Abstract
The kidneys are among the most commonly transplanted organs in the world. Transplant patients, as a consequence of their kidney disease and other risk factors which make it difficult for the surgeon to perform vascular anastomosis during kidney transplantation, often have numerous arterial calcifications. The preoperative assessment for transplantation includes an abdominal and pelvic CT scan without contrast that allows for the mapping of atheromatous calcification. However, non-contrast enhanced CT is not optimal and the surgeon is obliged to palpate the arteries during the operation to choose the anatomical site of the vascular anastomosis. This technical note reports the development of a new technique of preoperative reproduction of atherosclerotic arteries, owing to advancements in 3D multi-tissue printing technology. We used scans of four patients with varying degrees of calcified atheroma to model and print their arteries for their pre-surgical planning.
Keywords: 3D multi-tissue printing, kidney transplantation, pre-surgical planning
Introduction
The kidneys are among the most commonly transplanted organs in the world, evidenced by the abundant number of kidney transplants—19,849—in the United States in 2017, a figure which is continuing to rise (1). As the prevalence of arterial calcification is high in patients with end-stage kidney disease due to many risk factors such as age, duration of dialysis, and hyperparathyroidism, vascular assessment for this condition is part of the pre-transplant assessment. The purpose of this vascular assessment is to remove the temporary contraindication of transplant in relation to the feasibility of the surgical procedure. More specifically, vascular calcifications can make it difficult to perform anastomosis (arterial clamping, suture, calcium emboli migration) on the recipient's iliac vessels during the operation, and their presence may require an adaptation of the surgical procedure (bypass, alternate anastomosis site), or even make the procedure impracticable (2).
Previously used abdominal X-rays without contrast and arteriography have been replaced by the CT scan, which has become the standard reference examination for this type of indication. Thus, it is currently recommended to carry out one of the following examination types:
❖ An abdominal and pelvic CT scan without iodinated contrast medium in order to preserve the remaining renal function in non-dialysis patients or patients with peritoneal dialysis;
❖ An abdominal and pelvic CT scan with and without iodinated contrast medium in hemodialysis patients.
The CT scan is expected to provide information about the location and length of the plaques, their circumferential character or lack thereof, their degree of stenosis, and their thickness and density (3). When a CT scan without contrast is performed, only the calcified plaques are visible, allowing the radiologist to describe their location, length, and, if applicable, their circumferential character. The scan without contrast has an advantage over the scan performed after injection in enabling a better analysis of the thin vascular calcifications. Thanks to 3D volume rendering technique (VRT) reconstruction and maximum intensity projection (MIP) imaging, the CT scan at the arterial phase after intravenous injection of contrast medium makes it possible to analyze the atheroma plaques in greater detail, and to make global representations of the aorta and its branches. In the absence of intravenous iodinated contrast injection however, MIP only shows a global view of the arterial calcifications (Figure 1).
Figure 1.
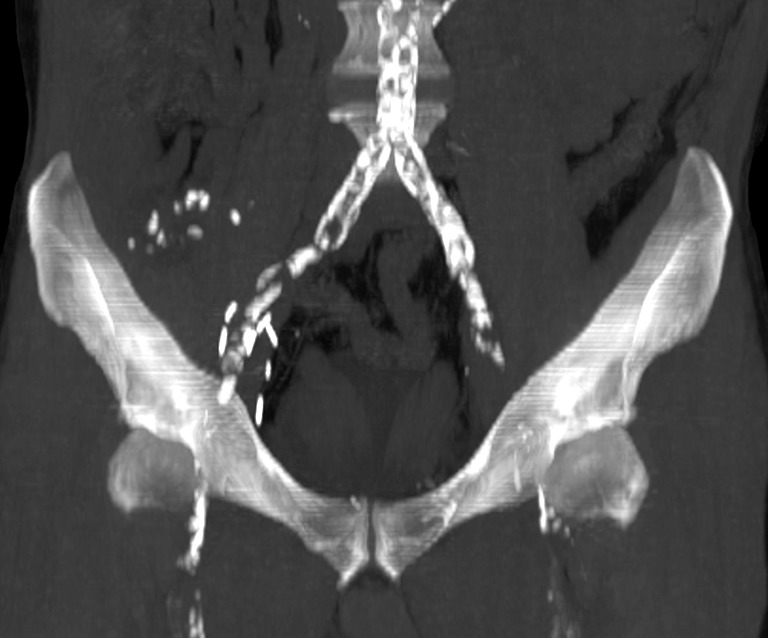
Coronal MIP image made from an abdominal pelvic CT scan without intravenous iodinated contrast injection. This type of image gives the surgeon a global view of the size and distribution of calcified atheromatous plaques; however, it is not precise enough and does not replace the intraoperative palpation of vessels during kidney transplantation. MIP, maximum intensity projection.
