Abstract
Resveratrol (RSV) extends the lifespan of various organisms through activation of sirtuin. However, whether RSV‐mediated longevity is entirely dependent upon sirtuin is still controversial. Thus, understanding additional mechanisms concerning the genetic requirements for the biological activity of RSV needs to be clarified to utilize the beneficial effects of RSV. In this study using Caenorhabditis elegans as a model system, we found that MPK‐1 (an ERK homolog) signaling is necessarily required for RSV‐mediated longevity of sir‐2.1/sirtuin mutants as well as for wild‐type worms. We demonstrated that MPK‐1 contributes to RSV‐mediated longevity through nuclear accumulation of SKN‐1 in a SIR‐2.1/DAF‐16 pathway‐independent manner. The positive effect of RSV in regulating lifespan was completely abolished by RNA interference against mpk‐1 in the sir‐2.1 and daf‐16 mutants, strongly indicating that the MPK‐1/SKN‐1 pathway is involved in RSV‐mediated longevity, independently of SIR‐2.1/DAF‐16. We additionally found that RSV protected worms from oxidative stress via MPK‐1. In addition to organismal aging, RSV prevented the age‐associated loss of mitotic germ cells, brood size, and reproductive span through MPK‐1 in C. elegans germline. Therefore, our findings not only provide new mechanistic insight into the controversial effects of RSV on organismal longevity, but additionally have important implications in utilizing RSV to improve the outcome of aging‐related diseases.
Keywords: ERK/MPK‐1, longevity, reproductive span, resveratrol, Sirtuin/SIR‐2.1, SKN‐1/NRF2
1. INTRODUCTION
Increasing evidence has shown that small molecules can affect the lifespan positively or negatively in a variety of organisms, including humans (Hubbard & Sinclair, 2014; Kennedy & Lamming, 2016). In particular, caloric restriction mimetics, including resveratrol (RSV), rapamycin, and metformin, have been shown to exert beneficial effects on longevity and health (Lamming, Sabatini, & Baur, 2012). Rapamycin was the first chemical identified which extends lifespan in mammals through the inhibition of mammalian target of rapamycin (mTOR; Harrison et al., 2009). Metformin, which can extend the lifespan of C57BL6 mice and short‐lived tumorigenic mice (Anisimov et al., 2005; Martin‐Montalvo et al., 2013), exerts its effects through inhibition of the mTOR signaling pathway (Dowling, Zakikhani, Fantus, Pollak, & Sonenberg, 2007). However, the mechanisms of RSV remain controversial even though its positive effects on longevity have been reported over the past decade. RSV was first identified as an activator of sirtuin (mammalian SIRT1/nematode SIR‐2.1, a family of NAD+‐dependent deacetylases; Figure 1a, Model I) and has been found to extend the lifespan of various organisms by mimicking dietary restriction and to improve the health of mice on a high‐fat diet (Baur et al., 2006; Wood et al., 2004). In addition, Herranz et al. (2010) reported the anti‐aging features of Sirt1 by showing its suppressive effects on aging and metabolic disease using Sirt1 transgenic mice. Thus, sirtuin activation has been thought to comprise an important mechanism of RSV‐mediated longevity. However, recent studies have highlighted the SIR‐2.1‐independent effects of RSV‐mediated longevity (Figure 1a, Model II). For example, (a) caloric restriction extends the lifespan independently of sirtuin in worms (Lee et al., 2006); (b) RSV has multiple targets including STAT3, JNK, AMPK, and ERK (Pirola & Frojdo, 2008). Among these, AMPK (5′‐AMP‐activated protein kinase) has been relatively well‐established as a target of RSV (Dasgupta & Milbrandt, 2007). RSV activates AMPK as its central target and acts indirectly on SIRT1 (Um et al., 2010). Therefore, sirtuin‐independent/indirect pathways or other RSV targets might be involved in RSV‐mediated longevity (Viswanathan, Kim, Berdichevsky, & Guarente, 2005); (c) SRT1720, known as a specific sirtuin activator which can ameliorate type 2 diabetes and metabolic diseases, can extend the lifespan and improve the health of mice (Mitchell et al., 2014). However, a contradictory effect of SRT1720 on longevity has additionally been reported, as SRT1720 cannot extend the lifespan and does not mimic the effect of RSV on lifespan extension in worms (Zarse et al., 2010). Therefore, the effect of RSV on lifespan extension may not function entirely in a sirtuin‐dependent manner. Moreover, it is necessary to identify other pathways or factors that respond to RSV (Figure 1a, Model III).
Figure 1.
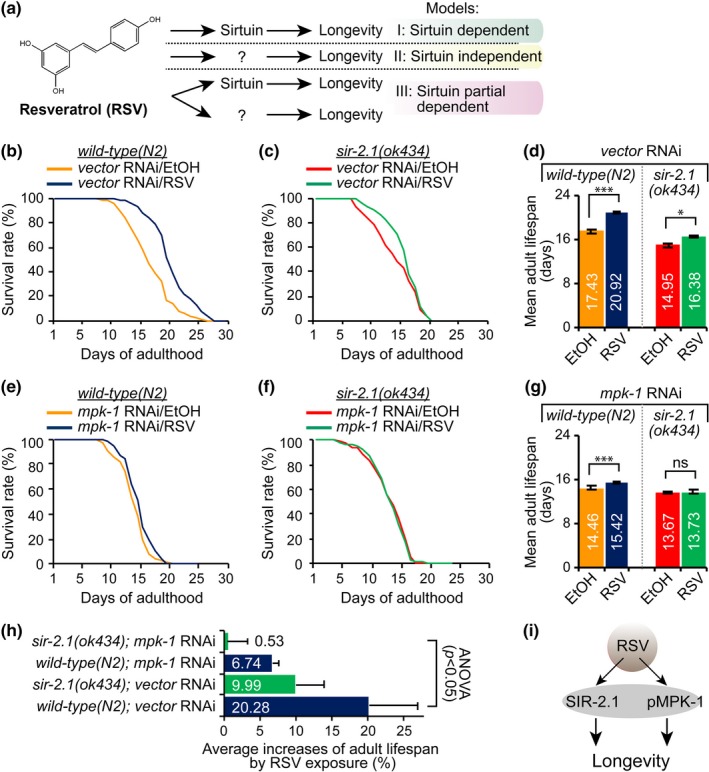
MPK‐1 is required for RSV‐mediated longevity of wild‐type (N2) and sir‐2.1(ok434) worms. (a) Potential action models for resveratrol (RSV)‐mediated longevity. (b−g) Adult lifespan curves and mean adult lifespan of wild‐type (b, d), sir‐2.1(ok434) (c, d), mpk‐1 RNAi in wild‐type (e, g), and mpk‐1 RNAi in sir‐2.1(ok434) null mutant worms (f, g) in the absence (0.1% EtOH as a vehicle) and presence of 100 μM RSV. (see Supporting Information Table S1; logrank test, *p < 0.05, **p < 0.01, and ***p < 0.001 RSV‐exposed worms compared with EtOH counterparts). (h) Average increases (%) of lifespan in worms exposed to RSV. ANOVA test (p < 0.05). See Supporting Information Table S3 for detailed statistical analysis. (i) This model shows that RSV exerts its longevity effect through two independent pathways, SIR‐2.1 and MPK‐1
