d-Galactonate is a widely prevalent aldonic sugar acid. Despite the proposed significance of the d-galactonate metabolic pathway in the interaction of enteric bacteria with their hosts, there are no details on its regulation even in Escherichia coli, which has been known to utilize d-galactonate since the 1970s. Here, using multiple methodologies, we identified the promoter, operator, and effector of DgoR, the transcriptional repressor of d-galactonate metabolism in E. coli. We establish DgoR as a GntR family transcriptional regulator. Recently, a human urinary tract isolate of E. coli introduced in the mouse gut was found to accumulate missense mutations in dgoR. Our results show these mutants to be DNA binding defective, hence emphasizing the role of the d-galactonate metabolic pathway in bacterial colonization of the mammalian gut.
KEYWORDS: GntR family, carbohydrate metabolism, dgo operon, effector, gene regulation, protein-DNA interactions, site-directed mutagenesis, sugar acid, transcription repressor, wHTH domain
ABSTRACT
d-Galactonate, an aldonic sugar acid, is used as a carbon source by Escherichia coli, and the structural dgo genes involved in its metabolism have previously been investigated. Here, using genetic, biochemical and bioinformatics approaches, we present the first detailed molecular and functional insights into the regulation of d-galactonate metabolism in E. coli K-12 by the transcriptional regulator DgoR. We found that dgoR deletion accelerates the growth of E. coli in d-galactonate concomitant with the strong constitutive expression of dgo genes. In the dgo locus, sequence upstream of dgoR alone harbors the d-galactonate-inducible promoter that likely drives the expression of all dgo genes. DgoR exerts repression on the dgo operon by binding two inverted repeats overlapping the dgo promoter. Binding of d-galactonate induces a conformational change in DgoR to derepress the dgo operon. The findings from our work firmly place DgoR in the GntR family of transcriptional regulators: DgoR binds an operator sequence [5′-TTGTA(G/C)TACA(A/T)-3′] matching the signature of GntR family members that recognize inverted repeats [5′-(N)yGT(N)xAC(N)y-3′, where x and y indicate the number of nucleotides, which varies], and it shares critical protein-DNA contacts. We also identified features in DgoR that are otherwise less conserved in the GntR family. Recently, missense mutations in dgoR were recovered in a natural E. coli isolate adapted to the mammalian gut. Our results show these mutants to be DNA binding defective, emphasizing that mutations in the dgo-regulatory elements are selected in the host to allow simultaneous induction of dgo genes. The present study sets the basis to explore the regulation of dgo genes in additional enterobacterial strains where they have been implicated in host-bacterium interactions.
IMPORTANCE d-Galactonate is a widely prevalent aldonic sugar acid. Despite the proposed significance of the d-galactonate metabolic pathway in the interaction of enteric bacteria with their hosts, there are no details on its regulation even in Escherichia coli, which has been known to utilize d-galactonate since the 1970s. Here, using multiple methodologies, we identified the promoter, operator, and effector of DgoR, the transcriptional repressor of d-galactonate metabolism in E. coli. We establish DgoR as a GntR family transcriptional regulator. Recently, a human urinary tract isolate of E. coli introduced in the mouse gut was found to accumulate missense mutations in dgoR. Our results show these mutants to be DNA binding defective, hence emphasizing the role of the d-galactonate metabolic pathway in bacterial colonization of the mammalian gut.
INTRODUCTION
The common gut bacterium Escherichia coli can utilize a variety of sugar acids, i.e., hexonates, hexuronates, hexuronides, and aldarates, as carbon and energy sources (1). Utilization of sugar acids has been implicated in the colonization of E. coli in the mammalian gut. The gut microbiota liberates sugar acids from polysaccharides present in nutrients ingested by the host and the mucosal layer that lines the intestinal epithelial cells (2–4). Certain gut microbes also produce sugar acids from simple sugars as catabolic intermediates of metabolism (5, 6). Antibiotic treatment has also been reported to induce the host to oxidize sugars present in the gut into sugar acids (7). E. coli degrades sugar acids via the Entner-Doudoroff or Ashwell pathway into glyceraldehyde 3-phosphate and pyruvate, which further enter the central metabolism through glycolysis and the tricarboxylic acid cycle, respectively (3, 8). The sugar acid metabolic pathways are regulated by specific transcriptional regulators whose DNA-binding properties are influenced by binding to effectors, which could be either the sugar acid itself, its catabolic intermediate, or both (1, 9–11).
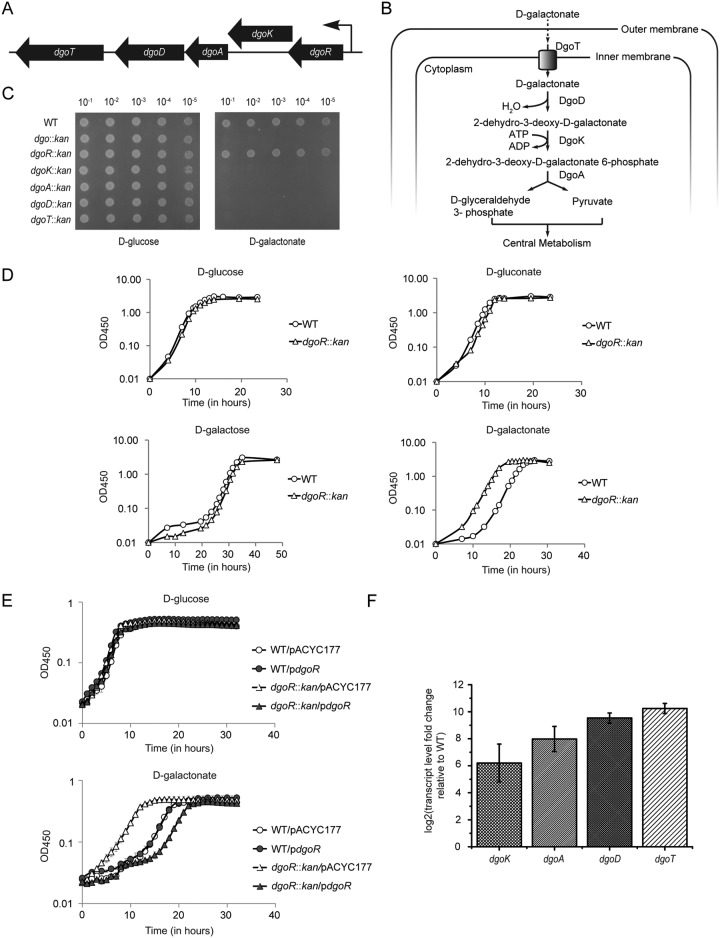
d-Galactonate, a hexonate sugar acid, was first reported as a carbon source for E. coli in studies conducted in the 1970s (12). Through classical mutagenesis and biochemical approaches, it was shown that E. coli metabolizes d-galactonate through a modified form of the Entner-Doudoroff pathway by a set of genes arranged in a putative d-galactonate operon (dgo) (Fig. 1A) (12, 13). Briefly, d-galactonate is transported into the cytoplasm by a putative transporter, DgoT, followed by dehydration by the dehydratase DgoD to form 2-dehydro-3-deoxy-d-galactonate. This intermediate is phosphorylated by the kinase DgoK to form 2-dehydro-3-deoxy-d-galactonate 6-phosphate, which is further cleaved by the aldolase DgoA to d-glyceraldehyde 3-phosphate and pyruvate (Fig. 1B). Further, a mutant that led to the constitutive expression of Dgo enzymes was isolated, and the mutation was mapped very close to the predicted operon. Thus, a regulator of the dgo operon, DgoR, was proposed (Fig. 1A) (13). In later studies, bioinformatics analysis of transcriptional regulators harboring N-terminal winged helix-turn-helix (wHTH) DNA-binding domains predicted DgoR to be a member of the GntR family (14, 15). On the basis of the similarity of the C-terminal effector-binding and oligomerization (E-O) domain, DgoR has been placed in the FadR subfamily within the GntR family (15, 16).
FIG 1.
DgoR negatively regulates d-galactonate metabolism. (A) Schematic of the chromosomal organization of the putative dgo operon in E. coli K-12. Filled arrows (not drawn to scale) show the direction of dgo genes, and the bent arrow indicates the direction of transcription. (B) Pathway of d-galactonate transport and degradation in E. coli K-12. (C) The dgo operon is involved in the metabolism of d-galactonate. Dilutions of the cultures were spotted on M9 minimal medium containing either d-glucose or d-galactonate as the carbon source. The experiment was repeated 3 times. A representative data set is shown. (D) Deletion of dgoR leads to faster growth of E. coli in d-galactonate. WT and dgoR::kan strains were grown in shake flasks in minimal medium containing one of the indicated carbon sources, and the OD450 was measured. The experiment was performed 3 times. A representative data set is shown. (E) dgoR cloned in plasmid complements the faster growth phenotype of the dgoR::kan strain in d-galactonate. The WT and dgoR::kan strains carrying either empty plasmid (pACYC177) or pACYC177 with dgoR (pdgoR: pBS13) were grown in 96-well plates in minimal medium containing either d-glucose or d-galactonate as the carbon source, and OD450 was measured. The experiment was done 3 times; each experiment had 3 technical replicates. A representative data set, with average and standard deviation (SD) of technical replicates, is shown. (F) DgoR negatively regulates transcription of dgo operon. WT and dgoR::kan strains were grown in minimal medium containing glycerol as the carbon source until exponential phase. RNA was isolated, and transcript abundance of dgo genes was assayed by qRT-PCR. Data were normalized to the transcript levels of dgo genes in WT and represent the average (±SD) from 3 independent experiments.
Several studies suggest the widespread occurrence of d-galactonate in nature. Bacteria such as Stenotrophomonas maltophilia (isolated from intestinal crypts and mucous membranes of mammalian hosts) (17), Gluconobacter liquefaciens (present in sugar-rich habitats) (18), and Pseudomonas saccharophila (a mud bacterium) (19) produce d-galactonate as an intermediate in d-galactose metabolism (6, 20, 21). Humans also produce small amounts of d-galactonate, and its levels increase in galactosemic patients, who lack the enzyme(s) of d-galactose metabolism. Various reports have demonstrated the presence of d-galactonate in mammalian tissues as well as body secretions (22–25). Genome-scale studies in the last couple of decades illustrate the importance of the d-galactonate metabolic pathway in the physiology of enteric bacteria. For example, the transcriptional profiles of Salmonella enterica strains grown in macrophages, soft-rotted leaves, or egg white and of an asymptomatic E. coli strain cultured in human urine showed upregulation of dgo genes (26–29), metabolic models of 55 fully sequenced E. coli and Shigella strains predicted 40 strains to be capable of growing on d-galactonate (30), and a mutant library screen of S. enterica serovar. Choleraesuis identified dgoT as a virulence determinant in pigs (31). Finally, in a recent in vivo evolution experiment where an E. coli strain isolated from a patient suffering from urinary tract infection was colonized in mouse gut for a year, a maximum number of mutations were found to accumulate in dgoR, indicating a role of dgo genes in bacterial colonization of the mammalian gut (32).
The above-mentioned examples of d-galactonate prevalence in nature and the proposed significance of dgo genes emphasize the importance of investigating d-galactonate metabolism in enteric bacteria. Although there are a few biochemical studies on Dgo enzymes (33–35), the molecular and functional characterization of DgoR is still lacking. Here, we show that deletion of dgoR leads to faster growth of E. coli K-12 in d-galactonate and results in a considerable increase in the expression of dgo genes. DgoR represses dgo genes by binding two closely spaced inverted repeats (IRs) in the cis-acting element that notably overlaps with the d-galactonate-inducible promoter. Mutational analysis of the predicted wHTH domain identified amino acid residues critical for DgoR-operator interactions. A comparison of DgoR and its operator with other characterized GntR family members revealed conserved and semiconserved features of protein-DNA recognition in the GntR family. Finally, we provide evidence that d-galactonate is a specific effector of DgoR.
RESULTS
The putative dgo operon is required for d-galactonate metabolism and is negatively regulated by DgoR.
