Abstract
This scientific commentary refers to ‘Polygenic risk score increases schizophrenia liability through cognition-relevant pathways’, by Toulopoulou et al.. (doi:10.1093/brain/awy279).
This scientific commentary refers to ‘Polygenic risk score increases schizophrenia liability through cognition-relevant pathways’, by Toulopoulou et al.. (doi:10.1093/brain/awy279).
Schizophrenia is a severe mental disorder with a lifetime prevalence of 6.35 per 1000 (Moreno-Küstner et al., 2018). Multiple treatments are available including antipsychotic drugs, psychological therapies and rehabilitation interventions. Although disease and treatment mechanisms are not fully understood, there is compelling evidence that schizophrenia is highly heritable with around 80% of its variance explained by genetic factors (Hilker et al., 2018). A mega-analysis of genome-wide association studies (GWAS) conducted by the Psychiatric Genomics Consortium identified more than a hundred genetic loci conferring susceptibility to schizophrenia (Ripke et al., 2014). While each individual single nucleotide polymorphism (SNP) carries only a subtle increase in schizophrenia risk (with odds ratios in the range of 1.1 to 1.2), their combination into a cumulative measure called the polygenic risk score (PRS) provides a stronger predictor of disease (Purcell et al., 2009). In this issue of Brain, Toulopoulou and co-workers explore the extent to which impairments in cognition mediate the influence of the PRS on schizophrenia liability (Toulopoulou et al., 2019).
One approach to identifying the mechanism(s) by which genes predispose to the development of psychotic symptoms is to examine the relationship between the PRS and endophenotypes—biomarkers of brain structure or function characterizing a disease. Deficits in global cognitive ability as well as in specific domains of working memory, attention, and executive function are associated with schizophrenia and have been consistently identified as endophenotypes. Several studies have shown that these cognitive deficits are heritable and are also present in unaffected family members at a higher rate than in the general population. Lencz et al. (2014) showed in a large study that higher PRSs for schizophrenia are associated with lower general cognitive ability, while Ranlund et al. (2018) investigated a range of neurophysiological, neuroimaging and cognitive endophenotypes for psychosis and found that higher PRSs for schizophrenia are associated with poorer spatial visualization skills. Recently, a large genome-wide association mega-analysis of human cognition revealed a bidirectional association with schizophrenia, showing that intelligence has a strong protective effect on schizophrenia risk (Savage et al., 2018).
Even if these studies demonstrate the existence of a genetic overlap between schizophrenia and cognitive deficits, they do not address the direction of causation. Toulopoulou et al. therefore set out to explore whether (and to what extent) cognitive impairments mediate the influence of schizophrenia PRSs on the disorder, or whether the cognitive deficits are instead a consequence of disease progression. They used causal modelling to analyse 1313 members of 1078 families (416 patients with schizophrenia, 290 of their unaffected siblings, and 607 healthy control subjects) who volunteered for extensive assessments of global intelligence and specific cognitive skills, as well as genotyping.
