting that differences in community composition could be driven by changes in low-abundance taxa.
Figure 2.
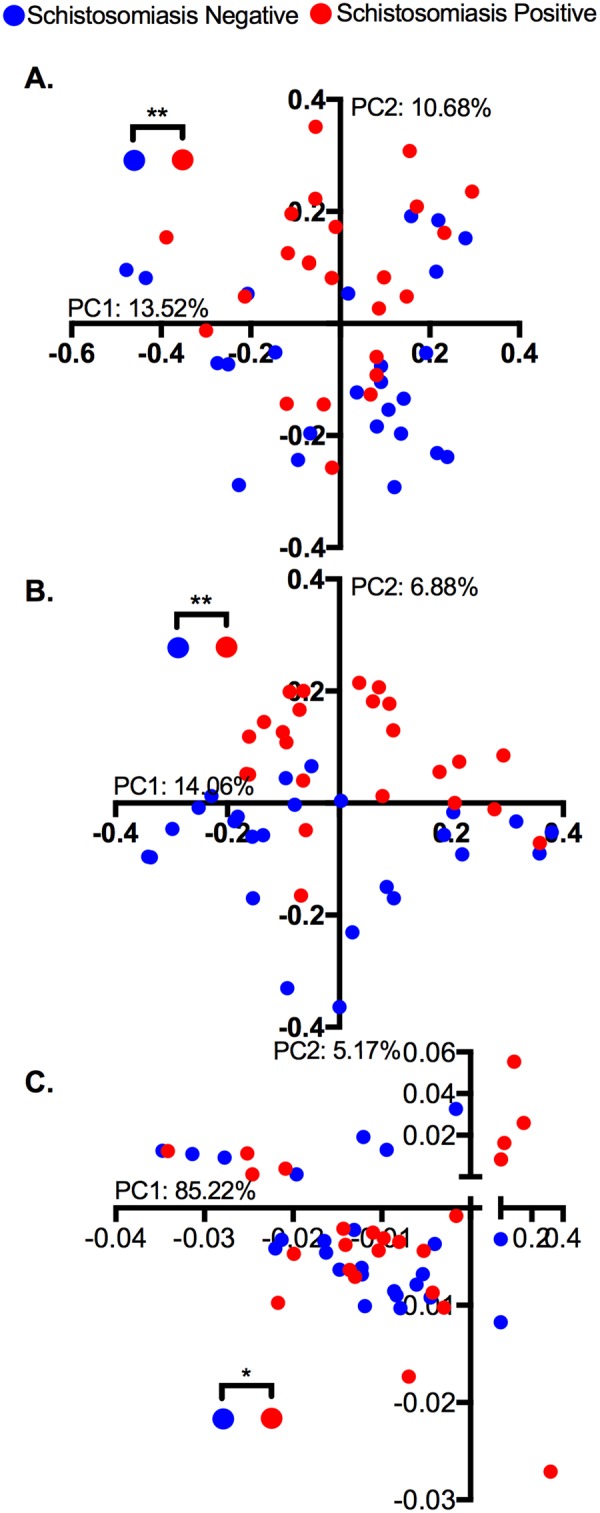
Principal Coordinate Analysis of community similarity by schistosomiasis infection status. Distance matrices were calculated using (A) Bray-Curtis Dissimilarity: p = 0.005. (B) Unweighted UniFrac: p = 0.003. (C) Weighted UniFrac: p = 0.012. Statistics: PERMANOVA through vegan package in R, *p < 0.05, **p < 0.01.
Significantly Different Genera by Infection Status
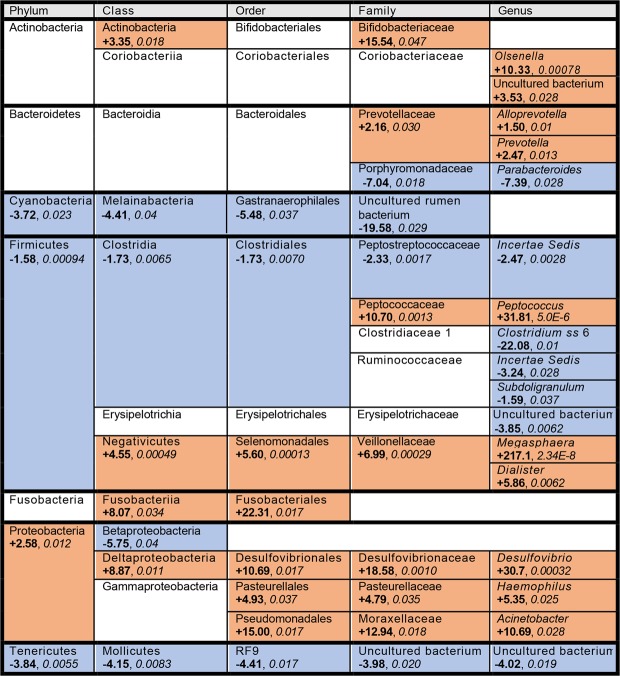
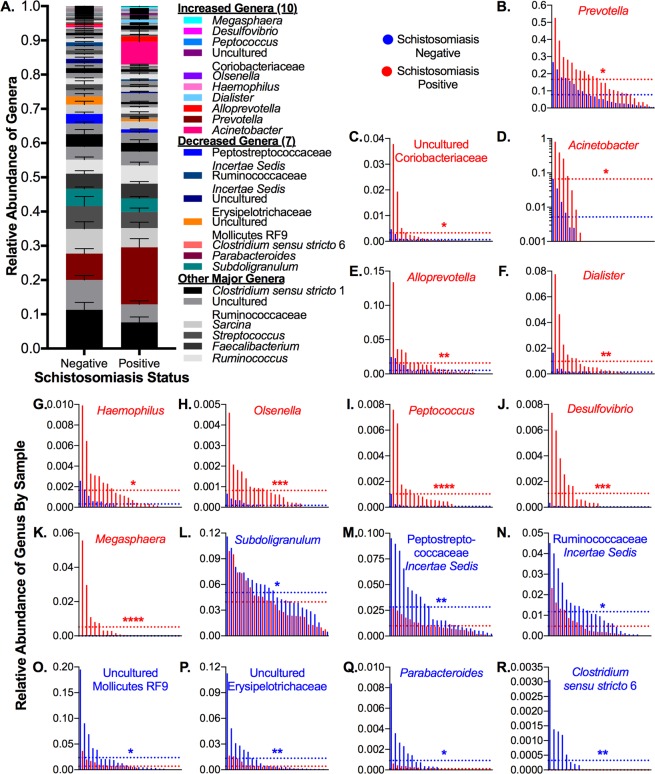
Given the significant differences in beta diversity, we examined the differential abundance of taxa between the infected and uninfected subjects. In total, 1,660 unique OTUs were identified across all samples. As most OTUs were not identified down to the species level, we agglomerated our samples at the genus level to perform differential abundance analysis. We detected significant differences in 17 genera: 10 increased (Megasphaera, Dialister, Acinetobacter, Prevotella, Alloprevotella, Desulfovibrio, Haemophilus, Peptococcus, Olsenella, and uncultured Coriobacteriaceae) and 7 decreased (Subdoligranulum, Parabacteroides, uncultured Erysipelotrichaceae, Ruminococcaceae incertae sedis, Peptostreptococcaceae incertae sedis, Clostridium sensu stricto 6, and uncultured Mollicutes RF9) in infected adolescents (Figs. 3, 4). Collectively, these 17 genera comprised an average of 23% of the relative abundance of the microbiota of uninfected subjects, and all have been previously specifically associated with or arise from lineages associated with the human gut microbiota72–85. Decreases were mainly found within the phylum Firmicutes, particularly in the class Clostridiales, while increases were mainly found within the phylum Proteobacteria and the family Veillonellaceae.