Earlier studies showed the involvement of the putative dgo operon (Fig. 1A) in d-galactonate metabolism by isolating mutants unable to grow on d-galactonate as the sole carbon source (13). However, the growth phenotype of strains carrying a clean deletion of dgo genes has not been reported. Before performing a detailed investigation on the role of DgoR, we validated the involvement of the predicted dgo operon in metabolizing d-galactonate. We examined the growth of dgo single-gene deletion strains obtained from the Keio library (dgoR::kan, dgoK::kan, dgoA::kan, dgoD::kan, and dgoT::kan strains) (36) and of a strain carrying deletion of the entire dgo operon (dgo::kan, constructed in this study) in solid M9 minimal medium containing either d-glucose or d-galactonate as the carbon source. In contrast to the normal growth of strains in d-glucose, none of the dgo strains except the dgoR::kan strain grew in d-galactonate (Fig. 1C). We therefore assessed the growth of the dgoR deletion strain in a liquid medium. The dgoR::kan strain exhibited faster growth in d-galactonate (Fig. 1D). This phenotype was specific to d-galactonate; the dgoR::kan strain showed a growth profile similar to that of the wild type (WT) in other tested carbon sources, i.e., d-glucose, d-galactose, and d-gluconate (Fig. 1D). Importantly, the accelerated growth phenotype was complemented by dgoR cloned with its putative promoter in pACYC177 (Fig. 1E). The faster growth of the dgoR::kan strain suggested that DgoR is a negative regulator of d-galactonate metabolism. Consistent with this, we observed constitutive expression of various dgo genes in the dgoR::kan strain grown in minimal medium supplemented with the non-catabolite-repressing carbon source glycerol (Fig. 1F).
dgo genes are transcriptionally induced by d-galactonate.
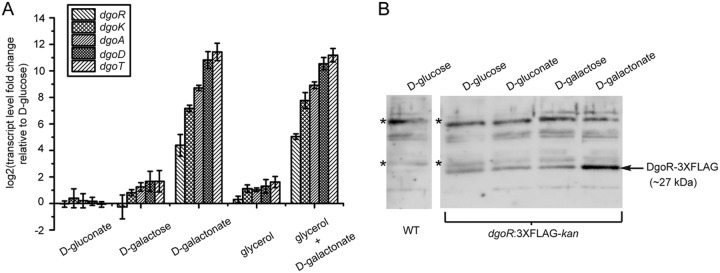
In an earlier study, enzymatic activities corresponding to d-galactonate dehydratase (DgoD), 2-dehydro-3-deoxygalactonate kinase (DgoK), and 2-dehydro-3-deoxygalactonate 6-phosphate aldolase (DgoA) were observed in extracts prepared from WT cells cultured in d-galactonate but were not detected in extracts prepared from glycerol-, d-gluconate-, or d-galactose-grown cells (12). In another study, the expression of a dgo promoter-reporter construct was induced in the presence of d-galactonate (32). Here we show that of all tested carbon sources, the dgo genes were significantly induced at a transcriptional level particularly by d-galactonate (Fig. 2A). Further, compared to cells grown in d-galactose or glycerol, cells grown in d-glucose and d-gluconate exhibited much lower dgo transcript levels, which can be attributed to the higher carbon catabolite repression exerted by the latter carbon sources (37, 38). The result with d-glucose is in accordance with an earlier finding that d-glucose represses the expression of d-galactonate catabolic enzymes (13). Consistent with the strong activation of dgo genes by d-galactonate, we observed higher levels of DgoR protein (expressed as a 3×FLAG-tagged fusion protein from the native chromosomal locus) in d-galactonate-grown cells (Fig. 2B).
FIG 2.
The dgo operon is significantly induced by d-galactonate. (A) The dgo operon is induced at a transcriptional level by d-galactonate. The WT strain was grown in minimal medium supplemented with carbon sources indicated. RNA was isolated in the exponential phase and processed for qRT-PCR. Data were normalized to the transcript levels of dgo genes in the WT grown in minimal medium containing d-glucose. Data represent the average (±SD) for three independent experiments. (B) DgoR protein levels are higher in d-galactonate-grown cells. The strain expressing C-terminally 3×FLAG-tagged DgoR from the chromosome was grown in minimal medium supplemented with one of the indicated carbon sources. Cells were harvested in the exponential phase and processed for Western blotting using anti-FLAG antibody. WT cells grown in d-glucose were used as negative control. *, nonspecific band detected by anti-FLAG antibody which served as a control for equal loading of samples.
dgo genes are transcribed from a d-galactonate-inducible promoter located upstream of dgoR.
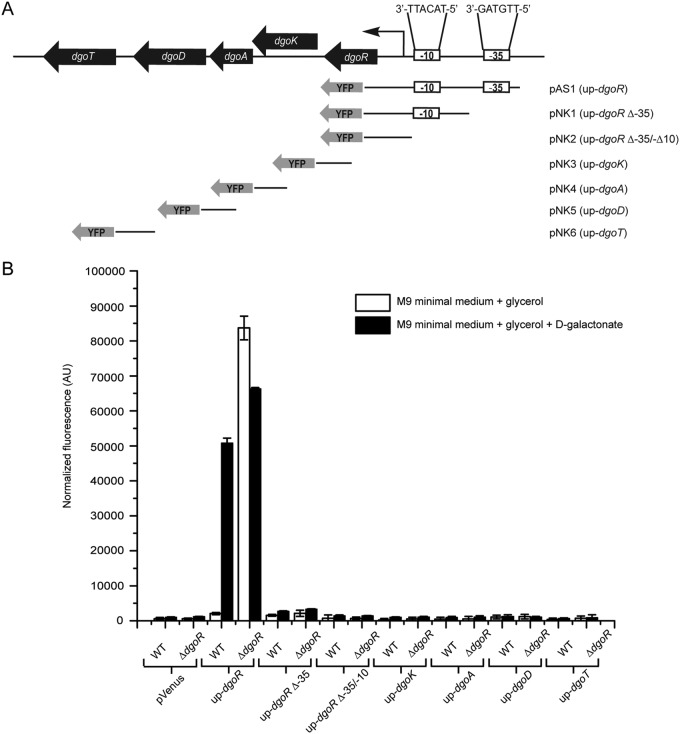
Because promoters in E. coli are usually located within ∼200 bp from the protein-coding sequence (39), to determine functional promoters in the putative dgo operon, we fused ∼200 to 300 bp of the upstream region of each individual dgo gene (up-dgoR, up-dgoK, up-dgoA, up-dgoD, and up-dgoT) with the fluorescent Venus reporter in the pVenus plasmid, generating constructs pAS1 and pNK3 to pNK6 (Fig. 3A). These transcriptional reporter constructs were integrated into the chromosomes of WT and ΔdgoR strains at the attλ site. The resulting reporter strains were grown either in noninducing (M9 minimal medium containing glycerol) or inducing (M9 minimal medium containing glycerol and d-galactonate) medium, and fluorescence was measured in the exponential phase. In the presence of d-galactonate, the increased expression of the reporter was observed only for the transcriptional fusion bearing the upstream region of dgoR (up-dgoR). Further, only the same reporter construct displayed constitutive fluorescence in noninducing medium in the ΔdgoR strain (Fig. 3B). We conclude that in the dgo locus, sequences upstream of dgoR alone harbor the d-galactonate-inducible promoter, which is normally repressed by DgoR. Because all dgo genes show increased expression in WT cells in the inducing medium and in a dgoR deletion strain in the noninducing medium (Fig. 1F and Fig. 2A), their expression must be from the dgo promoter located upstream of dgoR, indicating that the five dgo genes constitute an operon. These results are consistent with an earlier study where a mutation that simultaneously prevented the synthesis of d-galactonate catabolic enzymes was isolated, leading the authors to speculate that the mutation was in the common promoter of dgo genes (13).
FIG 3.
Sequence upstream of dgoR alone harbors the d-galactonate-inducible promoter. (A) Schematic of the transcriptional reporter fusions analyzed to identify functional promoters in the putative dgo operon. Reporter plasmids (pAS1 and pNK1 to pNK6) carrying ∼200 to 300 bp upstream of each individual dgo gene (up-dgoR, up-dgoK, up-dgoA, up-dgoD, and up-dgoT) and the up-dgoR truncated variants (up-dgoR Δ−35 and up-dgoR Δ−35/−10) were introduced as single-copy fusions in WT and ΔdgoR strains. The sequences of promoter elements (−35 and −10 boxes) in the upstream region of dgoR, predicted by both BPROM and bTSSfinder, are shown. Transcriptional fusions are not drawn to scale. (B) up-dgoR harbors the functional promoter of the dgo operon. The reporter strains described for panel A were grown in M9 minimal medium supplemented either with glycerol or with glycerol and d-galactonate to exponential phase. Fluorescence was measured and normalized to the OD450 of the samples. Data represent the average (±SD) from 3 independent experiments.
Next, we predicted −10 and −35 promoter elements in the sequence upstream of dgoR using the promoter prediction programs BPROM and bTSSfinder (39, 40) (Fig. 3A). To investigate whether the common promoter elements predicted by both the programs are indeed required for activity in vivo, the up-dgoR element was truncated to give rise to two variants, one that lacks the −35 element (up-dgoR Δ−35) and another that lacks both the −10 and −35 elements (up-dgoR Δ−35/−10) (Fig. 3A). The truncated variants were cloned in the pVenus plasmid, resulting in the constructs pNK1 and pNK2, which were integrated into the chromosome. Compared to the up-dgoR reporter construct, which displayed significant fluorescence in the presence of d-galactonate in the WT strain and constitutive fluorescence in the ΔdgoR strain, the Δ−35 and Δ−35/−10 reporter constructs showed near-background fluorescence under similar conditions (Fig. 3B). These data validate that Δ−35 and Δ−35/−10 constructs lack essential promoter elements.
DgoR binds inverted repeats IR1 and IR2, which overlap the dgo promoter.
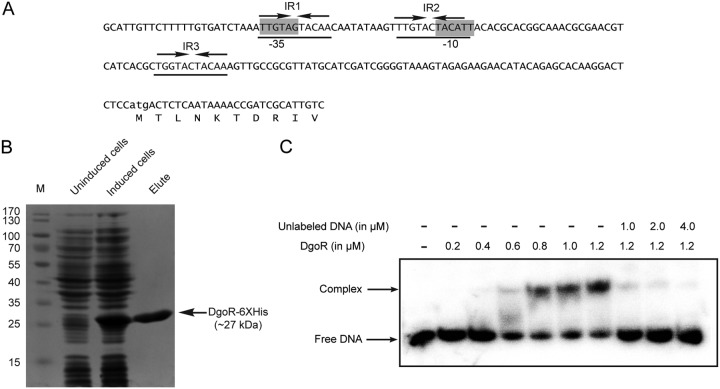
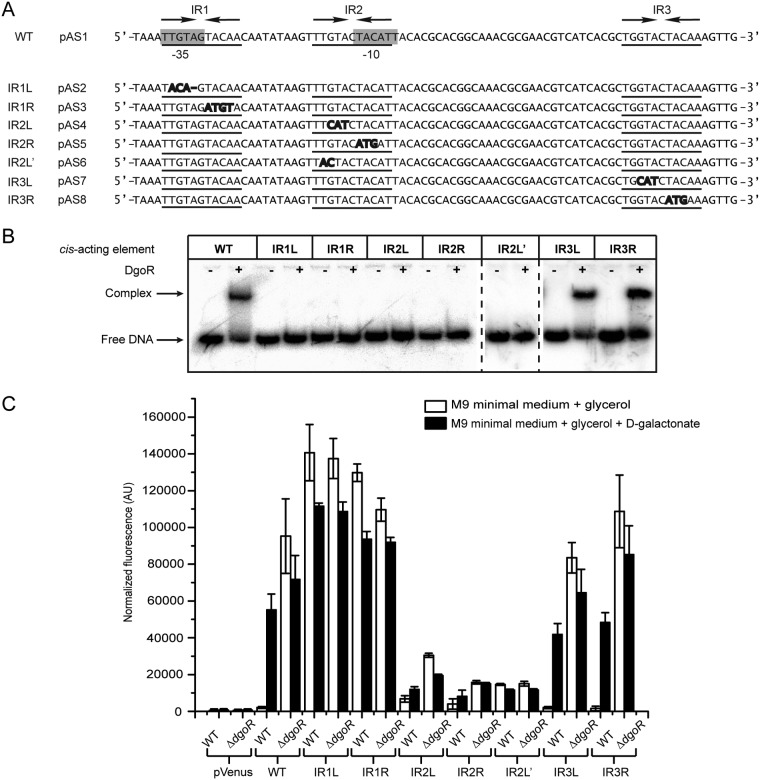
A majority of GntR family regulators bind inverted repeat sequences on DNA (15, 16, 41). A search for DgoR in the RegPrecise database (41, 42) showed three inverted repeats (IR1, IR2 and IR3) in the nucleotide sequence upstream of the dgo operon to be the probable DgoR-binding sites (Fig. 4A), suggesting that DgoR binds the up-dgoR element (hereafter referred to as the cis-acting element) to regulate itself and other dgo genes in the operon. IR1 is a perfect inverted repeat, whereas both IR2 and IR3 have one mismatch. To determine the binding of DgoR to the cis-acting element, an ∼200-bp DNA fragment encompassing the three inverted repeats (Fig. 4A) was PCR amplified from plasmid pAS1 using 32P-labeled primer and incubated with different concentrations of purified DgoR-6×His (Fig. 4B). DgoR shifted the labeled cis-acting element in a concentration-dependent manner (Fig. 4C). The addition of a 200-fold excess of the unlabeled cis-acting element completely abolished the interaction of DgoR with labeled DNA (Fig. 4C).
