Abstract
Particulate matter (PM) is a ubiquitous air pollutant that is a growing public health concern. Previous studies have suggested that PM is associated with asthma development and exacerbation of asthma symptoms. Although several studies have suggested increased risks of atopic dermatitis, allergic rhinitis, and allergic sensitization in relation to PM exposure, the evidence remains inconsistent. The plausible mechanisms underlying these effects are related to oxidative stress, enhancement of sensitization to allergens, inflammatory and immunological responses, and epigenetics. This review discusses the effect of PM on childhood allergic diseases, along with plausible mechanisms. Further studies are required to understand the role of PM exposure on childhood allergic diseases, to reduce these diseases in children.
Keywords: Particulate matter, Asthma, Allergy, Child
Introduction
Air pollution has been suggested as an important environmental risk factor for allergic diseases. Among air pollutants, particulate matter (PM) is a growing public health concern. PM is a complex and heterogeneous mixture of tiny solid or liquid particles suspended in a gas [1] that is generated from fossil fuel combustion by road transport and power plants; industrial processes, such as the production of metals, cement, lime, and chemicals; construction work; cigarette smoking; and wood stove burning [1]. PM contains acids, organic chemicals, hydrocarbons, metals, and biological material (e.g., endotoxins, allergens, and pollens) [1-3]. PM is categorized according to its aerodynamic diameter: PM10 (smaller than 10 μm), coarse PM (ranging from 2.5 to 10 μm), PM2.5 (smaller than 2.5 μm), and ultrafine PM (smaller than 0.1 μm) [2] Several studies have investigated the relationship between allergic diseases and PM. In this review, we discuss the effects of PM on childhood allergic diseases and the possible mechanisms.
Association of PM and childhood allergic diseases in epidemiologic studies
1. Asthma
1) Asthma incidence and prevalence (Table 1)
Table 1.
Particulate matter and asthma incidence and prevalence
| Study | Country | Pollutants | Exposure time | Outcomes |
|---|---|---|---|---|
| Gehring et al. 2010 [4] | Netherlands | PM2.5 | Birth addresses | Increased asthma incidence (aOR, 1.28; 95% CI, 1.10–1.49) and prevalence (aOR, 1.26; 95% CI, 1.04–1.51) during the first 8 years of life |
| Gruzieva et al. 2013 [5] | Sweden | PM2.5 | During the first year of life | Increased asthma incidence (OR, 2.39; 95% CI, 1.18–4.86) and prevalence (OR, 1.96; 95% CI, 1.08–3.53) during the first 12 years of life |
| Clark et al. 2010 [6] | Canada | PM2.5 | During pregnancy and the first year of life | Increased asthma incidence up to 3–4 years by the exposures during pregnancy (aOR,1.09; 95% CI, 1.05–1.13) and the first year of life (aOR,1.07; 95% CI, 1.03–1.12) |
| Tetreault et al. 2016 [7] | Canada | PM2.5 | Birth addresses | Increased asthma incidence (HR, 1.31; 95% CI, 1.28–1.33) |
| Hsu et al. 2015 [8] | USA | PM2.5 | During 16–25 weeks of gestation | Increased asthma onset by the age of 6 years in boys |
| Carlsten et al. 2011 [9] | Canada | PM2.5 | Birth addresses | Increased asthma onset (OR, 3.1; 95% CI, 1.3–7.4) and bronchial hyperresponsiveness at the age of 7 years in children with a family history of asthma |
| Hehua et al. 2017 [10] | Meta-analysis | PM2.5 | During pregnancy | Increased asthma development (OR, 1.08; 95% CI, 1.05–1.12) |
| Khreis et al. 2017 [11] | Meta-analysis | PM2.5, PM2.5 | Increased asthma development (overall risk estimate 1.03; 95% CI, 1.01–1.05 for PM2.5 and overall risk estimate 1.05; 95% CI, 1.02–1.08 for PM2.5) | |
| Sbihi et al. 2016 [12] | Canada | PM2.5 | During pregnancy | Increased asthma onset in pre-school children (age <6 years: aOR, 1.12; 95% CI, 1.05–1.19), not in school age children (age ≥6 years: aOR, 1.09; 95% CI, 0.96–1.24) |
| Nishimura et al. 2013 [13] | USA | PM2.5, PM2.5 | During the first year and first 3 years of life | Not associated with childhood asthma onset |
| McConnell et al. 2010 [14] | USA | PM2.5, PM2.5 | Current exposure | Not associated with new-onset asthma during 3 years of follow-up |
| Gehring et al. 2015 [15] | Four European birth cohort | PM2.5 | Birth and current addresses | Increased asthma incidence by the exposure at the birth addresses (aOR, 1.29; 95% CI, 1.00–1.66), but not at the current addresses. Not associated with asthma prevalence |
| Molter et al. 2014 [16] | UK | PM2.5 | During the first year of life and lifetime | Not associated with asthma prevalence |
| Hoek et al. 2012 [17] | Meta-analysis | PM2.5 | Not associated with asthma prevalence | |
| Molter et al. 2015 [18] | Meta-analysis | PM2.5, PM2.5 | Birth and current addresses | Not associated with asthma prevalence |
PM, particulate matter; aOR, adjusted odds ratio; CI, confidence interval; HR, hazard ratio.