Figure 3.
Taxa and associated lineages that significantly changed in schistosomiasis-positive subjects. Taxa that increased significantly in infected subjects are shown in orange cells, while taxa that decreased significantly are shown in blue cells. Taxa that did not change or with changes that did not reach significance are shown in white cells. Fold change values (shown in bold) were calculated from the log2(fold change) value output from DESeq2, and FDR values (shown in italics) were obtained from the same DESeq2 output.
Figure 4.
Differences in Relative Abundances of Genera Between Schistosomiasis-positive and -negative Subjects. (A) Average relative abundances of all genera, with genera showing significant differences between positive and negative samples highlighted in color. (B–R) Genera that changed in infected adolescents, with negative and positive samples interleaved by ranked abundance of each taxon and dotted lines representing the average relative abundance by group. Statistics: Wald test of differential abundance through DESeq2 package in R, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, error bars indicate SEM. Exact corrected p-values (FDR) can be found in Fig. 3.
Given that many of the genera that we found to be significant are of low abundance, we decided to use qPCR to independently verify the changes in several of these genera. We designed genus-specific 16S primers, validated their specificity against a mock community, and tested the abundances of each genus relative to total 16S rDNA present in the pooled genomic DNA of schistosomiasis-positive and -negative individuals. Despite differences in primers and methodologies between sequencing and qPCR, we were able to recapitulate differences in the abundances of Prevotella, Peptococcus, Megasphaera, Olsenella, Dialister, Alloprevotella, Haemophilus, and Parabacteroides, (Fig. 5), confirming that these genera did change in abundance in the schistosomiasis-positive individuals. For Subdoligranulum, which decreased very slightly in infected adolescents, qPCR did not detect a difference between the groups.
Figure 5.
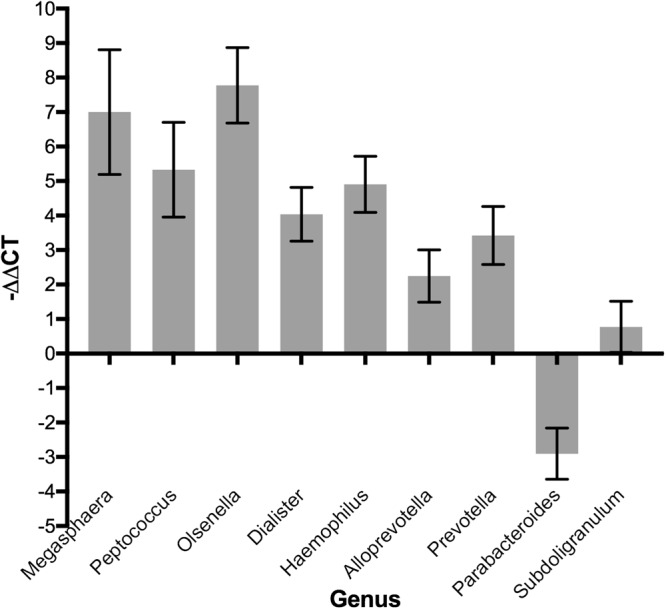
−ΔΔCT Values of Significant Genera Obtained from qPCR Using Genus-specific Primers. ΔΔCT, used to allow all genera to be shown on the same scale, is the corrected raw difference in CT values between infected and uninfected samples, and the sign change causes positive −ΔΔCT values to indicate a positive fold change. Error bars indicate SEM of technical replicates.
Changes Across Taxonomic Levels
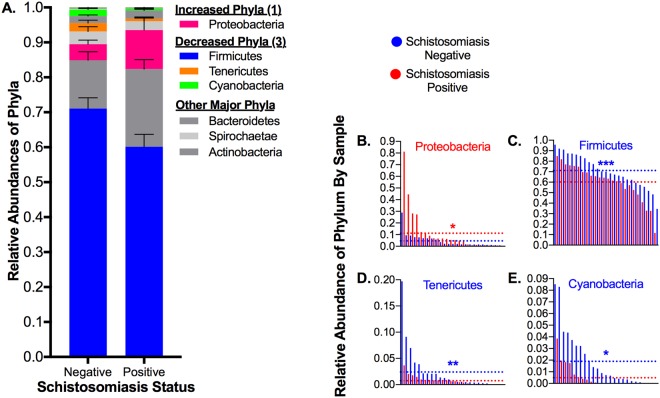
We then began to look at changes in community composition at higher taxonomic levels. At the phylum level, we noted that most phyla decreased in abundance in the schistosomiasis-positive group: we observed significant decreases in Firmicutes, Tenericutes, and Cyanobacteria, and a significant increase in Proteobacteria (Figs. 3, 6). We then analyzed differential abundances at the class, order, and family levels (Figs. S2, S3, S4) and identified several lineages that show significant differences across multiple taxonomic levels (Fig. S5).
Figure 6.
Differences in Relative Abundances of Phyla between Schistosomiasis-positive and -negative Subjects. (A) Average relative abundances of all phyla, with phyla showing significant differences between positive and negative samples highlighted in color. (B–E) Phyla that changed in infected adolescents, with negative and positive samples interleaved by ranked abundance of each taxon and dotted lines representing the average relative abundance by group. Statistics: Wald test of differential abundance through DESeq2 package in R, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, error bars indicate SEM. Exact corrected p-values (FDR) can be found in Fig. 3.
Within the phylum Proteobacteria, a number of lineages demonstrated significant increases in abundance across multiple taxonomic levels. For example, the lineage from which the genus Desulfovibrio arises shows significant increases across all taxonomic levels, including family (Desulfovibrionaceae), order (Desulfovibrionales), class (Deltaproteobacteria), and phylum (Proteobacteria) (Fig. S5A). However, as Deltaproteobacteria comprise a small proportion of the phylum, the increase in Proteobacteria is in fact largely driven by members of the class Gammaproteobacteria. While there was no significant difference at the class level, there were significant increases in two of its lineages: Haemophilus, including family Pasteurellaceae and order Pasteurellales, and Acinetobacter, including family Moraxellaceae and order Pseudomonadales (Fig. S5B). In the human gut, Proteobacteria are typically found at low abundances relative to the dominant phyla of Firmicutes and Bacteroidetes, but blooms in this phylum have been associated with dysbiosis86–88.
Similarly, there are significant increases throughout the taxonomic lineage of Megasphaera and Dialister, on the family (Veillonellaceae), order (Selenomonadales), and class (Negativicutes) levels (Fig. S5C). However, in this case, there is an overall decrease in the parent genus of Firmicutes. This may be related to the fact that Negativicutes, unlike the majority of Gram-positive Firmicutes, are diderms with distinct outer membranes containing lipopolysaccharides that cause them to stain Gram-negative89,90. Interestingly, it is hypothesized that these genes may have been laterally acquired from Proteobacteria91, which also increase in infected adolescents; it is possible that this similarity gives both groups a competitive advantage in the schistosomiasis-associated microbiota.
