Abstract
The laminin subunit alpha 2 (LAMA2) gene encodes an alpha 2 chain, which constitutes one of the subunits of laminin 2 (merosin) and laminin 4 (s-merosin). In the current study, we investigated the relationship between LAMA2 promoter methylation status and the invasiveness of clinically nonfunctioning pituitary adenomas (PitNETs). Specimens from patients with nonfunctioning PitNET were classified into three groups according to preoperative computed tomography (CT)/magnetic resonance imaging findings: a normal group (n = 6), non-invasive group (n = 11) and invasive group (n = 6). LAMA2 expression was assessed using quantitative real-time polymerase chain reaction (RT-qPCR) and western blotting, and the methylation status of the LAMA2 promoter region was observed using sodium bisulfite sequencing. Furthermore, 5-aza-2-deoxycytidine was used to explore the relationship between decreased LAMA expression and methylation in PitNET cells. According to the RT-qPCR and western blotting results, LAMA2 expression was downregulated in invasive PitNET, while the methylation of the LAMA2 promoter was increased. Methylation of the LAMA2 promoter decreased the expression of LAMA2. Thus, changes in LAMA2 expression due to promoter methylation were inversely correlated with the invasiveness of PitNET and the protein functions as a tumor suppressor. In addition, overexpression and demethylation of LAMA2 suppressed the invasion of PitNET cells, partially by exerting effects on the PTEN-PI3K/AKT signaling pathway and matrix metalloproteinase-9 (MMP-9). Furthermore, a xenograft model was also generated, and LAMA2 overexpression significantly suppressed tumor growth in vivo. Thus, LAMA2 expression and methylation patterns might be used as biomarkers to predict the prognosis of patients with PitNET.
Keywords: invasion, LAMA2, methylation, nonfunctioning pituitary adenoma
Introduction
Pituitary adenoma (PitNET) is currently one of the most common human intracranial tumors, accounting for 10–15% of intracranial tumors.1 Pituitary tumors are histologically benign, but approximately one third of PitNETs are invasive, making the complete removal of these tumors very difficult.2 Predictors of the likelihood of aggressive behavior or relapse of this type of tumor have not been identified, although matrix metalloproteinases (MMPs), such as a disintegrin and metalloproteinase proteins and various other markers, have shown potential for predicting tumor growth or invasiveness.3 Since current detection techniques do not accurately predict the malignancy of pituitary tumors at an early stage, or the invasiveness of PitNETs, effective methods for evaluating prognosis are urgently needed.4 Therefore, studies aiming to identify potential biomarkers associated with PitNET invasion are critical for timely diagnosis and treatment.
During carcinogenesis, epigenetic alterations in tumor suppressor genes are potentially important in regulating the pathogenesis of tumors. Hypermethylation of cytosine–phosphate–guanosine (CpG) islands in the promoter regions of tumor suppressor genes frequently occurs in early stages of various tumors.5 This modification has also been used as a potential biomarker for risk evaluations in patients with cancer.5 The dysregulation of extracellular-matrix-related genes has recently been shown to exert important effects on tumor invasiveness during tumor progression.6 Basal laminae are structural components of the extracellular matrix that influence cell proliferation and differentiation,7 and laminin subunit alpha 2 (LAMA2) is the major component of the basal laminae-α subunit of laminin. Interestingly, promoter hypermethylation is often associated with decreased expression of tumor suppressor genes.8 Dense CpG islands flank the first exon of LAMA2. These sequences have been reported as hypermethylated in many types of carcinoma.9
Decreased expression of LAMA2 caused by promoter hypermethylation has been confirmed in various cancers, including colon and bladder cancers.7 However, the role of LAMA2 in PitNET remains unknown. Thus, we seek to identify markers of the invasiveness and prognosis of pituitary tumors. We examined levels of the LAMA2 messenger ribonucleic acid (mRNA) and protein in non-invasive PitNET, invasive PitNET and normal pituitary tissues. Using sodium bisulfite sequencing, we also measured the methylation status of the LAMA2 promoter region. In addition, the relationship between the methylation of the LAMA2 promoter and decreased expression of LAMA2 in the GH3 PitNET cell line was also investigated.
Furthermore, the detailed mechanism of LAMA2 in PitNET invasiveness was also investigated. The PI3K/AKT signaling pathway has been associated with the occurrence of PitNET.10,11 PTEN, a tumor suppressor gene, has been reported to negatively regulate the PI3K-AKT signaling pathway to suppress tumor progression.12 The PTEN-PI3K/AKT signaling pathway plays important roles in mediating cancer cell migration or invasion.13–15 However, the relationship between the invasiveness of PitNET and the LAMA2/PTEN-PI3K/AKT signaling pathway requires further investigation.
Materials and methods
Sample collection
Written informed consent was obtained from patients, and the study was approved by the Ethics Committee of The Second Affiliated Hospital of Dalian Medical University, Dalian, China. We also obtained deceased written informed consent from patients’ relatives specifically for the use of donor material from patients with normal pituitary glands. The samples were classified into three groups: a normal pituitary control group, non-invasive group and invasive group.
Nineteen samples from patients with nonfunctioning PitNET (age range 33–83 years) were obtained from The Second Affiliated Hospital of Dalian Medical University, dating from 2014 to 2017. Invasive adenomas were defined as Hardy classification grade III and IV. Eight invasive and 11 non-invasive PitNETs were analyzed. The non-invasive group included six female patients and five male patients, and the median age was 58 years. The invasive group included three female patients and five male patients, with a median age of 51 years. All donors of normal pituitary glands died of non-neurological causes. The six control samples were rinsed with sterile saline and snap-frozen in liquid nitrogen. Suitable sections of each normal pituitary sample were embedded in paraffin.