FIG 4.
DgoR binds its cis-acting element. (A) Sequence of the ∼200-bp cis-acting element used in EMSAs. Lowercase letters denote the start codon of dgoR. The predicted promoter elements (−35 and −10 boxes) are highlighted in gray. The three inverted repeats (IR1, IR2, and IR3) are underlined. Arrows depict the left and right halves of the repeats. (B) SDS-PAGE of purified DgoR-6×His. The WT strain carrying pBS2 was induced with IPTG. Cells were harvested and processed for protein purification using Co-NTA resin. Lane M, molecular mass markers (in kilodaltons). The tagged protein could be purified to a final concentration of ∼15 µM. (C) EMSA shows the binding of purified DgoR to the cis-acting element. A 20 nM concentration of the 32P-labeled DNA fragment was incubated with different concentrations of purified DgoR-6×His with or without the unlabeled cis-acting element. Samples were resolved by native PAGE and subjected to autoradiography.
In order to investigate the importance of three inverted repeats in the cis-acting element for binding DgoR, the symmetry of each inverted repeat was broken by substitution or deletion mutations in the left and right halves of the repeat (Fig. 5A), and their binding to DgoR was examined in an electrophoretic mobility shift assay (EMSA). For this, similar to the case for the WT cis-acting element, we cloned mutated fragments upstream of the fluorescent Venus reporter in the pVenus plasmid, resulting in constructs pAS2 to pAS8. When required, cis-acting elements were amplified from the respective constructs using radiolabeled primer and incubated with DgoR-6×His. Whereas DgoR shifted the WT cis-acting element, the protein was unable to shift the mutant DNA fragments IR1L, IR1R, IR2L, IR2R, and IR2L'. On the contrary, both the IR3L and IR3R fragments formed protein-DNA complexes similar to that observed for the WT cis-acting element (Fig. 5B). Collectively, these data show that of the three inverted repeats, IR1 and IR2 are critical for DgoR to bind the cis-acting element.
FIG 5.
Inverted repeats IR1 and IR2 overlap the promoter and are critical for binding of DgoR to its cis-acting element. (A) Sequence of a region of the dgo cis-acting element, showing mutations created within the three inverted repeats. The sequences of a region of the WT cis-acting element and its various mutants (IR1L, IR1R, IR2L, IR2R, IR2L′, IR3L, and IR3R) are shown, where the predicted promoter core elements (−35 and −10 boxes) are highlighted in gray, the three inverted repeats (IR1, IR2, and IR3) are underlined, the left and right halves of the repeats are shown by arrows, nucleotide substitutions in the inverted repeats are shown in bold, and nucleotide deletions in the inverted repeats are shown by a line. L and R in the names of the inverted repeat mutants denote that the mutation is in the left or right half of the inverted repeat. For example, IR1L and IR1R denote that the mutations are in the left and right halves of IR1, respectively. (B) EMSA shows that IR1 and IR2 are critical for binding of DgoR to its cis-acting element in vitro. A 20 nM concentration of 32P-labeled WT and various mutant fragments was incubated with (+) or without (−) 1.2 µM purified DgoR-6×His. Samples were resolved by native PAGE and subjected to autoradiography. (C) Fluorescence assay reveals the requirement of IR1 and IR2 in the cis-acting element for binding of DgoR in vivo. The WT and various mutant fragments transcriptionally fused to the Venus reporter were integrated into the chromosome. Strains were grown in M9 minimal medium supplemented either with glycerol or with glycerol and d-galactonate to exponential phase. Fluorescence was measured and normalized to the OD450 of the samples. Data represent the average (±SD) from 3 independent experiments.
Next, we investigated the importance of inverted repeats in vivo. For this, we integrated the Venus reporter constructs, pAS2 to pAS8, into the chromosomes of the WT and ΔdgoR strains. In order to interpret the data from fluorescence assays for the inverted repeat mutants, it was important to consider that IR1 and IR2 sites overlap the predicted −35 and −10 promoter elements, respectively (Fig. 5A). The left half of IR1 overlaps the −35 element, whereas the right half of IR1 overlaps a few base pairs of the spacer region between the −35 and −10 elements. In the ΔdgoR background, both the IR1L and IR1R constructs exhibited fluorescence similar to that of the reporter construct harboring the WT cis-acting element (Fig. 5C). These data show that mutations in IR1 do not abrogate promoter activity. Importantly, both constructs showed constitutive fluorescence in the WT strain, suggesting a complete loss of DgoR repression (Fig. 5C). This result correlates with the defective binding of purified DgoR with the IR1L and IR1R fragments observed in EMSA (Fig. 5B). Together these data reveal that IR1 is essential for DgoR to bind the cis-acting element to repress transcription of the dgo operon.
The right half of IR2 overlaps the −10 element (Fig. 5A). In the ΔdgoR strain, the IR2R construct exhibited lower fluorescence than the construct harboring the WT cis-acting element, suggesting that mutations in the −10 box compromise promoter activity (Fig. 5C). However, the promoter was still active, because the fluorescence of IR2R was higher than that of the Δ−35 and Δ−35/−10 constructs, which lack essential promoter elements (compare Fig. 3B and Fig. 5C). Although the left half of IR2 lies outside the −10 box (Fig. 5A), its mutation in the IR2L and IR2L′ constructs resulted in low fluorescence in the ΔdgoR strain, suggesting that sequence adjacent to the −10 element also contributes to promoter activity. Further, in WT cells grown under noninducing conditions, all IR2 mutants exhibited higher fluorescence than the WT cis-acting element, indicating that mutations in IR2 compromise DgoR binding (Fig. 5C).
Consistent with the observation that IR3 does not overlap the promoter, in the ΔdgoR background, the IR3L and IR3R reporter constructs exhibited fluorescence comparable to that of the construct carrying the WT cis-acting element. In the WT strain as well, IR3L and IR3R showed regulatory behavior similar to that of the WT fragment (Fig. 5C). These data correlate with the ability of purified DgoR to bind IR3L and IR3R fragments in EMSA (Fig. 5B). Thus, the IR3 site is not critical for the interaction of DgoR with its cis-acting element.
Collectively, from our EMSAs and fluorescence assays, we conclude that DgoR binds IR1 and IR2 sites in the cis-acting element for repressing dgo genes. The information that DgoR-binding sites overlap the promoter suggests that DgoR represses transcription of the dgo operon by occluding the binding of RNA polymerase (RNAP) (43).
Mutations in the DNA-binding domain abrogate interaction of DgoR with its cis-acting element.
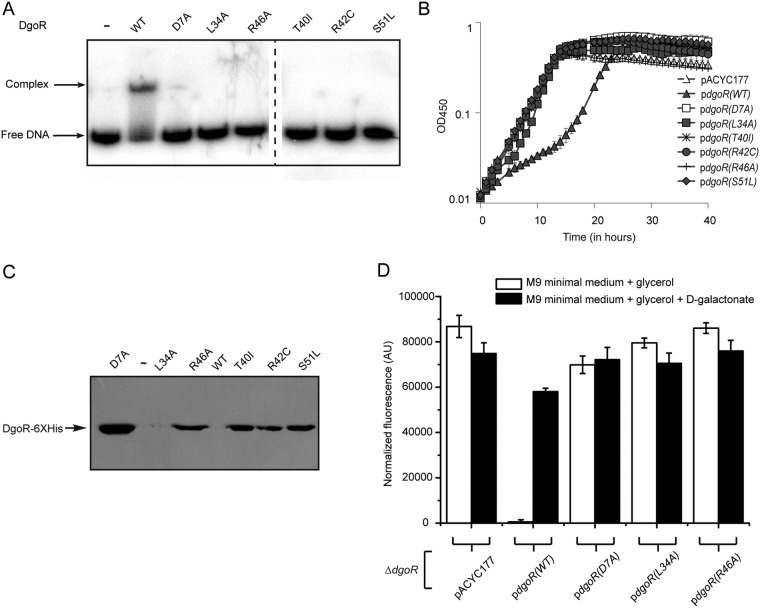
The structure of DgoR predicted by the Robetta server showed the presence of an N-terminal wHTH DNA-binding domain and a C-terminal seven-helix domain (see Fig. S1 in the supplemental material). Thus, based on its topology DgoR belongs to the FadR subgroup within the FadR subfamily of transcriptional regulators (15, 16). The far-UV circular dichroism (CD) spectrum of purified DgoR also showed a signature of a substantially helical protein (see Fig. S2 in the supplemental material).
The wHTH domain is composed of a tri-helical core followed by two beta strands connected through a small loop, designated the “wing” motif (Fig. S1). To ascertain whether secondary structure elements in the predicted DNA-binding domain of DgoR are involved in protein-DNA interaction, we created a missense mutation in each of the three α-helices (D7A in α1, L34A in α2, and R46A in α3), which are either conserved across the GntR family or reported to be functionally important in other characterized members (15) (see below). In addition, in a recent study where a human urinary tract isolate of E. coli was introduced into streptomycin-treated mouse gut and mutations in the evolved lineages were followed over a span of a year, three missense mutations were independently recovered in the predicted α2-α3 region of DgoR. These data suggested the importance of derepression of the dgo operon in bacterial adaptation in the mouse gut. Indeed, the evolved lineages grew faster than the parental strain in d-galactonate, implying a potential benefit to the evolved strains in utilizing this carbon source (32). However, because there were mutations at additional loci in the evolved strains, a reexamination of dgoR mutations in a clean background coupled with testing their DNA-binding ability is imperative to assess their true contribution to the upregulation of the dgo operon and adaptation in the host. To this end, we created mutations in dgoR corresponding to the three missense mutations (T40I, R42C, and S51L) isolated in the previous study.
We purified mutant DgoR as C-terminally His-tagged proteins. Far-UV CD showed that the mutants are folded (Fig. S2). As observed in EMSA, the interaction of mutants with the cis-acting element was completely abolished (Fig. 6A). Consistent with the loss of DNA-binding ability, the mutants could not complement the growth phenotype of the dgoR::kan strain in d-galactonate (Fig. 6B). Further, the expression of DgoR variants in the complementation strains in the noninducing medium largely correlated with their repressor ability (Fig. 6C). Whereas WT DgoR could not be detected due to autorepression, the D7A, T40I, R42C, R46A, and S51L mutants exhibited significant expression. Although the L34A mutant could not be detected, likely due to its rapid degradation, its low-level expression inside the cell is evident from the ability of this mutant to exhibit a weak dominant negative phenotype (see below [Fig. 7A]). We further tested the ability of a few of these mutants to repress expression from the dgo promoter in vivo. For this, we expressed either WT or mutant DgoR proteins from pACYC177 in a ΔdgoR reporter strain. In the noninducing medium, in contrast to the significant repression of the reporter in the ΔdgoR strain expressing WT DgoR from the plasmid, the mutants behaved similarly to the ΔdgoR strain carrying an empty plasmid; i.e., they exhibited constitutive expression of the reporter (Fig. 6D). In the inducing medium, as expected, all strains exhibited comparable reporter expression (Fig. 6D). Taken together, our results emphasize that the D7, L34, T40, R42, R46, and S51 amino acid residues are important for DgoR to bind its cis-acting element to repress the dgo operon.
FIG 6.
Mutations in the DNA-binding domain abrogate interaction of DgoR with its cis-acting element. (A) DNA-binding domain mutants do not bind the cis-acting element in vitro. A 20 nM concentration of 32P-labeled DNA fragment was incubated with either 1.2 µM WT DgoR-6×His or its various mutants. Samples were resolved by native PAGE and subjected to autoradiography. (B) DNA-binding domain mutants fail to complement the faster growth phenotype of dgoR deletion strain in d-galactonate. The WT and various dgoR mutants cloned in pACYC177 [pdgoR(WT), pBS13; pdgoR(D7A), pBS22; pdgoR(L34A), pBS14; pdgoR(R46A), pBS15; pdgoR(T40I), pBS37; pdgoR(R42C), pBS36; and pdgoR(S51L), pBS35] were individually transformed in a dgoR::kan strain. Cultures were grown in 96-well plates in minimal medium containing d-galactonate as the carbon source, and the OD450 was measured. The experiment was performed 3 times; each experiment had 3 technical replicates. A representative data set, with average and SD from technical replicates, is shown. (C) The expression of WT DgoR and various DNA-binding mutants from the native promoter largely correlates with their repressor ability. The strains described for panel B were grown in minimal medium supplemented with glycerol. Cells were harvested and processed for Western blotting using anti-His antibody. (D) Fluorescence assay shows that DNA-binding domain mutants are unable to repress expression from the dgo promoter in vivo. Plasmids [pdgoR(WT), pBS13; pdgoR(D7A), pBS22; pdgoR(L34A), pBS14; and pdgoR(R46A), pBS15] were individually transformed in a ΔdgoR strain carrying the fluorescent Venus reporter on the chromosome under the control of the dgo promoter. Strains were grown in minimal medium supplemented either with glycerol or with glycerol and d-galactonate to exponential phase. Fluorescence was measured and normalized to the OD450 of the samples. Data represent the average (±SD) from 3 independent experiments.
