Abstract
Based on the concept of the tripartite synapse, we have reviewed the role of glucose-derived compounds in glycolytic pathways in astroglial cells. Glucose provides energy and substrate replenishment for brain activity, such as glutamate and lipid synthesis. In addition, glucose metabolism in the astroglial cytoplasm results in products such as lactate, methylglyoxal, and glutathione, which modulate receptors and channels in neurons. Glucose has four potential destinations in neural cells, and it is possible to propose a crossroads in “X” that can be used to describe these four destinations. Glucose-6P can be used either for glycogen synthesis or the pentose phosphate pathway on the left and right arms of the X, respectively. Fructose-6P continues through the glycolysis pathway until pyruvate is formed but can also act as the initial compound in the hexosamine pathway, representing the left and right legs of the X, respectively. We describe each glucose destination and its regulation, indicating the products of these pathways and how they can affect synaptic communication. Extracellular L-lactate, either generated from glucose or from glycogen, binds to HCAR1, a specific receptor that is abundantly localized in perivascular and post-synaptic membranes and regulates synaptic plasticity. Methylglyoxal, a product of a deviation of glycolysis, and its derivative D-lactate are also released by astrocytes and bind to GABAA receptors and HCAR1, respectively. Glutathione, in addition to its antioxidant role, also binds to ionotropic glutamate receptors in the synaptic cleft. Finally, we examined the hexosamine pathway and evaluated the effect of GlcNAc-modification on key proteins that regulate the other glucose destinations.
Keywords: astrocyte, glycolysis, GSH, lactate, methylglyoxal, neurotransmission
Aim
Glucose is the major energetic source of neural cells. In addition to providing ATP via the glycolytic pathway, glucose provides metabolites that are key to brain activity, such as glutamate and NADPH for lipid and glutathione (GSH) synthesis, as well as recycling of ascorbic acid. This short review will focus on glucose-derived compounds from astrocytes that modulate neurotransmission, in addition to providing energetic and substrate replenishment for brain activity. Glutamate, for example, is the main excitatory neurotransmitter and originates from astroglial glucose, as it is synthetized de novo from alpha-ketoglutarate. Two specific astrocyte enzymes, pyruvate carboxylase and glutamine synthetase, are necessary for this process. We will restrict this review to the role of glucose-derived compounds (arising directly from the glycolytic pathway in the cytoplasm of astroglial cells) that modulate synaptic receptors or transporters by binding to them, such as lactate, methylglyoxal, and GSH. Moreover, we will review the regulatory role of uridine diphosphate-N-Acetylglucosamine (UDP-GlcNAc), which covalently regulates several astrocyte proteins, including glucose metabolism enzymes and related transcription factors, which in turn modulate synaptic communication.
Introduction
Preliminarily, it is important to highlight the importance of astrocytes in the synapse, particularly in glucose metabolism. Although there is no doubt about the significance of blood glucose for brain activity, the mode of entry of glucose to the brain and its cell distribution are still debated (e.g., Lundgaard et al., 2015; Barros et al., 2017). Once inside the cell, glucose is phosphorylated on carbon 6 by hexokinase (HK), generating glucose-6P, which is converted to fructose-6P via the action of an isomerase. These two compounds (glucose-6P and fructose-6P) are used in at least two different pathways. It is possible to propose a metaphorical “X” intersection of these reactions, as illustrated in Figure 1, to describe the destinations of glucose in the neural cells. Glucose-6P can be converted to fructose-6P, but can also be used for glycogen synthesis or in the pentose phosphate pathway (PPP) (left and right arm of the X, respectively). On the other hand, fructose-6P continues through the glycolysis pathway until pyruvate is formed, but can also act as the initial compound in the hexosamine pathway, as represented by the left and right legs of the X, respectively.
FIGURE 1.
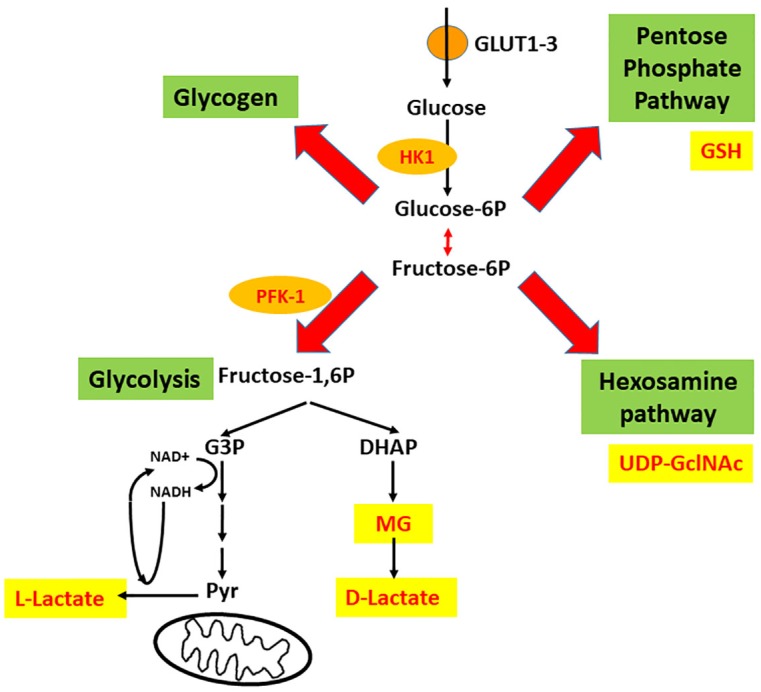
Four intracellular destinations of glucose that suggest an intersection in “X.” Glucose enters astrocytes mainly via GLUT1, and neurons mainly via GLUT3 and is trapped by phosphorylation (catalyzed by hexokinase 1, HK1). Afterward, four destinations are possible; these form a crossroads in the shape of an X, where glycogen synthesis and the pentose phosphate pathway (PPP) are the left and right arms of the X, and glycolysis (until pyruvate) and the hexosamine pathway (HP) are the left and right legs of the X. The deviation of glycolysis that generates methylglyoxal (MG) is also indicated. PFK-1, phosphofructokinase-1; G-3P, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate.
Glucose Transport and Phosphorylation
Before discussing the destinations of glucose, it is important to understand how it enters the neural cells, via the glucose transporter (GLUT) and sodium-glucose co-transporter (SGLT), which are blocked by phloretin and phlorizin, respectively (Shah et al., 2012). GLUTs are passive and bidirectional transporters. GLUT1 is the main isoform found in cells forming the blood-tissue barriers (endothelial and choroid plexus), astrocytes and the ependymal cells lining the cerebral ventricles. In addition to glucose, GLUT1 transports dehydroascorbic acid (the oxidized form of vitamin C) (Rivas et al., 2008) and glucosamine (Chopra, 2004). At the blood-brain barrier (BBB), GLUT1 works at about one-third of maximal capacity under basal conditions (Leybaert et al., 2007). Neurons express mainly GLUT3, although some neurons in the rodent brain also express the insulin-sensitive GLUT4 (in the hippocampus, the cerebellum and the hypothalamus) (Choeiri et al., 2002) and SGLT1 (in the hippocampus and the cerebral cortices) (Yu et al., 2010). However, glucose transportation by these carriers is not considered a rate-limiting step in brain energy metabolism. In contrast, the next step, glucose phosphorylation by hexokinase, represents the rate-limiting step.
Notably, more than 90% of non-fenestrated capillary brain vessels are covered by astrocytic end-feet (Jukkola and Gu, 2015). Moreover, the tight junctions between endothelial cells (responsible for non-fenestration) are actively regulated by astrocyte signals (Ballabh et al., 2004). These aspects indicate the importance of astrocytes in glucose distribution. However, this does not mean that glucose needs to pass through astrocytes to reach neurons. In fact, after crossing endothelial cells via GLUT 1, glucose can reach neurons directly via GLUT3, because there is room for molecular diffusion, since astrocytes form gap junctions among themselves instead of tight junctions.
Glucose phosphorylation on C6, catalyzed by HK, is the first rate-limiting step of glycolysis. All three isoforms of HK (of low Km) are present in brain tissue, but HK1 is the most abundant isoform in neurons and astrocytes and it is assumed to be “the brain hexokinase.” HKs 1 and 2 bind to the outer mitochondrial membrane by a hydrophobic sequence at their N-terminal, close to the pore that allows ATP output (Pastorino and Hoek, 2008). The activation of Akt kinase (or inhibition of glycogen synthase kinase 3, GSK-3) favors the binding of HK to the mitochondria by a mechanism that is still unclear. Glucose-6P induces a conformational change of the HK, displacing it from the mitochondria and decreasing its activity. Glucose-6P acts as a non-competitive inhibitor of HK, which under basal conditions is predominantly inhibited (DiNuzzo et al., 2015).
PFK-1 Catalyzes the Other Rate-Limiting Step of Glycolysis
Glucose-6P is isomerized to fructose-6P, which in turn is converted to fructose-1,6-bis-phosphate (F1,6BP, see Figure 1). This reaction is the second rate-limiting step of glycolysis and is catalyzed by PFK-1. HK has been reported to be higher expressed in neurons than in astrocytes (Lundgaard et al., 2015); however, the activity and regulation of PFK-1 in astrocytes suggest a higher glycolytic activity in these cells (Bolaños et al., 2010). PFK-1 is allosterically downregulated by metabolites ATP, citrate and long-chain fatty acids (Jenkins et al., 2011) and upregulated by fructose 2,6 bisphosphate (F2,6BP) (Mor et al., 2011). Moreover, lactate, at least in muscle cells, is able to disarrange the tetrameric structure and reduce the enzymatic activity of PFK-1 (Costa Leite et al., 2007). F2,6BP, the main allosteric activator, is present in astrocytes at higher concentrations than in neurons. In fact, the enzyme responsible for the generation of F2,6BP from fructose-6P in brain tissue, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB-3), has lower expression levels in neurons due to elevated proteosomal degradation (Herrero-Mendez et al., 2009). PFKFB-3 is the target of several kinases, including Akt and AMP-activated protein kinase (AMPK; Marsin et al., 2000). AMPK is able to phosphorylate/activate PFKFB-3 as well as PFK-1 (Bartrons et al., 2018), indicating a direct regulatory role of AMP.
The lower activities of PFK-1 and PFKFB-3 in neurons suggest that glucose uptake could be conducted to the PPP to generate NADPH, which is required for the regeneration of GSH in these cells (Bolaños et al., 2010). Neurons have low concentrations of GSH (Dringen et al., 2005) and activity of γ-glutamyl cysteine ligase (GCL; Makar et al., 1994) when compared to astrocytes. Interestingly, oxidative stress-mediated S-glutathionylation of PFKFB-3 decreases its catalytic activity in cancer cells, redirecting the glycolytic flux to the PPP (Seo and Lee, 2014). Understanding the metabolic regulation of these three enzymes (HK1, PFK-1, and PFKFB-3) is important for comprehending the journey of glucose to pyruvate (including the passage through glycogen) or to ribulose-5 (PPP), as well as the effects of products of these pathways (lactate, methylglyoxal, and GSH) on synaptic communication. All these regulatory enzymes of glucose flow are direct or indirect targets of GlnNAcylation, which in turn depends on glucose flow itself, as we will discuss below.
The Left Arm and Leg of Glucose Metabolism Modulate Synaptic Transmission Via Lactate
Lactate, directly derived from glucose or glycogen (in astrocytes), performs functions beyond energy supply. These functions are mediated by different mechanisms and newly presented pathways (already verified or still proposed), including a specific receptor and its signaling transduction pathways.
The Lactate Receptor: For Every Orphan, a Family
The lactate receptor, initially known as GPR81, belongs to a family of G protein-coupled receptors (GPRs). It was first mapped via a genomic sequence database and then identified in the human pituitary gland (Lee et al., 2001). At that time, in the absence of a specific ligand, the receptor was considered an orphan. Later, the receptor was shown to be highly expressed in adipose tissue (Liu et al., 2009). The subsequent pharmacological characterization of L-lactate as a ligand for GPR81 was initiated, taking into account the similarity of GPR81 with other receptors from the GPR family, GPR109a and GPR109b, which also have β-hydroxybutyrate as a ligand. L-lactate inhibits lipolysis via GPR81 in adipocytes from human, mouse, and rat adipose tissue (Cai et al., 2008; Liu et al., 2009). The suggestion that lactate may act in a hormone-like manner comes from the demonstration of an insulin-dependent inhibition of lipolysis via GPR81 by Ahmed et al. (2010).
As all GPR ligands are hydroxy-carboxylic acids, the GPRs are now HCA receptors (HCARs; Ahmed et al., 2009; Blad et al., 2011; Offermanns, 2017). GPR89/HCAR1 has received more attention during recent years and has been implicated in inflammation and cancer signaling [for reviews, see (Haas et al., 2016; Offermanns, 2017)]. A compound present in fruits (Liu et al., 2012; Bergersen, 2015), 3,5-dihydroxybenzoic acid (DHBA), was identified as an agonist for HCAR1, inhibiting lipolysis in wild-type mouse adipocytes, but not in HCAR1 knocked-down adipocytes (Liu et al., 2012). The HCAR2 ligand 3-hydroxy-butyrate has been considered as an antagonist for the HCA1 receptor and has been used experimentally as such (Shen et al., 2015).
To characterize HCAR1 signaling pathways, a study using a cell line designed to express human HCAR1 (CHO-K1) showed activation of extracellular signal-regulated kinases (ERK1/2) via HCAR1 in response to lactate and DHBA and sensitivity to the Gi protein inhibitor pertussis toxin (Li et al., 2014). Moreover, the Gαγ subunit dissociated from the activated Gi protein was central in the regulation of HCAR1-activated ERK1/2 phosphorylation via extracellular Ca2+, protein kinase C (PKC), and insulin-like growth factor-1 receptor (IGF-1R) activation. Arrestin-2 and 3 had no effect on ERK1/2 activation, whereas HCAR1 internalization was dependent on arrestin-3 (Li et al., 2014). Supposed non-canonical actions of the HCAR1 receptor, i.e., without involving cyclic AMP (cAMP) reduction, have been proposed based on β-arrestin actions but still await future confirmation (Bergersen, 2015; Morland et al., 2015) and a different yet unknown receptor has also been suggested (Tang et al., 2014).