Quantitative real-time PCR (RT-qPCR)
Total RNA was extracted from PitNETs and adjacent normal tissues using TRIzol reagent according to the manufacturer’s protocol (TaKaRa Bio, Dalian, China). The cDNA templates were reverse transcribed using a PrimeScript RT Reagent Kit (TaKaRa Bio, Dalian, China) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (RT-qPCR) was performed according to the manufacturer’s protocol (TaKaRa Bio, Dalian, China), and amplification was performed using an Mx3005P Real-Time PCR System (Agilent, CA, USA). The relative mRNA expression of each gene was normalized to GAPDH RNA levels and analyzed using the 2-∆∆CT/CT method.16 The primers were synthesized by Invitrogen (Shanghai, China). The following primers were used: LAMA2 forward primer 5′-CAAACCAGAGACACCCGAT-3′ and reverse primer 5′-ATCATCAAGA GAGCGTTCCAA-3′, and GAPDH forward primer 5′-TGACTTCAACAGCGACACCCA-3′ and reverse primer 5′-CACCCTGTTGCTGT AGCCAAA-3′.
Protein extraction and western blotting analysis
Total cellular proteins were extracted using RIPA lysis buffer containing phosphatase and protease inhibitors (Roche, Basel, Switzerland) in accordance with the manufacturer’s instructions. PitNETs and normal tissues were lysed in lysis buffer with shaking at 4°C for 30 min. Protein concentrations were quantified using a bicinchoninic acid (BCA) protein assay kit. Proteins were separated on SDS-PAGE gels and transferred to PVDF membranes that were blocked with 5% fat-free dry milk or 5% BSA in TBST and immunoblotted with primary antibodies at 4°C overnight. On the following day, membranes were incubated with secondary antibodies at room temperature for 2 h. The protein bands were detected using enhanced chemiluminescence. LAMA2 antibodies were purchased from Abcam (Pudong, Shanghai, China). Primary antibodies against PTEN, p-AKT, MMP-9, and β-actin and all the secondary antibodies were obtained from Cell Signaling Technology (Cell Signaling Technology, Inc., Danvers, MA, USA). A goat antirat antibody was used as the secondary antibody. The final data were subjected to grayscale scanning and a semiquantitative analysis using Quantity One software (Bio-Rad, Hercules, CA, USA).
Sodium bisulfite treatment and sequencing
Genomic DNA was extracted from samples using a DNA extraction kit (Huashun Co., Ltd, Shanghai, China). Samples of 1.5 µg of genomic DNA were treated with sodium bisulfite using a DNA Bisulfite Conversion Kit (TIANGEN, Beijing, China) according to the manufacturer’s protocol. One CpG-rich region was analyzed. PCR was performed using the following conditions: 94°C for 2 min; 94°C for 30 s, 64.5°C for 30 s, 68°C for 1 min for 40 cycles; and 68°C for 10 min. PCR products were subcloned into a pGEM®-T Easy Vector System (Promega, Madison, WI, USA), and six constructs representing each region from each sample were randomly selected for sequencing. The primers for the methylated region of the LAMA2 promoter were: forward primer: 5′-TATAAGTTAAGGTTAGGGGATAGGG-3′ and reverse primer: 5′-ATCATCAAGAGAGCGTTCCAA-3′
Cell culture and 5-aza-2-deoxycytidine treatment
GH3 cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco-BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS), which was referred to as FBS medium, at 37°C in a humidified atmosphere of 5% CO2. The basal serum-free (BSF) medium was composed of DMEM:Neurobasal™ (Gibco-BRL, Gaithersburg, MD) medium (1:1, v/v), supplemented with sodium pyruvate (1 mmol/l), GlutaMAX® (ThermoFisher Scientific, Waltham, MA, USA) (1 mmol/l), and bovine serum albumin (100 μg/ml), transferrin (100 μg/ml), putrescine (16 μg/ml), progesterone (60 ng/ml), sodium selenite (40 ng/ml) and NAC (5 mg/ml). A series of serum-free media were composed of BSF medium supplemented with insulin (5 ng/ml) and referred to as SF-I medium. The demethylating agent, 5-aza-2-deoxycytidine (Sigma, St. Louis, MO, USA), was freshly prepared in ddH2O. GH3 cells (3 × 105 cells/well) in the exponential growth phase were seeded in six-well plates. After 24 h of culture, cells were treated with 40 nmol/l 5-aza-2-deoxycytidine for 72 h. The culture medium was replaced every 24 h with fresh media containing 5-aza-2-deoxycytidine. Total proteins were extracted for western blotting, and total RNA was extracted for RT-qPCR analysis.
Cell viability assay
Cell proliferation was determined using the MTT assay (Roche Diagnosis, Indianapolis, IN, USA) and a hemocytometer. For the MTT assay, cells were plated at a certain density (1 × 103 cells per well) in 96-well plates. Cells were then incubated with complete media for an additional 1–3 days. Afterwards, cell growth was measured. The effect of 5-aza-2-deoxycytidine on cell viability was assessed as the percent cell viability compared with the untreated control group, which was set to 100% viability. The optical density (OD) values were measured at a wavelength of 490 nmol/l. All experiments were performed in triplicate. For the hemocytometer assay, GH3 cells at the second passages were incubated with 0, 10, 20, 30, and 40 nmol/l 5-aza-2-deoxycytidine in a 12-well plate comprising three wells per treatment, 2 ml of culture medium per well, and 100,000 cells per well at 37°C in a 95% air and 5% carbon dioxide atmosphere. After 72 h, the cells were trypsinized. Cell suspensions with a total volume of 4.5 ml each were centrifuged, and cells were resuspended in 450 μl of culture medium and counted three times using a hemocytometer.