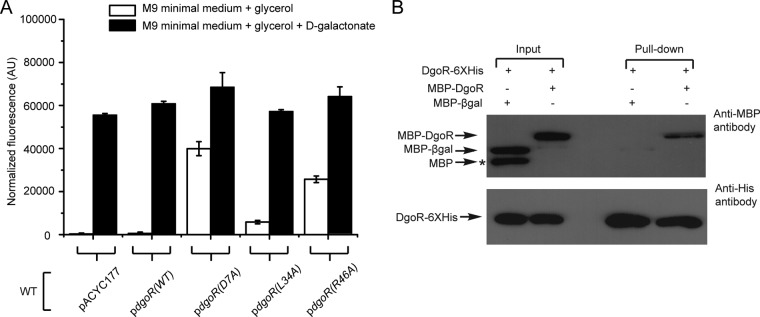
FIG 7.
DgoR forms oligomers. (A) DNA-binding-defective mutants exhibit a dominant negative phenotype. The empty plasmid pACYC177 and various constructs, described in the legend to Fig. 6D, were individually transformed in the WT strain carrying the Venus reporter (under the control of the native dgo promoter) on the chromosome. Strains were grown in minimal medium supplemented either with glycerol or with glycerol and d-galactonate to exponential phase. Fluorescence was measured and normalized to the OD450 of the samples. Data represent the average (±SD) from 3 independent experiments. (B) DgoR oligomerizes in vitro. DgoR-6×His from cell lysate was immobilized on Co-NTA beads to which either purified MBP-DgoR or MBP-βgal was added. A portion of these suspensions was saved as the input. The remaining suspension was incubated, and proteins were finally eluted as the pulldown. Samples were subjected to SDS-PAGE and processed for Western blotting using anti-MBP (upper panel) or anti-His (lower panel) antibody. *, band corresponding to MBP which likely arises due to spontaneous degradation of MBP-βgal.
DgoR forms oligomers.
Most GntR family proteins exist as oligomers (16). We made use of the DNA-binding-defective mutants, D7A, L34A, and R46A, to investigate whether DgoR oligomerizes in vivo. For this, we expressed either WT or mutant DgoR proteins from pACYC177 in a WT reporter strain. In the noninducing medium, whereas the WT protein from plasmid did not affect the expression of the Venus reporter, mutant DgoR proteins increased reporter expression (Fig. 7A). These results suggest that the DNA-binding-defective mutants expressed in trans oligomerize with WT DgoR from the chromosome, interfering with its binding to the cis-acting element and thereby exhibiting the dominant negative phenotype. On the other hand, as expected, under inducing conditions, all strains exhibited similar expression of the Venus reporter (Fig. 7A).
To determine whether DgoR forms oligomers in vitro, DgoR-6×His was immobilized on cobalt-nitrilotriacetic acid (Co-NTA) beads and incubated either with maltose-binding protein–β-galactosidase (MBP-βgal) or MBP-DgoR. DgoR-6×His was eluted using imidazole. Whereas MBP-βgal did not coelute with DgoR-6×His, MBP-DgoR was pulled down by DgoR-6×His, suggesting that DgoR molecules interact with each other to form oligomers (Fig. 7B).
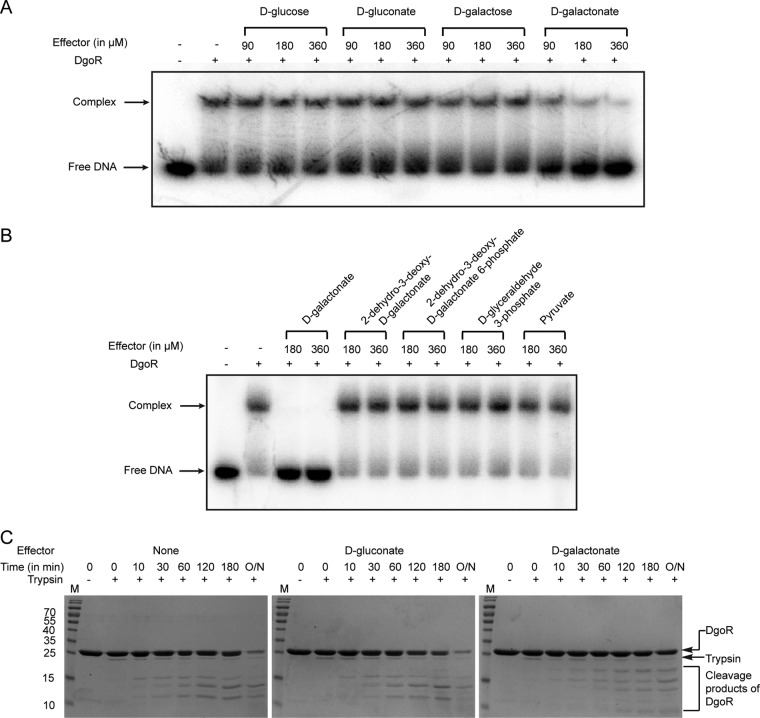
d-Galactonate is a specific effector of DgoR.
An earlier study reported the enzymatic activities of DgoK and DgoA to be significantly induced in cell extracts prepared from a dgoD mutant grown in d-galactonate-supplemented medium. Because DgoD catalyzes the first step in d-galactonate metabolism and hence no catabolic intermediate is expected to accumulate in a dgoD mutant, it was suggested that d-galactonate is the inducer of the dgo operon (13). However, since the catabolic pathways of sugar acids cross talk with each other either due to their enzymatic interconversion or shared transcriptional regulation (1, 9, 10), it is important to examine whether d-galactonate directly binds DgoR. To test this, we incubated DgoR with its cis-acting element in the presence of various concentrations of d-galactonate. To determine if d-galactonate is a specific effector of DgoR, additional sugars (d-glucose and d-galactose), a sugar acid (d-gluconate), and intermediates of the d-galactonate degradation pathway (Fig. 1B) were also examined. Whereas d-galactonate relieved DNA bound by DgoR in a concentration-dependent manner (KD [apparent dissociation constant] of 55 ± 6 µM), other tested carbohydrates did not affect the binding of DgoR to its target DNA (Fig. 8A and B; see Fig. S3 in the supplemental material).
FIG 8.
d-Galactonate directly interacts with DgoR. (A and B) d-Galactonate releases DgoR from its target DNA. A 1.2 µM concentration of DgoR-6×His was incubated with the indicated concentrations of various carbohydrates for 20 min, and then 20 nM 32P-labeled dgo cis-acting element was added to the samples and incubated for 30 min. Samples were resolved by native PAGE and subjected to autoradiography. (C) d-Galactonate induces a conformational change in DgoR. SDS-polyacrylamide gels show the results of trypsin-mediated digestion of DgoR-6×His alone (left panel), DgoR-6×His incubated with d-gluconate (middle panel), and DgoR-6×His incubated with d-galactonate (right panel). Lane M, molecular mass markers (in kilodaltons). O/N, the reaction mix was incubated overnight.
Limited proteolysis is widely used to monitor conformational changes induced in regulators upon interaction with their cognate effectors (44–47). To determine whether the interaction of d-galactonate with DgoR is specific and results in a conformational change in the regulator, we performed limited proteolysis of the DgoR protein alone and of the protein incubated with either d-gluconate or d-galactonate (Fig. 8C). Compared to DgoR alone or DgoR incubated with d-gluconate, the protein incubated with d-galactonate was less accessible to trypsin digestion and showed a different digestion pattern. These data show that the presence of d-galactonate protects DgoR against trypsin cleavage and suggest a conformational change in DgoR induced by its interaction with d-galactonate.
DISCUSSION
Here we investigated interactions between the dgo repressor, operator, and effector. The combined results provide evidence that DgoR represses the d-galactonate metabolic pathway by binding inverted repeats in the dgo cis-acting element and employs a derepression mechanism using d-galactonate as a specific effector molecule.
Interaction of the transcriptional repressor, DgoR, with its operator.
Our work shows that DgoR forms a complex at the dgo operator comprising of two inverted repeats that are likely on the same face of the DNA (the distance between the inverted repeats is 10 bp, i.e., 1 helix turn) (Fig. 4). Importantly, the inverted repeat sequences recognized by DgoR [5′-TTGTA(G/C)TACA(A/T)-3′] (Fig. 5), match the signature of GntR family regulators that also bind inverted repeats [5′-(N)y GT(N)x AC(N)y-3′, where x and y indicate the number of nucleotides, which varies] (16, 41, 48). The DgoR-binding sites overlap the promoter, indicating that DgoR represses transcription of the operon by occluding the binding of RNA polymerase (Fig. 5). Shortly after the submission of the manuscript for this article, a study using a high-throughput plasmid-based reporter assay reported a region in the dgo cis-acting element to harbor putative overlapping DgoR- and RNAP-binding sites (49). Importantly, the region identified in that study encompasses the sites determined by our single-copy reporter assays and EMSAs, providing strong support to our findings.
The mechanistic details of the interaction between the wHTH domain and the operator are derived mainly from the DNA-bound structures of GntR family regulators belonging to different subfamilies (AraR subfamily, Bacillus subtilis AraR [50]; FadR subfamily, E. coli FadR [51, 52] and Vibrio cholerae FadR [53]; and HutC subfamily, B. subtilis NagR [54]) and genetic/biochemical characterization of these and additional GntR family members (55–61). To understand how amino acid residues in DgoR mutated in this study determine its DNA-binding ability, we obtained the DNA-bound state of DgoR by superimposing the wHTH domain of a DgoR monomer onto the wHTH domain of E. coli FadR bound to its operator (PDB identification [ID] 1H9T) (see Fig. S4A in the supplemental material). In the characterized GntR family proteins, amino acid residues in α1 make nonspecific contacts with the phosphate backbone and support the specific contacts made by the downstream residues (50–54, 59). In the DgoR-DNA model, although the side chain of D7, an α1 residue, points toward the DNA, it does not make any contact (Fig. S4A). However, similar to the DNA-binding defect of the D7A mutant of DgoR, a previous study on another FadR subfamily member, AphS, reported a loss of repression by a mutant harboring a mutation in the corresponding α1 residue, E17 (56). A Glu or Asp residue is present at the analogous position in several GntR family members (15), and thus, an acidic residue at the start of α1 may play an important role in the repressor ability of these regulators.
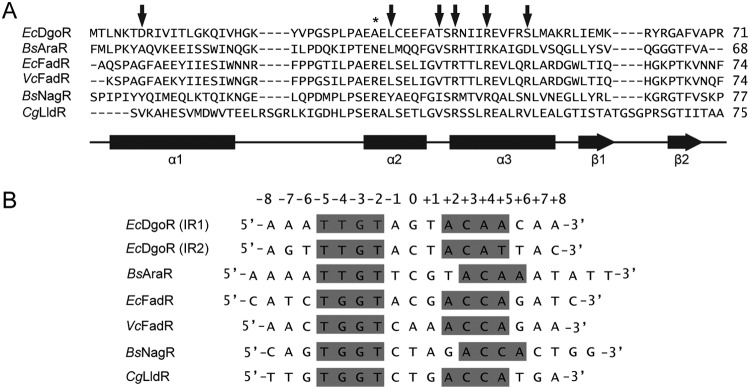
Residues in α2 and α3 form specific contacts in the major groove, where α3 defines a larger part of the specificity and hence termed the “recognition helix” (16). The information that amino acid substitution of several residues in α2-α3 abrogated the interaction of DgoR with its operator (Fig. 6) further underscores the importance of the wHTH motif in DNA recognition by GntR family regulators. Corresponding to the α2 residue, L34 in DgoR, a hydrophobic amino acid is present in a majority of the GntR family members (Fig. 9A) (15). Mutation of an equivalent Leu in FadR (L37A) and AraR (L33S) led to a weak dominant negative phenotype and poor expression of the protein, respectively, suggesting that this hydrophobic amino acid is important for protein stability (55, 57, 58). Along similar lines, we observed a weak dominant negative phenotype of the L34A mutant of DgoR and were unable to detect its expression from the native dgo promoter (Fig. 6C and Fig. 7A). In the DgoR-DNA model, we find that L34 is part of a hydrophobic core, further emphasizing that a hydrophobic amino acid at this position is structurally important in GntR family regulators (Fig. S4B, left panel). Additionally, the importance of the L34 residue in DNA binding is evident from both the inability of L34A to form protein-DNA complexes in vitro and negative dominance in vivo (Fig. 6A and Fig. 7A). In the DgoR-DNA model, L34 interacts with two hydrophobic residues, I45 and F49, located in α3 (Fig. S4B, left panel). Because mutating L34 to A will reduce hydrophobicity and increase the distance of I45 and F49 from α2, we suggest that the recognition helix might become reoriented, affecting its contact with DNA. Similar to the DNA-binding defect of R42C and R46A in DgoR, mutation of Arg residues at equivalent positions in α3 in several other GntR family members compromises their repressor ability (55, 57–59). From the available DNA-bound structures, it is evident that both Arg residues interact with a common Glu residue in α2, which orients them such that arginine corresponding to R42 makes a specific contact with guanine (G-3), while arginine equivalent to R46 interacts nonspecifically with the phosphate backbone (50–54, 59). In the DgoR-DNA model, we observe the interaction of R42 and R46 with an equivalent Glu residue, E31 in α2 (Fig. S4B, right panel), which leads us to speculate that these Arg residues make contacts with DNA similar to those observed in other characterized members (Fig. 9). GntR family regulators that recognize the TGGT motif in the operator make a specific contact with G-4 through an Arg residue conserved in these members (Fig. 9) (15, 51–54, 59). However, GntR family proteins, including DgoR, that recognize the TTGT motif lack the analogous Arg (Fig. 9) (15, 50), emphasizing that differences in the operator sequence are reflected in the amino acid sequences of their cognate regulators.