D-lactate, the stereoisomer of L-lactate, is produced at very low concentrations under physiological conditions from methylglyoxal (MG, see below). It is considered to be a partial agonist of the HCAR1 receptor with maximal stimulation significantly lower than that by L-lactate (Cai et al., 2008). As reported throughout this review, although some studies have shown D-lactate as a positive control for L-lactate HCAR1 signaling in neurons (Bozzo et al., 2013), many other studies have shown antagonistic (Tang et al., 2014), absent (Herrera-López and Galván, 2018), and controversial actions of the lactate enantiomer on neuroprotection and cognitive functions (Gibbs and Hertz, 2008; Castillo et al., 2015).
Synaptic and Vascular Modulation by Lactate
The presence of the – at that time – orphan GPR81 was first demonstrated in brain tissue in 2001 (Lee et al., 2001). HCAR1 is located at synaptic membranes of excitatory synapses in the hippocampus and the cerebellum. It is located predominantly at postsynaptic sites, but it is also present in astroglial end-feet processes and endothelial membranes, indicating that energy metabolism is associated with synaptic function (Lauritzen et al., 2014). The vascular endothelial density of the receptor is twice the density at the astrocytic end-feet, suggesting a lactate control of cerebral blood flow (see Figure 2). The effect of physical exercise on the density of capillaries via HCAR1 in the sensorimotor cortex and more markedly in the hippocampus, was reproduced by daily subcutaneous injections of L-lactate (about 10 mM in the blood). The regulation of angiogenesis via HCAR1 and downstream Erk1/2 and Akt signaling resulting in vascular endothelial growth factor (VEGF) production were confirmed by the extensive expression of the receptor at perivascular pial and pericyte-like cells (Morland et al., 2017).
FIGURE 2.
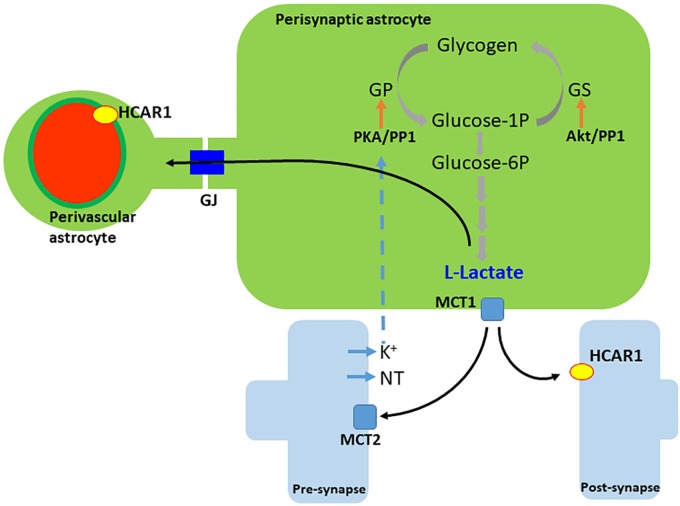
Generation and release of lactate in astrocytes. L-lactate is generated either from recently uptaken glucose or from glycogen. Neurotransmitters (NT), and/or extracellular K+, trigger glycogen breakdown until lactate, via cAMP/PKA signaling. Lactate leaves the cell by the monocarboxylate transporter 1 (MCT1) and enters neurons via the monocarboxylate transporter 2 (MCT2). Extracellular lactate also binds to hydroxy-carboxylic acid receptor 1 (HCAR1), which is found more abundantly in perivascular and post-synaptic membranes. Lactate travels among astrocytes through gap junctions (GJ). PKA, protein kinase A; GP, glycogen phosphorylase; GS, glycogen synthase; PP1, protein phosphatase 1.
Even before the deorphanization of the GPR81, several non-metabolic actions of lactate have been reported in neural cells. Lactate increases the action potential frequency of glucose-sensing neurons at the ventromedial hypothalamic nucleus via KATP and chloride channels (Song and Routh, 2005). Another study using cortical astrocyte cultures and SH-SY5Y neuronal cells incubated with lactate showed increased brain-derived neurotrophic factor (BDNF) and inducible nitric oxide synthase (iNOS) expression in astrocytes but not in SH-SY5Y cells. However, the authors centered their discussion only on the energetic aspect of lactate (Coco et al., 2013).
The first demonstration of a signaling pathway involving lactate and receptor interaction in the brain was demonstrated by Bozzo et al. (2013), who showed that L-lactate modulated the calcium spike frequency in primary mouse neuron cultures. The authors showed, for the first time, a brain non-energetic effect of L-lactate via HCAR1, since other metabolic substrates, such as pyruvate or glucose, could not mimic this effect of L-lactate. In addition, they used the agonist DHBA, reproducing the effects of lactate, and D-lactate, which has a poor affinity for the monocarboxylate transporter 2 (MCT2) and works as a partial agonist. On the other hand, a non-metabolic astrocyte-neuron signaling modulation by lactate, through a different and unknown mechanism, was demonstrated by Tang et al. (2014). This investigation elegantly showed the release of L-lactate by astrocytes employing in vitro optogenetics. They also demonstrated exogenous lactate in cultured and acute brain slices and showed that in vivo lactate administration modulates the excitability of noradrenergic neurons from the locus coeruleus. The authors suggested a possible receptor, other than HCAR1, since D-lactate acted as an inverse agonist and lactate concentrations used were about ten times lower than the IC50 for the Gi-coupled receptor.
A study revealed L-lactate upregulation of immediate early genes associated with N-methyl-D-aspartate (NMDA) transmission in neuronal cultures from the mouse neocortex and in vivo administration of L-lactate (Yang et al., 2014). Genes such as Arc, c-Fos, and Zif268 had an increased expression after lactate treatment in a range between 2.5 and 20 mM in a time-dependent manner, with a one-hour peak. An energetic effect was excluded after the ineffectiveness of D-lactate, pyruvate, and glucose in an equicaloric concentration at the same experimental conditions. Moreover, signaling of lactate on these plasticity-related genes was intracellular, since the MCT blocker UK5099 abolished this effect. Interestingly, after longer treatment periods, lactate also stimulated an increase in BDNF expression and the phosphorylation of Erk1/2. The authors showed lactate action via NMDA receptors, but not a specific lactate receptor.
More recently, one study showed the modulation by lactate of the action potential frequency in pyramidal cells from the CA1 region of the hippocampus, under stable energetic conditions. Both lactate and its agonist, DHBA, induced a biphasic modulation in neuronal excitability, inducing reduced excitability at lower concentrations (lactate at 5 mM and DHBA at 0.56 mM), while higher concentrations (lactate at 30 mM and DHBA at 3.1 mM) increased firing frequencies. Use of a neuronal MCT2 blocker did not abolish the lactate effect and neither did D-lactate alter the firing frequency of the cells; however, Gi protein inhibition via pertussis toxin confirmed the effect of lactate via HCA1R (Herrera-López and Galván, 2018).
Lactate release in response to glutamate uptake was described 20 years ago (Pellerin and Magistretti, 1994); however, an alternative molecular pathway for lactate efflux, induced by neuronal depolarization has been proposed (Choi et al., 2012). A soluble adenylyl cyclase (sAC) sensitive to HCO3- is found abundantly expressed in astrocytes and responds to extracellular K+ elevation. An increase in cAMP secondary to HCO3-influx (via HCO3-/Na+ transporter) was observed in cultured astrocytes and in brain slices; furthermore, sAC was found to be responsible for the production and release of lactate as a consequence of the glycogen breakdown coupled with K+ increase in astrocytes (see Figure 2). As cAMP levels stimulate glycogen breakdown (Pellerin et al., 2007), HCA1 receptor (coupled to Gi protein) activation via lactate could mediate glycogenolysis feedback control by lactate in astrocytes.
The lactate response during neurotransmission is fast and independent of the metabolic status or oxygen availability, leading to the observation by Sotelo-Hitschfeld et al. (2015) of a steady-state reservoir of lactate (Sotelo-Hitschfeld et al., 2015). The synaptic activity and consequent depolarization caused by extracellular K+ have been reported to elicit glycogen mobilization (Choi et al., 2012) and lactate generation (Sotelo-Hitschfeld et al., 2012). On the other hand, using a FRET lactate sensor, the group demonstrated that depolarization, via depletion of the astrocytic lactate reservoir in cultured astrocytes, may occur via a non-identified anion channel (Sotelo-Hitschfeld et al., 2015).
HCAR1-mediated lactate effects have been suggested to be neuroprotective in depression (Carrard et al., 2018) and cerebral ischemia (Berthet et al., 2009, 2012). More recently, in a middle cerebral artery occlusion stroke model, HCAR1 receptor expression was increased in the hippocampus, the cortex, and the striatum after ischemia (Castillo et al., 2015). Moreover, in hippocampal slices in an oxygen and glucose deprivation model, DHBA and D-lactate protected the CA1 region from the insult (Castillo et al., 2015). Although it is not possible to determine the definitive role for HCAR1 in the synaptic communication at this moment, the astrocyte lactate released during glycogenolysis is reportedly crucial for memory consolidation (see Hertz and Chen, 2018 for review). However, recent data indicate that an aging-associated shift of glycogen metabolism enzyme concentrations, and their localization in astrocytes and neurons, may occur (Drulis-Fajdasz et al., 2018). In addition to its role in synaptic signaling modulation, the astrocytic steady-state reservoir, and the rapid response to depolarization, lactate may act as a gliotransmitter molecule (Tang et al., 2014; Sotelo-Hitschfeld et al., 2015). However, synthesis rather than release should be considered as the limiting step for lactate signaling in the brain (Mosienko et al., 2015).
Methylglyoxal and D-Lactate Also Affect Neurotransmission
Methylglyoxal is a dicarbonyl compound (formula CH3C(O)CHO) derived from endogenous and exogenous sources and responsible for most of the glycation reactions in cell metabolism. The endogenous source of MG comes from enzymatic or non-enzymatic reactions of reducing sugars, lipids and amino acids in the cell. The main source of MG synthesis is from aldehydes, which are intermediates of the glycolysis pathway, such as glyceraldehyde 3-phosphate and dihydroxyacetone-phosphate (see Figures 1, 3). At physiological or pathological conditions, MG is produced through spontaneous dephosphorylation of dihydroxyacetone-phosphate (Angeloni et al., 2014; Muronetz et al., 2017). Coffee, alcoholic beverages, cigarette smoke and food are all exogenous sources of MG (Nemet et al., 2006; Angeloni et al., 2014).
FIGURE 3.
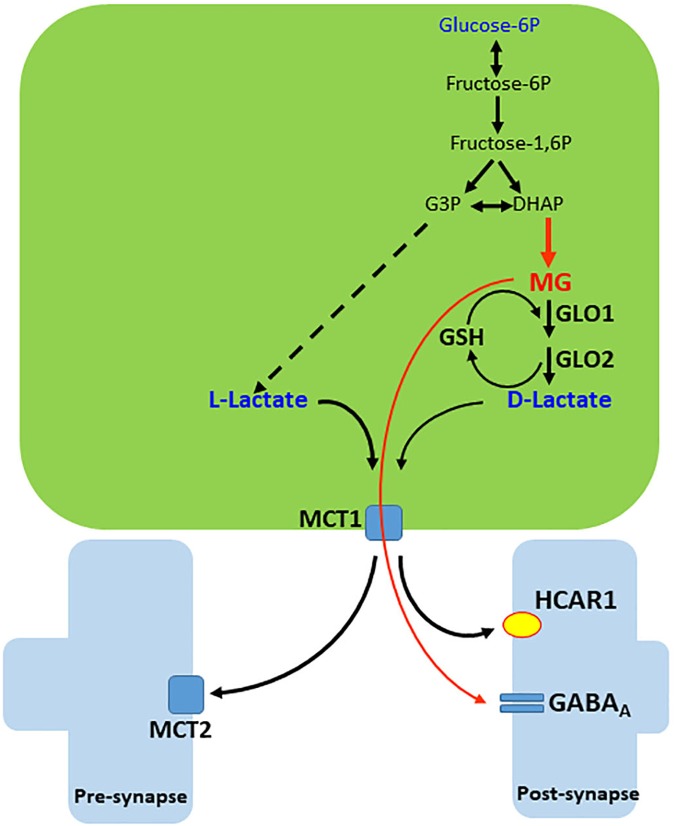
Generation and release of MG and D-lactate in astrocytes. Methylglyoxal (MG) is produced from dihydroxyacetone phosphate (DHAP) by a deviation of the glycolytic pathway. MG is condensed with glutathione (GSH) and then, by sequential action of glyoxalases 1 and 2 (GLO1 and 2), generates D-lactate and recycles GSH. D-lactate leaves the cell via monocarboxylate transporter 1 (MCT1) but disturbs lactate and pyruvate flows to mitochondria (not illustrated). MG and D-lactate leave the cell and act on the GABAA receptor and hydroxy-carboxylic acid receptor 1 (HCAR1), respectively. Extrasynaptic GABAA receptors of MG are not illustrated.
Methylglyoxal is also present in different biological materials (tissues, urine, plasma and the cerebrospinal fluid) and its concentration is related to the status of glucose metabolism (Nemet et al., 2006; Angeloni et al., 2014). It has been suggested that about 0.1–0.4% of the glycolysis pathway results in the formation of MG (Kalapos, 2008). More recently, it has been proposed that MG, at physiological levels (μM), acts as an agonist of the γ-aminobutyric acid type A (GABAA) receptor and is associated with anxiolytic behavior (Distler et al., 2012) and induction of sleep (Jakubcakova et al., 2013). However, due to the lower levels of MG in the synaptic cleft (compared to those of GABA), it has been proposed that MG has a modulatory effect on GABAA receptors in the extrasynaptic space (Tao et al., 2018).