Several birth cohort studies have reported a positive association between increased PM and asthma incidence [4-7]. A birth cohort study in the United States (US) demonstrated that increased prenatal exposure to PM2.5 during 16–25 weeks of gestation is associated with asthma onset by the age of 6 years in boys [8]. In addition, PM2.5 exposure at the birth address increases the risk of new-onset asthma (odds ratio [OR], 3.1; 95% confidence interval [CI], 1.3–7.4) and bronchial hyperresponsiveness at the age of 7 years in children with a family history of asthma [9]. In a systematic review, prenatal exposure to PM10 was found to be significantly related to asthma development (OR, 1.08; 95% CI, 1.05–1.12) but not exposure to PM2.5 (OR, 1.02; 95% CI, 0.97–1.03) [10]. Another meta-analysis also showed an association between increased PM2.5 and PM10 exposure and asthma development (for 1 μg/m3 PM2.5, overall risk estimate, 1.03; 95% CI, 1.01–1.05 and for 2 μg/m3 PM10, overall risk estimate 1.05; 95% CI, 1.02–1.08) [11].
Other cohort studies have not found a significant association between PM and asthma incidence. For example, a Canadian cohort study found that PM10 exposure during pregnancy increased asthma onset in young children (age <6 years: adjusted OR [aOR], 1.12; 95% CI, 1.05–1.19), whereas it did not affect school-aged children (age ≥6 years: aOR, 1.09; 95% CI, 0.96–1.24) [12]. Moreover, PM2.5 and PM10 exposure during the first year of life and the first 3 years of life were not associated with childhood asthma onset in Latino and African American populations [13]. In a cohort of 2,497 children in kindergarten and first grade in the US, new-onset asthma was not associated with PM2.5 and PM10 during 3 years of follow-up [14]. In a population-based birth cohort study, an increased risk of asthma incidence (up to age 14–16 years) was associated with increased exposure to PM2.5 absorbance at the birth address, but not at the current address [15]. There were also no significant associations between PM2.5 absorbance and asthma prevalence in that study [15]. A British birth cohort study showed that asthma prevalence from birth to 11 years of age was not associated with PM10 exposure during the first year of life and throughout the lifetime [16]. Further, a meta-analysis of cross-sectional studies conducted in 12 European countries showed no association between PM10 and asthma prevalence [17]. The European Study of Cohorts for Air Pollution Effects project found no significant association between PM exposure and asthma prevalence in 5 European birth cohorts [18]. The reasons for these differing results among epidemiologic studies may arise from differences in exposure and outcome measurement, study population, and study design.
2) Asthma exacerbation (Table 2)
Table 2.
Particulate matter and asthma exacerbation
| Study | Country | Pollutants | Outcomes |
|---|---|---|---|
| Son et al. 2013 [19] | Korea | PM10 | Increased asthma-related hospitalization (2.4%) |
| Mazenq et al. 2017 [20] | France | PM10 | Increased asthma-related hospitalization (aOR, 1.02; 95% CI, 1.01–1.04) |
| Silverman et al. 2010 [21] | USA | PM2.5 | Increased ICU admissions (26%) and general hospitalizations (19%) |
| O'Connor et al. 2008 [22] | USA | PM2.5 | Lower FEV1 and PEF in children with moderate-to-severe asthma |
| Orellano et al. 2017 [23] | Meta-analysis | PM2.5 | Exacerbation of asthma (OR, 1.022; 95% CI, 1.000–1.045) |
| Zheng et al. 2015 [24] | Meta-analysis | PM2.5, PM10 | Increased emergency room visits and hospital admissions (aOR, 1.025; 95% CI, 1.013–1.037 for PM2.5 and aOR, 1.013; 95% CI, 1.008–1.018 for PM10) |
| Weinmayr et al. 2010 [25] | Meta-analysis | PM10 | Increased asthma symptoms (2.8%) and decreases PEF (-0.082 L/min) |
PM, particulate matter; aOR, adjusted odds ratio; CI, confidence interval; ICU, intensive care unit; FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow.
Several studies have reported an association between increased PM levels and the exacerbation of asthma-related symptoms, with a high degree of consistency [19-25]. In a multicity study in Korea, an interquartile range (IQR) increase in PM10 (30.7 μg/m3) resulted in an increase of 2.4% in asthma-related hospitalization among children aged 0–14 years [19]. PM10 was also found to increase the risk of asthma-related hospitalization (aOR, 1.02; 95% CI, 1.01–1.04) in a nested case–control study of children aged 3–18 years in France [20]. An observational time-series analysis in the US showed a 26% increase in intensive care unit admissions and a 19% increase in general hospitalizations for each 12-μg/m3 increase in PM2.5 [21]. The Inner-City Asthma Study in the US revealed that higher PM2.5 is associated with significantly lower forced expiratory volume in 1 second and peak expiratory flow (PEF) in children with moderateto-severe asthma [22]. A meta-analysis revealed a significant association between PM2.5 levels and moderate or severe exacerbation of asthma (OR, 1.022; 95% CI, 1.000–1.045) [23]. Another meta-analysis showed that a significant relationship exists between exposure to PM10 (aOR, 1.013; 95% CI, 1.008–1.018) and PM2.5 (aOR, 1.025; 95% CI, 1.013–1.037),with increased emergency room visits and hospital admissions [24]. Another meta-analysis found that an increase of 10 μg/m3 in PM10 increases asthma symptoms by 2.8% and decreases PEF by 0.082 L/min [25].