Lentivirus construction, production and infection
The LAMA2 lentivirus plasmid was constructed, produced and used to infect pituitary adenoma cells using a previously described method.17 Forty-eight hours after infection, the virus-infected cells were cultured in medium containing 2.5 μg/ml puromycin (Sigma, St. Louis, MO, USA) for selection. The surviving cells were used in subsequent experiments. The LAMA2-overexpressing cells were named Lenti-LAMA2; the corresponding control cells were named Lenti-vector.
Transwell invasion assay
The motility of GH3 cells was assessed in 24-well transwell plates. The upper surfaces of polycarbonate filters with 8 μm pores were coated with 75 μL of Matrigel® (Corning, New York, USA) (Matrigel:DMEM = 1:3) and incubated for 0.5 h at 37°C to induce gelling. Then, cells were seeded into the upper chambers at a density of 5 × 104 cells per chamber, and the bottom chamber was filled with 600 μl of DMEM supplemented with 10% FBS. Both the top and bottom chambers contained the same concentrations of dopamine. After a 24 h incubation, non-invasive cells on the upper membrane surfaces were removed by wiping with cotton swabs. Migrated cells were fixed with methanol and stained with a 0.1% crystal violet staining solution. The membrane was dried in air. Images were captured using a Leica DM 14000B (Leica Microsystem Ltd., Germany) microscope. Cell invasion was counted in five independent areas per membrane, and the results are presented as the means calculated from five replicates of each experiment.
Animal studies
All animals were maintained in the SPF Laboratory Animal Center at Dalian Medical University, which was also the site at which all the animal experiments were performed. Female nu/nu mice (4–6-weeks old) were used in these experiments. The mice were randomly divided into three groups, each of which contained five mice. We subcutaneously injected GH3 cells (2 × 106 in 100 μl PBS), with or without LAMA2 overexpression, near the axillary fossae of the nude mice using a 27-gauge needle to evaluate the effect of LAMA2 on pitNET growth. Tumor sizes were measured with calipers every 3 days, and tumor volumes were calculated using the following formula: V = 0.5(width2 × length). On day 21 after tumor cell inoculation, all experimental mice were euthanized with ether anesthesia, and their total tumor weights were measured.
All animals were purchased from the Experimental Animal Center, Dalian Medical University [Certificate of Conformity: no. SYXK (Liao) 2013-0006]. The animals were acclimated to laboratory conditions (23°C, 12/12 h light/dark cycle, 50% humidity, with ad libitum access to food and water) for 2 weeks prior to the experiments, and no animals died before the experiments. In the animal study, all efforts were made to minimize the suffering of the mice. All mice were humanely sacrificed following the inhalation of ether anesthesia.
Statistical analysis
Statistical analyses were performed using Student’s t test or the nonparametric Mann–Whitney U test (for the results of western blotting, RT-qPCR, and methylation analyses). GraphPad Prism 6.0 (San Diego, CA, USA) software was used for statistical analyses. All data are presented as means ± standard error. p values less than 0.05 were considered to indicate statistical significance: *p < 0.05; **p < 0.01; and ***p < 0.001.
Results
LAMA2 is downregulated in invasive PitNET
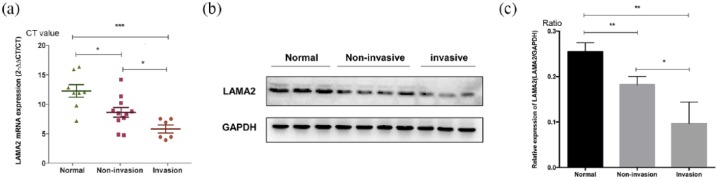
Quantitative measurements of the LAMA2 mRNA in PitNETs and normal pituitary tissues were performed using RT-PCR. A significantly lower level of the LAMA2 mRNA was detected in non-invasive PitNETs than in normal pituitary tissues (∆Ct: 6.387 ± 0.692 versus 9.218 ± 0.815, p = 0.0356, n = 11 versus n = 6). The LAMA2 mRNA level was even lower in the invasive PitNET group than in the non-invasive PitNET group (∆Ct: 9.218 ± 0.815 versus 12.86 ± 1.064, p = 0.0133, n = 8 versus n = 11) [Figure 1(a)]. The level of the LAMA2 protein was also evaluated using western blotting [Figure 1(b)]. Invasive PitNETs exhibited decreased levels of the LAMA2 protein compared with normal tissues (0.097 ± 0.02 versus 0.255 ± 0.01, p = 0.0058, n = 8 versus n = 6) and non-invasive PitNETs (0.097 ± 0.02 versus 0.182 ± 0.01, p = 0.019, n = 8 versus n = 11). A significantly lower level of the LAMA2 protein was observed in non-invasive PitNETs than in normal pituitary tissues (0.182 ± 0.01 versus 0.255 ± 0.01, p = 0.0038, n = 11 versus n = 6), as shown in Figure 1(c).