Additionally, while the Peptococcaceae family from which the genus Peptococcus stems is significantly increased, the order (Clostridiales), class (Clostridia), and phylum (Firmicutes) are significantly decreased (Fig. S5D). In fact, Peptococcus is the only significant genus within the Clostridiales lineage that increases in infected individuals, while the several other significant genera all decrease. For example, the related lineage of Peptostreptococcaceae incertae sedis shows reductions in abundance at all levels, reflecting the more typical pattern of members of Clostridiales and Firmicutes in general (Fig. S5D).
Finally, there are two lineages from less-common phyla that demonstrate reductions in abundance in schistomiasis-positive subjects: the Gastranaerophilales-Melainabacteria lineage of Cyanobacteria and the RF9-Mollicutes lineage of Tenericutes. Unlike most Cyanobacteria, the Melainabacteria are non-photosynthetic and rely on fermentation92,93, while Mollicutes are distinguished from most other bacteria by their lack of a cell wall94.
Functional Potential of the Microbial Communities
While taxonomic classifications of the microbial communities of the two groups is useful, we were also interested in the functional potential of the gut microbiome and how it might vary between infected and uninfected individuals95,96. In general, while there are often significant inter-individual differences in the taxonomic composition of the gut microbiome, the functionality of the resident taxa is relatively stable97. Recently, methods have become available to use the 16S content of a microbial community to infer the genomes present, and therefore the potential functionality of that community, in the absence of whole-genome sequencing data98–100. Importantly, it should be noted that this methodology is based on inference from known genomes, and therefore may not fully recapitulate the existing metagenomic content, relevant strain differences, and the contributions of understudied microbiota members.
We used the web-based tool Piphillin to predict changes in the functional potential of the microbiome by inferring the metagenomes from 16S sequences99 and the tool MicrobiomeAnalyst to analyze differential abundance of both KEGG Orthologs and Pathways101. We identified two KEGG pathways that were enriched in schistosomiasis-positive individuals (Table S3), as well as 35 KEGG orthologs that were significantly different between the two groups (Table S4). We were particularly interested to see that the top enriched pathway, “atrazine degradation”, was populated by the three subunits of bacterial urease (ureA, ureB, and ureC). Furthermore, there were increases in the ureD, ureE, ureF, ureG, and ureH orthologs, all urease accessory proteins, although these were not categorized into any KEGG pathways. Full metagenomics, metatranscriptomics, or functional assays could determine whether the increases observed here reflect a true increase in urease production or function in these microbial communities.
Discussion
We observed a general shift in the gut microbiome of adolescents infected with S. haematobium towards a state consistent with dysbiosis, with decreases in the dominant phylum Firmicutes and increases in the prevalence of the minor phylum Proteobacteria (Fig. 6). At the genus level, where we focused our analysis, we observed significant changes in seventeen genera collectively comprising over 20% of the gut microflora (Figs. 3, 4, Data S1). Interestingly, many of the changes we observed have been associated with gut inflammation. This was surprising, as previously helminth infection has been shown to reduce inflammation, and has even been investigated as a therapy to ameliorate symptoms of inflammatory bowel disease34,35. The apparent contrast could result from the distant location of S. haematobium; gut-resident helminths may exert local anti-inflammatory effects that are not observed in urogenital schistosomiasis. In general, however, these changes are consistent with our hypothesis that S. haematobium infection may impact the gut microbiota.
Several of the changes we found are similar to those observed in other studies of the microbiota of humans infected with helminths. Most directly, a study of Zimbabwean children found significant increases in several OTUs belonging to the genus Prevotella in S. haematobium-infected subjects50, a change we also observed on the genus level. Additionally, several studies investigating the impacts of gut-resident soil-transmitted helminths (STH) demonstrated some of the same taxonomic changes we observed. For example, a study in a Malaysian population infected with multiple STH found increases in the order Bacteroidales40. Similarly, we observed increases in the Prevotella and Alloprevotella genera within Bacteroidales, although we also observed a decrease in the Parabacteroides genus in this order. Another study found increases in Olsenella and the Desulfovibrio lineage in individuals infected with STH in both Indonesian and Liberian populations, as well as associations between STH infection and the Dialister lineage in the Indonesian group and Megasphaera and Peptococcus in the Liberian group39. However, this study also generally found increased abundances of Clostridiales members associated with helminths, which is largely contrary to what we observed39. Finally, researchers studying an Ecuadorean population generally found minimal differences in the microbiota of children with STH infections, but did find significant reductions in members of Clostridiales in children with mixed Trichuris trichiura and Ascaris lumbricoides infections41.
In addition to similarities with other studies of helminths and the human microbiome, we noted that some of the changes we observed were reminiscent of those seen in dysbiosis and inflammation. On the phylum level, decreases in the prevalence of Firmicutes have previously been associated with gut inflammation86,102,103. Firmicutes typically make up a significant proportion of the human gut microbiota, and some members are associated with immunoregulatory impacts. Firmicutes – particularly Clostridia – are associated with regulatory T-cell activation104–106, which is important for the prevention of intestinal inflammation. Additionally, some members of the phylum – such as Faecalibacterium prausnitzii, a member of the Clostridiales-Ruminococcaceae lineage – have been shown to have anti-inflammatory effects due to production of the short-chain fatty acid butyrate and have been negatively correlated with inflammatory bowel disease107.
Furthermore, increased levels of Proteobacteria have been associated with gut inflammation in a number of studies86–88, although whether they are causative or symptomatic remains unclear. Inflammation is associated with increased levels of oxygen and production of nitrate by the gut epithelium; Proteobacteria are generally aerotolerant and some have the capacity to utilize nitrate, potentially allowing them to outcompete other members of the microbiota – such as Clostridia – and bloom during inflammatory conditions87,88,108,109. In addition to thriving in an inflammatory environment, Proteobacteria themselves may contribute to inflammation. Relevant to our study, Desulfovibrio and other sulfate-reducing bacteria have been associated with gut inflammation and colitis, potentially through their production of cytotoxic hydrogen sulfide110–115. Additionally, Gram-negative bacteria, such as Proteobacteria and Negativicutes, can exacerbate existing gut inflammation through lipopolysaccharide infiltration into circulation116,117. Finally, it was recently shown that urease producers, potentially enriched in schistosomiasis-infected subjects, may contribute to a dysbiotic environment, favoring Proteobacteria at the expense of Clostridia and potentially promoting inflammation through increased nitrogen flux118.
In addition to these phylum-level shifts, we observed some changes on lower taxonomic levels that were also associated with gut inflammation. A large study of the gut mucosal and stool microbiota in new-onset pediatric Crohn’s disease patients revealed a number of changes that were similar to what we observed, including reductions in the order Clostridiales and the family Erysipelotrichaceae and increases in the families Veillonellaceae and Pasteurellaceae in patients with disease119. Similarly, we saw reductions in Clostridiales and a genus within Erysipelotrichaceae and increases in both Veillonellaceae and Pasteurellaceae; the exception is Peptococcus, which we saw increased within the Clostridiales family (Fig. 3). Additionally, researchers observed reductions in the order Bacteroidales and increases in the family Fusobacteriaceae in the Crohn’s disease patients119; we noted both increases (Prevotella and Alloprevotella within Prevotellaceae) and decreases (Parabacteroides within Porphyromonadaceae) within Bacteroidales in infected subjects, as well as increases in higher taxonomic levels (Fusobacteriia, Fusobacteriales) of the Fusobacteriaceae lineage (Fig. 3).