2. Allergic rhinitis (Table 3)
Table 3.
Particulate matter and allergic rhinitis
| Study | Country | Pollutants | Exposure time | Outcomes |
|---|---|---|---|---|
| Wang et al. 2016 [26] | Taiwan | PM2.5 | Lifetime | Increased lifetime AR (OR, 1.54; 95% CI, 1.03–2.32) |
| Wood et al. 2015 [27] | UK | PM2.5, PM10 | Annual mean concentrations | Increased current AR (OR, 1.38; 95% CI, 1.08–1.78 for PM2.5 and OR, 1.16; 95% CI, 1.04–1.28 for PM10) |
| Penard-Morand et al. 2010 [28] | France | PM10 | 3-year average concentration | Increased lifetime AR (OR, 1.20; 95% CI, 1.01–1.44) |
| Deng et al. 2016 [29] | China | PM10 | During the first year of life | Increased lifetime AR (OR, 1.54; 95% CI, 1.07–2.21) |
| Fuertes et al. 2013 [30] | meta-analysis | PM2.5 | Birth addresses | Increased AR at the age of 7–8 years (OR, 1.37; 95% CI, 1.01-1.86) |
| Liu et al. 2013 [31] | China | PM10 | 3-year average concentration | Not associated with lifetime AR |
| Hwang et al. 2006 [32] | Taiwan | PM10 | Annual mean concentrations | Not associated with AR prevalence |
| Deng et al. 2016 [33] | China | PM10 | During pregnancy | Not associated with AR incidence |
| Hoek et al. 2012 [17] | Meta-analysis | PM10 | Not associated with AR diagnosis | |
| Gehring et al. 2015 [15] | Four European birth cohort | PM2.5, PM10 | Birth and current addresses | Not associated with AR incidence and prevalence |
PM, particulate matter; AR, allergic rhinitis; OR, odds ratio; CI, confidence interval.
The role of PM in childhood allergic rhinitis (AR) is not well established. Although some epidemiologic studies suggest increased risks of AR in relation to PM exposure, the evidence remains inconsistent. Different AR definitions, exposure assessments, and study designs might explain these inconsistent results. In a crosssectional study of 2,661 kindergarten children in Taiwan, lifetime PM2.5 exposure increased the risk of lifetime AR (OR, 1.54; 95% CI, 1.03–2.32) [26]. Annual mean exposure to PM10 (OR, 1.16; 95% CI, 1.04–1.28) and PM2.5 (OR, 1.38; 95% CI, 1.08–1.78) was associated with current AR in children aged 8–9 years, in a cross-sectional study in England [27]. Another cross-sectional study in France showed that the 3-year average concentration of PM10 (OR, 1.20; 95% CI, 1.01–1.44) was associated with increased lifetime AR in children aged 9–11 years [28]. A cohort study of 2,598 children aged 3–6 years in China showed that PM10 exposure during the first year of life increased lifetime AR (OR, 1.54; 95% CI, 1.07–2.21) [29]. A pooled meta-analysis of 6 birth cohorts showed a significant association between PM2.5 exposure at the birth address and AR at the age of 7–8 years (OR, 1.37 per 5 μg/m3 PM2.5) [30].
Some studies have reported no association between PM and AR. Lifetime AR was not associated with PM10 in a study of 6,730 Chinese children aged 3–7 years [31]. AR prevalence was also found to not be associated with PM10 in a nationwide cross-sectional study among Taiwanese children aged 6–15 years [32]. No association between PM10 exposure during pregnancy and AR incidence in children aged 3–6 years was found in a prospective cohort study in China [33]. A meta-analysis of cross-sectional studies determined that there was no association between PM10 and AR diagnosis (OR, 1.20; 95% CI, 0.99–1.46) [17]. A population-based birth cohort study reported that no associations were identified between PM at the birth or current addresses and AR incidence or prevalence up to the age of 14–16 years [15].
3. Atopic dermatitis (Table 4)
Table 4.