On the other hand, the relationship between GABAA and glucose metabolism (more precisely, glucose uptake) has been investigated over the last 30 years, but findings are not clear and sometimes conflicting (Ito et al., 1994; Peyron et al., 1994; Parthoens et al., 2015). Currently, measurement of glucose metabolism (based on deoxyglucose uptake) is associated with glutamatergic activity, mainly because glutamate is the predominant neurotransmitter. However, other neurotransmitters such as noradrenaline and adenosine, as well as K+ itself, released by neuronal activity, modulate energetic metabolism (Hertz et al., 2015; Waitt et al., 2017). In this scenario, MG is a glucose-derivative molecule that putatively connects energetic metabolism and the GABAergic system.
Methylglyoxal is metabolized predominantly by the cytoplasmic glyoxalase system, formed by two enzymes, glyoxalases 1 and 2 (GLO1 and 2), which act sequentially. GLO1 depends on GSH. GSH reacts directly with MG and produces hemithioacetal and GLO1 converts this metabolite to S-Lactoylglutathione. Subsequently, this compound is hydrolyzed by GLO2 into D-lactate and GSH is regenerated (Figure 3). Notice that, due to the lower activity of the left leg of the destination of glucose in neurons, it may be assumed that extracellular MG and D-lactate, as well as L-lactate, originate predominantly from astroglial cells.
D-lactate is oxidized to pyruvate by a D-isomer-specific lactate dehydrogenase (D-LDH; Cristescu et al., 2008). Mitochondrial D-LDH activity in rat brain tissue is about 60% lower than in liver (Ling et al., 2012). High levels of D-lactate inhibit the membrane L-lactate transport and pyruvate transport to mitochondria in astrocytes (Gibbs and Hertz, 2008) and neurons (Ros et al., 2001), which explains the neurotoxicity of this compound. However, a neuroprotective effect of D-lactate has been proposed in seizures (Angamo et al., 2017), possibly due to energy impairment. More recently, in addition to confirming the presence D-LDH activity in mouse brain tissue, it was shown that D-lactate is a partial agonist of HCAR1 (Castillo et al., 2015).
Astrocyte Dysfunction, MG-Induced Protein Glycation, and Neurodegenerative Diseases
It is well known that in aging, diabetes mellitus, and neurodegenerative diseases, MG is elevated to sub-millimolar levels, working as a potent glycant agent (Srikanth et al., 2013; Maessen et al., 2015). Elevated D-lactate levels from liver metabolism are observed in diabetic animals (Kondoh et al., 1994) and the accumulation of this compound could contribute to memory impairment, dependent on lactate flow (Suzuki et al., 2011). However, when the detoxifying system fails due to a reduction in glyoxalase activity or GSH deficiency, MG and advanced glycation end-products (AGEs) formation increases, but D-lactate levels can be reduced, as has been observed in endothelial cells (Li et al., 2013).
Methylglyoxal promotes glycation on lipids, nucleic acid and proteins (Allaman et al., 2015). MG mainly promotes post-translation modifications on proteins by the Maillard reaction on amino acid residues. The most common glycated amino acids are arginine and lysine and consequently the formation of AGEs such as argpyrimidine, hydroimidazolone (MG-H1), Nε-(1-carboxyethyl)-L-lysine (CEL), and Nε-(1-carboxymethyl)-L-lysine (CML) adducts, respectively (Rabbani and Thornalley, 2012). AGEs are ligands of the receptor for advanced glycation end products (RAGE) and induce inflammation by activation of the nuclear factor κB (NF-κB) pathway in all neural cells (Chavakis et al., 2004; Donato et al., 2009).
Elevated MG seems to increase the expression of astrocyte markers (glial fibrillary acidic protein – GFAP and S100B) and cytokines in astrocyte culture and in vivo, leading to astrogliosis and neuroinflammation (Chu et al., 2016). However, cognitive impairment has also been reported without changes in classical parameters of astrogliosis (Hansen et al., 2016b). Impairment in glucose flow and/or dysfunction of the glyoxalase system is a common and early event in neurodegenerative diseases, such as Alzheimer’s (AD) and Parkinson’s diseases (PD).
Elevated MG and AGEs levels play a key role in protein misfolding and oxidative stress, and are also involved in AD (Angeloni et al., 2014). The increases in glycation reactions and MG levels are suggested as a possible link in diabetic individuals that develop AD (Janson et al., 2004). In different in vitro models, MG induced glutamatergic excitotoxicity by promoting glutamate release (Arriba et al., 2006) and glutamate uptake disturbance (Hansen et al., 2016a, 2017). Elevated MG and AGEs stimulate apoptosis and reduce neurogenesis as well as neuronal survival in the hippocampus by downregulation of BDNF expression and its signaling pathways (Di Loreto et al., 2008; Falone et al., 2012; Chun et al., 2016). MG is involved in tau hyperphosphorylation through activation of GSK-3β and p38 mitogen-activated protein kinases (p38 MAPK) (Li et al., 2012). Moreover, AGEs and hyperphosphorylated tau are co-localized in the cytoplasm of neurons, possibly contributing to neurofibrillary tangle formation (Lüth et al., 2005; Fawver et al., 2012). AGE immunoreactivity has also been observed in amyloid plaques (Münch et al., 1997; Krautwald and Münch, 2010); recently, MG was shown to glycate Lys-16 and Arg-5 residues on β-amyloid, resulting in glycated Aβ (Fica-Contreras et al., 2017). The Aβ-AGE form is more insoluble, neurotoxic and resistant to protease reactions (Angeloni et al., 2014).
In PD, a predictor event is the low activity of neuronal PPP enzymes (Dunn et al., 2014) and mitochondrial dysfunction (Dranka et al., 2012; Hipkiss, 2014), which changes the redox cell status and increases the anaerobic glycolysis pathway and MG formation. In addition, dysregulation of the glyoxalase system (Joe et al., 2018) also leads to high MG levels and an increase in glycation reactions. MG reacts directly with dopamine, reducing its concentration and generating the salsolinol-like compound, 1-acetyl-6.7- dihydroxy-1,2,3,4-tetrahydroisoquinoline, which promotes mitochondrial dysfunction (Hipkiss, 2014). Moreover, the glycation of the N-terminal region of α-synuclein reduces its ability to remain attached to the plasma membrane. In fact, the accumulation of aggregated glycated-α-synuclein in the cytoplasm results in neurotoxic effects on dopaminergic neurons (Vicente Miranda et al., 2016).
The Right Arm Modulates Glutathione Homeostasis and Glutamatergic Neurotransmission
Glucose metabolism, in addition to producing energy, supports other important functions, for example the generation of reducing equivalents for antioxidant defenses and biosynthetic pathways, in both astrocytes and neurons (Magistretti and Allaman, 2018). The PPP, which constitutes the right arm of the glucose destinations (Figure 1), is the main cytosolic source of NADPH and is essential for regeneration of GSH. This pathway decarboxylates glucose-6-phosphate into ribulose-5-phosphate, a precursor for the nucleotide biosynthesis, conserving the redox energy as NADPH. Glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme for the PPP (see Figures 4, 6), which in the resting brain represents a minor pathway for glucose metabolism (Gaitonde et al., 1983; Wamelink et al., 2008; Bouzier-Sore and Bolaños, 2015). However, under several conditions, for example in response to injury, the PPP can be markedly upregulated (Bartnik et al., 2005; Jalloh et al., 2015; Rosa et al., 2015) and has demonstrated protective roles because it provides precursors for tissue repair, as well as increases GSH to avoid oxidative stress and neuroinflammation.
FIGURE 4.
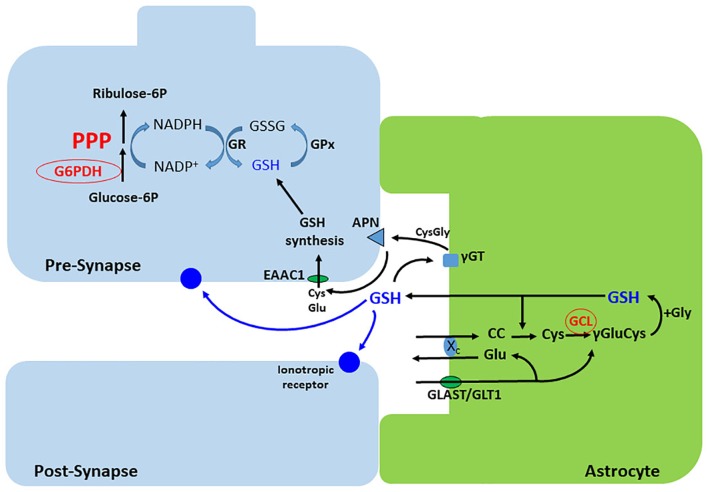
Synthesis and release of GSH in astrocytes. Cystin (cisteinyl-cystein, CC) is uptaken via the xc- exchanger, which releases glutamate. This exchanger is functionally coupled to the Na+- dependent glutamate transporters, GLAST or GLT-1. Cystin is reduced by GSH to two cysteines. The gamma acid group of glutamate then condenses with the amine group of cysteine, forming gamma-glutamyl-cysteine (γ Glu-Cys), by the action of the glutamate cysteine ligase (GCL). Addition of glycine completes the synthesis of GSH that, in part, is exported to modulate ionotropic receptors and as a source of cysteine for GSH synthesis in neurons. The extracellular cysteine from astroglial GSH is generated after sequential action of the extracellular peptidases, γGT and APN. Neurons synthesize low levels of GSH compared with astrocytes, but exhibit a high capacity of GSH regeneration, which depends on NADPH synthesis in the PPP. γGT, gamma peptidyl transpeptidase; APN, aminopeptidase neuronal; GPx, glutathione peroxidase; GR, glutathione reductase; EAAC, excitatory amino acid carrier.
FIGURE 6.
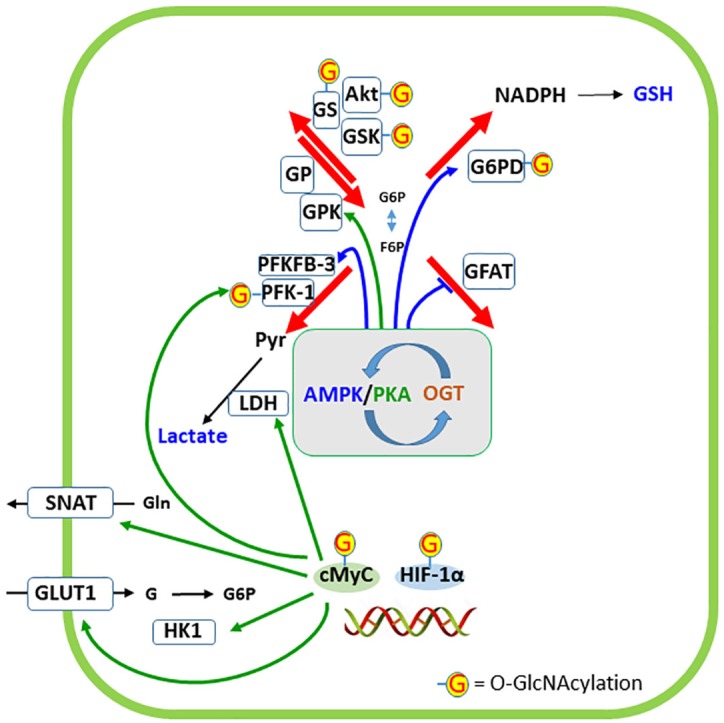
Regulation of cellular destinations of glucose by O-GlcNAc modification of proteins. AMP-activated protein kinase (AMPK) and protein kinase A (PKA) have a narrow interaction in the regulation of glucose metabolism as well as phosphorylate and are targets of O-GlcNAc transferase (OGT). The phosphorylation of specific targets of AMPK or PKA in the four cellular destinations of glucose are indicated by the respective colored arrows. Targets of OGT are indicated by G. Two transcription factors closely related to glucose metabolism (cMyc and HIF-1α) are represented at the double-strand DNA and five proteins whose expressions are regulated by these transcription factors are indicated. G6PD, glucose-6-phosphate dehydrogenase; GFAT, glutamine:fructose-6P aminotransferase; GLUT1, glucose transporter 1; GP, glycogen phosphorylase; GPK, glycogen phosphorylase kinase; GS, glycogen synthase; GSK, glycogen synthase kinase; HIF-1α, hypoxia-inducible factor-1α; HK1, hexokinase 1; LDH, lactate dehydrogenase; PFK-1, phosphofructokinase 1; PFKFB-3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3; SNAT, sodium-neutral amino acid transporter (type 3 or 5, in astrocytes).
In astrocytes, the PPP is essential for maintaining an adequate pool of reduced GSH, since these cells export this antioxidant molecule to neurons. Activation of the PPP in astrocytes protects neurons from oxidative stress by increasing astrocytic GSH levels (Takahashi et al., 2012). In neurons, the low expression of PFKFB-3 (Figure 6) results in a low glycolytic rate, and glucose is diverted to the PPP (Almeida et al., 2004; Herrero-Mendez et al., 2009). This is particularly important for generating GSH, since neuronal cells have a lower synthesis and, consequently, GSH concentrations, compared to astrocytes (Makar et al., 1994; Dringen et al., 2005).
Glutathione is an essential molecule for cellular antioxidant defense and detoxification processes, conferring neuroprotection (Dringen, 2000b; Dringen et al., 2015; Kinoshita et al., 2018). Impaired GSH metabolism is associated with oxidative stress and inflammatory responses, which have been linked to cerebral diseases and neurodegeneration (Dringen, 2000a; Lee et al., 2010; Aoyama and Nakaki, 2013; Arús et al., 2017). An age-dependent reduction in resting NADPH concentration, accompanied by a decrease in GSH levels, has been reported in cultured neurons, making them more susceptible to glutamate exposure (Parihar et al., 2008). This impaired reducing power can lead to pathological aging, since the inability of neurons to regenerate GSH is a hallmark of AD and PD (Currais and Maher, 2013).