Resveratrol can bind to the integrin αVβ3 receptor to activate extracellular signal‐regulated kinases 1 and 2 (ERK1/2) in a human breast cancer cell line (Lin et al., 2006). In our previous study, using human mesenchymal stem cells, we observed that RSV increased the phosphorylation level of ERK, although this could vary depending on the number of cell passages (Yoon, Choi, Choi, Park, & Lee, 2015). It was reported that mitogen‐activated protein kinase‐1 (MPK‐1, known as human ERK homolog) extended the lifespan of Caenorhabditis elegans through SKN‐1 (the mammalian nuclear factor erythroid‐related factor; Okuyama et al., 2010). However, it has not yet been determined whether the RSV‐mediated lifespan extension in C. elegans can be regulated through MPK‐1 activity. In addition, to date, there have been no genetic studies clarifying the relationship between RSV and MPK‐1. Thus, the purpose of this study was to re‐evaluate the longevity effect of RSV‐mediated SIR‐2.1 and then to test whether MPK‐1/ERK, one of the candidate genes that respond to RSV, was involved with the longevity effect. Here, we demonstrate that RSV‐mediated longevity largely relies on two independent pathways, SIR‐2.1/DAF‐16 and MPK‐1/SKN‐1. Specifically, mpk‐1 RNA interference (RNAi) completely abolished the longevity effect of RSV in sir‐2.1 single null mutants. RSV exposure increased the level of phosphorylated MPK‐1 (pMPK‐1) and maintained the level of pMPK‐1 during aging in the wild‐type (WT) and sir‐2.1 single null mutant nematodes. The RSV‐mediated MPK‐1 activation largely depended on the presence of SKN‐1 in a SIR‐2.1/DAF‐16‐independent manner. We additionally found that RSV‐mediated MPK‐1 activation increased reproductive span as well as delayed germline aging by maintaining mitotic germ cells.
2. RESULTS
2.1. mpk‐1 is required for RSV‐mediated longevity of sir‐2.1 mutant as well as WT worms
To re‐evaluate whether RSV‐mediated longevity depends entirely on SIR‐2.1, WT and sir‐2.1(ok434) null mutant worms on day 4 from embryos were cultured on nematode growth media (NGM) plates containing 100 μM RSV or 0.1% ethanol (EtOH) control at 20°C. The sir‐2.1(ok434) mutant worms were outcrossed four times prior to the main experiments (Supporting Information Figure S1a‐d). In agreement with a previous report (Bass, Weinkove, Houthoofd, Gems, & Partridge, 2007), RSV significantly extended the lifespan of WT worms (Figure 1b). RSV additionally extended the lifespan of sir‐2.1(ok434) mutants (Figure 1c). However, the RSV‐increased lifespan of sir‐2.1(ok434) mutants was less than that of WT worms (Figure 1d), suggesting that RSV‐mediated longevity is not entirely dependent upon sir‐2.1. A previous study demonstrated that the MPK‐1 pathway regulates longevity through SKN‐1 in C. elegans (Okuyama et al., 2010). To investigate whether MPK‐1 is involved in RSV‐mediated longevity, we examined the lifespan of WT worms in the absence or presence of RSV (vector or mpk‐1 RNAi). Exposure to RSV led to an increase in the lifespan of mpk‐1 RNAi‐treated WT worms (Figure 1e). However, the increased lifespan of mpk‐1 RNAi‐treated worms treated with RSV was likewise significantly less than that of WT worms (Figure 1e). This finding led us to test whether RSV‐mediated longevity may require both sir‐2.1 and mpk‐1. We measured the lifespan of sir‐2.1(ok434); mpk‐1 (RNAi) worms. RSV failed to extend the lifespan of sir‐2.1(ok434); mpk‐1 (RNAi) worms (Figure 1f); no significant change in the mean lifespan (Figure 1g) and no increase in lifespan by RSV were observed (Figure 1h). We therefore concluded that both sir‐2.1 and mpk‐1 are critical for the full activity of RSV in regulating the lifespan of C. elegans (Figure 1i).
2.2. RSV maintains MPK‐1 activity throughout the lifespan of C. elegans
We recently reported that RSV could activate ERK in human mesenchymal stem cells, depending on cell passage (Choi et al., 2018; Yoon et al., 2015). MPK‐1 has been shown to extend the lifespan of C. elegans (Okuyama et al., 2010). These findings led us to test whether MPK‐1 activity is regulated by RSV exposure. We confirmed that the levels of pMPK‐1 were significantly increased in WT worms exposed to 100 and 200 μM RSV (Supporting Information Figure S2). Next, WT and sir‐2.1(ok434) mutants were collected at three different time points. The results show that, upon RSV exposure, the levels of pMPK‐1 remained comparatively higher during aging in WT and sir‐2.1(ok434) mutant worms (Figure 2a,b). Next, to phenotypically reaffirm whether RSV induces MPK‐1 activation, we employed a temperature‐sensitive (ts) mpk‐1(ga111) loss‐of‐function mutant. The mpk‐1(ts) mutants are fertile at the permissive temperature (20°C) and have a sterile pachytene exit defect (PAC) phenotype that is caused by low levels of active MPK‐1 at the restrictive temperature (25°C; Leacock & Reinke, 2006). To test whether RSV rescues the PAC phenotype of mpk‐1(ts) by increasing MPK‐1 activity, L1‐stage mpk‐1(ts) mutants were grown on NGM agar plates containing RSV or EtOH control plates for four days at an intermediate temperature (23.5°C). The cellular morphology of meiotic germ cells was visualized by staining dissected gonads with HIM‐3 antibodies (a marker for meiotic cells; Figure 2c,d). A PAC phenotype was exhibited by 58.7% of mpk‐1(ts) mutant worms exposed to EtOH control, whereas 100 µM RSV exposure significantly reduced the percentage to 40.7% (Figure 2e). To confirm whether this reduction was a result of increased MPK‐1 protein activity, we utilized mpk‐1 (RNAi) in RSV‐ and EtOH‐treated mpk‐1(ts) mutant worms. The results show that mpk‐1(RNAi) inhibited RSV‐induced MPK‐1 activation and increased the percentage of worms with the PAC phenotype (Figure 2e). Thus, these findings strengthen the evidence that RSV promotes the activation of MPK‐1 in C. elegans.
Figure 2.