Particulate matter and atopic dermatitis
| Study | Country | Pollutants | Exposure time | Outcomes |
|---|---|---|---|---|
| Kim et al. 2013 [34] | Korea | PM10 | Previous day | 1-μg/m3 increase in PM10 increased AD symptoms by 0.44% |
| Kim et al. 2017 [35] | Korea | PM10 | Same day exposure | 10-μg/m3 increase in PM10 increased AD symptoms by 3.2% |
| Kim et al. 2018 [36] | Korea | PM2.5, PM10 | Up to the previous 5 days | Increased AD symptoms (OR, 1.078; 95% CI, 1.018–1.141 for PM2.5 and OR, 1.063; 95% CI, 1.006–1.123 for PM10) |
| Song et al. 2011 [37] | Korea | Ultrafine particles | Previous day | Increased itch symptom score by 3.1% |
| Penard-Morand et al. 2010 [28] | France | PM10 | 3-year average concentration | Increased lifetime AD (OR, 1.13; 95% CI, 1.01–1.24) and 1-year history of AD (OR, 1.15; 95% CI, 1.03–1.33) |
| Lee et al. 2018 [38] | Korea | PM10 | First trimester of pregnancy | Increased AD development at the age of 6 months (OR, 1.219; 95% CI, 1.023–1.452) |
| Kathuria et al. 2016 [39] | US | PM2.5, PM10 | Mean annual concentrations | PM2.5 was associated with moderate-to-severe eczema (OR, 1.070; 95% CI, 1.013–1.130), but inversely associated with eczema prevalence (OR, 0.993; 95% CI, 0.989–0.998 for PM2.5 and OR, 0.847; 95% CI, 0.739–0.971 for PM10) |
| Lee et al. 2008 [40] | Taiwan | PM10 | Mean annual concentrations | PM10 was inversely associated with eczema among girls (OR, 0.79; 95% CI, 0.70–0.89) |
| Wang et al 2016 [26] | Taiwan | PM2.5, PM10 | Lifetime | Not associated with lifetime AD |
| Gehring et al. 2010 [4] | Netherlands | PM2.5 | Birth addresses | Not associated with AD at the age of 8 years |
| Morgenstern et al. 2008 [41] | Germany | PM2.5 | During the first 6 years of life | Not associated with AD at the age of 6years |
| Huang et al. 2015 [42] | Taiwan | PM10 | During pregnancy and 3 months after birth | Not associated with AD at the age of 6 months |
| Lu et al. 2017 [43] | China | PM10 | During pregnancy | Not associated with AD incidence at the age of 3–6 years |
PM, particulate matter; AD, allergic dermatitis; OR, odds ratio; CI, confidence interval.
Longitudinal studies examining the effects of PM on exacerbation of atopic dermatitis (AD) symptoms reported similar findings. In a prospective study of 22 Korean children diagnosed with AD aged 16–85 months, for every 1-μg/m3 increase in PM10, AD symptoms were increased by 0.44% on the following day [34]. A 10-μg/m3 increase in PM10 increased AD symptoms by 3.2% in children aged <5 years in a panel study in Korea [35]. Another study in Korea showed that an IQR increase in PM10 (24.0 μg/m3) and PM2.5 (12.7 μg/m3) increased AD symptoms (OR, 1.063; 95% CI, 1.006–1.123 and OR, 1.078; 95% CI, 1.018–1.141, respectively) in children aged <6 years [36], and another longitudinal study among 41 children aged 8–12 years in Korea found that pruritus severity was significantly associated with ultrafine PM [37].
There is little evidence on the association between PM exposure and AD prevalence and incidence, and the available results are inconsistent. Further research is needed to investigate the impact of PM on AD prevalence and incidence. One study showed that the 3-year average concentration of PM10 was associated with both lifetime AD (OR, 1.13; 95% CI, 1.01–1.24) and 1-year history of AD (OR, 1.15; 95% CI, 1.03–1.33) in a cross-sectional study among 4,907 French children aged 9–11 years [28]. A prospective Korean birth cohort study found an association of PM10 exposure in the first trimester with AD development at the age of 6 months (OR, 1.219; 95% CI, 1.023–1.452, per 10 μg/m3 PM10) [38].
A cross-sectional study in the US found a positive association between mean annual PM2.5 and moderate-to-severe eczema (OR, 1.070; 95% CI, 1.013–1.130) in children aged 0–17 years.39) However, PM2.5 (OR, 0.993; 95% CI, 0.989–0.998) and PM10 (OR, 0.847; 95% CI, 0.739–0.971) have been found to be inversely associated with eczema prevalence in another study [39]. A nationwide survey of middle-school students in Taiwan also showed a negative association between mean annual PM10 exposure and eczema among girls (OR, 0.79; 95% CI, 0.70–0.89) [40]. The inverse association between outdoor PM and AD might be explained by protective climate factors and a difference in the measured and actual levels of PM exposure [39,40]. A cross-sectional study on 2,661 kindergarten children in Taiwan showed no association between PM2.5 or PM10 and lifetime AD [26]. This is consistent with German and Dutch birth cohort studies that found no association between long-term PM2.5 exposure and AD at the age of 6 or 8 years [4,41]. Additionally, PM10 during the prenatal period and 3 months after birth was not associated with AD at the age of 6 months in a Taiwan birth cohort study [42]. Finally, a prospective cohort study in China showed that PM10 exposure during pregnancy was not associated with AD incidence in children aged 3–6 years [43].
4. Allergic sensitization (Table 5)
Table 5.