Despite these benefits, preoperative vascular CT analysis has never been able to completely replace the intraoperative palpation of the recipient’s arteries on which the clamps are placed on either side of the anastomosis site (4) (Figure 2). This palpation allows the surgeon to differentiate both between a normal, supple and compressible artery, and an atheromatous, hard, and incompressible artery. It can also sometimes reveal the pathological conditions that cannot otherwise be detected by CT scan. This deficiency in CT sometimes requires the surgeon to adapt their procedure, to make an emergency request for vascular surgeons to perform a bypass, or to even cancel the transplantation altogether.
Figure 2.
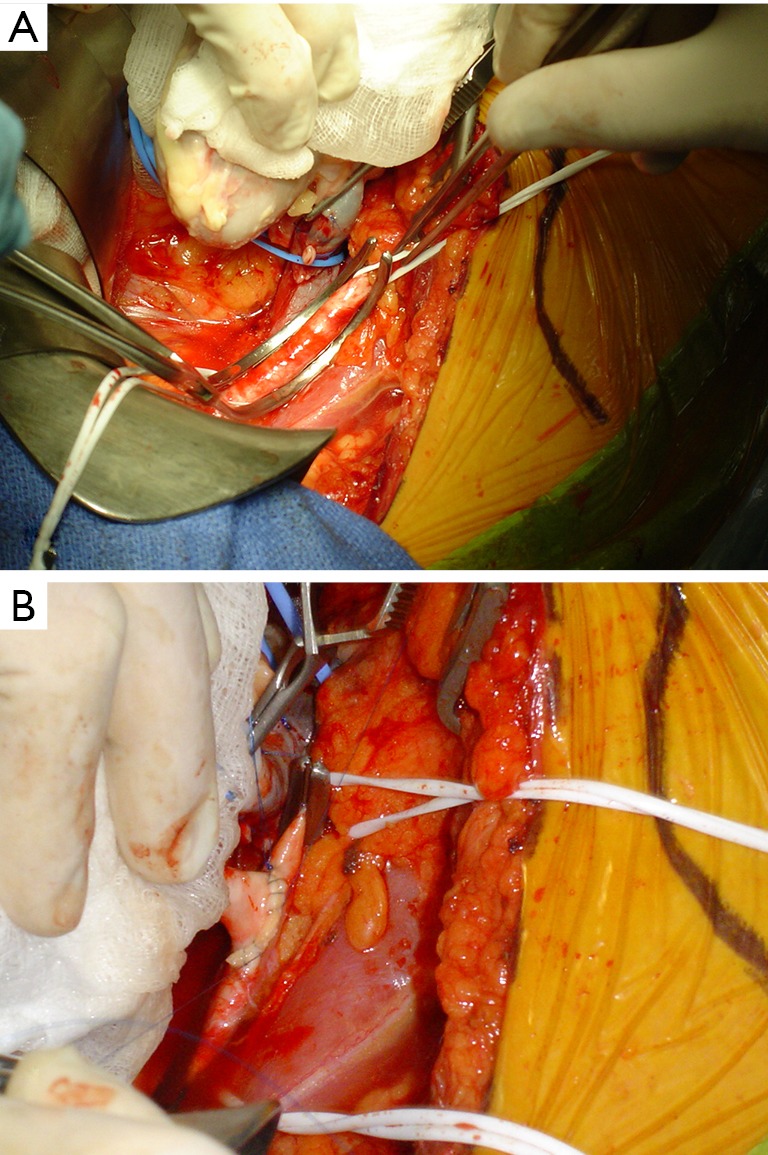
Photographs taken during the clamping of an external iliac artery during anastomosis with the renal artery of the graft (A), and after completion of the latero-terminal surgical anastomosis between the external iliac artery and the transplant renal artery (B).