FIG 9.
Structure-based sequence alignment of the N-terminal wHTH DNA-binding domains of GntR family members and comparison of their operator sequences. (A) The amino acid sequence of the N-terminal wHTH domain of DgoR is aligned with those of GntR family members for which either the DNA-bound structures are solved (BsAraR, PDB ID 4EGY; EcFadR, 1H9T; VcFadR, 4P9U; and BsNagR, 4WWC) or the structure of their apo form is modeled on DNA (CgLldR, 2DI3), using the DALI server (79). Arrows indicate amino acid residues in the wHTH domain of DgoR mutated in this study. Secondary structure elements are indicated by bars (α-helices) and horizontal arrows (β-strands). *, presence of an Arg residue at this position in GntR family members that recognize the TGGT motif in the operator (see panel B). Abbreviations: Ec, E. coli; Bs, B. subtilis; Vc, V. cholerae; and Cg, Corynebacterium glutamicum. (B) Comparison of the binding sites of DgoR (IR1 and IR2) with the operator sequences of GntR family members used to either solve the DNA-bound structures of their wHTH domains (BsAraR, EcFadR, VcFadR, and BsNagR) or biochemically test the binding to its transcriptional regulator (CgLldR). Nucleotides in the inverted repeat that are conserved and/or involved in making specific contacts with the protein are highlighted in gray. Abbreviations: Ec, E. coli; Bs, B. subtilis; Vc, V. cholerae; and Cg, C. glutamicum.
The DNA-binding defect of the T40I and S51L mutants cannot be explained from the DgoR-DNA model. Although the side chain of T40 points toward the DNA, there is no interaction with the DNA (Fig. S4A). In a majority of the GntR family regulators, a hydrophobic amino acid is present at a similar position (Fig. 9A) (15). The residue S51 does not face the DNA (Fig. S4A), and the nature of the amino acid at a similar position is highly variable across the GntR family (Fig. 9A) (15). Future studies aimed at solving the structure of DgoR alone and in complex with its operator might explain the importance of these amino acids in the functioning of the repressor.
d-Galactonate is the physiological effector of DgoR.
Besides DgoR, which regulates d-galactonate metabolism in E. coli, FadR subfamily members also control the metabolism of sugar acids such as d-gluconate, d-glucuronate, d-galacturonate, and d-glucarate (1, 10, 62, 63). In addition, FadR subfamily regulators also govern the metabolism of a variety of other carbon sources, such as fatty acids, sialic acids, l-lactate, and substrates emerging from central metabolism (59, 64–66). These regulators employ substrates (e.g., E. coli NanR, N-acetylneuraminic acid; B. subtilis GntR, d-gluconate), metabolic intermediates (e.g., E. coli FadR, long-chain fatty acyl coenzyme A [acyl-CoA]; Polaromonas sp. strain JS666 GguR, 5-keto-4-deoxy-d-glucarate/galactarate), or both (e.g., Rhizobium leguminosarum MatR, malonate and citrate; E. coli UxuR, d-glucuronate and d-fructuronate) as their effectors (9, 10, 62, 63, 66–69). We find that similar to the case for B. subtilis GntR, the substrate of the metabolic pathway, d-galactonate, serves as the effector of DgoR. d-Galactonate binds DgoR, brings about a conformational change in the protein, and interferes with its binding to the target DNA (Fig. 8). To date, within the FadR subfamily, the details of effector-induced allosteric changes are known for E. coli, V. cholerae, and Vibrio alginolyticus FadR proteins through a comparison of their apo, DNA-bound, and effector-bound structures (51, 53, 70). Such studies on DgoR will be important in the future, especially because the structural and molecular details of effector-regulator interactions have not been investigated for any FadR subfamily member that regulates sugar acid metabolism.
A bioinformatics study has predicted the presence of DgoR and its binding sites in the genomes of 13 different bacterial species, with the majority of members belonging to the family Enterobacteriaceae (41). The recovery of missense mutations in dgoR in a human urinary tract isolate of E. coli that inactivate the repressor, enabling faster growth of bacteria in d-galactonate and its adaptation in the mouse gut (Fig. 6A and B) (32), suggests that mutations in the regulatory elements are preferably selected to allow simultaneous induction of structural dgo genes. Considering this, it will be interesting to investigate the similarities/differences in the regulation of the d-galactonate metabolic pathway in enteric bacteria and understand their implications in the relative colonization of these bacteria in their environmental niches.
MATERIALS AND METHODS
Media and growth conditions.
The media had the following compositions: lysogeny broth (LB), 5 g/liter yeast extract, 10 g/liter Bacto tryptone, and 5 g/liter NaCl; and M9 minimal medium, 5.3 g/liter Na2HPO4, 3 g/liter KH2PO4, 0.5 g/liter NaCl, 1 g/liter NH4Cl, 0.12 g/liter MgSO4, 2 mg/liter biotin, 2 mg/liter nicotinamide, 0.2 mg/liter riboflavin, and 2 mg/liter thiamine. Where required, M9 minimal medium was supplemented with one of the following carbon sources: d-glucose (10 mM), sodium d-gluconate (10 mM), d-galactose (10 mM), d-galactonate (10 mM), glycerol (0.4%, vol/vol), or both glycerol and d-galactonate. Except for d-galactonate, which was prepared from calcium d-galactonate (MP Biomedicals) (see below), all carbon sources were used as procured from commercial sources (d-glucose from Fisher Scientific and sodium d-gluconate, d-galactose, and glycerol from Sigma). 2-Dehydro-3-deoxy-d-galactonate, 2-dehydro-3-deoxy-d-galactonate 6-phosphate, d-glyceraldehyde 3-phosphate, and pyruvate were procured from Sigma. Media were solidified using 1.5% (wt/vol) Bacto agar. When required, ampicillin (100 µg/ml) or kanamycin (30 µg/ml) was used. Cultures were incubated at 37°C unless otherwise specified. Primary cultures were grown in 3 ml LB liquid medium. Secondary cultures were set up either in LB or in M9 minimal medium containing the desired carbon source with an initial optical density (OD) of ∼0.01, unless indicated otherwise.
Preparation of d-galactonate.
d-Galactonate was prepared from its calcium salt as described previously (71). Briefly, equivalent amounts of calcium d-galactonate and oxalic acid were mixed in boiling water and vortexed for 3 min. The milky solution was filtered through a 0.2-µm filter. The filtrate was immediately transferred to 4°C for 15 min. Crystals were collected and dried overnight at room temperature on a Whatman filter paper. Crystals of d-galactonate were stored at room temperature.
Strains, plasmids, and primers.
Table 1 provides a list of bacterial strains and plasmids, and Table S1 in the supplemental material provides a list of primers used in the study. E. coli BW25113 and various deletion strains in this background were used for dilution spotting experiments, growth curve assays, quantitative reverse transcription-PCR (qRT-PCR), and fluorescence reporter assays. For deletion strains obtained from the Keio collection (36), the two independent clones and/or their fresh transductants (made by P1 transduction) were analyzed to rule out genetic errors. Strain DH5α was used for cloning in plasmids pACYC177, pRC10, and pMAL-c2, while strain BW25142 (pir+) was used for cloning in the pVenus plasmid. BL21(DE3) was used for protein expression and purification.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source and/or reference |
|---|---|---|
| Strains | ||
| DH5α | F− Δ(argF-lac)169 ϕ80dlacZ58(M15) glnX44(AS) λ−
rfbC1 gyrA96(Nalr) recA1 endA1 spoT1 thiE1 hsdR17 |
New England Biolabs |
| BL21(DE3) | F−
lon-11 Δ(ompT-nfrA)885 Δ(galM-ybhJ)884 λDE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5] Δ46 [mal+]K-12(λs) hsdS10 |
New England Biolabs |
| BW25142 |
lacIq
rrnB3 ΔlacZ4787 hsdR514 Δ(araBAD)567 Δ(rhaBAD)568 ΔphoBR580 rph-1 galU95
ΔendA9 uidA(ΔMluI)::pir-116 recA1 |
Rao lab (73) |
| BW25113 | F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− rph-1 Δ(rhaD-rhaB)568 hsdR514 | CGSCa (36) |
| dgoR::kan | BW25113 dgoR::kan Kanr | Keio collection (36) |
| dgoK::kan | BW25113 dgoK::kan Kanr | Keio collection (36) |
| dgoA::kan | BW25113 dgoA::kan Kanr | Keio collection (36) |
| dgoD::kan | BW25113 dgoD::kan Kanr | Keio collection (36) |
| dgoT::kan | BW25113 dgoT::kan Kanr | Keio collection (36) |
| RC2132 | BW25113 dgo::kan Kanr | This work |
| RC3067 | BW25113 dgoR:3×FLAG-kan Kanr | This work |
| RC2069 | ΔdgoR (Kan cassette flipped from BW25113 dgoR::kan) | This work |
| RC12022 | BW25113 attλ::[Kan promoterless-venus oriR6K] Kanr | This work |
| RC12023 | ΔdgoR attλ::[Kan promoterless-venus oriR6K] Kanr | This work |
| RC12018 | BW25113 attλ::[Kan Pdgo-venus oriR6K] Kanr | This work |
| RC12020 | ΔdgoR attλ::[Kan Pdgo-venus oriR6K] Kanr | This work |
| RC14029 | BW25113 attλ::[Kan Pdgo(Δ-35)-venus oriR6K] Kanr | This work |
| RC14030 | ΔdgoR attλ::[Kan Pdgo(Δ-35)-venus oriR6K] Kanr | This work |
| RC14031 | BW25113 attλ::[Kan Pdgo(Δ-35/-10)-venus oriR6K] Kanr | This work |
| RC14032 | ΔdgoR attλ::[Kan Pdgo(Δ-35/-10)-venus oriR6K] Kanr | This work |
| RC14033 | BW25113 attλ::[Kan Pup-dgoK-venus oriR6K] Kanr | This work |
| RC14034 | ΔdgoR attλ::[Kan Pup-dgoK-venus oriR6K] Kanr | This work |
| RC14036 | BW25113 attλ::[Kan Pup-dgoA-venus oriR6K] Kanr | This work |
| RC14037 | ΔdgoR attλ::[Kan Pup-dgoA-venus oriR6K] Kanr | This work |
| RC14038 | BW25113 attλ::[Kan Pup-dgoD-venus oriR6K] Kanr | This work |
| RC14039 | ΔdgoR attλ::[Kan Pup-dgoD-venus oriR6K] Kanr | This work |
| RC14040 | BW25113 attλ::[Kan Pup-dgoT-venus oriR6K] Kanr | This work |
| RC14041 | ΔdgoR attλ::[Kan Pup-dgoT-venus oriR6K] Kanr | This work |
| RC12035 | BW25113 attλ::[Kan Pdgo(IR1L)-venus oriR6K] Kanr | This work |
| RC12036 | ΔdgoR attλ::[Kan Pdgo(IR1L)-venus oriR6K] Kanr | This work |
| RC12040 | BW25113 attλ::[Kan Pdgo(IR1R)-venus oriR6K] Kanr | This work |
| RC12041 | ΔdgoR attλ::[Kan Pdgo(IR1R)-venus oriR6K] Kanr | This work |
| RC12028 | BW25113 attλ::[Kan Pdgo(IR2L)-venus oriR6K] Kanr | This work |
| RC12029 | ΔdgoR attλ::[Kan Pdgo(IR2L)-venus oriR6K] Kanr | This work |
| RC12031 | BW25113 attλ::[Kan Pdgo(IR2R)-venus oriR6K] Kanr | This work |
| RC12032 | ΔdgoR attλ::[Kan Pdgo(IR2R)-venus oriR6K] Kanr | This work |
| RC12043 | BW25113 attλ::[Kan Pdgo(IR2L')-venus oriR6K] Kanr | This work |
| RC12047 | ΔdgoR attλ::[Kan Pdgo(IR2L')-venus oriR6K] Kanr | This work |
| RC14053 | BW25113 attλ::[Kan Pdgo(IR3L)-venus oriR6K] Kanr | This work |
| RC14056 | ΔdgoR attλ::[Kan Pdgo(IR3L)-venus oriR6K] Kanr | This work |
| RC12025 | BW25113 attλ::[Kan Pdgo(IR3R)-venus oriR6K] Kanr | This work |