Particulate matter and allergic sensitization
| Study | Country | Pollutants | Exposure time | Outcomes |
|---|---|---|---|---|
| Penard-Morand et al. 2010 [28] | France | PM10 | 3-year average concentration | Increased pollen sensitization (OR, 1.35; 95% CI, 1.09–1.68) |
| Oftedal et al. 2007 [44] | Norway | PM10 | First year of life and lifetime | Not associated with sensitization |
| Morgenstern et al. 2008 [41] | Germany | PM2.5 | During the first 6 years of life | Increased sensitization to any inhalant (OR, 1.45; 95% CI, 1.21– 1.74) and pollen (OR, 1.52; 95% CI, 1.23–1.87) at the age of 6 years |
| Brauer et al. 2007 [45], Gehring et al. 2010 [4] | Netherlands | PM2.5 | Birth addresses | Increased sensitization to food allergens at the age of 4 years but not at 8 years. Not associated with sensitization to inhalant allergens at neither 4 or 8years |
| Gruzieva et al. 2012 [46] | Sweden | PM10 | During the first year of life | Not associated with sensitization |
| Gruzieva et al. 2014 [47] | Meta-analysis | PM2.5, PM10 | Birth and current addresses | Not associated with sensitization |
| Bowatte et al. 2015 [48] | Meta-analysis | PM2.5 | From birth to 5 years of age | Increased sensitization to outdoor aeroallergens (OR, 1.36; 95% CI, 1.14-1.62) but not indoor aeroallergens (OR, 1.00; 95% CI, 0.80-1.24) |
PM, particulate matter; OR, odds ratio; CI, confidence interval.
The association between PM and allergic sensitization is not well established due to the inconsistent results across studies. Pollen sensitization was significantly associated with 3-year average concentration of PM10 (OR, 1.35; 95% CI, 1.09–1.68) in a cross-sectional study among French children aged 9–11 years [28]. A population-based study including children aged 9–10 years in Oslo did not show a significant association between PM10 exposure in the first year of life or lifetime PM10 exposure and sensitization to any allergen [44]. However, PM2.5 during the first 6 years of life increased sensitization to any inhalant (OR, 1.45; 95% CI, 1.21–1.74) and pollen (OR, 1.52; 95% CI, 1.23–1.87) at the age of 6 years in a German birth cohort study [41]. In a Dutch birth cohort study, PM2.5 exposure at the birth address was associated with sensitization to food allergens at the age of 4 years but not at 8 years; associations between sensitization and inhalant allergens were not significant at either 4 or 8 years [4,45]. A Swedish birth cohort study also found no clear associations between PM10 exposure during the first year of life and overall sensitization at the age of 4 and 8 years [46]. A meta-analysis of 5 European birth cohorts showed no clear associations between PM2.5 and PM10 exposure and allergic sensitization in children up to 10 years of age [47]. Finally, another meta-analysis of birth cohort studies reported significant associations between early childhood PM2.5 exposure and sensitization to outdoor aeroallergens but not indoor aeroallergens [48].
Mechanisms
The mechanisms by which PM induces effects on childhood allergic diseases remain unclear. Oxidative stress and damage, enhancement of sensitization to allergens, inflammatory pathways and immunological responses, and epigenetics may have roles in childhood allergic diseases caused by PM. The plausible mechanisms by which PM affects childhood allergic diseases are summarized in Fig. 1.
Fig. 1.
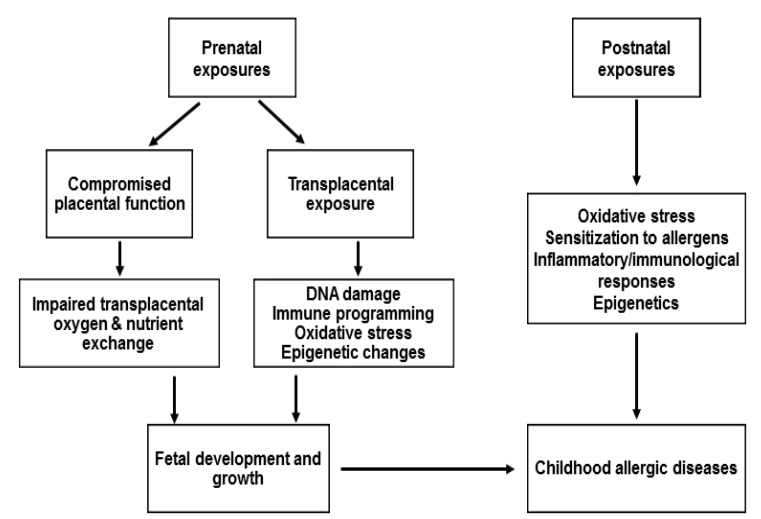
Plausible mechanisms by which PM affects the childhood allergic diseases
1. Oxidative stress and damage
PM forms reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide, and hydroxyl radicals [2]. These ROS may directly damage proteins, lipids, and DNA [49]. For example, ROS modifies surfactant protein, which enhances lipid peroxidation, inflammation, and oxidative damage within the lung. Lipid peroxidation, or the oxidative degeneration of lipids, can damage cell membranes and can ultimately lead to cell death. In addition, an end-product of lipid peroxidation, 4-hydroxy-2-nonenal, can lead to airway remodeling by increasing fibronectin production and activating epidermal growth factor receptor. PM-induced DNA damage alters gene and protein expression as well as increases cell death. In addition, oxidative stress activates mitogen-activated protein kinase signaling, activator protein 1, and nuclear factor-kB, which is responsible for the expression or activation of proinflammatory cytokines (e.g., interleukin [IL]-4, IL-6, IL-8, and tumor necrosis factor [TNF]-α), chemokines, and adhesion molecules [3,50-53].