In another study, researchers compared the microbiota associated with inflamed mucosa with normal tissue in ulcerative colitis patients, finding that inflamed mucosa was enriched in Proteobacteria and reduced in Firmicutes. Furthermore, these changes were driven largely by increases in the abundance of the Pseudomonadales-Moraxellaceae-Acinetobacter lineage of Proteobacteria and decreases in the Clostridia-Clostridiales lineage, particularly Ruminococcaceae120. Reductions in Ruminococcaceae have also been observed in other studies of inflammatory bowel disease121,122. This is quite similar to our observations in the microbiota of schistosomiasis-infected adolescents, where we saw an enrichment in the Acinetobacter lineage (Figs. 3, S5B) and reductions in many members of Clostridiales, including two Ruminococcaceae (Subdoligranulum and an incertae sedis) (Fig. 3). Taken together, these results suggest that the gut microbiota of S. haematobium-infected adolescents may reflect an inflammatory environment.
Importantly, it should be noted that while some of our observations have been seen in inflammation-related contexts in other individuals, they are not diagnostic of inflammation and it is unknown whether urogenital schistosomiasis-infected adolescents actually experience intestinal inflammation. In the future, it may be prudent to profile gut inflammation in this population in conjunction with microbiome analysis, potentially through measuring fecal biomarkers such as calprotectin123. If, in fact, there is intestinal inflammation associated with schistosomiasis and microbiome alterations, the directionality of this effect would remain unclear; infection-mediated immunological shifts might allow a bloom of pro-inflammatory microflora or might cause inflammation that allows dysbiotic microbes to proliferate.
Additionally, a potential confounder is the presence of co-infection with enteric helminths. While we selected our region of study due to its low rates of these infections58 and ruled out subjects with gastrointestinal symptoms, we also used PCR to check for the presence of these organisms in extracted fecal DNA. Using previously published species-specific primers124,125, we did not detect Ascaris spp, Ancyclostoma spp, Necator americanus, Trichuris trichiura, or S. mansoni in samples from either infected or uninfected subjects (Fig. S6). Additionally, while S. haematobium primarily excretes eggs through the bladder, in a small percentage of cases it can also take up residence in the enteric system and extrude eggs through the intestinal wall51,52. Therefore, we also used PCR to check for the presence of S. haematobium in the fecal samples, and did not detect it in samples from either group (Fig. S6). Importantly, while more sensitive than microscopic methods such as Kato-Katz8,126, even PCR is not perfectly sensitive on a single stool sample for detection of infection with gastrointestinal helminths or schistosomes; thus, while unlikely, it is still possible that some subjects may have those underlying infections and that a portion of our dysbiotic signal may originate from such intestinal morbidity.
Additionally, while we hypothesized that S. haematobium infection could lead to alterations in the gut microbiota due to its impacts on immune function, we cannot discount the possibility that adolescents with pre-existing dysbiosis may be more susceptible to successful schistosome infections, potentially due to immunological changes mediated by the gut microflora. For example, there is evidence that the gut microbiome influences the course of infection with S. mansoni, potentially via immunoregulatory effects; abolishing the gut microbiome of mice infected with S. mansoni reduces gut inflammation and egg excretion, although this may be due to local interactions as this parasite lives proximal to the gut itself127. It is even possible that the “uninfected” microbiome reflects the status of individuals who have acquired immunity to reinfection. In this observational study, it is not possible to determine whether S. haematobium infection is antecedent to changes in gut microbiota. We envision that a longitudinal study of the microbiome of children in schistosomiasis-endemic areas that profiles the same individuals before, during, and after clearance of S. haematobium infection via praziquantel treatment, could help to elucidate cause and effect in the system. In addition, our study was relatively small and subjects were recruited from a single site. It would be prudent to replicate our results in a larger, multi-site study to determine whether our findings are applicable to a wider community. Similarly, profiling the microbiota in a younger cohort may also be sensible, as the potential for gut inflammation could contribute to malnutrition and growth inhibition observed in infected children128.
In general, we have found that the adolescent gut microbiome may be shifted towards a dysbiotic state by infection with S. haematobium, with some similarities to prior observations of the gut microbiota in inflammatory contexts. Such a broad dysbiosis would be an interesting observation in urogenital schistosomiasis, building on the increased abundance of Prevotella OTUs associated with infection previously observed by Kay et al.50. Given the endemic nature of infection in tropical and subtropical regions, it is important to assess how potential dysbiosis may contribute to disease morbidity. In particular, infected adolescents should be assessed for the presence of intestinal inflammation to determine whether these observed microbiome changes truly reflect an inflammatory state or are associated with any of the known morbidities of urogenital schistosomiasis. Additionally, even the schistosomiasis-negative individuals in the current study are likely to have been infected in the past, due to the endemic nature of the infection, but their microbiomes were significantly different than those of currently-infected individuals. Therefore, it would be useful to track subjects long-term after curative praziquantel administration to see whether and how quickly their microbiota returns to an uninfected state; this would additionally help to clarify the causality of the observed changes.
Methods
Ethics Statement
This study was approved by the Kebbi State Ministry of Health and permission to visit the Gotomo Primary School in the Argungu Local Government Area was obtained from the local government education department. All research was undertaken in accordance with the the relevant guidelines and regulations of the Kebbi State Ministry of Health. The study and its risks were explained to the students, who then verbally assented to participation if they were interested. To reduce the risk of co-infection with gastrointestinal helminths, any potential subjects who reported recent gastrointestinal distress were excluded. Prior to sample collection, the parents/guardians of students who had assented to participate were informed of the study as well as the associated risks. Parents of assented students gave informed approval for their child’s participation by signing the study consent form; in the case of illiteracy, thumbprinting was used, as approved by the State Ministry of Health Ethics Committee. All identified cases of schistosomiasis were reported to the Department of Neglected Tropical Diseases at the Kebbi State Ministry of Health. All children at the school were treated with 40 mg/kg praziquantel by the state government as part of the routine national schistosomiasis control program within two weeks after our study.
Subject Characteristics
All samples were collected from Gotomo Primary School, which draws students from seven local villages in rural Nigeria. Six of the villages are within 1.5 km of the school, while the seventh is within 4 km; as is common in this rural area, most students walk to the school. Compared to other Nigerian states, this area is less developed and less influenced by Western culture and diet; the communities surrounding the school is primarily small-scale farmers of low socioeconomic status. As a result, the residents of this area share similar lifestyle and dietary characteristics, which reduces potential confounders compared with more developed regions of the country.
90 subjects were screened for S. haematobium, with 40 (44.4%) identified as infected. 50 adolescents aged 11–15 years were included in the study (Table S1). All samples were collected between July and August 2017. Questionnaires were administered to all participants, covering questions on demographics including age, biological sex, maternal occupation, drinking water source, and exposure to river water (Table S2). All biological samples collected were immediately transported to the Federal University Birnin Kebbi Microbiology Laboratory for processing.
Sample Processing
Urine samples were collected between 10 AM and 2 PM in labelled sample containers and placed in black polyethylene bags. The sedimentation technique was applied for examination of S. haematobium eggs in the urine. A minimum of 7 mL of urine was collected per subject. Urine was spun down at 1,000 × g for 5 minutes, the supernatant was decanted, and the sediments were examined by an experienced technician under the 40X objective of a brightfield microscope (Olympus, USA) to identify S. haematobium eggs, which are characterized by a terminal spine. The number of eggs in each sample was divided by the provided volume and multiplied by 10 to obtain normalized counts of eggs/10 mL (Table S1). The presence of eggs in the urine was used to identify cases of adolescents with urogenital schistosomiasis; for subjects with no eggs in the urine, a second sample was obtained and assessed the following day by a different technician to confirm the lack of eggs and reduce the risk of false negatives. The first 25 samples collected in each group were used in the study; cases and controls were not otherwise matched.