| RC12026 | ΔdgoR attλ::[Kan Pdgo(IR3R)-venus oriR6K] Kanr | This work |
| Plasmids | ||
| pKD13 | oriR6K, FRT-flanked Kanr, pANTSγ PS1 PS4 Kanr | 72 |
| pKD46 | pSC101 ori araC repA101(Ts) ParaBAD-λred Ampr | 72 |
| pCP20 | pSC101 ori cI857 λ-PR flp ts Ampr Camr | 72 |
| pACYC177 | p15A ori Ampr Kanr | New England Biolabs |
| pBS13 | dgo promoter and dgoR-6×His in pACYC177, Ampr Kanr | This work |
| pBS14 | dgo promoter and dgoR(L34A)-6×His in pACYC177, Ampr Kanr | This work |
| pBS15 | dgo promoter and dgoR(R46A)-6×His in pACYC177, Ampr Kanr | This work |
| pBS22 | dgo promoter and dgoR(D7A)-6×His in pACYC177, Ampr Kanr | This work |
| pBS35 | dgo promoter and dgoR(S51L)-6×His in pACYC177, Ampr Kanr | This work |
| pBS36 | dgo promoter and dgoR(R42C)-6×His in pACYC177, Ampr Kanr | This work |
| pBS37 | dgo promoter and dgoR(T40I)-6×His in pACYC177, Ampr Kanr | This work |
| pRC10 | pBR322 ori, −10 box of Ptrc changed to Plac in pTrc99a, ΔNcoI Ampr | 80 |
| pBS2 | dgoR-6×His in pRC10, Ampr | This work |
| pBS19 | dgoR(L34A)-6×His in pRC10, Ampr | This work |
| pBS20 | dgoR(R46A)-6×His in pRC10, Ampr | This work |
| pBS25 | dgoR(D7A)-6×His in pRC10, Ampr | This work |
| pBS32 | dgoR(S51L)-6×His in pRC10, Ampr | This work |
| pBS33 | dgoR(R42C)-6×His in pRC10, Ampr | This work |
| pBS34 | dgoR(T40I)-6×His in pRC10, Ampr | This work |
| pMAL-c2 | pBR322 ori, leaderless malE MCS,b Ampr | New England Biolabs |
| pBS11 | dgoR cloned in pMAL-c2, Ampr | This work |
| pINT-ts | oriR6K int Ampr | Rao lab (73) |
| pVenus | oriR6K, MCS venus t0 attλ, Kanr | Rao lab (74) |
| pAS1 | oriR6K, MCS Pdgo-venus t0 attλ, Kanr | This work |
| pAS2 | oriR6K, MCS Pdgo(IR1L)-venus t0 attλ, Kanr | This work |
| pAS3 | oriR6K, MCS Pdgo(IR1R)-venus t0 attλ, Kanr | This work |
| pAS4 | oriR6K, MCS Pdgo(IR2L)-venus t0 attλ, Kanr | This work |
| pAS5 | oriR6K, MCS Pdgo(IR2R)-venus t0 attλ, Kanr | This work |
| pAS6 | oriR6K, MCS Pdgo(IR2L')-venus t0 attλ, Kanr | This work |
| pAS7 | oriR6K, MCS Pdgo(IR3L)-venus t0 attλ, Kanr | This work |
| pAS8 | oriR6K, MCS Pdgo(IR3L')-venus t0 attλ, Kanr | This work |
| pNK1 | oriR6K, MCS Pdgo(Δ-35)-venus t0 attλ, Kanr | This work |
| pNK2 | oriR6K, MCS Pdgo(Δ-35/-10)-venus t0 attλ, Kanr | This work |
| pNK3 | oriR6K, MCS Pup-dgoK-venus t0 attλ, Kanr | This work |
| pNK4 | oriR6K, MCS Pup-dgoA-venus t0 attλ, Kanr | This work |
| pNK5 | oriR6K, MCS Pup-dgoD-venus t0 attλ, Kanr | This work |
| pNK6 | oriR6K, MCS Pup-dgoT-venus t0 attλ, Kanr | This work |
CGSC, E. coli Genetic Stock Center.
MCS, multiple cloning site.
Strains carrying a deletion of the entire dgo operon or expressing C-terminally 3×FLAG-tagged DgoR (DgoR-3×FLAG) from the chromosome were constructed using the λ Red recombinase method (72). For the deletion of the dgo operon, a kanamycin cassette was PCR amplified from the plasmid pKD13, while for tagging the C terminus-encoding end of dgoR on the chromosome with 3×FLAG, the sequence of 3×FLAG and the kanamycin cassette was PCR amplified from a sequential peptide affinity (SPA)-tagged strain obtained from the Keio collection (36). The amplified cassettes flanked by regions homologous to the target sites were transformed into BW25113 expressing λ Red recombinase enzymes from the pKD46 helper plasmid. Recombinants were selected on LB-kanamycin plates. Strains were PCR verified. The dgo operon deletion and dgoR:3×FLAG-kan were subsequently transduced into a clean BW25113 background using P1 phage, yielding strains RC2132 and RC3067, respectively.
For complementation experiments, dgoR encoding a C-terminally 6×His-tagged protein was cloned in pACYC177. The coding region of dgoR along with its putative promoter (446 bp upstream of the dgoR start codon) was PCR amplified and cloned in AatII and BamHI sites of pACYC177 to generate plasmid pBS13. Various dgoR mutations in pACYC177 were created by overlap extension PCR using pBS13 as the template.
Plasmid pBS2 was constructed for high-level expression and purification of C-terminally 6×His-tagged DgoR (DgoR-6×His). Briefly, the coding region of dgoR was PCR amplified and cloned in the EcoRI and BamHI sites of IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible plasmid pRC10. For expression and purification of various DgoR mutant proteins, mutations were generated by overlap extension PCR using pBS2 as the template. Plasmid pBS11 was constructed for expression and purification of MBP-DgoR by amplifying the coding region of dgoR and cloning in the EcoRI and SalI sites of pMAL-c2 to create an in-frame fusion with MBP.
To determine functional promoters and DgoR-binding sites in the dgo region, single-copy transcriptional fusions were constructed in the pVenus integration vector by a previously described method (73, 74). Briefly, to identify functional promoters in the putative dgo operon, sequences upstream of dgoR and its truncated variants were PCR amplified from pBS13, while sequences upstream of other dgo genes (dgoK, dgoA, dgoD, and dgoT) were amplified from genomic DNA. To identify DgoR-binding sites, mutations were created in the dgo cis-acting element by overlap extension PCR using pBS13 as the template. The DNA fragments were cloned upstream of the fluorescent Venus reporter at the KpnI and EcoRI sites of the pVenus plasmid. The reporter constructs were integrated at the attλ site of BW25113 with the help of integrase expressed from the pINT-ts helper plasmid. Strains were PCR verified for single-copy integration. Reporter integrations were P1 transduced into clean BW25113 or ΔdgoR strains. The ΔdgoR strain (derived by flipping out the kanamycin cassette from the dgoR::kan strain) was used since the Venus reporter construct carried a kanamycin marker.
Dilution spotting.
Overnight cultures were pelleted and resuspended in M9 minimal medium without any carbon source to a similar OD at 450 nm (OD450). Serial dilutions of resuspended cells were spotted on M9 minimal agar plates containing the desired carbon source. Plates were incubated at 37°C and imaged at definite time intervals using the Gel Doc XR+ imaging system from Bio-Rad.
RNA isolation, cDNA preparation, and qRT-PCR.
Secondary cultures were grown in 25 ml M9 minimal medium with the desired carbon source to an OD450 of ∼0.5, and 20-ml samples were collected and processed for RNA isolation by the hot-phenol method as described previously (75). RNA suspended in nuclease-free water was treated with DNA-free Turbo DNase (Applied Biosystems, USA) to avoid genomic DNA contamination, and 4 µg RNA was used to prepare cDNA using SuperScript III reverse transcriptase (Applied Biosystems, USA) using the protocol described previously (76). qRT-PCR was carried out with Power Sybr green PCR master mix (Applied Biosystems, USA) in accordance with the manufacturer’s recommended protocol using 50 ng cDNA as the template and 5 pmol of each forward and reverse primer listed in Table S1. qRT-PCR was performed using the Quant Studio 6 Flex system (Applied Biosystems, USA). Data were analyzed as described previously (77) using recA and gyrA as internal controls.
Western blotting.
The expression of DgoR-3×FLAG, DgoR-6×His, and MBP-DgoR was monitored by Western blot analysis. Samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked with 5% (wt/vol) skimmed milk at 4°C overnight and probed with anti-FLAG (1:1,000; Sigma) or anti-His (1:1,000; Thermo Fisher Scientific) primary antibody and horseradish peroxidase (HRP)-conjugated anti-mouse (1:5,000; Sigma) secondary antibody. Where required, anti-MBP conjugated to HRP (1:5,000; New England Biolabs) was used. Blots were developed with the SuperSignal West Dura extended-duration substrate (Pierce). Signal was captured with an LAS4000 instrument (GE Health Care) or on X-ray film.
Growth curve experiments in shake flasks and 96-well plates.
Overnight cultures were pelleted, washed, and resuspended in M9 minimal medium. For growth curves in shake flasks, cells were reinoculated in 25 ml M9 minimal medium supplemented with the desired carbon source (in 125-ml flasks) to an initial OD450 of ∼0.01. Cultures were grown at 37°C, and the OD450 was measured at defined time intervals. For growth curves determined in 96-well plates, cells were reinoculated in 200 µl medium to a starting OD450 of ∼0.03, using a robotic liquid handling system (Tecan). Plates were incubated in a shaker at 37°C, and the OD450 of the cultures was measured at hourly intervals (Tecan Infinite M200 monochromator). The incubator shaker and microplate reader were integrated with the liquid handling system, and the transfer of plates between shaker and reader was automated.
Overexpression and purification of DgoR-6×His, MBP-βgal, and MBP-DgoR.
For the purification of WT and mutant DgoR-6×His proteins, cultures were grown in 400 ml LB at 37°C to an OD600 of ∼0.5. Cultures were induced with 0.5 mM IPTG and grown overnight at 18°C. The cell pellet was resuspended in 20 ml lysis buffer containing 50 mM sodium phosphate (pH 7.5), 300 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 20 mM imidazole and disrupted by sonication. Cell debris was removed by centrifugation. The supernatant was incubated with Co-NTA beads (Pierce) on ice for 1 to 1.5 h with continuous shaking. Beads were washed with 40 ml wash buffer A containing 50 mM sodium phosphate (pH 7.5), 1 M NaCl, and 20 mM imidazole, followed by washing with 40 ml wash buffer B containing 50 mM sodium phosphate (pH 7.5), 300 mM NaCl, and 60 mM imidazole, and finally the protein was eluted in elution buffer containing 50 mM sodium phosphate (pH 7.5), 300 mM NaCl, and 500 mM imidazole. The eluted protein was dialyzed against buffer containing 20 mM Tris (pH 8.0), 300 mM NaCl, 1 mM dithiothreitol (DTT), and 10% (wt/vol) glycerol.
For the purification of MBP-βgal and MBP-DgoR, cultures were grown in 400 ml LB supplemented with 2 g/liter d-glucose at 37°C until the OD600 reached 0.5. Cultures were induced with 1 mM IPTG for 4 h. The cell pellet was resuspended in column buffer containing 20 mM HEPES (pH 7.5), 300 mM NaCl, and 1 mM EDTA (5 ml/g cell pellet) and lysed by sonication. The lysate was centrifuged to remove cell debris, and the supernatant was incubated with amylose resin for 1.5 to 2 h at 4°C. Amylose beads were washed with five bed volumes of column buffer and eluted with one bed volume of the same buffer containing 10 mM maltose. Proteins were analyzed by SDS-PAGE to assess purity and stored at −80°C until use. The concentrations of the proteins were determined by the Bradford assay using bovine serum albumin as the standard, as per the manufacturer’s protocol (Sigma).
EMSA.