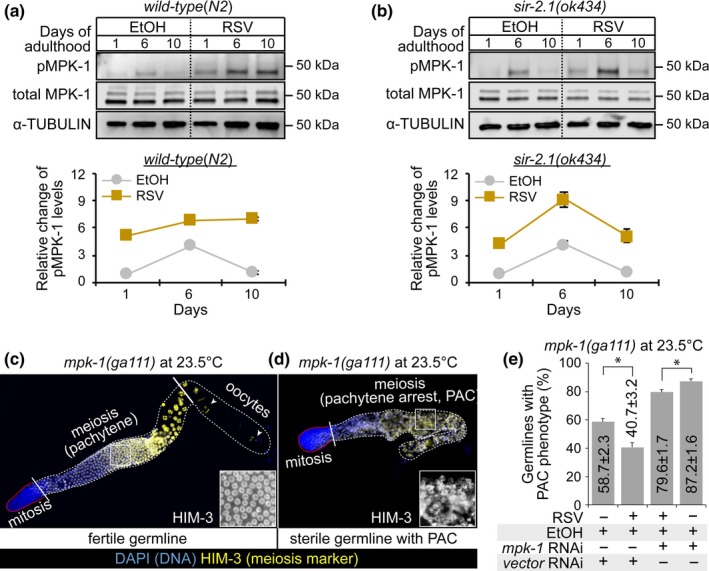
Resveratrol (RSV) activates MPK‐1 and rescues a sterile pachytene exit defect (PAC) phenotype in temperature‐sensitive mpk‐1 mutants. Levels of total MPK‐1 and pMPK‐1 proteins were analyzed using extracts from wild‐type (a) and sir‐2.1(ok434) mutant worms (b) grown on NGM plates containing 100 μM RSV or vehicle (0.1% EtOH) at different time points (4, 10, and 14 days). α‐tubulin was used as a loading control. (c, d) Dissected germlines of adult hermaphrodites were stained with an anti‐HIM‐3 antibody (a marker for meiotic cells). Images were captured using consistent acquisition settings and under the same magnification. (c) Fertile mpk‐1(ts) germline. (d) Sterile mpk‐1(ts) germline with a PAC phenotype. The PAC germline exhibits abnormal pachytene cell morphology. (e) RSV rescued the PAC phenotype of mpk‐1(ts) mutants at 23.5°C. Gonads with the PAC phenotype were counted. The graph shows average percent of mpk‐1(ts) mutant worms with the PAC phenotype grown with EtOH‐ or RSV (100 μM)‐containing NGM agar plates under control or mpk‐1 RNAi. The percentage of control (RNAi)‐treated mpk‐1(ts) worms with the PAC phenotype in the absence and presence of RSV was 58.70 ± 2.25 (EtOH control, triplicate, n = 84) and 40.68 ± 3.16 (RSV, triplicate, n = 114), respectively (p = 0.028). The percentage of mpk‐1(RNAi)‐treated mpk‐1(ts) worms with the PAC phenotype in the absence and presence of RSV was 79.56 ± 1.70 (EtOH, triplicate, n = 69) and 87.19 ± 1.60 (RSV, triplicate, n = 62) (p = 0.028). p‐Values for each experimental group were calculated using the two‐tailed Student’s t test, and the error bars represent SD
2.3. MPK‐1 and SIR‐2.1 may have different downstream targets to promote RSV‐mediated longevity
It has been reported that active MPK‐1 phosphorylates key residues of SKN‐1 protein, which is required for normal lifespan in C. elegans (An & Blackwell, 2003). Furthermore, MPK‐1/SKN‐1‐mediated longevity in C. elegans is shown to be independent of DAF‐16, which is known as a downstream regulator of SIR‐2.1 in regulating lifespan (Berdichevsky, Viswanathan, Horvitz, & Guarente, 2006; Tissenbaum & Guarente, 2001; Wang et al., 2006). Thus, we wanted to identify whether SKN‐1 and DAF‐16 are involved together or separately in RSV‐mediated longevity. To evaluate this, we employed WT and sir‐2.1(ok434) mutant worms for RNAi experiments involving skn‐1 and daf‐16. RSV led to an increased lifespan in each skn‐1 or daf‐16 knockdown worms (Figure 3a−c). However, the increased lifespan of skn‐1 or daf‐16 RNAi‐treated worms exposed to RSV was significantly less than that of WT worms (Figure 3d,i). Thus, this result indicates that both SKN‐1 and DAF‐16 are partially involved in RSV‐mediated longevity. Next, we measured the lifespan of sir‐2.1(ok434); skn‐1(RNAi) and sir‐2.1(ok434); daf‐16(RNAi) worms. RSV failed to extend the lifespan of sir‐2.1(ok434); skn‐1(RNAi) worms (Figure 3e,f); no significant change in the mean lifespan (Figure 3h) and no increase in lifespan by RSV were observed (Figure 3j). In contrast, RSV increased the lifespan of sir‐2.1(ok434); daf‐16(RNAi) worms to a level similar to that of sir‐2.1(ok434); control vector (RNAi) (Figure 3e,g,h,j). At this point, we concluded that SKN‐1 is required for RSV‐mediated longevity in a SIR‐2.1‐independent manner, whereas DAF‐16 has been shown to be involved in the RSV‐mediated SIR‐2.1 activation.
Figure 3.