Stool samples were also collected from each child that provided a urine sample; at the school, stool was delivered on sterile paper, collected with sterile plastic spatulas, and stored in sterile bottles. All samples were frozen at −20 degrees Celsius within one hour of production until DNA extraction. Microbial DNA was extracted from stool samples of 25 adolescents infected with urinary schistosomiasis and 25 uninfected controls. 1 g of stool was removed from the center of defrosted fecal samples and was processed following the manufacturer’s protocol using the ZR Fungal/Bacterial DNA Kit™ ZR D6005 (Zymo Research), which utilizes robust mechanical lysis. All samples were extracted at once using the same kit. Extracted DNA was shipped frozen to Brown University for 16S sequencing.
While the prevalence of confounding co-infections in Kebbi State is low58, species-specific PCR was nevertheless used to help rule out the presence of gastrointestinal helminths in our subjects. We also used PCR to assess whether S. haematobium eggs were present in the stool, which can occasionally occur due to unusual placement of adult worms. Extracted fecal DNA was pooled in equimolar amounts by infection status, and PCR for S. mansoni, S. haematobium, Ascaris species, Ancyclostoma species, T. trichiura, and N. americanus was performed using previously published primers (Fig. S6)124,125. For positive controls, parasite genomic DNA was used; S. mansoni and S. haematobium DNA was obtained from BEI Resources, while A. lumbricoides, A. duodenale, T. trichiura, and N. americanus DNA was graciously gifted to us by the Williams Laboratory at Smith College. Primer sequences and PCR conditions are listed in Table S5.
16S Sequencing
Extracted genomic DNA was quantified using a Qubit2 (Invitrogen) to ensure sufficient quantity for amplification. Amplification was performed in triplicate according to the Earth Microbiome Project protocols129, using a library of barcoded adaptor primers (515F) and a single reverse primer (806R) to amplify the V4 region of the 16S gene (Table S6)130. 240 ng of each amplicon was pooled together for sequencing.
Sequencing was performed on the Illumina MiSeq platform using the paired-end 2 × 250 bp protocol. Sample 42 was of poor quality, producing only 14 reads and was therefore removed from further analysis. This left 49 samples for analysis. A total of 1,939,065 reads were obtained across all samples, with a t-test (Prism 7) indicating no significant difference in read depth between infected and uninfected samples (Table S7, p = 0.112).
Data Processing
Reads were demultiplexed using the idemp utility, allowing for 2 barcode mismatches. Demultiplexed reads were imported into the software package Quantitative Insights Into Microbial Ecology 2 (QIIME2 version 2017-8)131,132. Within QIIME2, sequences were quality-filtered and denoised using the Divisive Amplicon Denoising Algorithm 2 (DADA2) pipeline133. A total of 1,660 ribosomal sequence variants (RSVs) were identified across all samples. Taxonomy was assigned using the 99% identity SILVA (release 119) V4 classifier71. RSVs are analogous to the Operational Taxonomic Units (OTUs) generated through traditional clustering methods, and we have used this more familiar terminology throughout the paper. The OTU table, rooted phylogenetic tree, representative sequences, and metadata from QIIME2 were then exported for further analysis in R (V3.3.1). Demultiplexed reads, metadata, and code are available from the Brown Digital Repository (10.7301/Z0K35RVK, https://repository.library.brown.edu/studio/item/bdr:698310/).
Diversity Analyses
Alpha diversity metrics were calculated using the phyloseq (V1.19.1) package (Shannon and Simpson Diversity indices and Observed OTUs) and btools (V0.0.1) packages (Faith’s Phylogenetic Diversity)134,135. Two-tailed Welch’s t-tests (Prism 7) were used to determine the significance of differences in alpha diversity between infection groups. Rarefaction curves were generated to ensure that potential differences in OTU counts were not attributable to increased read depth (Fig. S2). Beta diversity (Bray-Curtis dissimilarity and weighted and unweighted UniFrac) was analyzed using the VEGAN (V2.4-4) package136, and PERMANOVA was used to analyze the significance of differences in beta diversity. Principal coordinate analysis (PCoA) was then performed on the beta diversity distance matrices to visualize any relationships between microbiome composition and infection status.
Differential Abundance Analyses
To reduce noise, data was trimmed to include only genera that were present in at least two samples. Differential abundance analysis between infection groups was conducted using the DESeq2 (V1.14.1) package in R with agglomeration at various taxonomic levels137. To account for multiple hypothesis testing, a Benjamini-Hochberg correction was applied to obtain the false discovery rate (FDR), and taxa with FDR values below 0.05 were considered significant. Abundances by sample at all taxonomic levels are provided in Data S1.
Inferred Metagenomics
To predict the functional potential of the positive and negative communities, we used the web-based tool Piphillin, which infers metagenomes from 16S content99 and matches them to orthologs and pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The OTU abundance table and representative sequences file were exported from QIIME2 and uploaded to Piphillin with required formatting, and the analysis was run using the following parameters: Database – KEGG; Database Version – KEGG May 2017; % Identity Cutoff – 99. The Features output from Piphillin was formatted and uploaded to the web-based tool MicrobiomeAnalyst to identify differentially abundant KEGG orthologs and pathways101. The following MicrobiomeAnalyst parameters were used to analyze differential abundance in orthologs: Data filtering – None; Data Normalization – Relative Log Expression; Analysis Overview – RNASeq Methods (DESeq 2). To assess pathway enrichment, the Network Mapping function on the DESeq2 output was used. In both cases, a FDR less than 0.05 was considered significant.
Confirmatory qPCR of Specific Genus-Level Changes
Genus-specific primers were used to confirm that the changes seen in differential abundance analysis were reflected in the original templates. Many of the significant genera were taxonomically classified as “uncultured” or “incertae sedis” members of a higher taxonomic level, and therefore these were excluded. To design genus-specific primers, at least one 16S sequence from each of several major species in each significant genus was downloaded from the National Center for Biotechnology Information, as well as 16S sequences of representative species from other genera in the same family. When a large number of other genera were present, those also present in the samples were prioritized. For each genus, the relevant species were aligned using Muscle in UGENE (V1.28)138. Alignments were visually scanned for regions where the species within the relevant genus were very similar but were different from species in related genera.
After selecting a few potential regions, primer pairs were tested in NCBI’s Primer-BLAST, which combines Primer3 and BLAST139–141. Briefly, the 16S sequence of a representative species from the relevant genus was used as the template, and the potential primer pairs were input with the following parameters: Search Mode – Automatic; Database – Refseq Representative Genomes; Organism – Bacteria (taxid:2); Primer must have at least 3 total mismatches to unintended targets; At least 2 mismatches within the last 5 bps; Ignore targets that have 6 or more mismatches to the primer. Only primer pairs with unintended targets that matched with other species in the genus but not with species in related genera present in the sample were accepted. Primer pairs were validated by robust amplification from schistosomiasis-positive gDNA samples compared to a mock community that did not contain the genera of interest (ZymoBIOMICS Microbial Community DNA Standard). The exception is Desulfovibrio, which despite apparent strong specificity in PrimerBLAST did show some amplification from the mock community. Primers are listed in Table S7.