2. Enhancement of sensitization to allergens
PM can increase sensitization to allergens in several ways. Deposition of allergens in the airways increases owing to allergen carriage by PM. PM induces oxidative stress, leading to an increase in epithelial permeability and subsequent recruitment of cells involved in the allergic response. PM also modifies antigenic protein expression, leading to an exaggerated allergenic response. Furthermore, PM increases sensitization to allergens by acting as an adjuvant, thereby preventing antigens from dispersing, activating antigen-presenting cells, and stimulating the division of type 2 helper T-cells [3,51].
3. Inflammatory pathways and immunological responses
PM induces the release of inflammatory cytokines (e.g., IL-6, IL-8, granulocyte-macrophage colony-stimulating factor, and TNF-α) from immune and bronchial epithelial cells [52]. PM are recognized by pathogen recognition receptors (PRRs) on antigen-presenting cells and Toll-like receptors and nucleotide-binding oligomerization domain-like receptor families. Once PM is detected by PRRs, different signaling pathways are activated to upregulate the expression of inflammatory genes (e.g., IFN-α, IFN-β, and TNF-α). PM also promotes Th2 responses [52]. PM upregulates the expression of the dendritic cell (DC) maturation marker CD86, which is involved in priming Th2 responses. PM also activates DCs and basophils in vivo to produce Th2-associated cytokines IL-4 and IL-5 [52].
4. Epigenetics
Epigenetic changes may play a role in regulating the expression of genes related to allergic diseases. Exposure to ambient polycyclic aromatic hydrocarbons during pregnancy is associated with increased DNA methylation of the acyl-coenzyme A synthetase long-chain family member 3 promoter in cord blood DNA and is related to increased asthma risk in children [54]. Increased air pollution is also associated with hypermethylation of the FOXP3 gene in the peripheral blood of children with asthma, impairing regulator T-cell function and increasing asthma morbidity [55].
5. Prenatal exposure to PM
PM can act directly through translocation via the placenta or indirectly by compromising placental function [56]. Transplacental exposure to PM-induced oxidative stress and by-products of PM metabolism damage DNA, alter gene function, and subsequently affect fetal development [56]. Prenatal PM exposure may also influence immune programming. One study showed that increased PM2.5 levels during 14 days prior to birth was associated with decreased levels of T lymphocytes and increased B lymphocytes in cord blood [57]. Higher prenatal PM10 exposure decreased the expression of cytokines IL-10 and IL-1β and increased the percentage of CD4+ CD25+T-cells in cord blood [58,59]. Higher exposures to PM2.5 during midpregnancy were found associated with a higher prevalence of elevated cord IgE [60]. Increased levels of ROS, placental inflammation, maternal blood viscosity, and endothelial dysfunction could be associated with inadequate placental perfusion, which would subsequently impair transplacental oxygen and nutrient exchange and consequently affect fetal development [56,61].
Conclusions
There is considerable evidence that PM is an important risk factor in childhood allergic diseases. Oxidative stress, enhanced sensitization to allergens, inflammatory and immunological responses, and epigenetics are considered possible mechanisms. PM exposure during the prenatal period may also affect childhood allergic disease by way of direct transplacental exposure or possibly by compromising placental function. Further studies are needed to identify the mechanisms by which PM causes allergic diseases and to identify factors that increase the susceptibility of children to PM and allergic diseases, such as genetic polymorphisms and critical exposure windows. Effective new preventive and therapeutic approaches are also needed. Precise exposure assessments, as well as biomarkers of exposure and health effects, are required to accurately evaluate the association between PM and childhood allergic diseases. Strict monitoring and avoidance of PM exposure, as well as new clinical and therapeutic strategies, could helpto prevent childhood allergic diseases.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ. 2012;60:504–26. [Google Scholar]
- 2.de Kok TM, Driece HA, Hogervorst JG, Briedé JJ. Toxicological assessment of ambient and traffic-related particulate matter: a review of recent studies. Mutat Res. 2006;613:103–22. doi: 10.1016/j.mrrev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–92. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181:596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 5.Gruzieva O, Bergström A, Hulchiy O, Kull I, Lind T, Melén E, et al. Exposure to air pollution from traffic and childhood asthma until 12 years of age. Epidemiology. 2013;24:54–61. doi: 10.1097/EDE.0b013e318276c1ea. [DOI] [PubMed] [Google Scholar]
- 6.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–90. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tétreault LF, Doucet M, Gamache P, Fournier M, Brand A, Kosatsky T, et al. Childhood exposure to ambient air pollutants and the onset of asthma: an administrative cohort study in Québec. Environ Health Perspect. 2016;124:1276–82. doi: 10.1289/ehp.1509838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med. 2015;192:1052–9. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlsten C, Dybuncio A, Becker A, Chan-Yeung M, Brauer M. Trafficrelated air pollution and incident asthma in a high-risk birth cohort. Occup Environ Med. 2011;68:291–5. doi: 10.1136/oem.2010.055152. [DOI] [PubMed] [Google Scholar]