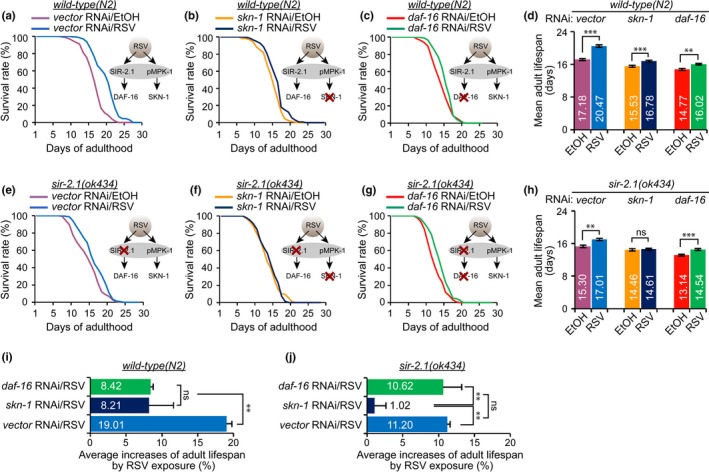
Knockdown of skn‐1 completely abolishes the longevity effect of RSV in sir‐2.1(ok434)‐deficient worms. (a−c) Adult lifespan curves of wild‐type with RNAi vector control (a), wild‐type with skn‐1 RNAi (b), and wild‐type with daf‐16 RNAi (c) with vehicle (0.1% EtOH) and 100 μM resveratrol (RSV). (see Supporting Information Table S1). (d) Representative data are the means of three independent experiments (logrank test, *p < 0.05, **p < 0.01, and ***p < 0.001 RSV‐exposed worms compared to EtOH counterparts). (e−g) Adult lifespan curves of sir‐2.1(ok434) with RNAi vector control (e), sir‐2.1(ok434) with skn‐1 RNAi (f), and sir‐2.1(ok434) with daf‐16 RNAi (g) with vehicle (0.1% EtOH) and 100 μM RSV. (see Supporting Information Table S1). (h) Representative data are the means of three independent experiments (logrank test, *p < 0.05, **p < 0.01, and ***p < 0.001 RSV‐exposed worms compared to EtOH counterparts). (i, j) Average increases (%) of adult lifespan in worms exposed to RSV. ANOVA test (p < 0.05). See Supporting Information Table S3 for detailed statistical analysis
2.4. MPK‐1 contributes to RSV‐mediated longevity via SKN‐1, independently of SIR‐2.1/DAF‐16 pathway
DAF‐16 can be activated by SIR‐2.1 to extend C. elegans lifespan (Berdichevsky et al., 2006; Mouchiroud et al., 2013), whereas RSV‐mediated MPK‐1 extends the lifespan of C. elegans through SKN‐1 regulation (Okuyama et al., 2010; Figures 1f and 3f). However, whether MPK‐1 and SIR‐2.1 have independent downstream targets under RSV stimulation has not been studied. To examine the possible mechanisms of MPK‐1 in RSV‐mediated lifespan extension, daf‐16(mu86) and skn‐1(zj15) mutant worms were employed. RSV led to a small increase in lifespan extension of daf‐16(mu86) mutant worms (Figure 4a,e,f). RNAi‐mediated knockdown of sir‐2.1 did not affect the increase in the lifespan effected by RSV in daf‐16(mu86) worms (Figure 4b,e,f). However, knockdown of mpk‐1 or skn‐1 completely blocked RSV‐induced lifespan extension in daf‐16(mu86) worms (Figure 4c‐f). Thus, we conclude that MPK‐1 and SKN‐1 influence RSV‐mediated longevity by acting independently of DAF‐16, whereas SIR‐2.1 has been shown to be dependent on the presence of DAF‐16 under RSV stimulation. RSV additionally extended the lifespan of skn‐1(zj15) mutant worms (Figure 4g,k,l). As expected, knockdown of mpk‐1 did not affect the RSV‐mediated increase in the average lifespan (Figure 4h,k,l), whereas knockdown of sir‐2.1 or daf‐16 completely blocked the RSV‐mediated lifespan extension in skn‐1(zj15) mutant worms (Figure 4i‐l), thereby strongly indicating that the MPK‐1‐mediated longevity effect is dependent upon the presence of skn‐1 under RSV stimulation, but independent of the presence of sir‐2.1 and daf‐16. Reactive oxygen species (ROS) generate byproducts of normal oxidative metabolism which cause an accumulation of molecular damage, resulting in acceleration of aging processes (Horne & Ricciardo, 1988). It is well‐known that SIR‐2.1 binds nuclear DAF‐16 to promote DAF‐16‐dependent transcription, stress resistance, and longevity (Berdichevsky et al., 2006). Although Okuyama et al. (2010) found that MPK‐1 phosphorylates the key site of SKN‐1 required for nuclear accumulation and SIR‐2.1, DAF‐16, and SKN‐1 have been implicated in the ROS‐mediated aging processes (Berdichevsky et al., 2006; Blackwell, Steinbaugh, Hourihan, Ewald, & Isik, 2015; Liu et al., 2015; Ye et al., 2010), whether MPK‐1 is involved in regulating oxidative stress under RSV stimulation remains undetermined. To clarify whether MPK‐1 activation is involved in the RSV‐mediated protection of worms from oxidative stress, a time‐course experiment was performed to measure the level of intracellular ROS using the molecular probe H2DCF‐DA with a 32°C heat shock. The results revealed that RSV attenuated ROS accumulation in WT worms and that silencing of sir‐2.1 partially abolished the RSV effect in preventing ROS accumulation from heat shock (Supporting Information Figure S3a,b). Knockdown of mpk‐1 additionally partially weakened the RSV effect in WT worms, whereas in sir‐2.1(ok434) mutants, the effect was completely abolished by mpk‐1 knockdown (Supporting Information Figure S3c,d). These data show that RSV‐activated MPK‐1 plays a role in RSV‐enhanced oxidative stress resistance. Thus, we suggest that the MPK‐1/SKN‐1 pathway is activated in a SIR‐2.1‐independent manner under RSV stimulation, but the role may be identical, at least in this part, for protecting the worms from ROS accumulation.
Figure 4.
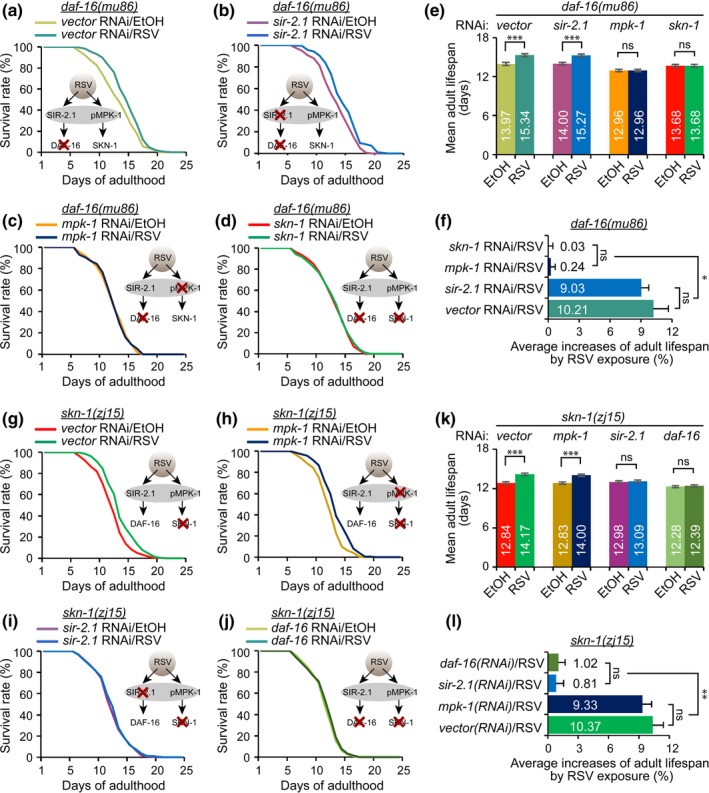