To solve this problem, 3D printing could be used in the surgical planning of kidney transplants to improve the transplantation procedure. 3D printing has already proven its usefulness in surgical planning (5), and is most often implemented in the specialties of orthopedics (guides for knee arthroplasties) and maxillofacial surgery (implant shaping) (6). However, until recently, 3D prints were made of hard plastic and could only reproduce the texture of bones. Technological advances in 3D printers now make it possible to print structures with different levels of transparency and hardness (7). If these technological innovations were applied to the vessels, it could print vessels whose normal areas would be soft and compressible, and whose calcified atheromatous plaques would be hard and incompressible. Thus, the surgeon could benefit from a preoperative 3D reproduction of the patient’s aortoiliac branch, in which the palpation would replicate the sensation found in the operating room. This would enable the surgeon to choose, in advance, the best site for anastomosis using models obtained from non-contrast CT scans.
3D printing has already been used in urology (8), and even in renal transplantation (9,10), but to our knowledge, our new application of 3D printing in the surgical planning of kidney transplantation has not been widely reported. The objective of our study is to describe a new 3D modeling and printing technique of the recipient’s aortoiliac axis from a non-contrast CT scan, with hardness and transparency being modulated according to the differing tactility of healthy and calcified areas.
Technical note
Prior to kidney transplantation, abdominal and pelvic non-contrast CT scans were performed in all patients. We selected four patients with various atherosclerotic damage (very low to very high, from type A to D of TASC II classification) (11) among transplant patients or patients waiting for transplantation for 2 years. Characteristics of the CT scanner scanning protocols and patients are detailed in Table 1.
Table 1. Characteristics of study population and of patient’s CT-scanner acquisition protocols. Atheroma has been graded according to the Trans-Atlantic Inter-Society Consensus for the management of peripheral arterial disease (11). 2-mm-slice-thickness axial reconstructions were obtained from various CT-scanner scanning protocols and imported into the 3D-slicer software.
| Patient | Birth date | Etiology of chronic renal failure | Degree of calcification | TASC II classification | CT-scanner acquisition protocol |
|---|---|---|---|---|---|
| Case 1 | 1966 | Diabetic nephropathy | Very high | Type D | 64-slice, 1-mm helical, 120 kV |
| Case 2 | 1948 | IgA nephropathy | High | Type D | 64-slice, 1-mm helical, 140 kV |
| Case 3 | 1963 | Diabetic nephropathy | Moderate | Type C | 160-slice, 0.5-mm helical, 120 kV |
| Case 4 | 1972 | IgA nephropathy | Very low | Type A | 64-slice, 1-mm helical, 120 kV |
CT DICOM (Digital Imaging and Communications in Medicine format) data were imported into the open source 3D-slicer software (version 4.8) to generate a 3D surface modeling saved in STL (STereoLithography) format.