- 10.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: a systematic review. Environ Res. 2017;159:519–30. doi: 10.1016/j.envres.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31. doi: 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Sbihi H, Tamburic L, Koehoorn M, Brauer M. Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur Respir J. 2016;47:1062–71. doi: 10.1183/13993003.00746-2015. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura KK, Galanter JM, Roth LA, Oh SS, Thakur N, Nguyen EA, et al. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;188:309–18. doi: 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118:1021–6. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. 2015;3:933–42. doi: 10.1016/S2213-2600(15)00426-9. [DOI] [PubMed] [Google Scholar]
- 16.Mölter A, Agius R, de Vocht F, Lindley S, Gerrard W, Custovic A, et al. Effects of long-term exposure to PM10 and NO2 on asthma and wheeze in a prospective birth cohort. J Epidemiol Community Health. 2014;68:21–8. doi: 10.1136/jech-2013-202681. [DOI] [PubMed] [Google Scholar]
- 17.Hoek G, Pattenden S, Willers S, Antova T, Fabianova E, Braun-Fahrländer C, et al. PM10, and children's respiratory symptoms and lung function in the PATY study. Eur Respir J. 2012;40:538–47. doi: 10.1183/09031936.00002611. [DOI] [PubMed] [Google Scholar]
- 18.Mölter A, Simpson A, Berdel D, Brunekreef B, Custovic A, Cyrys J, et al. A multicentre study of air pollution exposure and childhood asthma prevalence: the ESCAPE project. Eur Respir J. 2015;45:610–24. doi: 10.1183/09031936.00083614. [DOI] [PubMed] [Google Scholar]
- 19.Son JY, Lee JT, Park YH, Bell ML. Short-term effects of air pollution on hospital admissions in Korea. Epidemiology. 2013;24:545–54. doi: 10.1097/EDE.0b013e3182953244. [DOI] [PubMed] [Google Scholar]
- 20.Mazenq J, Dubus JC, Gaudart J, Charpin D, Nougairede A, Viudes G, et al. Air pollution and children's asthma-related emergency hospital visits in southeastern France. Eur J Pediatr. 2017;176:705–11. doi: 10.1007/s00431-017-2900-5. [DOI] [PubMed] [Google Scholar]
- 21.Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in New York City. J Allergy Clin Immunol. 2010;125:367–73. doi: 10.1016/j.jaci.2009.10.061. e5. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121:1133–9. doi: 10.1016/j.jaci.2008.02.020. e1. [DOI] [PubMed] [Google Scholar]
- 23.Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS One. 2017;12:e0174050. doi: 10.1371/journal.pone.0174050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng XY, Ding H, Jiang LN, Chen SW, Zheng JP, Qiu M, et al. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PLoS One. 2015;10:e0138146. doi: 10.1371/journal.pone.0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Shortterm effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and metaanalysis. Environ Health Perspect. 2010;118:449–57. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang IJ, Tung TH, Tang CS, Zhao ZH. Allergens, air pollutants, and childhood allergic diseases. Int J Hyg Environ Health. 2016;219:66–71. doi: 10.1016/j.ijheh.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Wood HE, Marlin N, Mudway IS, Bremner SA, Cross L, Dundas I, et al. Effects of air pollution and the introduction of the london low emission zone on the prevalence of respiratory and allergic symptoms in schoolchildren in East London: a sequential cross-sectional study. PLoS One. 2015;10:e0109121. doi: 10.1371/journal.pone.0109121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pénard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36:33–40. doi: 10.1183/09031936.00116109. [DOI] [PubMed] [Google Scholar]
- 29.Deng Q, Lu C, Yu Y, Li Y, Sundell J, Norbäck D. Early life exposure to traffic-related air pollution and allergic rhinitis in preschool children. Respir Med. 2016;121:67–73. doi: 10.1016/j.rmed.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Fuertes E, Brauer M, MacIntyre E, Bauer M, Bellander T, von Berg A, et al. Childhood allergic rhinitis, traffic-related air pollution, and variability in the GSTP1, TNF, TLR2, and TLR4 genes: results from the TAG Study. J Allergy Clin Immunol. 2013;132:342–52. doi: 10.1016/j.jaci.2013.03.007. e2. [DOI] [PubMed] [Google Scholar]
- 31.Liu MM, Wang D, Zhao Y, Liu YQ, Huang MM, Liu Y, et al. Effects of outdoor and indoor air pollution on respiratory health of Chinese children from 50 kindergartens. J Epidemiol. 2013;23:280–7. doi: 10.2188/jea.JE20120175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang BF, Jaakkola JJ, Lee YL, Lin YC, Guo YL. Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respir Res. 2006;7:23. doi: 10.1186/1465-9921-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng Q, Lu C, Li Y, Sundell J, Dan Norbäck. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 2016;150:119–27. doi: 10.1016/j.envres.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, et al. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132:495–8. doi: 10.1016/j.jaci.2013.04.019. e1. [DOI] [PubMed] [Google Scholar]