Figure 1.
Differential expression of LAMA2 in the normal pituitary and nonaggressive and aggressive pituitary adenomas.
(a) RT-qPCR analysis of LAMA2 mRNA expression in the normal pituitary and nonaggressive and aggressive pituitary adenomas; (b) western blotting analysis of the LAMA2 protein in the normal pituitary and nonaggressive and aggressive pituitary adenomas; the GAPDH protein was used as an internal control for western blotting; (c) quantitative analyses of western blotting results.
*p < 0.05; **p < 0.01; and ***p < 0.001.
CT, ; GAPDH, ; LAMA2, laminin subunit alpha 2; mRNA, messenger ribonucleic acid; RT-qPCR, quantitative real-time polymerase chain reaction.
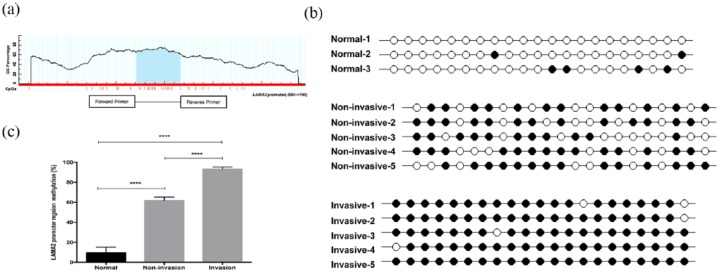
Promoter region of LAMA2 is hypermethylated in invasive PitNET
The promoter region of LAMA2 is rich in CpG dinucleotides [Figure 2(a)]. Genomic DNA from normal pituitary tissues, noninvasive PitNET and invasive PitNET were treated with sodium bisulfite and the methylation pattern in this promoter region was examined. In the current study, all cytosines other than those in CpGs were converted to thymines, excluding the possibility of the presence of cytosines in CpGs due to incomplete treatment. The results indicated a representative methylation pattern of the 21 CpGs from normal pituitary, non-invasive PitNET and invasive PitNET samples [Figure 2(b)]. In normal samples, most CpGs were unmethylated, while in tumors, the majority of CpGs were methylated, particularly in invasive PitNETs. The methylation status of CpGs in the LAMA2 promoter region of genomic DNA from normal pituitary, non-invasive PitNET and invasive PitNET samples lacking LAMA2 was examined using this method. According to the results of the quantitative analysis [Figure 2(c)], more CpGs showed hypermethylation in the LAMA2 promoter in invasive PitNET samples than in normal pituitary tissues (93.33% ± 1.91% versus 9.52% ± 5.5%, p < 0.0001, n = 8 versus n = 6) or non-invasive PitNET samples (93.33% ± 1.91% versus 61.9% ± 3.37%, p < 0.0001, n = 8 versus n = 11). In conclusion, more methylated CpGs were observed in the genomic DNA from invasive PitNET samples than in normal pituitary or noninvasive PitNET samples.
Figure 2.
Methylation status of LAMA2 in the normal pituitary and nonaggressive and aggressive pituitary adenomas.
(a) Description of the methylated region of the LAMA2 promoter; (b) a representative methylation pattern of the CpGs in tumor tissues with different levels of invasiveness after bisulfite treatment; each line represents one PCR product, and the PCR products are shown for each sample. ●: methylated CpG; ○: unmethylated CpG; (c) percent methylation of the LAMA2 promoter region.
All data are presented as means ± SEM; ***p < 0.001.
CpG, cytosine–phosphate–guanosine islands; GC, ; LAMA2, laminin subunit alpha 2; PCR, polymerase chain reaction; SEM, standard error of the mean.
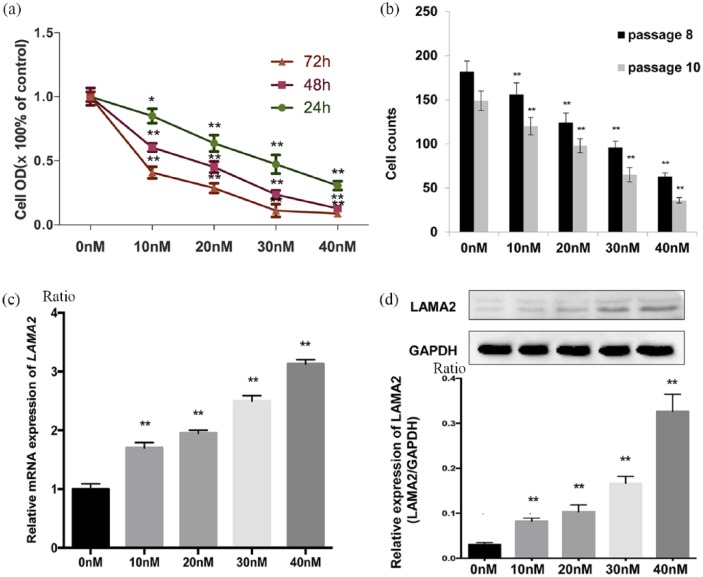
5-Aza-2-deoxycytidine markedly inhibits growth of GH3 cells and increases the expression of LAMA2
5-Aza-2-deoxycytidine is known to promote DNA demethylation. In the current study, the MTT analysis showed that 5-aza-2-deoxycytidine significantly inhibited GH3 cell growth. Cell growth was significantly inhibited by all concentrations of 5-aza-2-deoxycytidine (0, 10, 20, 30 and 40 nmol/l) after 3 days of exposure [Figure 3(a) and (b)], indicating that 5-aza-2-deoxycytidine suppressed cell growth in a dose- and time-dependent manner. We further evaluated the levels of the LAMA2 mRNA and protein in GH3 cells treated with 5-aza-2-deoxycytidine using RT-qPCR and western blotting [Figure 3(c) and (d)]. The treatment with 10 nmol/l 5-aza-2-deoxycytidine increased