Resveratrol (RSV)‐activated MPK‐1 contributes to longevity in a SKN‐1‐dependent manner, but not in SIR‐2.1 and DAF‐16‐dependent fashions. Adult lifespan curves of daf‐16(mu86) with RNAi vector control (a), daf‐16(mu86) with sir‐2.1 RNAi (b), daf‐16(mu86) with mpk‐1 RNAi (c), and daf‐16(mu86) with skn‐1 RNAi (d) with vehicle (0.1% EtOH) and 100 μM RSV. (see Supporting Information Table S1). (e) The representative data are the means of three independent experiments (logrank test, *p < 0.05, **p < 0.01, and ***p < 0.001 RSV‐exposed worms compared with EtOH counterparts). (f) Average increases (%) of adult lifespan in the worms exposed to RSV. ANOVA test (p < 0.05). See Supporting Information Table S3 for detailed statistical analysis. Adult lifespan curves of skn‐1(zj15) with RNAi vector control (g), skn‐1(zj15) with mpk‐1 RNAi (h), skn‐1(zj15) with sir‐2.1 RNAi (i), and skn‐1(zj15) with daf‐16 RNAi (j) in the absence (0.1% EtOH as a vehicle) and presence of 100 μM RSV (see Supporting Information Table S1). (k) The representative data are the means of three independent experiments (logrank test, *p < 0.05, **p < 0.01, and ***p < 0.001 RSV‐exposed worms compared with EtOH counterparts). (l) Average increases (%) of adult lifespan in the worms exposed to RSV. ANOVA test (p < 0.05)
2.5. MPK‐1 is involved in the RSV‐mediated nuclear accumulation of SKN‐1 independently of SIR‐2.1
It is well‐known that SIR‐2.1 binds nuclear DAF‐16 to promote DAF‐16‐dependent transcription, stress resistance, and longevity (Berdichevsky et al., 2006). Although Okuyama et al. found that MPK‐1 phosphorylates the key site of SKN‐1 required for nuclear accumulation (Okuyama et al., 2010), it has not been determined yet whether MPK‐1 induces nuclear import of SKN‐1. To clarify whether MPK‐1 regulates cellular localization of SKN‐1 under RSV stimulation, we employed a SKN‐1::GFP [ldIs7;skn‐1b/c::GFP+rol‐6(su1006)] strain. First, we checked whether SKN‐1::GFP localization was changed under RSV stimulation through immunostaining. RSV significantly induced the nuclear accumulation of SKN‐1 in the intestine (Figure 5a,b). We then examined whether sir‐2.1 and mpk‐1 are involved in the RSV‐induced accumulation of nuclear SKN‐1. To test this, worms were synchronized at L1 stage and maintained under normal growth conditions until they reached adults (at 4 days after embryo stage), and then the adult worms were transferred to EtOH‐ or RSV‐containing RNAi plates. The transferred worms were kept on the EtOH‐ or RSV‐containing RNAi plates for 10 days and then subjected to heat stress for 4 hr at 32°C (Figure 5c). We first checked the mRNA and protein levels of skn‐1 using real‐time qPCR and western blot analysis. Neither RSV nor RNAi of mpk‐1 or sir‐2.1 affected the mRNA and protein levels of skn‐1 (Figure 5d,e). Knockdown of sir‐2.1 by RNAi did not affect the nuclear accumulation of SKN‐1 induced by RSV (Figure 5f), which indicates that the nuclear import of SKN‐1 does not depend on the presence of sir‐2.1. However, RNAi‐mediated knockdown of mpk‐1 completely blocked RSV‐induced nuclear accumulation of SKN‐1::GFP (Figure 5f). Quantitative analysis of SKN‐1::GFP translocation following RSV stimulation in mpk‐1 RNAi‐treated worms showed no difference between EtOH‐ and RSV‐treated groups (Figure 5g). To investigate whether RSV‐induced MPK‐1 is related to the presence of DAF‐16, we examined the mRNA and protein levels of DAF‐16. Neither RSV nor RNAi knockdown of mpk‐1 or sir‐2.1 affected the mRNA expression of daf‐16 (Supporting Information Figure S4a). Interestingly, RSV increased the protein level of DAF‐16 in WT worms treated with vector or mpk‐1 RNAi, whereas sir‐2.1 inhibition by RNAi completely blocked the RSV‐mediated increase in DAF‐16 protein (Supporting Information Figure S4b,c). In addition, RSV treatment increased the mRNA levels of sod‐3 and hsp‐16.2, which are well‐known downstream targets of DAF‐16 (Wang, Zhang, Lu, & Zhou, 2015). As expected, RSV increased the expression of the DAF‐16 target mRNAs in the worms treated with vector control or mpk‐1 RNAi, which was blocked by knockdown of sir‐2.1 by RNAi (Supporting Information Figure S4d). These results suggest that RSV‐mediated MPK‐1 action is independent of the SIR‐2.1/DAF‐16 pathway. However, we demonstrated that the RSV‐induced accumulation of SKN‐1 is dependent on mpk‐1.
Figure 5.
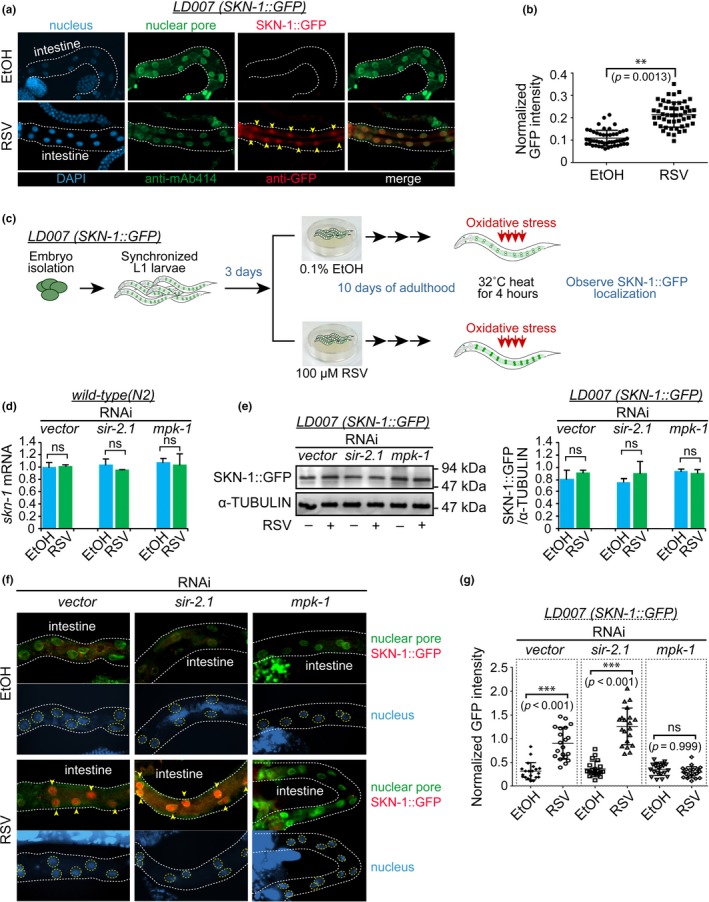
Nuclear accumulation of SKN‐1 by resveratrol (RSV) depends on the presence of mpk‐1. (a) SKN‐1(b/c)::GFP transgenic worms treated with 0.1% EtOH or 100 µM RSV were dissected, and the worms were stained with antibodies against GFP or nuclear pore complex proteins (Mab414). All images were acquired using consistent settings and under the same magnification. (b) Quantitative analysis of the GFP‐positive nuclei was performed using ImageJ. **p < 0.01 compared with EtOH‐treated SKN‐1::GFP transgenic worms. The p‐values were calculated using the two‐tailed unpaired Student's t test, and the error bars indicate SD n = 50 for each group. (c) Embryos in SKN‐1(b/c)::GFP transgenic worms were isolated and then maintained for 4 days on OP50 NGM plates. On day 4 after embryo isolation, the worms were transferred to each RNAi pate containing 0.1% EtOH or 100 µM RSV and then maintained for an additional 10 days. At day 10 of adulthood, the worms were placed in an incubator at 32°C for 4 hr, then collected for western blotting, and dissected and fixed for immunostaining. (d) mRNA expression of skn‐1 was determined in wild‐type (N2) worms on day 10 of