Equivalent amounts of genomic DNA from all schistosomiasis-positive and schistosomiasis-negative samples were pooled into two samples for analysis. All qPCR was run on a Roche Lightcycler 480, using the SYBRGreen-based 2X Fast Start Essential DNA Green Master Mix in the following preparation: 7.5 µL Master Mix, 6.35 µL H2O, 0.075 µL each primer, and 1 ng of template gDNA. Each qPCR run was performed in triplicate technical replicates on pooled positive gDNA, pooled negative gDNA, a negative control mock community, and a no template control. Reactions were performed in parallel. Cycling conditions are listed in Table S8. Changes were calculated using the ΔΔCT method, using total 16S DNA amplified from the pooled samples with universal primers to normalize data.
Electronic supplementary material
Acknowledgements
We wish to acknowledge the Gotomo primary school headmaster, teachers, and parents for their support during the study. We also thank the Director of Public Health of the Kebbi State Ministry of Health for his support and guidance during the project. We acknowledge the University of Rhode Island Genomics and Sequencing Center, which performed the quality control and 16S sequencing, and the Brown University Statistical Consulting Group and the Brown COBRE Computational Biology Core for their advice on statistics. We also thank the Williams Laboratory at Smith College for their generous gift of genomic DNA from several helminth species. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number AI097493 awarded to SO), the Bill and Melinda Gates Foundation (grant number OPP1034619 awarded to SO), and the National Institute of General Medical Science (IDeA grant PM20GM109035 from the COBRE Center for Computational Biology of Human Disease and grant P20GM121344 from the COBRE Center for Antimicrobial Resistance and Therapeutic Discovery, awarded to PB).
Author Contributions
O.A. and P.B. designed and oversaw the study. S.O. advised during the study. O.A., C.O.O., M.B.M., and A.A.E. contributed to participant recruitment and sample collection and processing. A.D.R. performed data analysis and generated figures. D.J.C. advised during data analysis. A.D.R. and O.A. wrote the manuscript. All authors reviewed and revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Olumide Ajibola and Aislinn D. Rowan contributed equally.
Contributor Information
Olumide Ajibola, Email: olumide.ajibola@fubk.edu.ng.
Peter Belenky, Email: peter_belenky@brown.edu.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36709-1.
References
- 1.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiper, R. T. Researches on Egyptian bilharziosis (a report to the War Office on the results of the Bilharzia Mission in Egypt, 1915). (Bale & Danielsson, 1918).
- 3.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 4.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 5.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dew HR. Observations on the Pathology of Schistosomiasis (S. haematobium and S. mansoni) in the Human Subject. J Pathol Bacteriol. 1923;26:27–39. doi: 10.1002/path.1700260104. [DOI] [Google Scholar]
- 7.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 8.Weerakoon KG, Gobert GN, Cai P, McManus DP. Advances in the Diagnosis of Human Schistosomiasis. Clin Microbiol Rev. 2015;28:939–967. doi: 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montresor A, et al. Preventive chemotherapy and the fight against neglected tropical diseases. Expert Rev Anti Infect Ther. 2012;10:237–242. doi: 10.1586/eri.11.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everts B, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J Exp Med. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girgis NM, Gundra UM, Loke P. Immune regulation during helminth infections. PLoS Pathog. 2013;9:e1003250. doi: 10.1371/journal.ppat.1003250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138:666–675. doi: 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips SM, Lammie PJ. Immunopathology of granuloma formation and fibrosis in schistosomiasis. Parasitol Today. 1986;2:296–302. doi: 10.1016/0169-4758(86)90123-7. [DOI] [PubMed] [Google Scholar]
- 16.Davies, S. J. & McKerrow, J. H. In Biology of Parasitism (eds Tschudi, C. & Pearce, E. J.) 273-289 (Springer US, 2000).
- 17.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 18.Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunol. 2014;36:347–357. doi: 10.1111/pim.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra I, et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–6848. [PubMed] [Google Scholar]
- 21.Marshall AJ, et al. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-alpha and early death in C57BL/6 mice. J Immunol. 1999;163:2089–2097. [PubMed] [Google Scholar]
- 22.Actor JK, et al. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melhem RF, LoVerde PT. Mechanism of interaction of Salmonella and Schistosoma species. Infect Immun. 1984;44:274–281. doi: 10.1128/iai.44.2.274-281.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LoVerde PT, Amento C, Higashi GI. Parasite-parasite interaction of Salmonella typhimurium and Schistosoma. J Infect Dis. 1980;141:177–185. doi: 10.1093/infdis/141.2.177. [DOI] [PubMed] [Google Scholar]
- 25.Feldmeier H, Krantz I, Poggensee G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. Int J STD AIDS. 1994;5:368–372. doi: 10.1177/095646249400500517. [DOI] [PubMed] [Google Scholar]
- 26.Mbabazi PS, et al. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl Trop Dis. 2011;5:e1396. doi: 10.1371/journal.pntd.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallestrup P, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis. 2005;192:1956–1961. doi: 10.1086/497696. [DOI] [PubMed] [Google Scholar]
- 28.Brown M, et al. Schistosoma mansoni, nematode infections, and progression to active tuberculosis among HIV-1-infected Ugandans. Am J Trop Med Hyg. 2006;74:819–825. doi: 10.4269/ajtmh.2006.74.819. [DOI] [PubMed] [Google Scholar]
- 29.DiNardo AR, et al. Schistosome Soluble Egg Antigen Decreases Mycobacterium tuberculosis-Specific CD4+ T-Cell Effector Function With Concomitant Arrest of Macrophage Phago-Lysosome Maturation. J Infect Dis. 2016;214:479–488. doi: 10.1093/infdis/jiw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monin L, et al. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J Clin Invest. 2015;125:4699–4713. doi: 10.1172/JCI77378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Awady MK, et al. Soluble egg antigen of Schistosoma Haematobium induces HCV replication in PBMC from patients with chronic HCV infection. BMC Infect Dis. 2006;6:91. doi: 10.1186/1471-2334-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sipahi AM, Baptista DM. Helminths as an alternative therapy for intestinal diseases. World J Gastroenterol. 2017;23:6009–6015. doi: 10.3748/wjg.v23.i33.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rook GA, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol. 2014;177:1–12. doi: 10.1111/cei.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helmby H. Human helminth therapy to treat inflammatory disorders - where do we stand? BMC Immunol. 2015;16:12. doi: 10.1186/s12865-015-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruszewska-Cheruiyot M, Donskow-Lysoniewska K, Doligalska M. Helminth therapy: Advances in the use of parasitic worms against Inflammatory Bowel Diseases and its challenges. Helminthologia. 2018;55:1–11. doi: 10.1515/helm-2017-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloomfield SF, et al. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health. 2016;136:213–224. doi: 10.1177/1757913916650225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiemsma LT, Reynolds LA, Turvey SE, Finlay BB. The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther. 2015;4:143–157. doi: 10.2147/ITT.S61528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutapi F, et al. Age-related and infection intensity-related shifts in antibody recognition of defined protein antigens in a schistosome-exposed population. J Infect Dis. 2008;198:167–175. doi: 10.1086/589511. [DOI] [PubMed] [Google Scholar]