Glutathione is a tripeptide, consisting of glutamate, cysteine and glycine, that is synthesized by two enzymatic steps. First, GCL catalyzes the reaction between glutamate and cysteine to form the dipeptide γ-glutamylcysteine. The second step is the reaction of GSH synthase, which mediates the addition of glycine to γ-glutamylcysteine to form GSH (Lu, 2013). While the first step catalyzed by GCL is considered the rate-limiting reaction, the intracellular content of cysteine is the rate-limiting precursor for GSH biosynthesis (Griffith, 1999; Figure 4).
Glutathione in the brain is present in both intra- and extracellular compartments and acts as a dynamic buffer of the redox state. Non-enzymatically, GSH is able to react directly with free radicals including superoxide, hydroxyl radical, nitric oxide, peroxynitrite and MG (Clancy et al., 1994; Winterbourn and Metodiewa, 1994; Aoyama and Nakaki, 2015). Moreover, GSH can react with protein thiol groups, leading to a reversible formation of mixed disulfides (S-glutathionylation), which are important for preventing protein oxidation, thus preserving and modulating its functions (Giustarini et al., 2004). GSH also participates in enzymatic reactions, such as those catalyzed by GSH peroxidase (GPx) and glutathione-S-transferase (GST). GPx detoxifies hydrogen peroxides and other endogenous hydroperoxides using GSH as an electron donor. In this reaction, GSH is oxidized to glutathione disulfide (GSSG; Dringen et al., 2015); GSSG is then reduced back to GSH via glutathione reductase (GR) using NADPH (Dringen and Gutterer, 2002; Ren et al., 2017).
The high oxidative metabolic rate can increase mitochondrial reactive oxygen species (ROS) production, rendering the brain vulnerable to oxidative stress. Astrocytes and neurons exhibit differences in GSH metabolism and different mechanisms maintain GSH homeostasis between these cell types. In astroglial cells, the basal levels of GSH are higher than in neuronal cells, showing their pivotal antioxidant role in the central nervous system (Dringen et al., 2005). Neurons, in turn, depend on astrocytic GSH release for providing extracellular cysteine for their synthesis of GSH (Figure 4). As previously mentioned, cysteine is the limiting precursor for synthesis of GSH.
Astrocytes have a Cys-Glu exchanger (system xc-) that mediates the uptake of cystine, the bioavailable form of cysteine, in exchange for glutamate (Bridges R.J. et al., 2012; Ottestad-Hansen et al., 2018). Moreover, they express the glutamate/aspartate transporter GLAST (also known as excitatory amino acid transporter 1, EAAT1 in humans) and glutamate transporter 1 (GLT1, or EAAT2 in humans) (Lehre and Danbolt, 1998), which provide intracellular glutamate for GSH synthesis and for system xc- operation (Reichelt et al., 1997). Importantly, these glutamate transporters also account for the majority of glutamate removal from the synaptic cleft, maintaining extracellular glutamate concentrations low to avoid excitotoxicity (Anderson and Swanson, 2000; Rose et al., 2017). In this regard, system xc- and glutamate transporters are associated with both GSH biosynthesis and modulation of glutamatergic neurotransmission, as xc- mediates glutamate release. Altered function of these transporters can result in GSH depletion and/or, consequently, glutamate excitotoxicity in pathological conditions (Ré et al., 2003; Yi and Hazell, 2006; Bridges R. et al., 2012; Thorn et al., 2015). At the same time, GLAST/GLT1 are also related to glucose metabolism; as they are sodium-dependent, their activity increases the intracellular sodium concentration, consequently activating the Na+/K+ ATPase pump, which consumes ATP in astrocytes. ATP, in turn, can be supplied by the glycolytic pathway (for a review, see Bélanger et al., 2011).
Glutamate is also involved in the mechanism by which astrocytes are able to readily release GSH in response to neural activity, to maintain neuronal GSH levels via the astrocyte-neuronal GSH shuttle. In astrocytes, glutamate triggers a cascade of signals that promote the expression of antioxidant genes through activation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2), leading to the biosynthesis of GSH (Frade et al., 2008; Jimenez-Blasco et al., 2015; McGann and Mandel, 2018). In the extracellular space, GSH can be hydrolyzed by γ-glutamyl transpeptidase forming γ-glutamyl and the dipeptide CysGly. CysGly is, in turn, cleaved by the neuronal aminopeptidase N into cysteine and glycine, which serve as precursors for neuronal GSH synthesis (Dringen et al., 1999, 2001; Hertz and Zielke, 2004; Figure 4). Neuronal cells are less capable of importing cystine, but the sodium-dependent excitatory amino acid carrier 1 (EAAC1, also known as EAAT3 in humans) is able to uptake cysteine in addition to glutamate (Zerangue and Kavanaugh, 1996; Shanker et al., 2001). EAAC1/EAAT3 supplies neurons with the rate-limiting precursor for GSH synthesis, directly influencing their redox homeostasis (Paul et al., 2018). Furthermore, EAAC1/EAAT3 acts as a bridge between astrocytic and neuronal GSH metabolism by importing cysteine released from the extracellular breakdown of astrocytic GSH.
Glucose metabolism, GSH synthesis and glutamatergic homeostasis are closely associated processes and share extracellular and intracellular regulatory mechanisms. Among these, the neurotrophic factor BDNF has recently been demonstrated as a key regulator of central energy homeostasis (Marosi and Mattson, 2014). BDNF increases glucose transport in neurons by inducing the expression of GLUT3 through phosphatidylinositol-3 kinase (PI3K) and Akt kinase (Burkhalter et al., 2003). Because of the low neuronal content of PFKFB-3, glucose can be used for the generation of GSH through the PPP. Additionally, BDNF can activate hypoxia-inducible factor-1 (HIF-1) and Nrf2, which are related to the induction of enzymes that participate in glucose metabolism in both astrocytes and neurons. Importantly, Nrf2 regulates G6PD, GCL, GSH synthase, system xc-, and EAAC1/EAAT3 (Thimmulappa et al., 2002; Shih et al., 2003; Escartin et al., 2011; Niture et al., 2014), thus facilitating both regeneration and synthesis of GSH.
Experimental data have suggested a role for GSH as a neuromodulator. As a thiol-containing compound, GSH may regulate the redox sites of several ionotropic receptors and ion channels, altering their functional characteristics (Gozlan and Ben-Ari, 1995; Pan et al., 1995). In this regard, both GSH and GSSG have been demonstrated to modulate neuronal depolarization, calcium ion influx and second messenger activity (Janáky et al., 1993; Varga et al., 1997) through the glutamatergic receptors NMDA and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA). Such effects appear to be dependent on GSH concentrations. At micromolar levels, GSH is inhibitory via its interaction with glutamate binding sites. In contrast, at millimolar concentrations, GSH activates NMDA receptors by reducing functional thiol groups (Janáky et al., 1993). Interestingly, as a consequence, GSH becomes oxidized to GSSG, which triggers an increase in the PPP to generate GSH (Delgado-Esteban et al., 2000).
In retinal glial cells, GSH induces calcium shifts in a P2X7 (a purinergic receptor subtype), but not ionotropic glutamate receptor dependent manner. In contrast, GSSG did not reproduce this effect, indicating that the antioxidant and/or structural features of GSH are essential to promote elevations in cytoplasmic calcium levels (Freitas et al., 2016). In cortical brain slices, GSH was able to evoke a depolarizing potential that appears to be mediated by sodium ions. As this potential was not blocked by antagonists of glutamate receptors, GSH may act through its own receptor-mediated channels (Shaw et al., 1996). In this regard, radioligand binding assays have shown the presence of binding sites for GSH in different neural cell preparations, including brain synaptosomal membranes (Janáky et al., 2000) and astrocytes (Guo and Shaw, 1992). GSH also seems to be released upon calcium-dependent depolarization in brain slices (Zängerle et al., 1992) and is able to modulate the release of neurotransmitters, including GABA (Janáky et al., 1994; Freitas et al., 2016) and dopamine (Janáky et al., 2007). Interestingly, GSH can reverse aging-associated hippocampal synaptic plasticity deficits (Yang et al., 2010). Together, these data support an emerging role of GSH in signal transduction and synaptic transmission.
The Right Leg Controls the Other Destinations of Glucose in Neural Cells by Protein Glcnacylation
The Protein GlcNAcylation and Hexosamine Pathway
The post-translational modification of proteins by O-linked-N-acetyl-D-glucosamine (O-GlcNAc) is assumed to be a glucose-responsive mechanism that modulates cellular signaling (see Nagel and Ball, 2014 for a review). O-GlcNAc rapidly cycles onto the serine or threonine residues of target proteins. This process is equivalent to phosphorylation and occurs via the activity of two enzymes: O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which add and remove GlcNAcetyl, respectively (see Figure 5). GlcNAcetyl is derived from the hexosamine biosynthetic pathway (HP), the right leg in our X of metabolic glucose destination. It is estimated that 2–5% of incoming glucose goes to the HP, which has UDP-GlcNAc as its final product, the donor of GlcNAc (Ozcan et al., 2010). In the liver, the levels of O-GlcNAc-modified proteins fluctuate according to the nutrient status, i.e., they are regulated by intracellular concentrations of UDP-GlcNAc that rise with feeding and are increased in diabetes mellitus patients (Hanover et al., 2010; Nagel and Ball, 2014). In the brain, under conditions of hypometabolism of glucose, as observed in AD, the levels of O-GlcNAc-modified proteins are reduced (Dos Santos et al., 2018). However, it is necessary to identify specific changes in protein GlcNAcylation to understand particular protein alterations in physiological and pathological conditions. Herein, we will discuss some aspects of HP regulation and specific targets of O-GlcNAcylation in astrocytes, which modulate glucose metabolism and synaptic communication.
FIGURE 5.
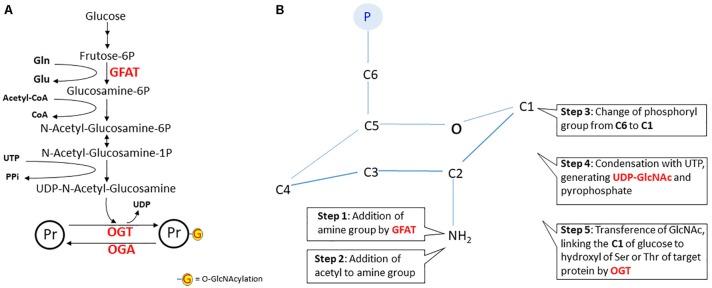
The hexosamine pathway. In panel A, the steps of the hexosamine pathway (HP) from frutose-6P to UDP-N-acetyl-glucosamine. Notice that glutamine (Gln), Acetyl-CoA and UTP are key substrates in this pathway. The rate-limiting step is catalyzed by glutamine:fructose-6P aminotransferase (GFAT). In panel B, a schematic representation of the structural changes during N-acetyl-glucosamine synthesis. The first reaction is the amination of fructose-6P (fructofuranose) to glucosamine-6P (glucopyranose). It is worth noting that, in step six, carbon 1 of glucopyranose binds to the hydroxyl of serine or threonine of the protein target. In fact, this is a reaction of O-linked β-N-acetylglucosaminylation, but for simplification, the nomenclature widely used is O-GlcNAcylation. However, this gives the wrong idea that linking occurs at the acyl group. It would be better to use NAGylation, since NAG is another (and the simplest) abbreviation of N-AcetylGlucosamine, found in some polymers. Herein, we will maintain the use of the term “O-GlcNAcylation.” Abbreviations: OGT, O-GlcNAc transferase; OGA, O-GlcNAcase; Pr, protein.
The first reaction of HP is catalyzed by the glutamine:fructose-6P aminotransferase (GFAT) enzyme. The glutamine transfers the amine group to carbon 2 of fructose-6P, converting it to glucosamine-6P (Yuzwa and Vocadlo, 2014). Note that neurons depend on astroglial glutamine, since glutamine synthetase is a glial enzyme. In the next step, acetyl-CoA transfers acetyl to the amine group and then the phosphate from carbon 6 is transferred to carbon 1. This compound, N-acetyl-glucosamine-1P, reacts with UTP to release the end products UDP-GlcNAc and PPi. The rate-limiting step of HP is the reaction catalyzed by GFAT, which is negatively modulated by AMPK (Eguchi et al., 2009), like other glycolytic key enzymes such as PFK-1 and PFKFB-3. Therefore, besides glucose flow, GlcNAcylation of proteins is regulated by GFAT, and also by the activities of OGT and OGA (Worth et al., 2017).
The OGT and OGA enzymes are evolutionarily well conserved and have many targets involved in signal transduction, transcription, translation, cell cycle control and apoptosis. However, since these are just two enzymes (in contrast to the hundreds of protein kinases and phosphatases), there is still little understanding of how their targets are recognized (Yang and Qian, 2017). Moreover, Ser and Thr sites for GlcNAcylation and phosphorylation co-exist in the same protein and these modifications often share the same sites, establishing a complex functional relationship (Wang et al., 2007; Hu et al., 2010).
Glucose Flow Regulates Its Own Fate and Its Derivatives
The main protein targets of O-GlcNAcylation, which modulate glucose flow and/or destination, are indicated in Figure 6. GFAT, the regulatory enzyme of HP, is inhibited by phosphorylation, catalyzed by AMPK, at Ser 243 (Eguchi et al., 2009). Therefore, activated AMPK decreases UDP-GlcNAc levels. However, AMPK is also able to phosphorylate (activate in this case) the OGT at Thr 444, which in turn could lead to O-GlcNAcylation of AMPK, resulting in a complex regulation that involves changes in the activities of enzymes as well as their cellular localizations (Bullen et al., 2014). It is possible, but has not yet been determined, that this triangular interaction among AMPK, GFAT, and OGT occurs in neural cells, particularly in astrocytes, where AMPK has a crucial role (Bolaños, 2016).