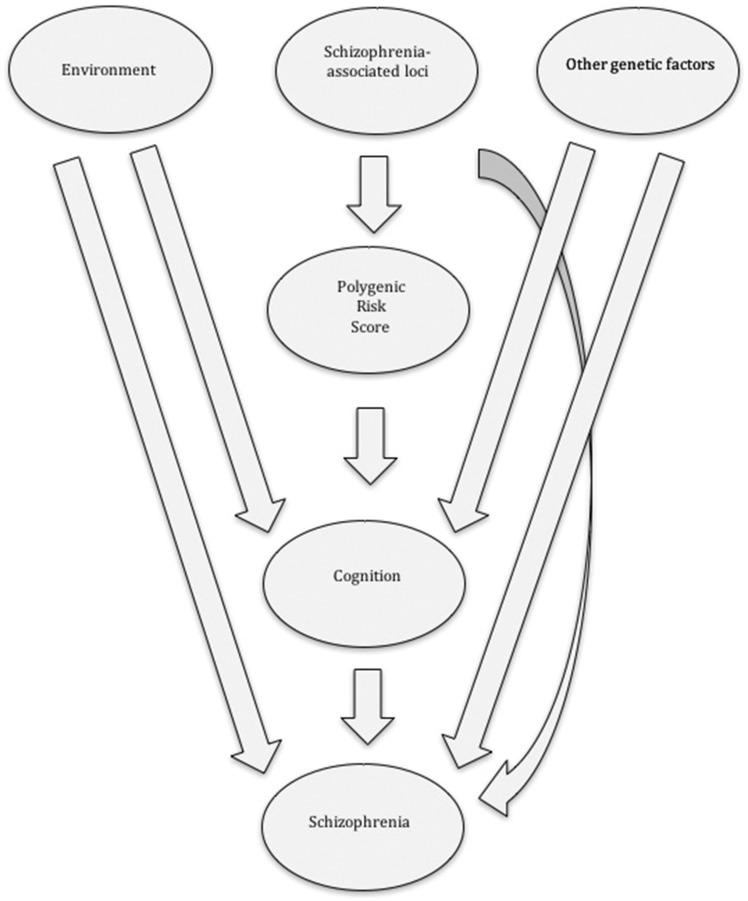
Toulopoulou and colleagues confirmed previous findings that the PRS alone can explain around 8% of interindividual variation in schizophrenia risk (Ripke et al., 2014; Calafato et al., 2018). More than a third (2.7%) of this PRS influence is mediated through cognition-related pathways. Furthermore, almost 27% of the genetic liability to schizophrenia is associated with cognition-related pathways not captured by the PRS. Overall, Toulopoulou et al. estimate that around a third (34%) of the genetic risk of developing schizophrenia is mediated by influences on cognition. Fig. 1 summarizes the findings by Toulopoulou et al. Despite cognitive impairments consistently being identified in patients with schizophrenia as well as in their unaffected relatives, the underlying pathophysiology remains to be explained. Cognitive deficits alone are probably not sufficient to lead to the development of psychotic symptoms. Other factors, both genetic and/or environmental, are involved, with cannabis use, obstetric complications, malnutrition, childhood maltreatment, trauma, migration and urbanicity some of the best studied environmental risk factors.
Figure 1.
Proposed model whereby genetic and environmental factors influence schizophrenia risk in part through effects on cognition. By modelling both molecular and phenotypic data from families, Toulopoulou et al. show that cognition is an important mediator trait (endophenotype) that lies between known genetic factors captured by PRSs and the disease and that cognition can explain about a third of the known polygenic risk liability for schizophrenia. Environmental and genetic factors also influence schizophrenia risk through alternative cognitive-independent pathways.
Although only a modest 7% to 9% of schizophrenia genetic variance is explained by the PRS, the score is notable for its ability to capture individual genetic liability in a single quantitative variable (Ripke et al., 2014; Calafato et al., 2018). As the costs of genotyping blood or saliva samples have reduced dramatically, the PRS is increasingly used to incorporate molecular data into large cohort studies and other experimental designs. There is also growing interest in developing the PRS as a screening tool, one that may facilitate prompt access to treatment (Purcell et al., 2009; Wray et al., 2014).
While heritability estimates for schizophrenia from twin, family, adoption and other epidemiological studies range from 60% to 80%, the estimates obtained from genome-wide association studies are only about 25% (Hilker et al., 2018; Anttila et al., 2018). This discrepancy, known as schizophrenia’s ‘missing heritability’, is attributed to as yet undiscovered common genetic variants as well as to rare genetic mutations and copy number variants (Wray et al., 2014). Indeed, a large exome sequencing study of 12 332 individuals, including 4877 with schizophrenia, found that putatively protein-damaging rare variants were more abundant in individuals with schizophrenia compared to controls (Genovese et al., 2016). Similarly, a recent analysis conducted by the Psychiatric Genomics Consortium (36 573 schizophrenia cases and 112 468 controls) identified eight regions harbouring rare copy number variants conveying large odds ratios for schizophrenia (Marshall et al., 2017). Further studies using even larger samples are still needed if we are to capture more of the elusive heritability of schizophrenia, and uncover complex gene-environment interactions.