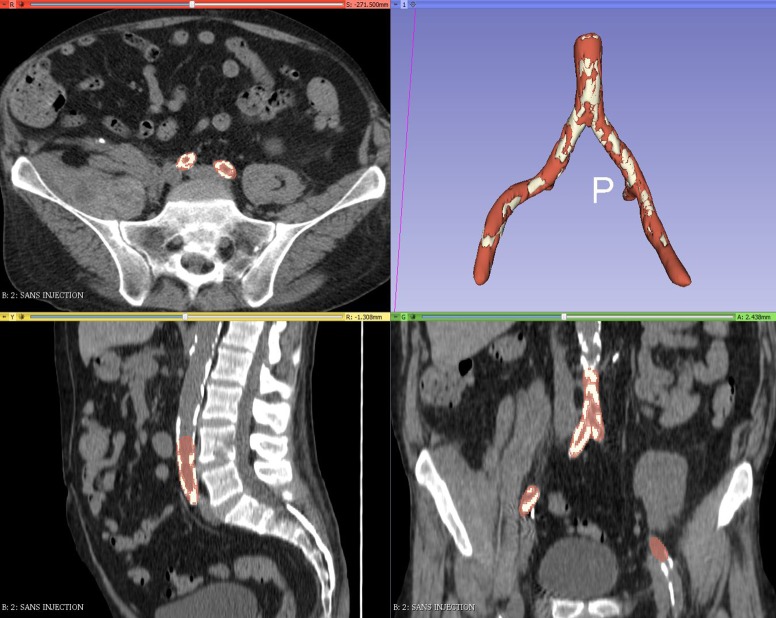
Every 3D-sliced module used for the study were natively included in the software, and we did not use an additional plugin. An inferior threshold was set at −20 Hounsfield units (HU) to model the aorta and the iliac vessels with the segment editor paint tool, while excluding perivascular fat. For calcified atheromatous plaques, the threshold was set at +300 HU. Residual artifacts were partially corrected by software, specifically the smoothing tool from the segment editor (median, opening, closing and joint smooth) (Figure 3). The working time required to obtain a printable file was 30 to 45 minutes. The printer created a hollow object from the solid object to reproduce the vascular wall with a thickness of 1.5 mm.
Figure 3.
Result of the 3D modeling of the arteries created from the abdominal pelvic CT scan without contrast medium.
3D printing was performed on a multi-jet printer (Scalia®, Cryla Group, Besancon, France), which produced 0.028-mm-thick layers of translucent soft resin with a hardness of 28 shore A, elastomeric type for the normal vascular wall, and rigid, opaque acrylonitrile butadiene styrene (ABS) resin for the calcified atheroma. The total printing time of an aortoiliac axis was 10 to 14 hours. The printing cost was estimated to be between 300 to 400 euros.
A kidney transplant surgeon with more than 20 years of experience performed a qualitative evaluation by giving a rating from 0 (worst) to 10 (best) to the characteristics of the printed models. The characteristics and ratings were as follows: visual appearance, 3/10; haptic anatomy, 8/10; preoperative anatomy, 8/10; would you use it yourself? 10/10. Despite noting that improvements could be made to the visual appearance, the evaluator was highly satisfied with the result both visually, in precisely locating the calcified plaques (Figure 4), and tactually, in simulating palpation and clamping of the arterial anastomosis area (Figure 5).
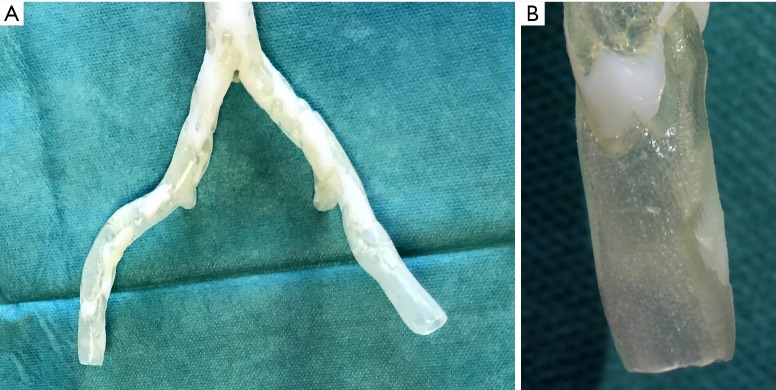
Figure 4.
Front view of the 3D printing results of the aortic and external iliac vessels (A). The end of the right iliac artery was zoomed in to better visualize the transparency of the normal arterial wall, and the calcified plaques were printed with an opaque and rigid white material (B).