Glutathione, as discussed above, has an important antioxidant role in neurons. However, at higher concentrations in astrocytes, it is exported not only to support neuronal synthesis, but also to modulate ionotropic synaptic receptors. Glucose generates NADPH in the PPP to recycle GSH in neurons and astrocytes. The G6PD enzyme is the rate-limiting step of this pathway. G6PD is modulated by O-GlcNAcylation, as demonstrated in several cell lines (Rao et al., 2015). In contrast to O-GlcNAcylation of GFAT, this modification of G6PD activates the enzyme, increasing the activity of the PPP and NADPH formation. Although this is of importance in neurons to regenerate GSH, the effect of O-GlcNAcylation on neuronal G6PD has not yet been analyzed. However, it is possible to realize the importance of glutamine from astrocytes to neuronal synthesis of glucosamine-6P, the precursor of UDP-GlcNAc. Another important aspect of GSH synthesis, particularly in astrocytes, is its modulation by AMPK (Guo et al., 2018). AMPK positively regulates the expression of the modulatory subunit of enzyme GCL through the transcriptional co-activator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), (Figure 4), which catalyzes the first step (and regulatory step) of GSH synthesis.
Glycogen synthase (GS) is covalently modified by phosphorylation (induced by catecholamines and insulin) and O-GlcNAcylation, at least in adipocytes (Parker et al., 2004). Phosphorylated GS is less sensitive to the allosteric activator, glucose-6P. Insulin phosphorylates both GSK3 and the glycogen-targeting protein through the PI3K/Akt pathway, in turn activating protein phosphatase 1, PP1. Phosphorylation of both GSK-3 and PP1 activates GS, which leads to glycogen formation (Obel et al., 2012). Similarly, glucose flow in the HP leads to O-GlcNAcylation and activation of the GS. This is an example of mutual exclusivity, where O-GlcNAcylation acts similarly to dephosphorylation, which cannot be generalized to other conditions. Moreover, this relationship is more complex because the upstream enzymes, Akt and GSK3, are also targets of O-GlcNAcylation (e.g., Park et al., 2005).
Glycogen breakdown is also regulated by phosphorylation of glycogen-targeting protein by protein kinase A (PKA) at a different site of Akt (Bak et al., 2018). This PKA-induced phosphorylation is triggered by neurotransmitters. The resulting phosphorylation of glycogen-targeting protein at the glycogen granule dissociates PP1, glycogen phosphorylase (GP), glycogen phosphorylase kinase (GPK), and GS. PKA phosphorylates/activates GPK, which in turn phosphorylates/activates GP. This dissociation is an important step because both GP and GPK are targets of PP1. Moreover, PKA phosphorylates/activates the inhibitor 1 of PP1. It was recently reported that the catalytic subunit of PKA is a target of O-GlcNAcylation (Xie et al., 2016), but the direct effect on glycogen breakdown remains unclear. Interestingly, O-GlcNAc protein modification increases in tumor cells, in response to glucose deprivation, through glycogen degradation (Kang et al., 2009), contradicting the idea that an increase in O-GlcNAcylation acts as a negative feed-back signal to ATP generation from glucose. This phenomenon may involve changes in GS/GP balance, due to changes in OGT and/or OGA, and not UDP-GlcNAc levels (Taylor et al., 2008).
Lactate generation is strongly regulated by O-GlcNAcylation because PFK-1 and PFKFB-3 are direct and indirect targets of OGT, respectively. O-GlcNAc modification of PFK-1 at Ser529 is induced by hypoxia in cancer cells and this modification inhibits enzyme activity and redirects the flux of glucose to PPP (Yi et al., 2012). The authors also observed a modest O-GlcNAcylation of HK. PFKFB-3, which regulates PFK-1, is phosphorylated by AMPK and Akt, which are targets of OGT, as mentioned above. Therefore, it is possible to conceive an interaction between AMPK, PFKFB-3, and OGT, that is just as complex or even more than the interaction between AMPK-GFAT-OGT (Bullen et al., 2014). Moreover, at least two transcription factors that have been well studied in tumor cells and are connected to glycolysis are modified by O-GlcNAcylation: c-Myc and HIF-1α. Akt/c-Myc activation induces expression of GLUT-1, HK 1 and 2, PFK-1, lactate dehydrogenase A and glutamine transporters (Miller et al., 2012; Swamy et al., 2016; Zhang et al., 2017). HIF-1α induces GLUT-1 and 3, hexokinases, and PFK-1 (Chen and Russo, 2012). Interestingly, also in cancer cells, lactate is able to trigger changes in glutamine uptake and metabolism (Pérez-Escuredo et al., 2016), which are necessary not only for cell proliferation but also for protein O-GlcNAcylation. Considering the importance of glutamine/glutamate in brain tissue and lactate in neuron/astrocyte communication, it would seem that this mechanism is worthy of investigation in the nervous system.
Finally, the interplay between AMPK and PKA in glucose metabolism should be considered. Microdomains of cAMP have been characterized to explain the localized action of this intracellular messenger, at the membrane or on soluble adenylyl cyclase. cAMP acts on PKA and is inactivated by phosphodiesterase. Both enzymes (PKA and sAC) bind to A-kinase anchoring protein (AKAP; Zippin et al., 2004; Oliveira et al., 2010). A physical connection between PKA and AMPK, via AKAP, has been proposed in muscle cells, where AMPK phosphorylates AKAP, releasing PKA (Hoffman et al., 2015). In hepatocytes, PKA phosphorylates and reduces AMPK activity (Hurley et al., 2006). Again, although most data regarding protein O-GlcNAcylation derive from tumor and/or non-neural cells, protein O-GlcNAcylation may also occur in the brain. Therefore, it is possible that a general cross-talk occurs between PKA and AMPK (involving O-GlcNAcylation, because both are targets of OGT) with a role in the metabolic regulation of glucose destination and synaptic plasticity.
Summary
The importance of glucose for brain activity is very clear, since glucose provides ATP and replenishment of substrates, such as glutamate and cholesterol. Additionally, glucose metabolism provides derivatives such as lactate, MG and GSH, which regulate synaptic communication. Herein, we propose an intersection in an “X” that defines the four destinations of glucose in neural cells, where astrocytes work as integrative and modulatory elements in the synaptic communication. Such destinations depend on the metabolic arrangement in each cell type, which in turn depends on the glucose supply and neural activity. Extracellular L-lactate released by astrocytes, either generated from recently captured glucose or from glycogen, binds to HCAR1, a specific perivascular and post-synaptic receptor, regulating synaptic plasticity. Currently, lactate is being considered as a putative gliotransmitter. MG results from a deviation of the glycolytic pathway and is metabolized to D-lactate. Both MG and D-lactate are released and modulate neuronal activity, possibly through GABAA and HCAR1, respectively. The main cellular antioxidant GSH, whose regeneration depends on the PPP, is also released by astrocytes and alters the synaptic response by modulating the redox and non-redox sites of ionotropic receptors and ion channels. O-GlcNAcylation is an important post-translational protein modification for cell signaling, and the glucose flow through the HP regulates the content of UDP-GlcNAc. The levels of UDP-GlcNAc, in part, are determinant for the GlcNAc-modification of proteins, including the proteins that modulate the glucose destinations. As discussed above, glutamine from astrocytes is essential, literally, for the neuronal synthesis of UDP-GlcNAc. Although the coupling between astrocytes and neurons most often addresses the relationship of glutamate or GABA with the glycolytic pathway, we should not forget that other neurotransmitters also modulate glucose metabolism, which then regulates neurotransmission through glycolysis-derived products.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and National Institute of Science and Technology for Excitotoxicity and Neuroprotection (INCTEN/MCT).
References
- Ahmed K., Tunaru S., Offermanns S. (2009). GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol. Sci. 30 557–562. 10.1016/j.tips.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Ahmed K., Tunaru S., Tang C., Müller M., Gille A., Sassmann A., et al. (2010). An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11 311–319. 10.1016/j.cmet.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Allaman I., Bélanger M., Magistretti P. J. (2015). Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 9:23. 10.3389/fnins.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A., Moncada S., Bolaños J. P. (2004). Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 6 45–51. 10.1038/ncb1080 [DOI] [PubMed] [Google Scholar]
- Anderson C. M., Swanson R. A. (2000). Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32 1–14. [DOI] [PubMed] [Google Scholar]
- Angamo E. A., Haq R. U., Rösner J., Gabriel S., Gerevich Z., Heinemann U., et al. (2017). Contribution of intrinsic lactate to maintenance of seizure activity in neocortical slices from patients with temporal lobe epilepsy and in rat entorhinal cortex. Int. J. Mol. Sci. 18 1–15. 10.3390/ijms18091835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeloni C., Zambonin L., Hrelia S. (2014). Role of methylglyoxal in Alzheimer’s disease. Biomed Res. Int. 2014:238485. 10.1155/2014/238485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K., Nakaki T. (2013). Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 14 21021–21044. 10.3390/ijms141021021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K., Nakaki T. (2015). Glutathione in cellular redox homeostasis: association with the excitatory amino acid carrier 1 (EAAC1). Molecules 20 8742–8758. 10.3390/molecules20058742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriba S. G., De Krügel U., Regenthal R., Vissiennon Z., Verdaguer E., Lewerenz A., et al. (2006). Carbonyl stress and NMDA receptor activation contribute to methylglyoxal neurotoxicity. Free Radic. Biol. Med. 40 779–790. 10.1016/j.freeradbiomed.2005.09.038 [DOI] [PubMed] [Google Scholar]
- Arús B. A., Souza D. G., Bellaver B., Souza D. O., Gonçalves C.-A., Quincozes-Santos A., et al. (2017). Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Mol. Cell. Biochem. 428 67–77. 10.1007/s11010-016-2917-5 [DOI] [PubMed] [Google Scholar]
- Bak L. K., Walls A. B., Schousboe A., Waagepetersen H. S. (2018). Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 293 7108–7116. 10.1074/jbc.R117.803239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P., Braun A., Nedergaard M. (2004). The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16 1–13. 10.1016/J.NBD.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Barros L. F., San Martín A., Ruminot I., Sandoval P. Y., Fernández-Moncada I., Baeza-Lehnert F., et al. (2017). Near-critical GLUT1 and neurodegeneration. J. Neurosci. Res. 95 2267–2274. 10.1002/jnr.23998 [DOI] [PubMed] [Google Scholar]
- Bartnik B. L., Sutton R. L., Fukushima M., Harris N. G., Hovda D. A., Lee S. M. (2005). Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma 22 1052–1065. 10.1089/neu.2005.22.1052 [DOI] [PubMed] [Google Scholar]
- Bartrons R., Simon-Molas H., Rodríguez-García A., Castaño E., Navarro-Sabaté À., Manzano A., et al. (2018). Fructose 2,6-bisphosphate in cancer cell metabolism. Front. Oncol. 8:331. 10.3389/fonc.2018.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M., Allaman I., Magistretti P. J. (2011). Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 14 724–738. 10.1016/j.cmet.2011.08.016 [DOI] [PubMed] [Google Scholar]
- Bergersen L. H. (2015). Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J. Cereb. Blood Flow Metab. 35 176–185. 10.1038/jcbfm.2014.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet C., Castillo X., Magistretti P. J., Hirt L. (2012). New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc. Dis. 34 329–335. 10.1159/000343657 [DOI] [PubMed] [Google Scholar]
- Berthet C., Lei H., Thevenet J., Gruetter R., Magistretti P. J., Hirt L. (2009). Neuroprotective role of lactate after cerebral ischemia. J. Cereb. Blood Flow Metab. 29 1780–1789. 10.1038/jcbfm.2009.97 [DOI] [PubMed] [Google Scholar]
- Blad C. C., Ahmed K., IJzerman A. P., Offermanns S. (2011). Biological and pharmacological roles of HCA receptors. Adv. Pharmacol. 62 219–250. 10.1016/B978-0-12-385952-5.00005-1 [DOI] [PubMed] [Google Scholar]