For electrophoretic mobility shift assay (EMSA), the dgo cis-acting elements were PCR amplified with primers BS104 and 32P-labeled BS105, using appropriate pVenus constructs as the templates, and gel purified. A 20-µl reaction mixture was set up in protein dialysis buffer supplemented with 10 mM MgCl2 and 1 mM PMSF, to which 20 nM labeled DNA, 1 µg herring sperm DNA, and either WT or mutant DgoR-6×His proteins were added. The reaction mix was incubated at 27°C for 30 min, and samples were subjected to 8% native PAGE at 60 V for 6 h. The signal was visualized using a PhosphorImager (Fuji or Bio-Rad). For experiments involving effectors, DgoR-6×His was incubated with effectors at 37°C for 20 min before incubation with DNA.
Fluorescence assay.
Overnight cultures of reporter strains in LB were pelleted and resuspended in M9 minimal medium. Secondary cultures were set up in 96-well, black, clear-bottom plates (Costar, Corning) in M9 minimal medium supplemented with either glycerol or glycerol and d-galactonate, followed by incubation at 37°C with shaking. Fluorescence was measured in exponential phase using a microplate reader (Tecan Infinite M200 monochromator) in the top mode with excitation and emission wavelengths of 498 nm and 568 nm, respectively. Fluorescence normalized to the OD450 of the samples was plotted in a bar graph.
Pulldown experiments.
Culture expressing DgoR-6×His was harvested, and the cell pellet was resuspended in lysis buffer (50 mM sodium phosphate [pH 7.5], 300 mM NaCl, 1 mM PMSF, and 20 mM imidazole). Cells were lysed by sonication and centrifuged to remove the debris. The supernatant was incubated with 60 µl Co-NTA beads at 4°C for 90 min. Beads were washed with 35 bed volumes of lysis buffer. The beads were equally split into two fractions. Purified MBP-DgoR was added to one fraction, while to the other fraction purified MBP-βgal was added, and immediately a portion of these suspensions was saved as “input.” The remaining suspension was incubated for 20 min at 4°C. Beads were washed with 25 bed volumes of column buffer (20 mM Tris [pH 7.4], 200 mM NaCl, and 1 mM EDTA). Proteins were finally eluted with 120 µl elution buffer (50 mM sodium phosphate [pH 7.5], 300 mM NaCl, and 300 mM imidazole). The eluted sample was saved as the “pulldown.”
Limited proteolysis assay.
Trypsin digestion was performed following the procedure described previously (45, 47) with modifications. A 200-µl reaction mixture was set up at 37°C in protein dialysis buffer supplemented with 500 mM NaCl, 10 mM MgCl2, and 0.02 mg/ml trypsin (Sigma), to which either DgoR-6×His (10 µM) alone or both protein and effectors (2 mM) were added. Twenty-microliter samples were withdrawn at various time intervals. The reaction was quenched by adding SDS sample buffer (4× stock: 10% [wt/vol] glycerol, 0.6% [wt/vol] Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 0.02% [wt/vol] bromophenol blue, and 1.5% [wt/vol] DTT). Samples were boiled at 95°C for 5 min and subjected to 15% SDS-PAGE followed by staining with Coomassie brilliant blue.
Bioinformatics approaches.
The amino acid sequence of full-length DgoR was submitted to the Robetta server (78) for model building. The top model build by Robetta was used for analysis. The PDB database was searched for proteins with a structure similar to that of the N-terminal wHTH domain of DgoR (from amino acids 1 to 73) using the DALI server (79). The DALI server was also used to generate structure-based sequence alignment of DgoR with selected members of the GntR family. ICM-Browser (www.molsoft.com) was used for model visualization, structure comparison and analysis, and preparation of related figures.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Dipak Dutta lab (Institute of Microbial Technology, India) for help with radioactive EMSAs, Vishal Agrawal (Panjab University, India) and Shashi Bhushan Pandit (IISER-Mohali, India) for guidance with bioinformatics studies, and Samrat Mukhopadhyay (IISER-Mohali, India) for help with CD spectroscopy. We acknowledge technical assistance from Yatendra Arya in making the chromosomal 3×FLAG-tagged dgoR construct. We thank Christopher V. Rao (University of Illinois at Urbana-Champaign, USA) for strain BW25142 and plasmids pVenus and pINT-ts. We thank members of the Chaba lab for discussions.
B.S. and N.K. acknowledge fellowship support from IISER-Mohali for doctoral work. G.A. is a recipient of a University Grants Commission (UGC) fellowship for doctoral work. A.S. and S.N. acknowledge the support of Inspire fellowships from the Department of Science and Technology (DST) for undergraduate studies. R.C. acknowledges financial support from the Department of Biotechnology (DBT) (grant number BT/PR11559/BRB/10/1285/2014) and IISER-Mohali.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We declare that we have no conflicts of interest with the contents of this article.
B.S. and R.C. designed the study. B.S., G.A., N.K., A.S., and S.N. performed experiments and analyzed the data. B.S., G.A., N.K., A.S., and R.C. interpreted the data. B.S. and R.C. wrote the manuscript. B.S., G.A., N.K., and R.C. edited the manuscript. All authors reviewed the manuscript. R.C. supervised the project.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00281-18.
REFERENCES
- 1.Mandrand-Berthelot MA, Condemine G, Hugouvieux-Cotte-Pattat N. 2004. Catabolism of hexuronides, hexuronates, aldonates, and aldarates. EcoSal Plus 2004. doi: 10.1128/ecosalplus.3.4.2. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney NJ, Laux DC, Cohen PS. 1996. Escherichia coli F-18 and E. coli K-12 eda mutants do not colonize the streptomycin-treated mouse large intestine. Infect Immun 64:3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peekhaus N, Conway T. 1998. What's for dinner? Entner-Doudoroff metabolism in Escherichia coli. J Bacteriol 180:3495–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway T, Cohen PS. 2015. Commensal and pathogenic Escherichia coli metabolism in the gut. Microbiol Spectr 3. doi: 10.1128/microbiolspec.MBP-0006-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hommes RW, Postma PW, Tempest DW, Neijssel OM. 1989. The influence of the culture pH value on the direct glucose oxidative pathway in Klebsiella pneumoniae NCTC 418. Arch Microbiol 151:261–267. doi: 10.1007/BF00413140. [DOI] [PubMed] [Google Scholar]
- 6.Brechtel E, Huwig A, Giffhorn F. 2002. l-Glucitol catabolism in Stenotrophomonas maltophilia Ac. Appl Environ Microbiol 68:582–587. doi: 10.1128/AEM.68.2.582-587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faber F, Tran L, Byndloss MX, Lopez CA, Velazquez EM, Kerrinnes T, Nuccio SP, Wangdi T, Fiehn O, Tsolis RM, Baumler AJ. 2016. Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature 534:697–699. doi: 10.1038/nature18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashwell A, Wahba AJ, Hickman J. 1958. A new pathway of uronic acid metabolism. Biochim Biophys Acta 30:186–187. doi: 10.1016/0006-3002(58)90257-9. [DOI] [PubMed] [Google Scholar]
- 9.Bates Utz C, Nguyen AB, Smalley DJ, Anderson AB, Conway T. 2004. GntP is the Escherichia coli fructuronic acid transporter and belongs to the UxuR regulon. J Bacteriol 186:7690–7696. doi: 10.1128/JB.186.22.7690-7696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tutukina MN, Potapova AV, Cole JA, Ozoline ON. 2016. Control of hexuronate metabolism in Escherichia coli by the two interdependent regulators, ExuR and UxuR: derepression by heterodimer formation. Microbiology 162:1220–1231. doi: 10.1099/mic.0.000297. [DOI] [PubMed] [Google Scholar]
- 11.Rodionov DA, Mironov AA, Rakhmaninova AB, Gelfand MS. 2000. Transcriptional regulation of transport and utilization systems for hexuronides, hexuronates and hexonates in gamma purple bacteria. Mol Microbiol 38:673–683. doi: 10.1046/j.1365-2958.2000.02115.x. [DOI] [PubMed] [Google Scholar]
- 12.Deacon J, Cooper RA. 1977. d-Galactonate utilisation by enteric bacteria. The catabolic pathway in Escherichia coli. FEBS Lett 77:201–205. doi: 10.1016/0014-5793(77)80234-2. [DOI] [PubMed] [Google Scholar]
- 13.Cooper RA. 1978. The utilisation of d-galactonate and d-2-oxo-3-deoxygalactonate by Escherichia coli K-12. Biochemical and genetical studies. Arch Microbiol 118:199–206. doi: 10.1007/BF00415730. [DOI] [PubMed] [Google Scholar]
- 14.Haydon DJ, Guest JR. 1991. A new family of bacterial regulatory proteins. FEMS Microbiol Lett 63:291–295. [DOI] [PubMed] [Google Scholar]
- 15.Rigali S, Derouaux A, Giannotta F, Dusart J. 2002. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J Biol Chem 277:12507–12515. doi: 10.1074/jbc.M110968200. [DOI] [PubMed] [Google Scholar]
- 16.Jain D. 2015. Allosteric control of transcription in GntR family of transcription regulators: a structural overview. IUBMB Life 67:556–563. doi: 10.1002/iub.1401. [DOI] [PubMed] [Google Scholar]
- 17.Saffarian A, Mulet C, Naito T, Bouchier C, Tichit M, Ma L, Grompone G, Sansonetti PJ, Pedron T. 2015. Draft genome sequences of Acinetobacter parvus CM11, Acinetobacter radioresistens CM38, and Stenotrophomonas maltophilia BR12, isolated from murine proximal colonic tissue. Genome Announc 3:e01089-15. doi: 10.1128/genomeA.01089-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Singh VK, Qazi GN, Kumar A. 2001. Gluconobacter oxydans: its biotechnological applications. J Mol Microbiol Biotechnol 3:445–456. [PubMed] [Google Scholar]
- 19.Gomila M, Bowien B, Falsen E, Moore ER, Lalucat J. 2007. Description of Pelomonas aquatica sp. nov. and Pelomonas puraquae sp. nov., isolated from industrial and haemodialysis water. Int J Syst Evol Microbiol 57:2629–2635. doi: 10.1099/ijs.0.65149-0. [DOI] [PubMed] [Google Scholar]
- 20.De Ley J, Doudoroff M. 1957. The metabolism of d-galactose in Pseudomonas saccharophila. J Biol Chem 227:745–757. [PubMed] [Google Scholar]
- 21.Stouthamer AH. 1961. Glucose and galactose metabolism in Gluconobacter liquefaciens. Biochim Biophys Acta 48:484–500. doi: 10.1016/0006-3002(61)90046-4. [DOI] [PubMed] [Google Scholar]
- 22.Lai K, Klapa MI. 2004. Alternative pathways of galactose assimilation: could inverse metabolic engineering provide an alternative to galactosemic patients? Metab Eng 6:239–244. doi: 10.1016/j.ymben.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Yager C, Ning C, Reynolds R, Leslie N, Segal S. 2004. Galactitol and galactonate accumulation in heart and skeletal muscle of mice with deficiency of galactose-1-phosphate uridyltransferase. Mol Genet Metab 81:105–111. doi: 10.1016/j.ymgme.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Ficicioglu C, Yager C, Segal S. 2005. Galactitol and galactonate in red blood cells of children with the Duarte/galactosemia genotype. Mol Genet Metab 84:152–159. doi: 10.1016/j.ymgme.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Rancour NJ, Hawkins ED, Wells WW. 1979. Galactose oxidation in liver. Arch Biochem Biophys 193:232–241. doi: 10.1016/0003-9861(79)90027-4. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol 47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 27.Roos V, Ulett GC, Schembri MA, Klemm P. 2006. The asymptomatic bacteriuria Escherichia coli strain 83972 outcompetes uropathogenic E. coli strains in human urine. Infect Immun 74:615–624. doi: 10.1128/IAI.74.1.615-624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goudeau DM, Parker CT, Zhou Y, Sela S, Kroupitski Y, Brandl MT. 2013. The Salmonella transcriptome in lettuce and cilantro soft rot reveals a niche overlap with the animal host intestine. Appl Environ Microbiol 79:250–262. doi: 10.1128/AEM.02290-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron F, Bonnassie S, Alabdeh M, Cochet MF, Nau F, Guerin-Dubiard C, Gautier M, Andrews SC, Jan S. 2017. Global gene-expression analysis of the response of Salmonella enteritidis to egg white exposure reveals multiple egg white-imposed stress responses. Front Microbiol 8:829. doi: 10.3389/fmicb.2017.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monk JM, Charusanti P, Aziz RK, Lerman JA, Premyodhin N, Orth JD, Feist AM, Palsson BØ. 2013. Genome-scale metabolic reconstructions of multiple Escherichia coli strains highlight strain-specific adaptations to nutritional environments. Proc Natl Acad Sci U S A 110:20338–20343. doi: 10.1073/pnas.1307797110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ku YW, McDonough SP, Palaniappan RU, Chang CF, Chang YF. 2005. Novel attenuated Salmonella enterica serovar Choleraesuis strains as live vaccine candidates generated by signature-tagged mutagenesis. Infect Immun 73:8194–8203. doi: 10.1128/IAI.73.12.8194-8203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lescat M, Launay A, Ghalayini M, Magnan M, Glodt J, Pintard C, Dion S, Denamur E, Tenaillon O. 2017. Using long-term experimental evolution to uncover the patterns and determinants of molecular evolution of an Escherichia coli natural isolate in the streptomycin-treated mouse gut. Mol Ecol 26:1802–1817. doi: 10.1111/mec.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babbitt PC, Mrachko GT, Hasson MS, Huisman GW, Kolter R, Ringe D, Petsko GA, Kenyon GL, Gerlt JA. 1995. A functionally diverse enzyme superfamily that abstracts the alpha protons of carboxylic acids. Science 267:1159–1161. doi: 10.1126/science.7855594. [DOI] [PubMed] [Google Scholar]