By modelling molecular genetic data captured by PRSs from families—in which unaffected relatives share genetic and cognitive traits with affected individuals—Toulopoulou et al. infer causal relationships between cognition and schizophrenia. Using causal modelling, they convincingly show that a third of the genetic influence on schizophrenia risk is mediated by cognitive pathways and that cognitive deficits are not just an epiphenomenon of the disease. However, since the data available for modelling come from cross-sectional observations, they cannot absolutely prove causality. The timing and severity of any cognitive deficits and whether they are present before and/or after disease onset are key elements in establishing potentially complex cause-effect relationships. Further evidence is needed to corroborate their findings—ideally cohort studies with genomics and multiple prospective, longitudinal cognitive assessments before and after the onset of psychotic symptoms—and to disentangle the relationship between genetics, cognition and schizophrenia.
The PRSs used by Toulopoulou and colleagues in this study were calculated based on the latest available data from the Psychiatric Genomics Consortium. As the consortium expands this invaluable dataset, the largest international collaboration in mental health, the performance of the PRS will be further enhanced. In the near future, we can expect to explain a larger proportion of schizophrenia’s genetic liability and the extent to which it is mediated through cognition. Finally, endophenotypes such as cognition offer a unique opportunity to tackle the disease pathway both from research as well as treatment perspectives. Gaining a better understanding of the mechanisms leading from genes to the development of psychosis is key to developing new therapeutic interventions.
Funding
M.S.C. is funded by a National Institute for Health Research (NIHR) Academic Clinical Fellowship. E.B. holds the British Medical Association Margaret Temple Fellowship 2016. Further support to E.B: Medical Research Council-Korean Health Industry Development Institute Partnering Award MC_PC_16014. Medical Research Council (MRC) New Investigator Award and MRC Centenary Award G0901310. MRC grant G1100583. Wellcome Trust grants 085475/B/08/Z and 085475/Z/08/Z. National Institute of Health Research UK post-doctoral fellowship PDA/02/06/016. Brain and Behaviour Research Foundation NARSAD Young Investigator Awards 2005, 2008. Wellcome Trust Research Training Fellowship. NIHR Biomedical Research Centre at UCLH.
Competing interests
The authors report no competing interests.
References
- Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L et al. . Analysis of shared heritability in common disorders of the brain. Science 2018; 360. pii: eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafato MS, Thygesen JH, Ranlund S, Zartaloudi E, Cahn W, Crespo-Facorro B et al. . Use of schizophrenia and bipolar disorder polygenic risk scores to identify psychotic disorders. Br J Psychiatry 2018; 213: 535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G, Fromer M, Stahl EA, Ruderfer DM, Chambert K, Landén M et al. . Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci 2016; 19: 1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilker R, Helenius D, Fagerlund B, Skytthe A, Christensen K, Werge TM et al. . Heritability of schizophrenia and schizophrenia spectrum based on the nationwide danish twin register. Biol Psychiatry 2018; 83: 492–8. [DOI] [PubMed] [Google Scholar]
- Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM et al. . Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry 2014; 19: 168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS et al. . Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet 2017; 49: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Küstner B, Martín C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One 2018; 13: e0195687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF et al. . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460: 748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranlund S, Calafato S, Thygesen JH, Lin K, Cahn W, Crespo-Facorro B et al. . A polygenic risk score analysis of psychosis endophenotypes across brain functional, structural, and cognitive domains. Am J Med Genet Part B Neuropsychiatr Genet 2018; 177: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walter JTR, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, De Leeuw CA et al. . Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet 2018; 50: 912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulopoulou T, Zhang X, Cherny SS, Dickinson D, Berman K, Straub R et al. . Polygenic risk score increases schizophrenia liability through cognition-relevant pathways. Brain 2019; 142: 471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, Middeldorp CM. Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry 2014; 55: 1068–87. [DOI] [PubMed] [Google Scholar]