- 39.Rosa BA, et al. Differential human gut microbiome assemblages during soil-transmitted helminth infections in Indonesia and Liberia. Microbiome. 2018;6:33. doi: 10.1186/s40168-018-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SC, et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl Trop Dis. 2014;8:e2880. doi: 10.1371/journal.pntd.0002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper P, et al. Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS One. 2013;8:e76573. doi: 10.1371/journal.pone.0076573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holm JB, et al. Chronic Trichuris muris Infection Decreases Diversity of the Intestinal Microbiota and Concomitantly Increases the Abundance of Lactobacilli. PLoS One. 2015;10:e0125495. doi: 10.1371/journal.pone.0125495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins TP, et al. Infections by human gastrointestinal helminths are associated with changes in faecal microbiota diversity and composition. PLoS One. 2017;12:e0184719. doi: 10.1371/journal.pone.0184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li RW, et al. The effect of helminth infection on the microbial composition and structure of the caprine abomasal microbiome. Sci Rep. 2016;6:20606. doi: 10.1038/srep20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li RW, et al. Alterations in the porcine colon microbiota induced by the gastrointestinal nematode Trichuris suis. Infect Immun. 2012;80:2150–2157. doi: 10.1128/IAI.00141-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plieskatt JL, et al. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB J. 2013;27:4572–4584. doi: 10.1096/fj.13-232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broadhurst MJ, et al. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog. 2012;8:e1003000. doi: 10.1371/journal.ppat.1003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simcock DC, et al. Hypergastrinaemia, abomasal bacterial population densities and pH in sheep infected with Ostertagia circumcincta. Int J Parasitol. 1999;29:1053–1063. doi: 10.1016/S0020-7519(99)00065-X. [DOI] [PubMed] [Google Scholar]
- 49.McKenney EA, et al. Alteration of the rat cecal microbiome during colonization with the helminth Hymenolepis diminuta. Gut Microbes. 2015;6:182–193. doi: 10.1080/19490976.2015.1047128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kay GL, et al. Differences in the Faecal Microbiome in Schistosoma haematobium Infected Children vs. Uninfected Children. PLoS Negl Trop Dis. 2015;9:e0003861. doi: 10.1371/journal.pntd.0003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blair DM. The routes of schistosome egg passage from the human body. Cent Afr J Med. 1965;11:243–249. [PubMed] [Google Scholar]
- 52.Cunin P, Tchuem Tchuente LA, Poste B, Djibrilla K, Martin PM. Interactions between Schistosoma haematobium and Schistosoma mansoni in humans in north Cameroon. Trop Med Int Health. 2003;8:1110–1117. doi: 10.1046/j.1360-2276.2003.01139.x. [DOI] [PubMed] [Google Scholar]
- 53.Monaco CL, et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe. 2016;19:311–322. doi: 10.1016/j.chom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhat M, et al. Impact of Immunosuppression on the Metagenomic Composition of the Intestinal Microbiome: a Systems Biology Approach to Post-Transplant Diabetes. Sci Rep. 2017;7:10277. doi: 10.1038/s41598-017-10471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z, et al. Immunosuppressive effect of the gut microbiome altered by high-dose tacrolimus in mice. Am J Transplant. 2018;18:1646–1656. doi: 10.1111/ajt.14661. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, Zhang X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol Res. 2015;171:97–106. doi: 10.1016/j.micres.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Tourret J, et al. Immunosuppressive Treatment Alters Secretion of Ileal Antimicrobial Peptides and Gut Microbiota, and Favors Subsequent Colonization by Uropathogenic Escherichia coli. Transplantation. 2017;101:74–82. doi: 10.1097/TP.0000000000001492. [DOI] [PubMed] [Google Scholar]
- 58.Nigeria, F. M. o. H. o. Report on the Epidemiological Mapping of Schistosomiasis and Soil Transmitted Helminthiases in 19 States and the FCT, Nigeria. (Federal Ministry of Health of Nigeria, 2015).
- 59.Okwori AEJ, et al. Prevalence of Schistosomiasis among Primary School Children in Gadabuke District, Toto LGA, North Central Nigeria. Brit Microbiol Res J. 2014;4:255–261. doi: 10.9734/BMRJ/2014/5736. [DOI] [Google Scholar]
- 60.Nduka FO, Ajaero CM, Nwoke BE. Urinary schistosomiasis among school children in an endemic community in south-eastern Nigeria. Appl Parasitol. 1995;36:34–40. [PubMed] [Google Scholar]
- 61.Dawaki S, et al. Prevalence and Risk Factors of Schistosomiasis among Hausa Communities in Kano State, Nigeria. Rev Inst Med Trop Sao Paulo. 2016;58:54. doi: 10.1590/S1678-9946201658054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kapito-Tembo AP, et al. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Negl Trop Dis. 2009;3:e361. doi: 10.1371/journal.pntd.0000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdullahi M, Saidu TB. Prevalence of Urinary Schistosomiasis among School-Aged Children in Wushishi Local Government Area of Niger State, Nigeria. Bayero J Pure Appl Sci. 2012;4:53–55. [Google Scholar]
- 64.Mitchell KM, Mutapi F, Savill NJ, Woolhouse ME. Protective immunity to Schistosoma haematobium infection is primarily an anti-fecundity response stimulated by the death of adult worms. Proc Natl Acad Sci USA. 2012;109:13347–13352. doi: 10.1073/pnas.1121051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mutapi F, et al. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J Infect Dis. 1998;178:289–293. doi: 10.1086/517456. [DOI] [PubMed] [Google Scholar]
- 66.Healey GR, Murphy R, Brough L, Butts CA, Coad J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr Rev. 2017;75:1059–1080. doi: 10.1093/nutrit/nux062. [DOI] [PubMed] [Google Scholar]
- 67.Ingang-Etoh, P. C., Essien, U. C., Amama, S. A. & Useh, M. F. Prevalence of urinary schistosomiasis among school children in Ukwelo-Obudu and Abini communities in Cross River State, Nigeria. Port Harcourt Medical Journal3 (2009).
- 68.Okoli EI, Odaibo AB. Urinary schistosomiasis among schoolchildren in Ibadan, an urban community in south-western Nigeria. Trop Med Int Health. 1999;4:308–315. doi: 10.1046/j.1365-3156.1999.00388.x. [DOI] [PubMed] [Google Scholar]
- 69.Fatiregun AA, Osungbade KO, Olumide EA. Prevalence of Urinary Schistosomiasis among Secondary School Students in Ibadan, Nigeria. Tropical Journal of Health Sciences. 2006;13:6–10. doi: 10.4314/tjhc.v13i2.36690. [DOI] [Google Scholar]
- 70.Ivoke N, et al. Prevalence and transmission dynamics of Schistosoma haematobium infection in a rural community of southwestern Ebonyi State, Nigeria. Trop Biomed. 2014;31:77–88. [PubMed] [Google Scholar]
- 71.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agans R, et al. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol Ecol. 2011;77:404–412. doi: 10.1111/j.1574-6941.2011.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y, et al. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci Rep. 2017;7:3628. doi: 10.1038/s41598-017-03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kvasnovsky CL, et al. Clinical and symptom scores are significantly correlated with fecal microbiota features in patients with symptomatic uncomplicated diverticular disease: a pilot study. Eur J Gastroenterol Hepatol. 2018;30:107–112. doi: 10.1097/MEG.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 75.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suau A, et al. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belizario JE, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015;6:1050. doi: 10.3389/fmicb.2015.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bressa C, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. 2017;12:e0171352. doi: 10.1371/journal.pone.0171352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez-Cobas AE, et al. Structural and functional changes in the gut microbiota associated to Clostridium difficile infection. Front Microbiol. 2014;5:335. doi: 10.3389/fmicb.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3:637–682. doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhute S, et al. Molecular Characterization and Meta-Analysis of Gut Microbial Communities Illustrate Enrichment of Prevotella and Megasphaera in Indian Subjects. Front Microbiol. 2016;7:660. doi: 10.3389/fmicb.2016.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carbonero, F. & Gaskins, H. R. In Encyclopedia of Metagenomics (eds Highlander, S. K., Rodriguez-Valera, F., & White, B. A.) (Springer, Boston, MA, 2015).