- 34.Wieczorek SJ, Kalivoda KA, Clifton JG, Ringe D, Petsko GA, Gerlt JA. 1999. Evolution of enzymatic activities in the enolase superfamily: identification of a “new” general acid catalyst in the active site of d-galactonate dehydratase from Escherichia coli. J Am Chem Soc 121:4540–4541. doi: 10.1021/ja990500w. [DOI] [Google Scholar]
- 35.Walters MJ, Srikannathasan V, McEwan AR, Naismith JH, Fierke CA, Toone EJ. 2008. Characterization and crystal structure of Escherichia coli KDPGal aldolase. Bioorg Med Chem 16:710–720. doi: 10.1016/j.bmc.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hogema BM, Arents JC, Inada T, Aiba H, van Dam K, Postma PW. 1997. Catabolite repression by glucose 6-phosphate, gluconate and lactose in Escherichia coli. Mol Microbiol 24:857–867. doi: 10.1046/j.1365-2958.1997.3991761.x. [DOI] [PubMed] [Google Scholar]
- 38.Chan V, Dreolini LF, Flintoff KA, Lloyd SJ, Mattenley AA. 2002. The effects of glycerol, glucose, galactose, lactose and glucose with galactose on the induction of β-galactosidase in Escherichia coli. J Exp Microbiol Immunol 2:130–137. [Google Scholar]
- 39.Solovyev V, Salamov A. 2011. Automatic annotation of microbial genomes and metagenomic sequences, p 61–78. In Li RW. (ed), Metagenomics and its applications in agriculture, biomedicine and environmental studies. Nova Science Publishers, Hauppauge, NY. [Google Scholar]
- 40.Shahmuradov IA, Mohamad Razali R, Bougouffa S, Radovanovic A, Bajic VB. 2017. bTSSfinder: a novel tool for the prediction of promoters in cyanobacteria and Escherichia coli. Bioinformatics 33:334–340. doi: 10.1093/bioinformatics/btw629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suvorova IA, Korostelev YD, Gelfand MS. 2015. GntR family of bacterial transcription factors and their DNA binding motifs: structure, positioning and co-evolution. PLoS One 10:e0132618. doi: 10.1371/journal.pone.0132618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novichkov PS, Kazakov AE, Ravcheev DA, Leyn SA, Kovaleva GY, Sutormin RA, Kazanov MD, Riehl W, Arkin AP, Dubchak I, Rodionov DA. 2013. RegPrecise 3.0—a resource for genome-scale exploration of transcriptional regulation in bacteria. BMC Genomics 14:745. doi: 10.1186/1471-2164-14-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojo F. 1999. Repression of transcription initiation in bacteria. J Bacteriol 181:2987–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontana A, de Laureto PP, Spolaore B, Frare E, Picotti P, Zambonin M. 2004. Probing protein structure by limited proteolysis. Acta Biochim Pol 51:299–321. [PubMed] [Google Scholar]
- 45.Durante-Rodríguez G, Valderrama JA, Mancheño JM, Rivas G, Alfonso C, Arias-Palomo E, Llorca O, García JL, Díaz E, Carmona M. 2010. Biochemical characterization of the transcriptional regulator BzdR from Azoarcus sp. CIB. J Biol Chem 285:35694–35705. doi: 10.1074/jbc.M110.143503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resch M, Schiltz E, Titgemeyer F, Muller YA. 2010. Insight into the induction mechanism of the GntR/HutC bacterial transcription regulator YvoA. Nucleic Acids Res 38:2485–2497. doi: 10.1093/nar/gkp1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gourinchas G, Etzl S, Gobl C, Vide U, Madl T, Winkler A. 2017. Long-range allosteric signaling in red light-regulated diguanylyl cyclases. Sci Adv 3:e1602498. doi: 10.1126/sciadv.1602498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoskisson PA, Rigali S. 2009. Variation in form and function the helix-turn-helix regulators of the GntR superfamily. Adv Appl Microbiol 69:1–22. doi: 10.1016/S0065-2164(09)69001-8. [DOI] [PubMed] [Google Scholar]
- 49.Belliveau NM, Barnes SL, Ireland WT, Jones DL, Sweredoski MJ, Moradian A, Hess S, Kinney JB, Phillips R. 2018. Systematic approach for dissecting the molecular mechanisms of transcriptional regulation in bacteria. Proc Natl Acad Sci U S A 115:E4796–E4805. doi: 10.1073/pnas.1722055115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain D, Nair DT. 2013. Spacing between core recognition motifs determines relative orientation of AraR monomers on bipartite operators. Nucleic Acids Res 41:639–647. doi: 10.1093/nar/gks962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Aalten DM, DiRusso CC, Knudsen J. 2001. The structural basis of acyl coenzyme A-dependent regulation of the transcription factor FadR. EMBO J 20:2041–2050. doi: 10.1093/emboj/20.8.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Heath RJ, Li Z, Rock CO, White SW. 2001. The FadR.DNA complex. Transcriptional control of fatty acid metabolism in Escherichia coli. J Biol Chem 276:17373–17379. doi: 10.1074/jbc.M100195200. [DOI] [PubMed] [Google Scholar]
- 53.Shi W, Kovacikova G, Lin W, Taylor RK, Skorupski K, Kull FJ. 2015. The 40-residue insertion in Vibrio cholerae FadR facilitates binding of an additional fatty acyl-CoA ligand. Nat Commun 6:6032. doi: 10.1038/ncomms7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fillenberg SB, Grau FC, Seidel G, Muller YA. 2015. Structural insight into operator dre-sites recognition and effector binding in the GntR/HutC transcription regulator NagR. Nucleic Acids Res 43:1283–1296. doi: 10.1093/nar/gku1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raman N, Black PN, DiRusso CC. 1997. Characterization of the fatty acid-responsive transcription factor FadR. Biochemical and genetic analyses of the native conformation and functional domains. J Biol Chem 272:30645–30650. doi: 10.1074/jbc.272.49.30645. [DOI] [PubMed] [Google Scholar]
- 56.Arai H, Akahira S, Ohishi T, Kudo T. 1999. Adaptation of Comamonas testosteroni TA441 to utilization of phenol by spontaneous mutation of the gene for a trans-acting factor. Mol Microbiol 33:1132–1140. [DOI] [PubMed] [Google Scholar]
- 57.Franco IS, Mota LJ, Soares CM, de Sa-Nogueira I. 2006. Functional domains of the Bacillus subtilis transcription factor AraR and identification of amino acids important for nucleoprotein complex assembly and effector binding. J Bacteriol 188:3024–3036. doi: 10.1128/JB.188.8.3024-3036.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franco IS, Mota LJ, Soares CM, de Sa-Nogueira I. 2007. Probing key DNA contacts in AraR-mediated transcriptional repression of the Bacillus subtilis arabinose regulon. Nucleic Acids Res 35:4755–4766. doi: 10.1093/nar/gkm509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao YG, Suzuki H, Itou H, Zhou Y, Tanaka Y, Wachi M, Watanabe N, Tanaka I, Yao M. 2008. Structural and functional characterization of the LldR from Corynebacterium glutamicum: a transcriptional repressor involved in l-lactate and sugar utilization. Nucleic Acids Res 36:7110–7123. doi: 10.1093/nar/gkn827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalivoda KA, Steenbergen SM, Vimr ER. 2013. Control of the Escherichia coli sialoregulon by transcriptional repressor NanR. J Bacteriol 195:4689–4701. doi: 10.1128/JB.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lord DM, Uzgoren Baran A, Soo VW, Wood TK, Peti W, Page R. 2014. McbR/YncC: implications for the mechanism of ligand and DNA binding by a bacterial GntR transcriptional regulator involved in biofilm formation. Biochemistry 53:7223–7231. doi: 10.1021/bi500871a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miwa Y, Fujita Y. 1988. Purification and characterization of a repressor for the Bacillus subtilis gnt operon. J Biol Chem 263:13252–13257. [PubMed] [Google Scholar]
- 63.Bouvier JT, Sernova NV, Ghasempur S, Rodionova IA, Vetting MW, Al-Obaidi NF, Almo SC, Gerlt JA, Rodionov DA. 2018. Novel metabolic pathways and regulons for hexuronate utilization in proteobacteria. J Bacteriol doi: 10.1128/JB.00431-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujita Y, Matsuoka H, Hirooka K. 2007. Regulation of fatty acid metabolism in bacteria. Mol Microbiol 66:829–839. doi: 10.1111/j.1365-2958.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- 65.Quail MA, Haydon DJ, Guest JR. 1994. The pdhR-aceEF-lpd operon of Escherichia coli expresses the pyruvate dehydrogenase complex. Mol Microbiol 12:95–104. doi: 10.1111/j.1365-2958.1994.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 66.Kalivoda KA, Steenbergen SM, Vimr ER, Plumbridge J. 2003. Regulation of sialic acid catabolism by the DNA binding protein NanR in Escherichia coli. J Bacteriol 185:4806–4815. doi: 10.1128/JB.185.16.4806-4815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HY, An JH, Kim YS. 2000. Identification and characterization of a novel transcriptional regulator, MatR, for malonate metabolism in Rhizobium leguminosarum bv. trifolii. Eur J Biochem 267:7224–7230. doi: 10.1046/j.1432-1327.2000.01834.x. [DOI] [PubMed] [Google Scholar]
- 68.DiRusso CC, Heimert TL, Metzger AK. 1992. Characterization of FadR, a global transcriptional regulator of fatty acid metabolism in Escherichia coli. Interaction with the fadB promoter is prevented by long chain fatty acyl coenzyme A. J Biol Chem 267:8685–8691. [PubMed] [Google Scholar]
- 69.DiRusso CC, Tsvetnitsky V, Hojrup P, Knudsen J. 1998. Fatty acyl-CoA binding domain of the transcription factor FadR. Characterization by deletion, affinity labeling, and isothermal titration calorimetry. J Biol Chem 273:33652–33659. doi: 10.1074/jbc.273.50.33652. [DOI] [PubMed] [Google Scholar]
- 70.Gao R, Li D, Lin Y, Lin J, Xia X, Wang H, Bi L, Zhu J, Hassan B, Wang S, Feng Y. 2017. Structural and functional characterization of the FadR regulatory protein from Vibrio alginolyticus. Front Cell Infect Microbiol 7:513. doi: 10.3389/fcimb.2017.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Isbell HS, Frush HL. 1933. Preparation and properties of aldonic acids and their lactones and basic calcium salts. Bur Stand J Res 11:649–664. doi: 10.6028/jres.011.043. [DOI] [Google Scholar]
- 72.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol 183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saini S, Pearl JA, Rao CV. 2009. Role of FimW, FimY, and FimZ in regulating the expression of type I fimbriae in Salmonella enterica serovar Typhimurium. J Bacteriol 191:3003–3010. doi: 10.1128/JB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agrawal S, Jaswal K, Shiver AL, Balecha H, Patra T, Chaba R. 2017. A genome-wide screen in Escherichia coli reveals that ubiquinone is a key antioxidant for metabolism of long-chain fatty acids. J Biol Chem 292:20086–20099. doi: 10.1074/jbc.M117.806240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cummings CA, Bootsma HJ, Relman DA, Miller JF. 2006. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol 188:1775–1785. doi: 10.1128/JB.188.5.1775-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim DE, Chivian D, Baker D. 2004. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res 32:W526–W531. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holm L, Laakso LM. 2016. Dali server update. Nucleic Acids Res 44:W351–W355. doi: 10.1093/nar/gkw357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaba R, Grigorova IL, Flynn JM, Baker TA, Gross CA. 2007. Design principles of the proteolytic cascade governing the sigmaE-mediated envelope stress response in Escherichia coli: keys to graded, buffered, and rapid signal transduction. Genes Dev 21:124–136. doi: 10.1101/gad.1496707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.