Figure 5.
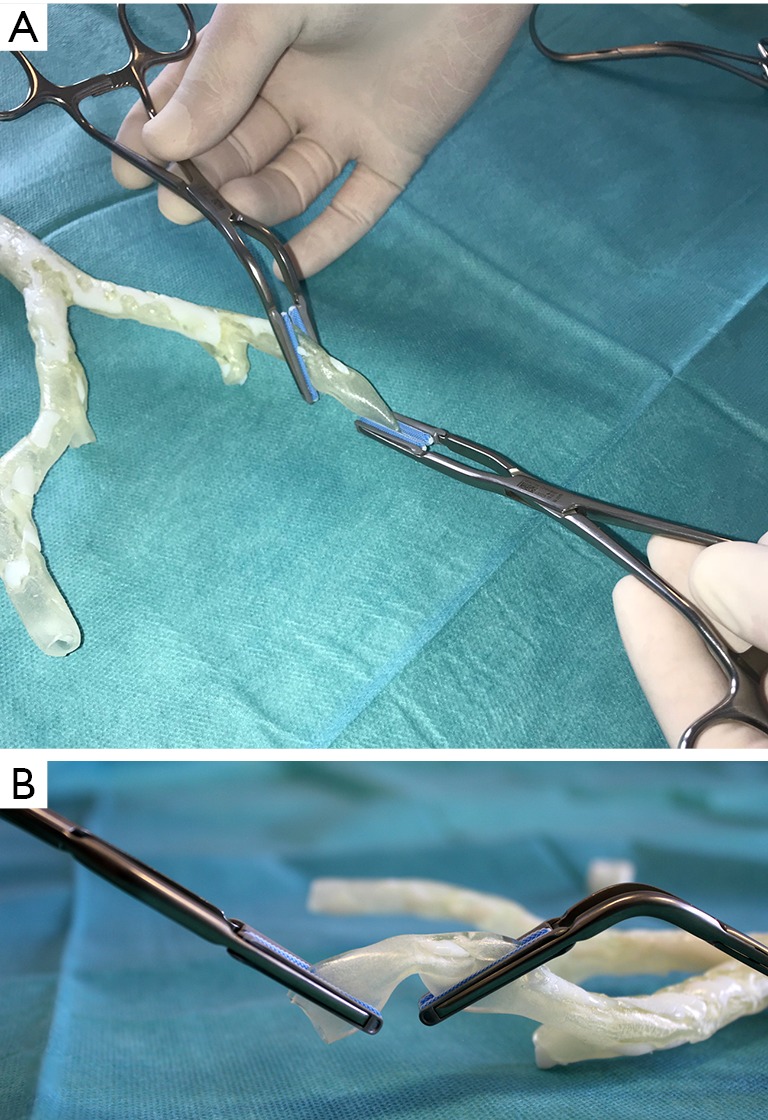
Simulation of clamping of the left external iliac artery in preparation for anastomosis of the left side (A). The normal 3D arterial wall has the same flexibility and is as compressible as the patient’s normal arterial wall (B).
Discussion
3D printing is a tool that is becoming increasingly important in the medical field. The new model described in our study can be a valuable aid for surgeons performing renal transplants by virtue of reducing operating time and the rate of complications at the anastomosis site, and even contraindicating certain procedures. We can envision extending this concept to various vascular bypass procedures (e.g., coronary arteries, femoral, etc.) in patients with calcified atheromas.
In fact, as precise as the CT scan report provided by the radiologist can be, it is sometimes insufficient in meeting the surgeon’s requirements for pre-surgical planning; 3D model innovation could advantageously complement the report.
Another benefit of this innovation is that, as a model, it does not require implantation or contact with the patient, and thus can be produced and used without specific authorization (12).