the levels of the LAMA2 mRNA and protein in GH3 cells with untreated GH3 cells (0.083 ± 0.004 versus 0.031 ± 0.002, p = 0.003, n = 8 versus n = 8). In addition, the level of the LAMA2 protein progressively increased as the 5-aza-2-deoxycytidine concentration increased, except for the lack of a significant difference between the 10 nmol/l group and the 20 nmol/l group (0.103 ± 0.009 versus 0.083 ± 0.004, p = 0.09, n = 8 versus n = 8) [Figure 3(c)]. Thus, the methylation status of the LAMA2 promoter regulated the growth of GH3 cells, and 5-aza-2-deoxycytidine markedly increased LAMA2 expression by promoting its demethylation and inhibiting the growth of GH3 cells. Additionally, GH3 cells survived without serum for a few days if a defined amount of insulin was added to the culture medium (5 ng/ml), which was referred to as SF-I medium (Supplementary Figure S1). Furthermore, serum-free medium might more closely resemble the in vivo brain microenvironment than FBS-containing medium. Although the differences in gene profiles between GH3 cells cultured in SF-I and FBS media require further investigation, our result indicated that GH3 cells cultured in SF-I medium are a useful cell culture model, and we plan to investigate these cells further in the future
Figure 3.
Demethylation of LAMA2 affects LAMA2 expression and the proliferation of pituitary adenoma cells.
(a) Relative optical density (OD) of GH3 pituitary adenoma cells at 24, 48 and 72 h after treatment with the indicated concentrations of 5-aza-2-deoxycytidine. OD values are reported relative to the control (the concentration of DAC: 0 nmol/l) and were determined using an MTT assay; (b) after 72 h of treatment, the viability of passage two GH3 cells was significantly decreased with increasing concentrations of 5-aza-2-deoxycytidine [passage 8 (black) versus passage 10 (gray)], p < 0.01); (c) RT-qPCR analysis of LAMA2 mRNA expression 72 h after treatment with the indicated concentrations of 5-aza-2-deoxycytidine; (d) levels of the LAMA2 and GAPDH proteins measured in GH3 pituitary adenoma cells at 72 h after treatment with the indicated concentrations of 5-aza-2-deoxycytidine; quantitative analyses of western blotting results in GH3 pituitary adenoma cells are shown.
All data are presented as means ± SEM, *p < 0.05 and **p < 0.01. PCR.
DAC, ; GAPDH, ; GH3, ; LAMA2, laminin subunit alpha 2; mRNA, messenger ribonucleic acid; MTT, ; RT-qPCR, quantitative real-time polymerase chain reaction; SEM, standard error of the mean.
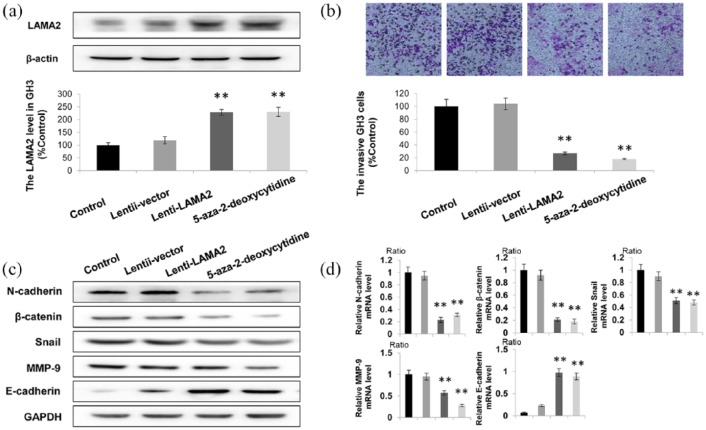
Overexpression or demethylation of LAMA2 suppresses the invasion of PitNET cells
Next, LAMA2 was overexpressed in GH3 cells. Lentiviral transfection of GH3 cells was used to increase the expression of LAMA2. As shown in Figure 4(a), LAMA2 expression was increased by more than 200% in both the Lenti-LAMA2 group and the 5-aza-2-deoxycytidine treatment group. Furthermore, the transwell assay revealed that increased expression of LAMA2 significantly suppressed PitNET cell invasion, which was negatively correlated with LAMA2 levels Figure 4(b). We further detected the changes in the expression of genes related to migration and invasion after upregulating LAMA2 [Figure 4(c) and (d)]. Compared with the scrambled group, the levels of N-cadherin, β-catenin, MMP-9 and Snail in shATP1A1-treated cells were significantly decreased, while the expression of E-cadherin was significantly increased [Figure 4(c)]. Similar results were also obtained from the RT-qPCR analysis [Figure 4(d)]. Based on these results, overexpression and demethylation of LAMA2 decreased the invasiveness of PitNET cells.
Figure 4.
Increased LAMA2 expression suppresses the invasion of GH3 cels.