- Bolaños J. P. (2016). Bioenergetics and redox adaptations of astrocytes to neuronal activity. J. Neurochem. 139 115–125. 10.1111/jnc.13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños J. P., Almeida A., Moncada S. (2010). Glycolysis: a bioenergetic or a survival pathway? Trends Biochem. Sci. 35 145–149. 10.1016/j.tibs.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore A.-K., Bolaños J. P. (2015). Uncertainties in pentose-phosphate pathway flux assessment underestimate its contribution to neuronal glucose consumption: relevance for neurodegeneration and aging. Front. Aging Neurosci. 7:89. 10.3389/fnagi.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzo L., Puyal J., Chatton J. Y. (2013). Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS One 8:e71721. 10.1371/journal.pone.0071721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R., Lutgen V., Lobner D., Baker D. A. (2012). Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol. Rev. 64 780–802. 10.1124/pr.110.003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges R. J., Natale N. R., Patel S. A. (2012). System xc- cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS: system xc- cystine/glutamate antiporter. Br. J. Pharmacol. 165 20–34. 10.1111/j.1476-5381.2011.01480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. W., Balsbaugh J. L., Chanda D., Shabanowitz J., Hunt D. F., Neumann D., et al. (2014). Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK). J. Biol. Chem. 289 10592–10606. 10.1074/jbc.M113.523068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter J., Fiumelli H., Allaman I., Chatton J.-Y., Martin J.-L. (2003). Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J. Neurosci. 23 8212–8220. 10.1523/JNEUROSCI.23-23-08212.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T. Q., Ren N., Jin L., Cheng K., Kash S., Chen R., et al. (2008). Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem. Biophys. Res. Commun. 377 987–991. 10.1016/j.bbrc.2008.10.088 [DOI] [PubMed] [Google Scholar]
- Carrard A., Elsayed M., Margineanu M., Boury-Jamot B., Fragnière L., Meylan E. M., et al. (2018). Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 23 392–399. 10.1038/mp.2016.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo X., Rosafio K., Wyss M. T., Drandarov K., Buck A., Pellerin L., et al. (2015). A probable dual mode of action for both L-and D-lactate neuroprotection in cerebral ischemia. J. Cereb. Blood Flow Metab. 35 1561–1569. 10.1038/jcbfm.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T., Bierhaus A., Nawroth P. P. (2004). RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 6 1219–1225. 10.1016/j.micinf.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Chen J.-Q., Russo J. (2012). Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim. Biophys. Acta 1826 370–384. 10.1016/j.bbcan.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Choeiri C., Staines W., Messier C. (2002). Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience 111 19–34. 10.1016/S0306-4522(01)00619-4 [DOI] [PubMed] [Google Scholar]
- Choi H. B., Gordon G. R., Zhou N., Tai C., Rungta R. L., Martinez J., et al. (2012). Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75 1094–1104. 10.1016/j.neuron.2012.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A. (2004). cypate-d: -(+)-Glucosamine (cyp-GlcN), and d: -(+)-Glucosamine-cypate-d: -(+)-Glucosamine (cyp-2GlcN). Available at: http://www.ncbi.nlm.nih.gov/pubmed/22993875 [accessed October 16, 2018]. [PubMed] [Google Scholar]
- Chu J. M., Lee D. K., Wong D. P., Wong G. T., Yue K. K. (2016). Methylglyoxal-induced neuroinflammatory response in in vitro astrocytic cultures and hippocampus of experimental animals. Metab. Brain Dis. 31 1055–1064. 10.1007/s11011-016-9849-3 [DOI] [PubMed] [Google Scholar]
- Chun H. J., Lee Y., Kim A. H., Lee J. (2016). Methylglyoxal causes cell death in neural progenitor cells and impairs adult hippocampal neurogenesis. Neurotox Res. 29 419–431. 10.1007/s12640-015-9588-y [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Levartovsky D., Leszczynska-Piziak J., Yegudin J., Abramson S. B. (1994). Nitric oxide reacts with intracellular glutathione and activates the hexose monophosphate shunt in human neutrophils: evidence for S-nitrosoglutathione as a bioactive intermediary. Proc. Natl. Acad. Sci. U.S.A. 91 3680–3684. 10.1073/pnas.91.9.3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco M., Caggia S., Musumeci G., Perciavalle V., Graziano A. C., Pannuzzo G., et al. (2013). Sodium L-lactate differently affects brain-derived neurothrophic factor, inducible nitric oxide synthase, and heat shock protein 70 kDa production in human astrocytes and SH-SY5Y cultures. J. Neurosci. Res. 91 313–320. 10.1002/jnr.23154 [DOI] [PubMed] [Google Scholar]
- Costa Leite T., Da Silva D., Guimarães Coelho R., Zancan P., Sola-Penna M. (2007). Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem. J. 408 123–130. 10.1042/BJ20070687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristescu M. E., Innes D. J., Stillman J. H., Crease T. J. (2008). D- and L-lactate dehydrogenases during invertebrate evolution. BMC Evol. Biol. 8:268. 10.1186/1471-2148-8-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A., Maher P. (2013). Functional consequences of age-dependent changes in glutathione status in the brain. Antioxid. Redox Signal. 19 813–822. 10.1089/ars.2012.4996 [DOI] [PubMed] [Google Scholar]
- Delgado-Esteban M., Almeida A., Bolaños J. P. (2000). D-Glucose prevents glutathione oxidation and mitochondrial damage after glutamate receptor stimulation in rat cortical primary neurons. J. Neurochem. 75 1618–1624. 10.1046/j.1471-4159.2000.0751618.x [DOI] [PubMed] [Google Scholar]
- Di Loreto S., Zimmitti V., Sebastiani P., Cervelli C., Falone S., Amicarelli F. (2008). Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int. J. Biochem. Cell Biol. 40 245–257. 10.1016/j.biocel.2007.07.019 [DOI] [PubMed] [Google Scholar]
- DiNuzzo M., Giove F., Maraviglia B., Mangia S. (2015). Monoaminergic control of cellular glucose utilization by glycogenolysis in neocortex and hippocampus. Neurochem. Res. 40 2493–2504. 10.1007/s11064-015-1656-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler M. G., Plant L. D., Sokoloff G., Hawk A. J., Aneas I., Wuenschell G. E., et al. (2012). Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J. Clin. Invest. 122 2306–2315. 10.1172/JCI61319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R., Sorci G., Riuzzi F., Arcuri C., Bianchi R., Brozzi F., et al. (2009). S100B’s double life: intracellular regulator and extracellular signal. Biochim. Biophys. Acta 1793 1008–1022. 10.1016/j.bbamcr.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Dos Santos J. P. A., Vizuete A., Hansen F., Biasibetti R., Goncalves C. A., Gonçalves C.-A. (2018). Early and persistent O-GlcNAc protein modification in the streptozotocin model of Alzheimer’s disease. J. Alzheimers Dis. 61 237–249. 10.3233/JAD-170211 [DOI] [PubMed] [Google Scholar]
- Dranka B. P., Zielonka J., Kanthasamy A. G., Kalyanaraman B. (2012). Alterations in bioenergetic function induced by Parkinson’s disease mimetic compounds: lack of correlation with superoxide generation. J. Neurochem. 122 941–951. 10.1111/j.1471-4159.2012.07836.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R. (2000a). Glutathione metabolism and oxidative stress in neurodegeneration. Eur. J. Biochem. 267:4903 10.1046/j.1432-1327.2000.01651.x [DOI] [PubMed] [Google Scholar]
- Dringen R. (2000b). Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62 649–671. 10.1016/S0301-0082(99)00060-X [DOI] [PubMed] [Google Scholar]
- Dringen R., Brandmann M., Hohnholt M. C., Blumrich E.-M. (2015). Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res. 40 2570–2582. 10.1007/s11064-014-1481-1 [DOI] [PubMed] [Google Scholar]
- Dringen R., Gutterer J. M. (2002). Glutathione reductase from bovine brain. Methods Enzymol. 348 281–288. 10.1016/S0076-6879(02)48646-6 [DOI] [PubMed] [Google Scholar]
- Dringen R., Gutterer J. M., Gros C., Hirrlinger J. (2001). Aminopeptidase N mediates the utilization of the GSH precursor CysGly by cultured neurons. J. Neurosci. Res. 66 1003–1008. 10.1002/jnr.10042 [DOI] [PubMed] [Google Scholar]
- Dringen R., Pawlowski P. G., Hirrlinger J. (2005). Peroxide detoxification by brain cells. J. Neurosci. Res. 79 157–165. 10.1002/jnr.20280 [DOI] [PubMed] [Google Scholar]
- Dringen R., Pfeiffer B., Hamprecht B. (1999). Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J. Neurosci. 19 562–569. 10.1523/JNEUROSCI.19-02-00562.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drulis-Fajdasz D., Gizak A., Wójtowicz T., Wiśniewski J. R., Rakus D. (2018). Aging-associated changes in hippocampal glycogen metabolism in mice. Evidence for and against astrocyte-to-neuron lactate shuttle. Glia 66 1481–1495. 10.1002/glia.23319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L., Allen G. F. G., Mamais A., Ling H., Li A., Duberley K. E., et al. (2014). Dysregulation of glucose metabolism is an early event in sporadic Parkinson’s disease. Neurobiol. Aging 35 1111–1115. 10.1016/j.neurobiolaging.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S., Oshiro N., Miyamoto T., Yoshino K.-I., Okamoto S., Ono T., et al. (2009). AMP-activated protein kinase phosphorylates glutamine: fructose-6-phosphate amidotransferase 1 at Ser243 to modulate its enzymatic activity. Genes Cells 14 179–189. 10.1111/j.1365-2443.2008.01260.x [DOI] [PubMed] [Google Scholar]
- Escartin C., Won S. J., Malgorn C., Auregan G., Berman A. E., Chen P.-C., et al. (2011). Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J. Neurosci. 31 7392–7401. 10.1523/JNEUROSCI.6577-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falone S., D’Alessandro A., Mirabilio A., Petruccelli G., Cacchio M., Di Ilio C., et al. (2012). Long term running biphasically improves methylglyoxal-related metabolism, redox homeostasis and neurotrophic support within adult mouse brain cortex. PLoS One 7:e31401. 10.1371/journal.pone.0031401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawver J. N., Schall H. E., Petrofes Chapa R. D., Zhu X., Murray I. V. J. (2012). Amyloid-β Metabolite sensing: biochemical linking of glycation modification and misfolding. J. Alzheimers Dis. 30 63–73. 10.3233/JAD-2012-112114 [DOI] [PubMed] [Google Scholar]
- Fica-Contreras S. M., Shuster S. O., Durfee N. D., Bowe G. J. K., Henning N. J., Hill S. A., et al. (2017). Glycation of Lys-16 and Arg-5 in amyloid-β and the presence of Cu2+play a major role in the oxidative stress mechanism of Alzheimer’s disease. J. Biol. Inorg. Chem. 22 1211–1222. 10.1007/s00775-017-1497-5 [DOI] [PubMed] [Google Scholar]
- Frade J., Pope S., Schmidt M., Dringen R., Barbosa R., Pocock J., et al. (2008). Glutamate induces release of glutathione from cultured rat astrocytes–a possible neuroprotective mechanism? J. Neurochem. 105 1144–1152. 10.1111/j.1471-4159.2008.05216.x [DOI] [PubMed] [Google Scholar]
- Freitas H. R., Ferraz G., Ferreira G. C., Ribeiro-Resende V. T., Chiarini L. B., do Nascimento J. L. M., et al. (2016). Glutathione-induced calcium shifts in chick retinal glial cells. PLoS One 11:e0153677. 10.1371/journal.pone.0153677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde M. K., Evison E., Evans G. M. (1983). The rate of utilization of glucose via hexosemonophosphate shunt in brain. J. Neurochem. 41 1253–1260. 10.1111/j.1471-4159.1983.tb00819.x [DOI] [PubMed] [Google Scholar]
- Gibbs M. E., Hertz L. (2008). Inhibition of astrocytic energy metabolism by d-lactate exposure impairs memory. Neurochem. Int. 52 1012–1018. 10.1016/j.neuint.2007.10.014 [DOI] [PubMed] [Google Scholar]
- Giustarini D., Rossi R., Milzani A., Colombo R., Dalle-Donne I. (2004). S-glutathionylation: from redox regulation of protein functions to human diseases. J. Cell. Mol. Med. 8 201–212. 10.1111/j.1582-4934.2004.tb00275.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozlan H., Ben-Ari Y. (1995). NMDA receptor redox sites: are they targets for selective neuronal protection? Trends Pharmacol. Sci. 16 368–374. 10.1016/S0165-6147(00)89077-X [DOI] [PubMed] [Google Scholar]
- Griffith O. W. (1999). Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 27 922–935. 10.1016/S0891-5849(99)00176-8 [DOI] [PubMed] [Google Scholar]
- Guo N., Shaw C. (1992). Characterization and localization of glutathione binding sites on cultured astrocytes. Brain Res. Mol. Brain Res. 15 207–215. 10.1016/0169-328X(92)90110-W [DOI] [PubMed] [Google Scholar]
- Guo X., Jiang Q., Tuccitto A., Chan D., Alqawlaq S., Won G.-J., et al. (2018). The AMPK-PGC-1α signaling axis regulates the astrocyte glutathione system to protect against oxidative and metabolic injury. Neurobiol. Dis. 113 59–69. 10.1016/j.nbd.2018.02.004 [DOI] [PubMed] [Google Scholar]
- Haas R., Cucchi D., Smith J., Pucino V., Macdougall C. E., Mauro C. (2016). Intermediates of metabolism: from bystanders to signalling molecules. Trends Biochem. Sci. 41 460–471. 10.1016/j.tibs.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Krause M. W., Love D. C. (2010). The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta 1800 80–95. 10.1016/j.bbagen.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen F., Battú C. E., Dutra M. F., Galland F., Lirio F., Broetto N., et al. (2016a). Methylglyoxal and carboxyethyllysine reduce glutamate uptake and S100B secretion in the hippocampus independently of RAGE activation. Amino Acids 48 375–385. 10.1007/s00726-015-2091-1 [DOI] [PubMed] [Google Scholar]
- Hansen F., Pandolfo P., Galland F., Torres F. V., Dutra M. F., Batassini C., et al. (2016b). Methylglyoxal can mediate behavioral and neurochemical alterations in rat brain. Physiol. Behav. 164 93–101. 10.1016/j.physbeh.2016.05.046 [DOI] [PubMed] [Google Scholar]
- Hansen F., Galland F., Lirio F., De Souza D. F., Da Ré C., Pacheco R. F., et al. (2017). Methylglyoxal induces changes in the glyoxalase system and impairs glutamate uptake activity in primary astrocytes. Oxid. Med. Cell. Longev. 2017:9574201. 10.1155/2017/9574201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-López G., Galván E. J. (2018). Modulation of hippocampal excitability via the hydroxycarboxylic acid receptor 1. Hippocampus 28 557–567. 10.1002/hipo.22958 [DOI] [PubMed] [Google Scholar]