A limitation of our multi-tissue 3D printing technique study has been the arbitrary choice to print calcified atheroma from a scan density greater than 300 HU; non-calcified atheromas whose density is often less than 300 HU can also be troublesome for surgeons performing arterial anastomosis. If CT scans with iodinated contrast were performed, we could extend our technique to printing non-calcified atheromatous plaques with a plastic of intermediate hardness. Arterial modeling has been complicated by the lack of intravenous injection of iodinated contrast on the pre-operative scans of our series: it has sometimes resulted in difficulties in differentiating the arteries from the veins and muscles with which they have contact, and led to the impossibility of carrying out an automatic segmentation of the vessels. This difficulty was reduced in patients in our study who had a relatively high number of calcified atheromas because calcifications allowed the radiologist to locate the vessel wall and to segment the vessel more easily. Fortunately, the interest of 3D printing for the surgeon is most important in these patients with a high prevalence of atheromas.
The qualitative and quantitative evaluation of the benefits of these 3D models in patient care will now have to be evaluated prospectively with a larger sample, in light of the results both from the surgeons' satisfaction questionnaires and from the comparison of the progress and the operative outcomes between the patients who benefited from a 3D impression of their arteries before the transplant and those who did not have one.
Acknowledgements
We thank Mr. Benoit for the technical implementation of the 3D printing.
Ethical Statement: This was a non-interventional data study. As a result, written informed consent was waived by the Institutional Review Board.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.United Network for Organ Sharing. Transplant trends. Available online: https://unos.org/data/transplant-trends/#transplants_by_organ_type+year+2017 [Accessed 18 Sep. 2018].
- 2.Descotes JL, Hubert J. Urology imaging: contribution of imaging in kidney transplantation. Prog Urol 2003;13:1093-114. [PubMed] [Google Scholar]
- 3.Renard-Penna R, Ayed A, Barrou B, Grenier P. Pre-kidney-transplant evaluation of donors and recipients. J Radiol 2011;92:358-66. 10.1016/j.jradio.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 4.Pillot P, Kleinclauss F. Kidney transplantion. Prog Urol 2009;19:254-9. 10.1016/j.purol.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 5.Martelli N, Serrano C, van den Brink H, Pineau J, Prognon P, Borget I, El Batti S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016;159:1485-500. 10.1016/j.surg.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 6.Tack P, Victor J, Gemmel P, Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016;15:115. 10.1186/s12938-016-0236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim GB, Lee S, Kim H, Yang DH, Kim YH, Kyung YS, Kim CS, Choi SH, Kim BJ, Ha H, Kwon SU, Kim N. Three-Dimensional Printing: Basic Principles and Applications in Medicine and Radiology. Korean J Radiol 2016;17:182-97. 10.3348/kjr.2016.17.2.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Z, Liu D. A systematic review of clinical value of three-dimensional printing in renal disease. Quant Imaging Med Surg 2018;8:311-25. 10.21037/qims.2018.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandak P, Byrne N, Coleman A, Karunanithy N, Carmichael J, Marks SD, Stojanovic J, Kessaris N, Mamode N. Patient-specific 3D Printing: A Novel Technique for Complex Pediatric Renal Transplantation. Ann Surg 2019;269:e18-e23. 10.1097/SLA.0000000000003016 [DOI] [PubMed] [Google Scholar]
- 10.Kusaka M, Sugimoto M, Fukami N, Sasaki H, Takenaka M, Anraku T, Ito T, Kenmochi T, Shiroki R, Hoshinaga K. Initial experience with a tailor-made simulation and navigation program using a 3-D printer model of kidney transplantation surgery. Transplant Proc 2015;47:596-9. 10.1016/j.transproceed.2014.12.045 [DOI] [PubMed] [Google Scholar]
- 11.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Rutherford RB; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. Int Angiol 2007;26:81-157. [PubMed] [Google Scholar]
- 12.Montmartin M, Meyer C, Euvrard E, Pazart L, Weber E, Benassarou M. 3D printing in health care facilities: What legislation in France? Rev Stomatol Chir Maxillofac Chir Orale 2015;116:302-7. 10.1016/j.revsto.2015.04.007 [DOI] [PubMed] [Google Scholar]