(a) Western blotting showing the LAMA2 levels in the control group, Lenti-LAMA2 group and 5-aza-2-deoxycytidine treatment group; (b) According to the results of the transwell assay, the number of invaded cells in the LAMA2 overexpression group was less than 40% of the number in the control group; (c, d) increased protein levels of epithelial markers (E-cadherin) was observed using western blotting (C), and decreased expression of genes related to migration and invasion (N-cadherin, β-catenin, MMP-9, and Snail) was observed using RT-qPCR (d) in cells overexpressing LAMA2 or treated with 5-aza-2-deoxycytidine; GAPDH was used as a positive control.
n = 3; *p < 0.05 and **p < 0.01.
GH3, ; GAPDH, ; LAMA2, laminin subunit alpha 2; MMP-9, metalloproteinase-9; RT-qPCR, quantitative real-time polymerase chain reaction.
Effects of LAMA2 on the PI3K/AKT signaling pathway and MMP-9 expression are mediated by PTEN
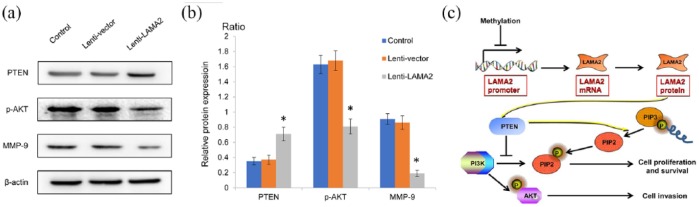
The PI3K/AKT signaling pathway induces matrix MMP-9 expression, thus promoting tumor cell invasion and migration, while PTEN negatively regulates the PI3K/AKT signaling pathway. As mentioned above, we already observed a significant decrease in the invasion of cells transfected with Lenti-LAMA2. Furthermore, we investigated whether LAMA2 affected the biological functions of GH3 cells by modulating the PTEN-PI3K/AKT pathway. The upregulation of LAMA2 increased the expression of the endogenous PTEN gene by approximately 200%, and the level of the PTEN protein was also substantially increased [Figure 5(a) and (b)]. However, the phosphorylation of the PI3K and AKT proteins was inhibited, accompanied by a decrease in the levels of the MMP-9 mRNA and protein. Thus, the effect of LAMA2 on the invasiveness of PitNET is mediated by the PTEN-PI3K/AKT signaling pathway [Figure 5(c)].
Figure 5.
LAMA2 affected the PTEN-PI3K/AKT signaling pathway and MMP-9 expression.
(a) Western blots showing protein expression; (b) quantitative analyses of western blotting results; (c) schematic depicting the mechanism by which LAMA may exert an effect on the PI3K/AKT signaling pathway and MMP-9 expression via PTEN.
n = 3; *p < 0.05.
AKT, ; LAMA2, laminin subunit alpha 2; MMP-9, metalloproteinase-9; mRNA, messenger ribonucleic acid; PTEN, ; PI3K, ; PIP, .
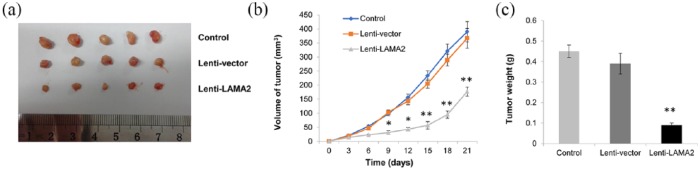
LAMA2 regulated GH3 cell growth in vivo
We generated a xenograft model of PitNET by subcutaneously injecting either GH3 empty vector control or cells overexpressing LAMA2 into nude mice to further evaluate the effects of LAMA2 on GH3 cells in vivo. The mice were divided into three groups (control, Lenti-vector/empty vector, and Lenti-LAMA2) according to the treatment. Tumor volumes of all mice were calculated every 3 days for 3 weeks. LAMA2 overexpression significantly suppressed tumor growth in vivo [Figure 6(a), (b), and (c)]. Thus, LAMA2 overexpression decreases PitNET cell proliferation both in vitro and in vivo.
Figure 6.
LAMA2 regulated the growth of GH3 cells in vivo.
(a) GH3 cells that had been transfected with the empty vector or LAMA2 vector were subcutaneously injected into nude mice, and the tumors that were excised from different groups are shown; (b) growth curve showing the changes in mouse tumor volumes after the administration of vehicle or Lenti-LAMA2; (c) the weights of the excised tumors in each group.
n = 5; **p < 0.01.
GH3, ; LAMA2, laminin subunit alpha 2.
Discussion
Although PitNETs are defined as benign tumors, some present invasive characteristics, and postoperative radiotherapy is generally recommended.18 Recently, aggressive pituitary adenomas have been diagnosed, particularly invasive adenomas.19,20 A new and comprehensive definition of aggressive adenoma has been proposed that considers several previously described features: clinical behaviors, radiological aspects, pathology results and responses to medical treatment.19 The invasiveness of pituitary tumors must be predicted to administer effective treatments. The functional LAMA2 protein in the extracellular matrix may regulate the proliferation of regenerating hepatocytes, and a loss of LAMA2 function might facilitate tumor progression.21 The promotion of tumor growth due to the loss of cell surface anchoring to basal laminae also supports this observation.7 Furthermore, soluble laminin suppresses the proliferation of cultured mammary epithelial cells.6
An evaluation of LAMA2 as a putative marker of invasive PitNET has not been reported previously. In this study, the western blotting and RT-qPCR results for LAMA2 showed the lowest LAMA2 expression in invasive PitNETs. Moreover, the downregulation of LAMA2 expression in human PitNETs could be associated with CpG methylation in the LAMA2 promoter. We have previously reported DNA methylation of the LAMA2 promoter region,9 which supports the targeting of LAMA2 in various cancers via epigenetic mechanisms.