adulthood. Likewise, on day 4 from embryo isolation, the worms were transferred to each RNAi pate containing 0.1% EtOH or 100 µM RSV and then maintained for an additional 10 days. The worms were placed in an incubator at 32°C for 4 hr and then collected for qRT–PCR. The qRT–PCR assay was performed in triplicate (ns denotes no significance). (e) Protein level of SKN‐1 was determined by western blot assay in triplicate, and the quantification was performed using the ImageJ program (ns, no significance). (f) The dissected and fixed worms were stained with antibodies against GFP or nuclear pore complex proteins (Mab414). All images were acquired using consistent settings under the same magnification. (g) Quantitative analysis of the GFP‐positive nuclei was performed using ImageJ. **p < 0.01 compared with EtOH‐treated SKN‐1::GFP transgenic worms. The p‐values were calculated using the one‐way ANOVA test, and the error bars indicate SD (n = 20 for each group)
2.6. RSV prevents germline aging via MPK‐1
Germline stem cell (GSC) capacity is diminished, and germ cell numbers are decreased during the aging process (Qin & Hubbard, 2015). Thus, we wanted to investigate the potential effect of RSV on the maintenance of GSC capacity during aging. For more detailed analysis, germline phenotypes were classified into three groups depending on (I) germline size, (II) GSC proliferation capacity, and (III) the presence of gametes (e.g., oocytes; see Figure 6a legend for more details). We found that WT worms exposed to RSV contained more class III germlines and significantly reduced percentages of class I and II germlines than those of worms treated with EtOH and at day 10 of the adult stage (Figure 6b). We next investigated the effect of RSV on sir‐2.1(ok434) germline aging. The results show that sir‐2.1(ok434) mutant worms exposed to RSV likewise had more class III and slightly reduced percentages of class I and II germlines compared to those of the EtOH‐treated worms at day 14 (Figure 6c). We then examined whether MPK‐1 is required for this RSV‐mediated effect delaying germline aging. RNAi‐mediated knockdown of mpk‐1 in WT and sir‐2.1(ok434) worms clearly blocked the effects of RSV, which can delay germline aging. There were no differences between EtOH‐ and RSV‐treated groups, especially in class III gonads (Figure 6d,e). This result suggests that the positive effects of RSV in maintaining mitotic GSCs depend entirely on the presence of MPK‐1 throughout the worm’s lifespan.
Figure 6.
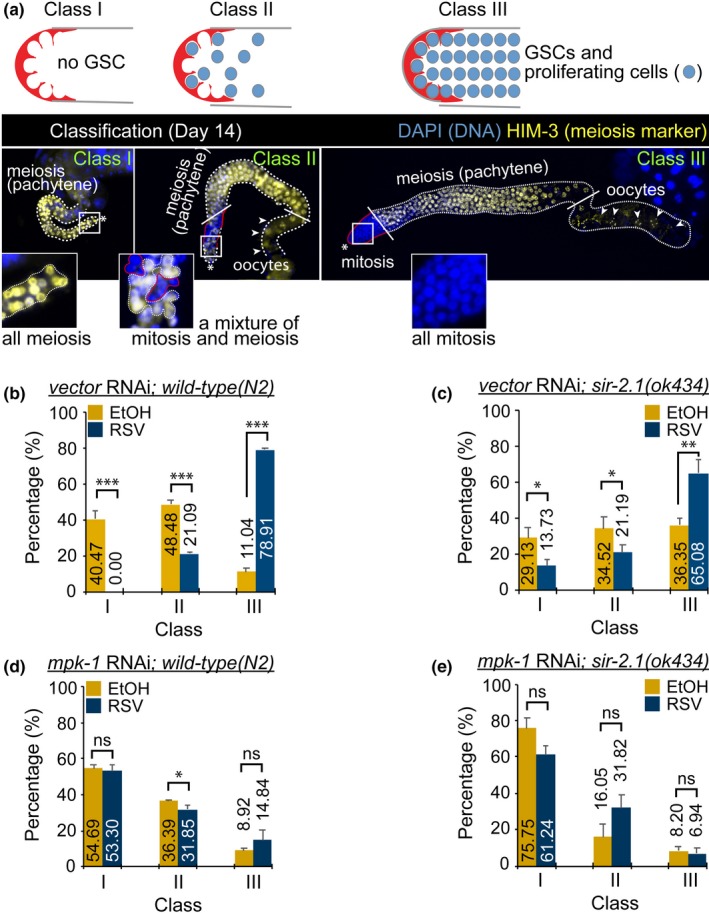
Effects of resveratrol (RSV) on Caenorhabditis elegans germline aging via MPK‐1. (a) Dissected germlines of 14‐day‐old adult hermaphrodites were stained with an antibody against HIM‐3. All images were acquired using consistent settings under the same magnification. A picture in the class I panel shows a smaller gonad and all HIM‐3‐negative/meiotic cells (see the magnified inset of the picture in Figure 6a). An image on the class II panel illustrates a middle‐sized gonad, in which oocytes are occasionally observed. The class II gonads possess a mixture of mitotically dividing cells (HIM‐3‐negative) and meiotic cells (HIM‐3‐positive) in the most distal germline (see the magnified image of the inset in Figure 5b; dashed white lines indicate HIM‐3‐positive cells, and red lines indicate HIM‐3‐negative cells). A picture in the class III panel shows gonads larger in size compared with the other two groups, class I and II. All of the class III gonads possess oocytes and HIM‐3 negative mitotic germ cells in the distal region of the germline. *, distal end; solid red lines, mitotic cells (HIM‐3‐negative); dashed white lines, meiotic cells (HIM‐3‐positive); solid white lines, a boundary between mitosis and meiosis as well as a boundary between meiotic pachytene and maturing oocytes region; arrowheads, individual oocytes. Percentages of each germline classified as class I, II, or III in wild‐type with RNAi vector control (b), sir‐2.1(ok434) with RNAi vector control (c), wild‐type with mpk‐1 RNAi (d), and sir‐2.1(ok434) with mpk‐1 RNAi (e) worms that were exposed to vehicle (0.1% EtOH) or to RSV during the 14 days after the embryonic stage. All scores shown in the graphs are the means of three independent experiments. p‐Values for each experimental group were calculated using the two‐tailed Student's t test, and the error bars indicate SD
2.7. mpk‐1 is required for RSV‐mediated reproductive longevity