- 83.Ricaboni D, et al. Olsenella provencensis sp. nov., Olsenella phocaeensis sp. nov., and Olsenella mediterranea sp. nov. isolated from the human colon. Hum. Microbiome J. 2017;4:22–23. doi: 10.1016/j.humic.2017.05.002. [DOI] [Google Scholar]
- 84.Rey FE, et al. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci USA. 2013;110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clavel T, et al. Intestinal microbiota in metabolic diseases: from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes. 2014;5:544–551. doi: 10.4161/gmic.29331. [DOI] [PubMed] [Google Scholar]
- 86.Major G, Spiller R. Irritable bowel syndrome, inflammatory bowel disease and the microbiome. Curr Opin Endocrinol Diabetes Obes. 2014;21:15–21. doi: 10.1097/MED.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int. 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Gupta RS. Origin of diderm (Gram-negative) bacteria: antibiotic selection pressure rather than endosymbiosis likely led to the evolution of bacterial cells with two membranes. Antonie Van Leeuwenhoek. 2011;100:171–182. doi: 10.1007/s10482-011-9616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marchandin H, et al. Negativicoccus succinicivorans gen. nov., sp. nov., isolated from human clinical samples, emended description of the family Veillonellaceae and description of Negativicutes classis nov., Selenomonadales ord. nov. and Acidaminococcaceae fam. nov. in the bacterial phylum Firmicutes. Int J Syst Evol Microbiol. 2010;60:1271–1279. doi: 10.1099/ijs.0.013102-0. [DOI] [PubMed] [Google Scholar]
- 91.Campbell C, Sutcliffe IC, Gupta RS. Comparative proteome analysis of Acidaminococcus intestini supports a relationship between outer membrane biogenesis in Negativicutes and Proteobacteria. Arch Microbiol. 2014;196:307–310. doi: 10.1007/s00203-014-0964-4. [DOI] [PubMed] [Google Scholar]
- 92.Soo RM, et al. An expanded genomic representation of the phylum cyanobacteria. Genome Biol Evol. 2014;6:1031–1045. doi: 10.1093/gbe/evu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di Rienzi SC, et al. The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria. eLife. 2013;2:e01102. doi: 10.7554/eLife.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown, D. R. & Bradbury, J. M. in Mollicutes: Molecular Biology and Pathogenesis (eds Browning G. F. & Citti, C.) (Caister Academic Press, 2014).
- 95.Turnbaugh PJ, et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature486, 207-214, 10.1038/nature11234 (2012). [DOI] [PMC free article] [PubMed]
- 98.Asshauer KP, Wemheuer B, Daniel R, Meinicke P. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 2015;31:2882–2884. doi: 10.1093/bioinformatics/btv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iwai S, et al. Piphillin: Improved Prediction of Metagenomic Content by Direct Inference from Human Microbiomes. PLoS One. 2016;11:e0166104. doi: 10.1371/journal.pone.0166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Langille MG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dhariwal A, et al. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hold GL, et al. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20:1192–1210. doi: 10.3748/wjg.v20.i5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 2017;10:63–73. doi: 10.2147/JIR.S116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 105.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J. 2013;7:1256–1261. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Winter SE, Baumler AJ. Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes. 2014;5:71–73. doi: 10.4161/gmic.27129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rooks MG, et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014;8:1403–1417. doi: 10.1038/ismej.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van den Elsen LW, Poyntz HC, Weyrich LS, Young W, Forbes-Blom EE. Embracing the gut microbiota: the new frontier for inflammatory and infectious diseases. Clin Transl Immunology. 2017;6:e125. doi: 10.1038/cti.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Loubinoux J, Bronowicki JP, Pereira IA, Mougenel JL, Faou AE. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol. 2002;40:107–112. doi: 10.1111/j.1574-6941.2002.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 113.Roediger WE, Duncan A, Kapaniris O, Millard S. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology. 1993;104:802–809. doi: 10.1016/0016-5085(93)91016-B. [DOI] [PubMed] [Google Scholar]
- 114.Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571–1579. doi: 10.1023/A:1018851723920. [DOI] [PubMed] [Google Scholar]
- 115.Rowan F, et al. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. 2010;53:1530–1536. doi: 10.1007/DCR.0b013e3181f1e620. [DOI] [PubMed] [Google Scholar]
- 116.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo S, et al. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J Immunol. 2015;195:4999–5010. doi: 10.4049/jimmunol.1402598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ni, J. et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med9, 10.1126/scitranslmed.aah6888 (2017). [DOI] [PMC free article] [PubMed]
- 119.Gevers D, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leung JM, et al. IL-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol. 2014;7:124–133. doi: 10.1038/mi.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Halfvarson J, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morgan XC, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841–845. doi: 10.1097/00042737-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 124.Sady H, et al. Detection of Schistosoma mansoni and Schistosoma haematobium by Real-Time PCR with High Resolution Melting Analysis. Int J Mol Sci. 2015;16:16085–16103. doi: 10.3390/ijms160716085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Llewellyn S, et al. Application of a Multiplex Quantitative PCR to Assess Prevalence and Intensity Of Intestinal Parasite Infections in a Controlled Clinical Trial. PLoS Negl Trop Dis. 2016;10:e0004380. doi: 10.1371/journal.pntd.0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gordon CA, Gray DJ, Gobert GN, McManus DP. DNA amplification approaches for the diagnosis of key parasitic helminth infections of humans. Mol Cell Probes. 2011;25:143–152. doi: 10.1016/j.mcp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 127.Holzscheiter M, et al. Lack of host gut microbiota alters immune responses and intestinal granuloma formation during schistosomiasis. Clin Exp Immunol. 2014;175:246–257. doi: 10.1111/cei.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McGarvey ST. Schistosomiasis: Impact on childhood and adolescent growth, malnutrition, and morbidity. Semin Pediatr Infect Dis. 2000;11:269–274. doi: 10.1053/spid.2000.9642. [DOI] [Google Scholar]
- 129.Thompson LR, et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature. 2017;551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walters, W. et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems1, 10.1128/mSystems.00009-15 (2016). [DOI] [PMC free article] [PubMed]
- 131.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Navas-Molina JA, et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013;531:371–444. doi: 10.1016/B978-0-12-407863-5.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Callahan BJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McMurdie, P. J. & Holmes, S. Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pac Symp Biocomput, 235-246 (2012). [PMC free article] [PubMed]
- 136.Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci. 2009;14:927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x. [DOI] [Google Scholar]
- 137.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq 2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okonechnikov K, Golosova O, Fursov M. & team, U. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 2012;28:1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- 139.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 140.Kumar A, Chordia N. In silico PCR Primer Designing and Validation. Methods Mol Biol. 2015;1275:143–151. doi: 10.1007/978-1-4939-2365-6_10. [DOI] [PubMed] [Google Scholar]
- 141.Coordinators NR. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2014;42:D7–17. doi: 10.1093/nar/gkt1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.