In the current study, the effect of 5-aza-2-deoxycytidine on LAMA2 expression was assessed in GH3 cell lines to explore the relationship between the methylation status of the LAMA2 promoter and the low LAMA2 expression observed in invasive PitNET. Methylation of the LAMA2 promoter was suggested to result in the downregulation of LAMA2 expression in vitro. Laminins also exert important effects on neoplastic tissues and normal tissues, including cell migration, adhesion, proliferation, maintaining the cell shape and differentiation.6 Capillaries expressing the laminin α2 chain in basement membranes, which is encoded by LAMA2, may be considered early developing vessels in normal and neoplastic human tissues.22,23 In addition, for the first time, the current study also revealed that the overexpression or demethylation of LAMA2 suppressed the invasiveness of PitNET cells; importantly, this suppressive effect was mediated by the PTEN-mediated effect of LAMA2 on the PI3K/AKT signaling pathway and MMP-9 expression.
Regrettably, the number of samples in this study was limited; thus, the reliability of these results must be confirmed in studies using a larger sample size. However, we believe that our results provide critical information for the design of larger and more focused studies that will be necessary to systematically and definitively evaluate the role of LAMA2 in treating PitNET. Another main concern is that the current study did not include hormone-producing tumor samples. The impact of the tumor subtype on its behavior remains unclear, but accumulating evidence suggests that characteristics such as the secretory profile and cytodifferentiation influence the behavior of PitNETs. LAMA expression could also be evaluated in somatotropinomas, which will fit better with cell lines available for in vitro functional studies. Importantly, our data are applicable to cancer types other than invasive PitNET and provide a rationale for the therapeutic targeting of LAMA2 in a broader context. Given the importance of PTEN signaling in various cancers, our findings not only provide a better understanding of the molecular mechanisms underlying the maintenance of invasiveness in PitNET but also identify a molecular target for therapeutic intervention. Furthermore, examining the expression level of LAMA2 in larger samples, as well as testing the standard human serum plasma levels or half-life of 5-aza-2’-deoxycytidine in human plasma might be helpful for revealing the therapeutic potential of LAMA2 in the treatment of invasive PitNET. In addition, the identification of LAMA2-specific activators might potentially create a new paradigm in the discovery and development of molecule-targeted therapeutics for cancers, including PitNET.
In conclusion, we are the first group to show that LAMA2 expression is inversely correlated with the invasiveness of PitNET, and the overexpression or demethylation of LAMA2 suppresses the invasion of PitNET cells, partially by exerting an effect on the PTEN-PI3K/AKT signaling pathway and MMP-9 expression. Thus, the LAMA2 expression pattern might be used as a biomarker to predict the prognosis of patients with PitNET. CpG methylation of the LAMA2 promoter downregulates LAMA2 expression and thus could also serve as a marker for evaluating the grade of cancer malignancy. However, more research should be performed to confirm these results.
Supplemental Material
Supplemental material, Supplemental_Material for Expression and methylation status of LAMA2 are associated with the invasiveness of nonfunctioning PitNET by Ruo-Qiang Wang, Yu-Long Lan, Jia-Cheng Lou, Yi-Zhu Lyu, Yu-Chao Hao, Qian-Fei Su, Bin-Bin Ma, Zhong-Bo Yuan, Zhi-Kuan Yu, Hong-Qiang Zhang, Dong-Sheng Wang, Ting-Zhun Zhu, Yan Ding, Ning Zhang and Bo Zhang in Therapeutic Advances in Endocrinology and Metabolism
Acknowledgments
Ruo-Qiang Wang, Yu-Long Lan and Jia-Cheng Lou contributed equally to this work. Yu-Long Lan, Ruo-Qiang Wang, Yi-Zhu Lyu and Bo Zhang designed the experiments. Ruo-Qiang Wang, Yu-Long Lan, Yi-Zhu Lyu, Yu-Chao Hao, Qian-Fei Su and Bin-Bin Ma performed the experiments. Zhong-Bo Yuan, Zhi-Kuan Yu, Hong-Qiang Zhang, and Dong-Sheng Wang analyzed the data. Ting-Zhun Zhu contributed materials/analysis tools. Yu-Long Lan, Jia-Cheng Lou, Yan Ding, Ning Zhang and Bo Zhang wrote the paper.
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Dalian Medical University (reference number KY2014-05). We confirm that study participants and relatives gave written informed consent before taking part in the research. Furthermore, animal housing experiments were conducted in accordance with international, national and institutional guidelines for humane animal treatment and complied with relevant legislation. The protocol was approved by the Institutional Animal Ethics Committee, in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experimentation on Animals (IAEC/IV/03/BCOP/2012).
Footnotes
Funding: This work is supported by grants from National Natural Science Foundation of China (nos. 81372714, 81672480, 81872065, 81802506), Liaoning Provincial Natural Science Foundation of China (no. 201602244), Distinguished Professor Project of Liaoning Province, Special Grant for Translational Medicine, Dalian Medical University (no. 2015002), Basic Research Projects in Colleges and Universities of Liaoning Province (no. LQ2017033).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Bo Zhang  https://orcid.org/0000-0001-6635-6577
https://orcid.org/0000-0001-6635-6577
Contributor Information
Ruo-Qiang Wang, Department of Neurosurgery, Shenzhen People’s Hospital, Shenzhen, China; The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Yu-Long Lan, Department of Neurosurgery, Shenzhen People’s Hospital, Shenzhen, China; The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Jia-Cheng Lou, Department of Neurosurgery, Shenzhen People’s Hospital, Shenzhen, China; The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Yi-Zhu Lyu, Department of Hematology, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Yu-Chao Hao, Institute of Cancer Stem Cell, Dalian Medical University, Dalian, China.