As mentioned above, RSV maintained healthy germ cells during aging. To clarify the physiological significance of this RSV effect in the maintenance of mitotic germ cells during aging, we examined the effects of RSV on progeny production on aging. WT and sir‐2.1(ok434) mutant worms were synchronized at the L1 stage by embryo isolation and then grown on until they reached the L4 stage. The worms were then transferred to a new NGM plate with a single worm per plate. The next day, worms capable of producing progeny were transferred to EtOH‐ or RSV‐containing plates in seeded with RNAi bacteria. Worms that died during the reproductive periods were excluded in this experiment. The worms were transferred to new plates daily and viable progenies on the plates were counted 2 days after the worms were moved to a new plate. The results show that RSV clearly increased the brood size and reproductive span of WT worms (Figure 7a,b). The number of viable progeny was likewise increased by RSV treatment during the reproductive period (Figure 7c). As expected, mpk‐1 inhibition by RNAi abolished the effects of RSV as well as decreased brood size, reproductive span, and number of viable progeny (Figure 7d−f). Similarly, RSV increased brood size, reproductive span, and number of viable progeny in the sir‐2.1(ok434) mutant worms (Figure 7g−i), but RNAi of mpk‐1 blocked this effect (Figure 7j−l). Collectively, these results indicate that MPK‐1 contributes to the prolonged reproductive span induced by RSV through maintenance of mitotic germ cells during reproductive aging. Thus, we suggest that the RSV/MPK‐1 pathway might be a new target for germline health.
Figure 7.
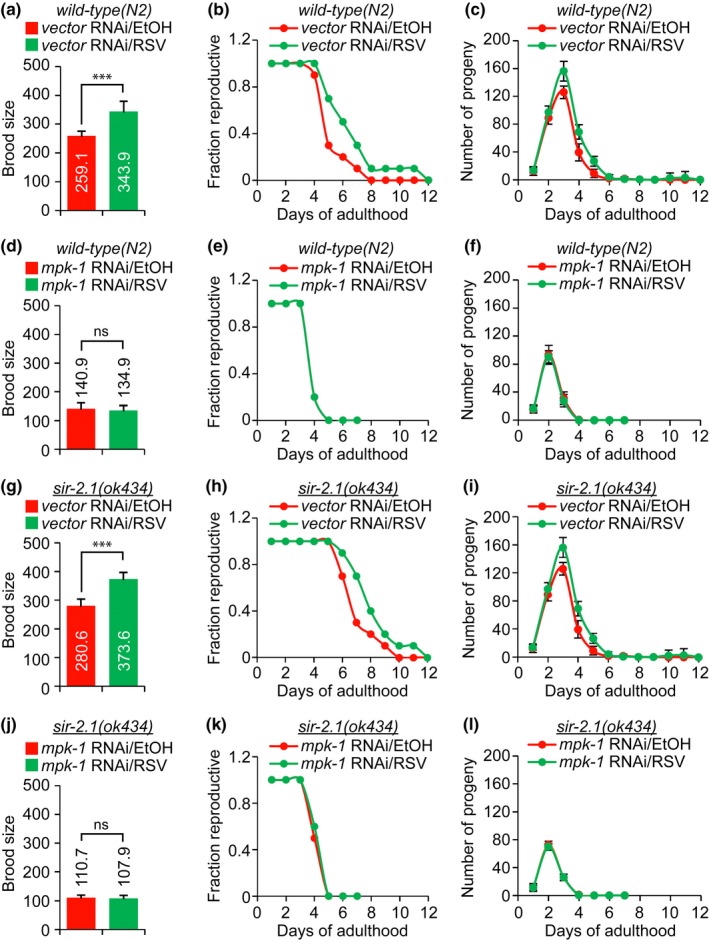
Physiological effects of resveratrol (RSV) on the reproductive system of Caenorhabditis elegans. (a) Brood size, (b) reproductive span curves, and (c) daily progeny production of wild‐type (N2) worms in the absence (0.1% EtOH as a vehicle) and presence of 100 μM RSV. (d) Brood size, (e) reproductive span curves (there is no difference between EtOH and RSV groups; hence, it only shows the mpk‐1 RNAi/RSV response; the EtOH and RSV curves are superimposed over each other), and (f) daily progeny production of the mpk‐1 RNAi‐treated wild‐type worms in the absence (0.1% EtOH as a vehicle) and presence of RSV. (g) Brood size, (h) reproductive span curves, and (i) daily progeny production of sir‐2.1 (ok434) mutant worms in the absence (0.1% EtOH as a vehicle) and presence of RSV. (j) Brood size, (k) reproductive span curves, and (l) daily progeny production of the mpk‐1 RNAi‐treated sir‐2.1 (ok434) mutant worms in the absence (0.1% EtOH as a vehicle) and presence of RSV. (a, d, g, e) The representative data are the means of three independent experiments (Student's t test, ***p < 0.001; RSV‐exposed worms compared with EtOH counterparts, ns; not significant). (b, e, h, k) The representative data are the means of three independent experiments (ANOVA test, b, h; p < 0.01, e, k; ns). (c, f, i, l) The representative data are the means of three independent experiments (ANOVA test, c, i; p < 0.05, f, l; ns)
3. DISCUSSION
Resveratrol was originally identified as an activator of sirtuin and its invertebrate homologs, and has been shown to extend lifespan in both invertebrates and vertebrates (Bhullar & Hubbard, 2015; Pallauf, Rimbach, Rupp, Chin, & Wolf, 2016). However, subsequent studies have suggested that RSV‐mediated longevity may be independent of sirtuin and may not stimulate sirtuin activity to promote longevity (Hu, Liu, Wang, & Liu, 2011). Therefore, the effects of RSV on longevity seem to be controversial. In this study, we provide important new insights into the effects of RSV on longevity and GSC aging using C. elegans. Previous findings have shown that RSV can extend the lifespan of yeast, worms, and mice in a sirtuin‐dependent manner (Wood et al., 2004). This positive effect of RSV‐mediated sirtuin activation on lifespan extension has been explored in various research fields, such as stem cell aging, diabetes, and cancer (Buhrmann, Shayan, Popper, Goel, & Shakibaei, 2016; Cote et al., 2015; Liu et al., 2012). For these reasons, the effects of RSV‐mediated sirtuin activity cannot be overlooked, although some studies reported that the role of RSV as a sirtuin activator still remains controversial and other alternative pathways need to be found and characterized. Viswanathan et al. (2005) reported that the RSV effect on C. elegans longevity is completely dependent upon sir‐2.1, but independent of daf‐16. Nevertheless, they suggested that RSV inhibits SIR‐2.1 activity to prevent sir‐2.1‐mediated abu‐11 repression. Other studies have shown that SRT1720, a sirtuin‐specific activator, did not extend the lifespan of C. elegans (Zarse et al., 2010). In this study, we observed that RSV partially induced the lifespan extension of C. elegans by sir‐2.1(ok434) mutation. RSV has multiple putative targets, including STAT3, JNK, AMPK, and ERK, among others (Pirola & Frojdo, 2008). Of these, AMPK has been relatively well‐established as an RSV target (Dasgupta & Milbrandt, 2007). One study showed that RSV activates AMPK as its central target and acts indirectly on SIRT1. Additionally, RSV‐mediated AMPK activation is dependent on SIRT1 (Um et al., 2010). Furthermore, RSV cannot induce SIRT1 activation in the absence of AMPK. Hence, it is thought that AMPK is a mediator of RSV‐induced sirtuin activation. In contrast, Dasgupta and Milbrandt (2007) showed that neuronal activation of AMPK by RSV does not require the presence of SIRT1. Thus, it is controversial whether RSV‐mediated AMPK action is dependent upon the presence of SIRT1. In C. elegans, lifespan can be regulated by aak‐2, a gene encoding