- Herrero-Mendez A., Almeida A., Fernández E., Maestre C., Moncada S., Bolaños J. P. (2009). The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat. Cell Biol. 11 747–752. 10.1038/ncb1881 [DOI] [PubMed] [Google Scholar]
- Hertz L., Chen Y. (2018). Glycogenolysis, an astrocyte-specific reaction, is essential for both astrocytic and neuronal activities involved in learning. Neuroscience 370 27–36. 10.1016/j.neuroscience.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Hertz L., Song D., Xu J., Peng L., Gibbs M. E. (2015). Role of the astrocytic Na(+), K(+)-ATPase in K(+) homeostasis in brain: K(+) uptake, signaling pathways and substrate utilization. Neurochem. Res. 40 2505–2516. 10.1007/s11064-014-1505-x [DOI] [PubMed] [Google Scholar]
- Hertz L., Zielke H. R. (2004). Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 27 735–743. 10.1016/j.tins.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Hipkiss A. R. (2014). Aging risk factors and Parkinson’s disease: contrasting roles of common dietary constituents. Neurobiol. Aging 35 1469–1472. 10.1016/j.neurobiolaging.2013.11.032 [DOI] [PubMed] [Google Scholar]
- Hoffman N. J., Parker B. L., Chaudhuri R., Fisher-Wellman K. H., Kleinert M., Humphrey S. J., et al. (2015). Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metab. 22 922–935. 10.1016/j.cmet.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Shimoji S., Hart G. W. (2010). Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 584 2526–2538. 10.1016/j.febslet.2010.04.044 [DOI] [PubMed] [Google Scholar]
- Hurley R. L., Barré L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., et al. (2006). Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J. Biol. Chem. 281 36662–36672. 10.1074/jbc.M606676200 [DOI] [PubMed] [Google Scholar]
- Ito K., Sawada Y., Sugiyama Y., Suzuki H., Hanano M., Iga T. (1994). Linear relationship between GABAA receptor occupancy of muscimol and glucose metabolic response in the conscious mouse brain. Clinical implication based on comparison with benzodiazepine receptor agonist. Drug Metab. Dispos. 22 50–54. [PubMed] [Google Scholar]
- Jakubcakova V., Curzi M. L., Flachskamm C., Hambsch B., Landgraf R., Kimura M. (2013). The glycolytic metabolite methylglyoxal induces changes in vigilance by generating low-amplitude non-REM sleep. J. Psychopharmacol. 27 1070–1075. 10.1177/0269881113495596 [DOI] [PubMed] [Google Scholar]
- Jalloh I., Carpenter K. L. H., Grice P., Howe D. J., Mason A., Gallagher C. N., et al. (2015). Glycolysis and the pentose phosphate pathway after human traumatic brain injury: microdialysis studies using 1,2-(13)C2 glucose. J. Cereb. Blood Flow Metab. 35 111–120. 10.1038/jcbfm.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janáky R., Dohovics R., Saransaari P., Oja S. S. (2007). Modulation of [3H]dopamine release by glutathione in mouse striatal slices. Neurochem. Res. 32 1357–1364. 10.1007/s11064-007-9315-z [DOI] [PubMed] [Google Scholar]
- Janáky R., Shaw C. A., Varga V., Hermann A., Dohovics R., Saransaari P., et al. (2000). Specific glutathione binding sites in pig cerebral cortical synaptic membranes. Neuroscience 95 617–624. 10.1016/S0306-4522(99)00442-X [DOI] [PubMed] [Google Scholar]
- Janáky R., Varga V., Oja S. S., Saransaari P. (1994). Release of [3H]GABA evoked by glutamate agonists from hippocampal slices: effects of dithiothreitol and glutathione. Neurochem. Int. 24 575–582. 10.1016/0197-0186(94)90010-8 [DOI] [PubMed] [Google Scholar]
- Janáky R., Varga V., Saransaari P., Oja S. S. (1993). Glutathione modulates the N-methyl-D-aspartate receptor-activated calcium influx into cultured rat cerebellar granule cells. Neurosci. Lett. 156 153–157. 10.1016/0304-3940(93)90461-S [DOI] [PubMed] [Google Scholar]
- Janson J., Laedtke T., Parisi J. E., O’Brien P., Petersen R. C., Butler P. C. (2004). Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53 474–481. 10.2337/diabetes.53.2.474 [DOI] [PubMed] [Google Scholar]
- Jenkins C. M., Yang J., Sims H. F., Gross R. W. (2011). Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J. Biol. Chem. 286 11937–11950. 10.1074/jbc.M110.203661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Blasco D., Santofimia-Castaño P., Gonzalez A., Almeida A., Bolaños J. P. (2015). Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 22 1877–1889. 10.1038/cdd.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe E.-H., Choi D.-J., An J., Eun J.-H., Jou I., Park S. (2018). Astrocytes, microglia, and Parkinson’s disease. Exp. Neurobiol. 27 77–87. 10.5607/en.2018.27.2.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukkola P., Gu C. (2015). Regulation of neurovascular coupling in autoimmunity to water and ion channels. Autoimmun. Rev. 14 258–267. 10.1016/j.autrev.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapos M. P. (2008). Methylglyoxal and glucose metabolism: a historical perspective and future avenues for research. Drug Metab. Drug Interact. 23 69–91. 10.1515/DMDI.2008.23.1-2.69 [DOI] [PubMed] [Google Scholar]
- Kang J. G., Park S. Y., Ji S., Jang I., Park S., Kim H. S., et al. (2009). O-GlcNAc protein modification in cancer cells increases in response to glucose deprivation through glycogen degradation. J. Biol. Chem. 284 34777–34784. 10.1074/jbc.M109.026351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita C., Aoyama K., Nakaki T. (2018). Neuroprotection afforded by circadian regulation of intracellular glutathione levels: a key role for miRNAs. Free Radic. Biol. Med. 119 17–33. 10.1016/j.freeradbiomed.2017.11.023 [DOI] [PubMed] [Google Scholar]
- Kondoh Y., Kawase M., Kawakami Y., Ohmori S. (1994). Concentrations of D-lactate and its related metabolic intermediates in liver, blood, and muscle of diabetic and starved rats. Res. Exp. Med. 192 407–414. 10.1016/S0300-9629(96)00166-1 [DOI] [PubMed] [Google Scholar]
- Krautwald M., Münch G. (2010). Advanced glycation end products as biomarkers and gerontotoxins - A basis to explore methylglyoxal-lowering agents for Alzheimer’s disease? Exp. Gerontol. 45 744–751. 10.1016/j.exger.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Lauritzen K. H., Morland C., Puchades M., Holm-Hansen S., Hagelin E. M., Lauritzen F., et al. (2014). Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb. Cortex 24 2784–2795. 10.1093/cercor/bht136 [DOI] [PubMed] [Google Scholar]
- Lee D. K., Nguyen T., Lynch K. R., Cheng R., Vanti W. B., Arkhitko O., et al. (2001). Discovery and mapping of ten novel G protein-coupled receptor genes. Gene 275 83–91. 10.1016/S0378-1119(01)00651-5 [DOI] [PubMed] [Google Scholar]
- Lee M., Cho T., Jantaratnotai N., Wang Y. T., McGeer E., McGeer P. L. (2010). Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J. 24 2533–2545. 10.1096/fj.09-149997 [DOI] [PubMed] [Google Scholar]
- Lehre K. P., Danbolt N. C. (1998). The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci. 18 8751–8757. 10.1523/JNEUROSCI.18-21-08751.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L., De Bock M., Van Moorhem M., Decrock E., De Vuyst E. (2007). Neurobarrier coupling in the brain: adjusting glucose entry with demand. J. Neurosci. Res. 85 3213–3220. 10.1002/jnr.21189 [DOI] [PubMed] [Google Scholar]
- Li G., Wang H. Q., Wang L. H., Chen R. P., Liu J. P. (2014). Distinct pathways of ERK1/2 activation by hydroxy-carboxylic acid receptor-1. PLoS One 9:e93041. 10.1371/journal.pone.0093041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Maloney R., Circu M., Alexander J., Aw T. (2013). Acute carbonyl stress induces occludin glycation and brain microvascular endothelial barrier dysfunction: role for glutathione-dependent metabolism of methylglyoxal. Free Radic. Biol. Med. 54 51–61. 10.1088/1367-2630/15/1/015008.Fluid [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Xie J. Z., Jiang X., Lv B. L., Cheng X. S., Du L. L., et al. (2012). Methylglyoxal induces tau hyperphosphorylation via promoting ages formation. NeuroMolecular Med. 14 338–348. 10.1007/s12017-012-8191-0 [DOI] [PubMed] [Google Scholar]
- Ling B., Peng F., Alcorn J., Lohmann K., Bandy B., Zello G. A. (2012). D-Lactate altered mitochondrial energy production in rat brain and heart but not liver. Nutr. Metab. 9:6. 10.1186/1743-7075-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Kuei C., Zhu J., Yu J., Zhang L., Shih A., et al. (2012). 3,5-Dihydroxybenzoic acid, a specific agonist for hydroxycarboxylic acid 1, inhibits lipolysis in adipocytes. J. Pharmacol. Exp. Ther. 341 794–801. 10.1124/jpet.112.192799 [DOI] [PubMed] [Google Scholar]
- Liu C., Wu J., Zhu J., Kuei C., Yu J., Shelton J., et al. (2009). Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 284 2811–2822. 10.1074/jbc.M806409200 [DOI] [PubMed] [Google Scholar]
- Lu S. C. (2013). Glutathione synthesis. Biochim. Biophys. Acta 1830 3143–3153. 10.1016/j.bbagen.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I., Li B., Xie L., Kang H., Sanggaard S., Haswell J. D. R., et al. (2015). Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat. Commun. 6:6807. 10.1038/ncomms7807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüth H. J., Ogunlade V., Kuhla B., Kientsch-Engel R., StahlÌ P., Webster J., et al. (2005). Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb. Cortex 15 211–220. 10.1093/cercor/bhh123 [DOI] [PubMed] [Google Scholar]
- Maessen D. E. M., Stehouwer C. D. A., Schalkwijk C. G. (2015). The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin. Sci. 128 839–861. 10.1042/CS20140683 [DOI] [PubMed] [Google Scholar]
- Magistretti P. J., Allaman I. (2018). Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19 235–249. 10.1038/nrn.2018.19 [DOI] [PubMed] [Google Scholar]
- Makar T. K., Nedergaard M., Preuss A., Gelbard A. S., Perumal A. S., Cooper A. J. (1994). Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J. Neurochem. 62 45–53. 10.1046/j.1471-4159.1994.62010045.x [DOI] [PubMed] [Google Scholar]
- Marosi K., Mattson M. P. (2014). BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 25 89–98. 10.1016/j.tem.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsin A. S., Bertrand L., Rider M. H., Deprez J., Beauloye C., Vincent M. F., et al. (2000). Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 10 1247–1255. 10.1016/S0960-9822(00)00742-9 [DOI] [PubMed] [Google Scholar]
- McGann J. C., Mandel G. (2018). Neuronal activity induces glutathione metabolism gene expression in astrocytes. Glia 66 2024–2039. 10.1002/glia.23455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Thomas S. D., Islam A., Muench D., Sedoris K. (2012). c-Myc and cancer metabolism. Clin. Cancer Res. 18 5546–5553. 10.1158/1078-0432.CCR-12-0977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor I., Cheung E. C., Vousden K. H. (2011). Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb. Symp. Quant. Biol. 76 211–216. 10.1101/sqb.2011.76.010868 [DOI] [PubMed] [Google Scholar]
- Morland C., Andersson K. A., Haugen Ø. P., Hadzic A., Kleppa L., Gille A., et al. (2017). Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 8:15557. 10.1038/ncomms15557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland C., Lauritzen K. H., Puchades M., Holm-Hansen S., Andersson K., Gjedde A., et al. (2015). The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J. Neurosci. Res. 93 1045–1055. 10.1002/jnr.23593 [DOI] [PubMed] [Google Scholar]
- Mosienko V., Teschemacher A. G., Kasparov S. (2015). Is L-lactate a novel signaling molecule in the brain? J. Cereb. Blood Flow Metab. 35 1069–1075. 10.1038/jcbfm.2015.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch G., Thome J., Foley P., Schinzel R., Riederer P. (1997). Advanced glycation endproducts in ageing and Alzheimer’s disease. Brain Res. Rev. 23 134–143. 10.1016/S0165-0173(96)00016-1 [DOI] [PubMed] [Google Scholar]
- Muronetz V. I., Melnikova A. K., Seferbekova Z. N., Barinova K. V., Schmalhausen E. V. (2017). Glycation, glycolysis, and neurodegenerative diseases: is there any connection? Biochemistry 82 874–886. 10.1134/S0006297917080028 [DOI] [PubMed] [Google Scholar]
- Nagel A. K., Ball L. E. (2014). O-GlcNAc transferase and O-GlcNAcase: achieving target substrate specificity. Amino Acids 46 2305–2316. 10.1007/s00726-014-1827-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet I., Varga-Defterdarović L., Turk Z. (2006). Methylglyoxal in food and living organisms. Mol. Nutr. Food Res. 50 1105–1117. 10.1002/mnfr.200600065 [DOI] [PubMed] [Google Scholar]
- Niture S. K., Khatri R., Jaiswal A. K. (2014). Regulation of Nrf2-an update. Free Radic. Biol. Med. 66 36–44. 10.1016/j.freeradbiomed.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel L. F., Müller M. S., Walls A. B., Sickmann H. M., Bak L. K., Waagepetersen H. S., et al. (2012). Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front. Neuroenergetics 4:3. 10.3389/fnene.2012.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S. (2017). Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol. Metab. 28 227–236. 10.1016/j.tem.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Oliveira R. F., Terrin A., Di Benedetto G., Cannon R. C., Koh W., Kim M., et al. (2010). The role of type 4 phosphodiesterases in generating microdomains of cAMP: large scale stochastic simulations. PLoS One 5:e11725. 10.1371/journal.pone.0011725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottestad-Hansen S., Hu Q. X., Follin-Arbelet V. V., Bentea E., Sato H., Massie A., et al. (2018). The cystine-glutamate exchanger (xCT, Slc7a11) is expressed in significant concentrations in a subpopulation of astrocytes in the mouse brain. Glia 66 951–970. 10.1002/glia.23294 [DOI] [PubMed] [Google Scholar]