Qian-Fei Su, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Bin-Bin Ma, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Zhong-Bo Yuan, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Zhi-Kuan Yu, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Hong-Qiang Zhang, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Dong-Sheng Wang, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Ting-Zhun Zhu, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Yan Ding, Department of Pediatrics, Children’s Hospital of Boston, Harvard Medical School, Boston, MA, USA.
Ning Zhang, Department of Pharmacology, The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
Bo Zhang, Department of Neurosurgery, Shenzhen People’s Hospital, Shenzhen, China; The Second Affiliated Hospital of Dalian Medical University, Dalian, China.
References
- 1. Melmed S. Update in pituitary disease. J Clin Endocrinol Metab 2008; 93: 331–338. [DOI] [PubMed] [Google Scholar]
- 2. Di Ieva A, Rotondo F, Syro LV, et al. Aggressive pituitary adenomas–diagnosis and emerging treatments. Nat Rev Endocrinol 2014; 10: 423–435. [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Voellger B, Benzel J, et al. Metalloproteinases ADAM12 and MMP-14 are associated with cavernous sinus invasion in pituitary adenomas. Int J Cancer 2016; 139: 1327–1339. [DOI] [PubMed] [Google Scholar]
- 4. Priola SM, Esposito F, Cannavò S, et al. Aggressive pituitary adenomas: the dark side of the moon. World Neurosurg 2017; 97: 140–155. [DOI] [PubMed] [Google Scholar]
- 5. Ning B, Li W, Zhao W, et al. Targeting epigenetic regulations in cancer. Acta Biochim Biophys Sin (Shanghai) 2016; 48: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol 2002; 12: 197–207. [DOI] [PubMed] [Google Scholar]
- 7. Akhavan A, Griffith OL, Soroceanu L, et al. Loss of cell-surface laminin anchoring promotes tumor growth and is associated with poor clinical outcomes. Cancer Res 2012; 72: 2578–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell 2013; 153: 38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee S, Oh T, Chung H, et al. Identification of GABRA1 and LAMA2 as new DNA methylation markers in colorectal cancer. Int J Oncol 2012; 40: 889–898. [DOI] [PubMed] [Google Scholar]
- 10. Di Pasquale C, Gentilin E, Falletta S, et al. PI3K/Akt/mTOR pathway involvement in regulating growth hormone secretion in a rat pituitary adenoma cell line. Endocrine 2018; 60: 308–316. [DOI] [PubMed] [Google Scholar]
- 11. Roof AK, Gutierrez-Hartmann A. Consider the context: Ras/ERK and PI3K/AKT/mTOR signaling outcomes are pituitary cell type-specific. Mol Cell Endocrinol 2018; 463: 87–96. [DOI] [PubMed] [Google Scholar]
- 12. Haddadi N, Lin Y, Travis G, et al. PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol Cancer 2018; 17: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng Z, Zhang Y, Zhang Z, et al. Effect of miR-106b on invasiveness of pituitary adenoma via PTEN-PI3K/AKT. Med Sci Monit 2017; 23: 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fong P, Meng LR. Effect of mTOR inhibitors in nude mice with endometrial carcinoma and variable PTEN expression status. Med Sci Monit Basic Res 2014; 20: 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Li X, Zhang Y. Correlation of NEDD4–1 and PTEN expression with the invasive capacity of pituitary adenomas. Mol Clin Oncol 2017; 6: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 17. Gao S, Wang J, Zhang T, et al. Low Expression of CAPON in glioma contributes to cell proliferation via the Akt signaling pathway. Int J Mol Sci 2016; 17. pii: E1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heaney A. Management of aggressive pituitary adenomas and pituitary carcinomas. J Neurooncol 2014; 117: 459–468. [DOI] [PubMed] [Google Scholar]
- 19. Ceccato F, Regazzo D, Barbot M, et al. Early recognition of aggressive pituitary adenomas: a single-centre experience. Acta Neurochir (Wien) 2018; 160: 49–55. [DOI] [PubMed] [Google Scholar]
- 20. Zada G, Kelly DF, Cohan P, et al. Endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions. J Neurosurg 2003; 98: 350–358. [DOI] [PubMed] [Google Scholar]
- 21. Jhunjhunwala S, Jiang Z, Stawiski EW, et al. Diverse modes of genomic alteration in hepatocellular carcinoma. Genome Biol 2014; 15: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn 2000; 218: 213–234. [DOI] [PubMed] [Google Scholar]
- 23. Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 2004; 20: 255–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Expression and methylation status of LAMA2 are associated with the invasiveness of nonfunctioning PitNET by Ruo-Qiang Wang, Yu-Long Lan, Jia-Cheng Lou, Yi-Zhu Lyu, Yu-Chao Hao, Qian-Fei Su, Bin-Bin Ma, Zhong-Bo Yuan, Zhi-Kuan Yu, Hong-Qiang Zhang, Dong-Sheng Wang, Ting-Zhun Zhu, Yan Ding, Ning Zhang and Bo Zhang in Therapeutic Advances in Endocrinology and Metabolism