- 35.Kim YM, Kim J, Han Y, Jeon BH, Cheong HK, Ahn K. Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: A panel study in Korea. PLoS One. 2017;12:e0175229. doi: 10.1371/journal.pone.0175229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YM, Kim J, Jung K, Eo S, Ahn K. The effects of particulate matter on atopic dermatitis symptoms are influenced by weather type: Application of spatial synoptic classification (SSC) Int J Hyg Environ Health. 2018;221:823–9. doi: 10.1016/j.ijheh.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Song S, Lee K, Lee YM, Lee JH, Lee SI, Yu SD, et al. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ Res. 2011;111:394–9. doi: 10.1016/j.envres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Lamichhane DK, Lee M, Ye S, Kwon JH, Park MS, et al. Preventive effect of residential green space on infantile atopic dermatitis associated with prenatal air pollution exposure. Int J Environ Res Public Health. 2018;15:102. doi: 10.3390/ijerph15010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kathuria P, Silverberg JI. Association of pollution and climate with atopic eczema in US children. Pediatr Allergy Immunol. 2016;27:478–85. doi: 10.1111/pai.12543. [DOI] [PubMed] [Google Scholar]
- 40.Lee YL, Su HJ, Sheu HM, Yu HS, Guo YL. Traffic-related air pollution, climate, and prevalence of eczema in Taiwanese school children. J Invest Dermatol. 2008;128:2412–20. doi: 10.1038/jid.2008.110. [DOI] [PubMed] [Google Scholar]
- 41.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, et al. Atopic diseases, allergic sensitization, and exposure to trafficrelated air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–7. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 42.Huang CC, Wen HJ, Chen PC, Chiang TL, Lin SJ, Guo YL. Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol. 2015;173:981–8. doi: 10.1111/bjd.14039. [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Deng L, Ou C, Yuan H, Chen X, Deng Q. Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J Dermatol Sci. 2017;85:85–95. doi: 10.1016/j.jdermsci.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Oftedal B, Brunekreef B, Nystad W, Nafstad P. Residential outdoor air pollution and allergen sensitization in schoolchildren in Oslo, Norway. Clin Exp Allergy. 2007;37:1632–40. doi: 10.1111/j.1365-2222.2007.02823.x. [DOI] [PubMed] [Google Scholar]
- 45.Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29:879–88. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 46.Gruzieva O, Bellander T, Eneroth K, Kull I, Melén E, Nordling E, et al. Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J Allergy Clin Immunol. 2012;129:240–6. doi: 10.1016/j.jaci.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Gruzieva O, Gehring U, Aalberse R, Agius R, Beelen R, Behrendt H, et al. Meta-analysis of air pollution exposure association with allergic sensitization in European birth cohorts. J Allergy Clin Immunol. 2014;133:767–76. doi: 10.1016/j.jaci.2013.07.048. e7. [DOI] [PubMed] [Google Scholar]
- 48.Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a metaanalysis of birth cohort studies. Allergy. 2015;70:245–56. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- 49.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–68. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–71. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 51.Gowers AM, Cullinan P, Ayres JG, Anderson HR, Strachan DP, Holgate ST, et al. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology. 2012;17:887–98. doi: 10.1111/j.1440-1843.2012.02195.x. [DOI] [PubMed] [Google Scholar]
- 52.Ristovski ZD, Miljevic B, Surawski NC, Morawska L, Fong KM, Goh F, et al. Respiratory health effects of diesel particulate matter. Respirology. 2012;17:201–12. doi: 10.1111/j.1440-1843.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 53.Huang SK, Zhang Q, Qiu Z, Chung KF. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J Thorac Dis. 2015;7:23–33. doi: 10.3978/j.issn.2072-1439.2014.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, et al. Relation of DNA methylation of 5'-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4:e4488. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126:845–52. doi: 10.1016/j.jaci.2010.08.008. e10. [DOI] [PubMed] [Google Scholar]
- 56.Veras MM, de Oliveira Alves N, Fajersztajn L, Saldiva P. Before the first breath: prenatal exposures to air pollution and lung development. Cell Tissue Res. 2017;367:445–55. doi: 10.1007/s00441-016-2509-4. [DOI] [PubMed] [Google Scholar]
- 57.Hertz-Picciotto I, Herr CE, Yap PS, Dostál M, Shumway RH, Ashwood P, et al. Air pollution and lymphocyte phenotype proportions in cord blood. Environ Health Perspect. 2005;113:1391–8. doi: 10.1289/ehp.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, et al. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLoS One. 2011;6:e23130. doi: 10.1371/journal.pone.0023130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baïz N, Slama R, Béné MC, Charles MA, Kolopp-Sarda MN, Magnan A, et al. Maternal exposure to air pollution before and during pregnancy related to changes in newborn's cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth. 2011;11:87. doi: 10.1186/1471-2393-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herr CE, Ghosh R, Dostal M, Skokanova V, Ashwood P, Lipsett M, et al. Exposure to air pollution in critical prenatal time windows and IgE levels in newborns. Pediatr Allergy Immunol. 2011;22(1 Pt 1):75–84. doi: 10.1111/j.1399-3038.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- 61.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114:1636–42. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]