- Ozcan S., Andrali S. S., Cantrell J. E. L., Özcan S., Andrali S. S., Cantrell J. E. L. (2010). Modulation of transcription factor function by O-GlcNAc modification. Biochim. Biophys. Acta 1799 353–364. 10.1016/j.bbagrm.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z. H., Bähring R., Grantyn R., Lipton S. A. (1995). Differential modulation by sulfhydryl redox agents and glutathione of GABA- and glycine-evoked currents in rat retinal ganglion cells. J. Neurosci. 15 1384–1391. 10.1523/JNEUROSCI.15-02-01384.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar M. S., Kunz E. A., Brewer G. J. (2008). Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J. Neurosci. Res. 86 2339–2352. 10.1002/jnr.21679 [DOI] [PubMed] [Google Scholar]
- Park S. Y., Ryu J., Lee W. (2005). O-GlcNAc modification on IRS-1 and Akt2 by PUGNAc inhibits their phosphorylation and induces insulin resistance in rat primary adipocytes. Exp. Mol. Med. 37 220–229. 10.1038/emm.2005.30 [DOI] [PubMed] [Google Scholar]
- Parker G., Taylor R., Jones D., McClain D. (2004). Hyperglycemia and inhibition of glycogen synthase in streptozotocin-treated mice. J. Biol. Chem. 279 20636–20642. 10.1074/jbc.M312139200 [DOI] [PubMed] [Google Scholar]
- Parthoens J., Servaes S., Verhaeghe J., Stroobants S., Staelens S. (2015). Prelimbic cortical injections of a GABA agonist and antagonist: in vivo quantification of the effect in the rat brain using [(18)F] FDG MicroPET. Mol. Imaging Biol. 17 856–864. 10.1007/s11307-015-0859-z [DOI] [PubMed] [Google Scholar]
- Pastorino J. G., Hoek J. B. (2008). Regulation of hexokinase binding to VDAC. J. Bioenerg. Biomembr. 40 171–182. 10.1007/s10863-008-9148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B. D., Sbodio J. I., Snyder S. H. (2018). Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol. Sci. 39 513–524. 10.1016/j.tips.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L., Bouzier-Sore A. K., Aubert A., Serres S., Merle M., Costalat R., et al. (2007). Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55 1251–1262. 10.1002/glia.20528 [DOI] [PubMed] [Google Scholar]
- Pellerin L., Magistretti P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U.S.A. 91 10625–10629. 10.1073/pnas.91.22.10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Escuredo J., Dadhich R. K., Dhup S., Cacace A., Van Hée V. F., De Saedeleer C. J., et al. (2016). Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle 15 72–83. 10.1080/15384101.2015.1120930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R., Le Bars D., Cinotti L., Garcia-Larrea L., Galy G., Landais P., et al. (1994). Effects of GABAA receptors activation on brain glucose metabolism in normal subjects and temporal lobe epilepsy (TLE) patients. A positron emission tomography (PET) study. Part I: brain glucose metabolism is increased after GABAA receptors activation. Epilepsy Res. 19 45–54. 10.1016/0920-1211(94)90087-6 [DOI] [PubMed] [Google Scholar]
- Rabbani N., Thornalley P. J. (2012). Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 42 1133–1142. 10.1007/s00726-010-0783-0 [DOI] [PubMed] [Google Scholar]
- Rao X., Duan X., Mao W., Li X., Li Z., Li Q., et al. (2015). O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat. Commun. 6:8468. 10.1038/ncomms9468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ré D. B., Boucraut J., Samuel D., Birman S., Kerkerian-Le Goff L., Had-Aissouni L. (2003). Glutamate transport alteration triggers differentiation-state selective oxidative death of cultured astrocytes: a mechanism different from excitotoxicity depending on intracellular GSH contents: astrocyte death by glutamate transport alteration. J. Neurochem. 85 1159–1170. 10.1046/j.1471-4159.2003.01752.x [DOI] [PubMed] [Google Scholar]
- Reichelt W., Stabel-Burow J., Pannicke T., Weichert H., Heinemann U. (1997). The glutathione level of retinal Müller glial cells is dependent on the high-affinity sodium-dependent uptake of glutamate. Neuroscience 77 1213–1224. 10.1016/S0306-4522(96)00509-X [DOI] [PubMed] [Google Scholar]
- Ren X., Zou L., Zhang X., Branco V., Wang J., Carvalho C., et al. (2017). Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid. Redox Signal. 27 989–1010. 10.1089/ars.2016.6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas C. I., Zúñiga F. A., Salas-Burgos A., Mardones L., Ormazabal V., Vera J. C. (2008). Vitamin C transporters. J. Physiol. Biochem. 64 357–375. 10.1007/BF03174092 [DOI] [PubMed] [Google Scholar]
- Ros J., Pecinska N., Alessandri B., Landolt H., Fillenz M. (2001). Lactate reduces glutamate-induced neurotoxicity in rat cortex. J. Neurosci. Res. 66 790–794. 10.1002/jnr.10043 [DOI] [PubMed] [Google Scholar]
- Rosa A. P., Jacques C. E. D., de Souza L. O., Bitencourt F., Mazzola P. N., Coelho J. G., et al. (2015). Neonatal hyperglycemia induces oxidative stress in the rat brain: the role of pentose phosphate pathway enzymes and NADPH oxidase. Mol. Cell. Biochem. 403 159–167. 10.1007/s11010-015-2346-x [DOI] [PubMed] [Google Scholar]
- Rose C. R., Felix L., Zeug A., Dietrich D., Reiner A., Henneberger C. (2017). Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 10:451 10.3389/fnmol.2017.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M., Lee Y.-H. (2014). PFKFB3 regulates oxidative stress homeostasis via its S-glutathionylation in cancer. J. Mol. Biol. 426 830–842. 10.1016/j.jmb.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K., DeSilva S., Abbruscato T. (2012). The role of glucose transporters in brain disease: diabetes and Alzheimer’s disease. Int. J. Mol. Sci. 13 12629–12655. 10.3390/ijms131012629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G., Allen J. W., Mutkus L. A., Aschner M. (2001). The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 902 156–163. 10.1016/S0006-8993(01)02342-3 [DOI] [PubMed] [Google Scholar]
- Shaw C. A., Pasqualotto B. A., Curry K. (1996). Glutathione-induced sodium currents in neocortex. Neuroreport 7 1149–1152. 10.1097/00001756-199604260-00010 [DOI] [PubMed] [Google Scholar]
- Shen Z., Jiang L., Yuan Y., Deng T., Zheng Y. R., Zhao Y. Y., et al. (2015). Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS Neurosci. Ther. 21 271–279. 10.1111/cns.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A. Y., Johnson D. A., Wong G., Kraft A. D., Jiang L., Erb H., et al. (2003). Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 23 3394–3406. 10.1523/JNEUROSCI.23-08-03394.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Routh V. H. (2005). Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 54 15–22. 10.2337/diabetes.54.1.15 [DOI] [PubMed] [Google Scholar]
- Sotelo-Hitschfeld T., Fernández-Moncada I., Barros L. F. (2012). Acute feedback control of astrocytic glycolysis by lactate. Glia 60 674–680. 10.1002/glia.22304 [DOI] [PubMed] [Google Scholar]
- Sotelo-Hitschfeld T., Niemeyer M. I., Mächler P., Ruminot I., Lerchundi R., Wyss M. T., et al. (2015). Channel-mediated lactate release by K+-stimulated astrocytes. J. Neurosci. 35 4168–4178. 10.1523/JNEUROSCI.5036-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth V., Westcott B., Forbes J., Phan T. G., Beare R., Venn A., et al. (2013). Methylglyoxal, cognitive function and cerebral atrophy in older people. J. Gerontol. A Biol. Sci. Med. Sci. 68 68–73. 10.1093/gerona/gls100 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Stern S. A., Bozdagi O., Huntley G. W., Ruth H., Magistretti P. J., et al. (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144 810–823. 10.1016/j.cell.2011.02.018.Astrocyte-neuron [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy M., Pathak S., Grzes K. M., Damerow S., Sinclair L. V., van Aalten D. M. F., et al. (2016). Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 17 712–720. 10.1038/ni.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Izawa Y., Suzuki N. (2012). Astroglial pentose phosphate pathway rates in response to high-glucose environments. ASN Neuro 4:AN20120002. 10.1042/AN20120002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Lane S., Korsak A., Paton J. F., Gourine A. V., Kasparov S., et al. (2014). Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 5:3284. 10.1038/ncomms4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H., Zhou X., Zhao B., Li K. (2018). Conflicting effects of methylglyoxal and potential significance of miRNAs for seizure treatment. Front. Mol. Neurosci. 11:70. 10.3389/fnmol.2018.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. P., Parker G. J., Hazel M. W., Soesanto Y., Fuller W., Yazzie M. J., et al. (2008). Glucose deprivation stimulates O-GlcNAc modification of proteins through up-regulation of O-linked N-acetylglucosaminyltransferase. J. Biol. Chem. 283 6050–6057. 10.1074/jbc.M707328200 [DOI] [PubMed] [Google Scholar]
- Thimmulappa R. K., Mai K. H., Srisuma S., Kensler T. W., Yamamoto M., Biswal S. (2002). Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 62 5196–5203. [PubMed] [Google Scholar]
- Thorn T. L., He Y., Jackman N. A., Lobner D., Hewett J. A., Hewett S. J. (2015). A cytotoxic, Co-operative interaction between energy deprivation and glutamate release from system xc- mediates aglycemic neuronal cell death. ASN Neuro 7:1759091415614301. 10.1177/1759091415614301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V., Jenei Z., Janáky R., Saransaari P., Oja S. S. (1997). Glutathione is an endogenous ligand of rat brain N-methyl-D-aspartate (NMDA) and 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors. Neurochem. Res. 22 1165–1171. 10.1023/A:1027377605054 [DOI] [PubMed] [Google Scholar]
- Vicente Miranda H., El-Agnaf O. M., Outeiro T. F. (2016). Glycation in Parkinson’s disease and Alzheimer’s disease. Mov. Disord. 31 782–790. 10.1002/mds.26566 [DOI] [PubMed] [Google Scholar]
- Waitt A. E., Reed L., Ransom B. R., Brown A. M. (2017). Emerging roles for glycogen in the CNS. Front. Mol. Neurosci. 10:73 10.3389/fnmol.2017.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamelink M. M. C., Struys E. A., Jakobs C. (2008). The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J. Inherit. Metab. Dis. 31 703–717. 10.1007/s10545-008-1015-6 [DOI] [PubMed] [Google Scholar]
- Wang Z., Pandey A., Hart G. W. (2007). Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol. Cell. Proteomics 6 1365–1379. 10.1074/mcp.M600453-MCP200 [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Metodiewa D. (1994). The reaction of superoxide with reduced glutathione. Arch. Biochem. Biophys. 314 284–290. 10.1006/abbi.1994.1444 [DOI] [PubMed] [Google Scholar]
- Worth M., Li H., Jiang J. (2017). Deciphering the functions of protein O -GlcNAcylation with chemistry. ACS Chem. Biol. 12 326–335. 10.1021/acschembio.6b01065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Jin N., Gu J., Shi J., Sun J., Chu D., et al. (2016). O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging Cell 15 455–464. 10.1111/acel.12449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ruchti E., Petit J. M., Jourdain P., Grenningloh G., Allaman I., et al. (2014). Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. U.S.A. 111 12228–12233. 10.1073/pnas.1322912111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Qian K. (2017). Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 18 452–465. 10.1038/nrm.2017.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-J., Wu P.-F., Long L.-H., Yu D.-F., Wu W.-N., Hu Z.-L., et al. (2010). Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging Cell 9 709–721. 10.1111/j.1474-9726.2010.00595.x [DOI] [PubMed] [Google Scholar]
- Yi J.-H., Hazell A. S. (2006). Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 48 394–403. 10.1016/j.neuint.2005.12.001 [DOI] [PubMed] [Google Scholar]
- Yi W., Clark P. M., Mason D. E., Keenan M. C., Hill C., Goddard W. A., III, et al. (2012). Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337 975–980. 10.1126/science.1222278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A. S., Hirayama B. A., Timbol G., Liu J., Basarah E., Kepe V., et al. (2010). Functional expression of SGLTs in rat brain. Am. J. Physiol. Cell Physiol. 299 C1277–C1284. 10.1152/ajpcell.00296.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa S. A., Vocadlo D. J. (2014). O-GlcNAc and neurodegeneration: biochemical mechanisms and potential roles in Alzheimer’s disease and beyond. Chem. Soc. Rev. 43 6839–6858. 10.1039/C4CS00038B [DOI] [PubMed] [Google Scholar]
- Zängerle L., Cuénod M., Winterhalter K. H., Do K. Q. (1992). Screening of thiol compounds: depolarization-induced release of glutathione and cysteine from rat brain slices. J. Neurochem. 59 181–189. 10.1111/j.1471-4159.1992.tb08889.x [DOI] [PubMed] [Google Scholar]
- Zerangue N., Kavanaugh M. P. (1996). Interaction of L-cysteine with a human excitatory amino acid transporter. J. Physiol. 493(Pt 2), 419–423. 10.1113/jphysiol.1996.sp021393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Ai Z., Chen J., Yi J., Liu Z., Zhao H., et al. (2017). Glycometabolic adaptation mediates the insensitivity of drug-resistant K562/ADM leukaemia cells to adriamycin via the AKT-mTOR/c-Myc signalling pathway. Mol. Med. Rep. 15 1869–1876. 10.3892/mmr.2017.6189 [DOI] [PubMed] [Google Scholar]
- Zippin J. H., Farrell J., Huron D., Kamenetsky M., Hess K. C., Fischman D. A., et al. (2004). Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell Biol. 164 527–534. 10.1083/jcb.200311119 [DOI] [PMC free article] [PubMed] [Google Scholar]