the AMPK protein (Apfeld, O'Connor, McDonagh, DiStefano, & Curtis, 2004). SIR‐2.1 extends the lifespan of C. elegans via both aak‐2‐dependent and aak‐2‐independent mechanisms (Curtis, O'Connor, & DiStefano, 2006). DAF‐16 is known to be a longevity factor in C. elegans (Lin, Hsin, Libina, & Kenyon, 2001). However, it was reported that RSV‐mediated lifespan extension was not dependent on DAF‐16 (Viswanathan et al., 2005). Thus, it is possible that RSV functions through an additional mechanism to extend the lifespan of C. elegans in a SIR‐2.1‐independent manner. Our results show that mpk‐1 is required for the RSV‐mediated lifespan extension in C. elegans. Knockdown of mpk‐1 in sir‐2.1(ok434) mutant worms completely abolished RSV‐mediated lifespan extension, indicating that MPK‐1 operates independently of SIR‐2.1 in RSV‐mediated lifespan extension. MPK‐1 was first identified as a longevity factor in C. elegans by Okuyama et al. (2010). They reported that active MPK‐1 phosphorylates key residues required for nuclear import of SKN‐1, which is required for normal lifespan (An & Blackwell, 2003). They additionally showed that MPK‐1 extended the lifespan of C. elegans independently of bar‐1 (β‐catenin human homolog), hsf‐1 (Heat shock protein human homolog), and sir‐2.1, which are known as regulators related to daf‐16‐dependent lifespan regulation (Essers et al., 2005; Hsu, Murphy, & Kenyon, 2003). Our lifespan results support this mechanism in that double mutation of sir‐2.1 and mpk‐1 completely abolished the RSV‐mediated lifespan extension, thereby indicating that mpk‐1 contributes to the RSV‐mediated lifespan extension independently of sir‐2.1. Our findings suggest that both sir‐2.1 and mpk‐1 are required for RSV‐mediated lifespan extension and that MPK‐1 is a longevity determinant which acts independently of SIR‐2.1 in C. elegans.
In addition to somatic aging, several studies have shown that the functioning of the reproductive system declines with age. During developmental processes in C. elegans, mitotic germ stem cells, termed GSCs, or progenitors, are located farthest from distal end. The cells enter meiosis and then are differentiated into sperm (L4 stage) or oocytes (adulthood stage). The number of these mitotic germ cells decreases with age (Qin & Hubbard, 2015). However, the biological mechanisms underlying this process remain poorly understood. Moreover, the effect of RSV on age‐associated stem cell loss has not yet been studied in other systems. Our current study demonstrates that RSV can delay germline aging by maintaining mitotic cells at distal regions and maintaining proliferative capacity (Figure 6). We did not observe mitotic germ cells (HIM‐3‐negative cells) in most WT or sir‐2.1(ok434) mutant worms by day 10 of adult stage. Therefore, it seems that mitotic germ cells of C. elegans gonads lose their self‐renewal potential with age. In addition, oocytes were not observed in the aged gonads, indicating that meiotic cells lose their potential to differentiate into the oocyte lineage. Most stem cells lose their self‐renewal and differentiation potentials with age (Oh, Lee, & Wagers, 2014). Thus, it is thought that the C. elegans germline is a good in vivo model system for study of stem cell aging (Hubbard, 2007). Our current study shows that RSV can prolong the in vivo maintenance of germline mitotic cells of C. elegans during aging, and this positive effect of RSV on germline mitotic cell maintenance is lost in mpk‐1 knockdown worms (Figure 6d,e). MPK‐1 has multiple functions in the C. elegans germline, such as germ cell fate specification and membrane organization of pachytene cells. Most processes during germline development of C. elegans appear to be regulated through sustained MPK‐1 activation (Lee et al., 2007). RSV maintained MPK‐1 activity during aging, whereas MPK‐1 activity dramatically decreased with age in EtOH‐treated worms (Figure 2a,b). Thus, we suggest that sustained MPK‐1 activation might be an important factor in the RSV‐mediated maintenance of germline mitotic cells during aging. These results suggest that sustained MPK‐1 activation might be an important factor in RSV‐mediated maintenance of GSCs and progenitor cells during aging. It is known that sir‐2.1(ok434) mutant worms are short‐lived and stress‐sensitive, while overexpression of sir‐2.1 induces lifespan extension in a daf‐16‐dependent manner (Tissenbaum & Guarente, 2001; Wang & Tissenbaum, 2006). Fertility can be reduced in short‐lived worms (Gems & Riddle, 2000). Although loss of germ cells extends C. elegans lifespan through regulation of DAF‐16 (Berman & Kenyon, 2006), the lifespan extension induced by certain mutations occasionally requires the presence of an intact adult germline and the continuous production of mature eggs (Greer et al., 2010). Thus, the relationship between lifespan and reproduction can be more complex than we might think. In this study, we observed that adult lifespan of sir‐2.1(ok434) mutant worms was shorter than that of WT worms. Nevertheless, the sir‐2.1(ok434) mutant worms had more class III germlines compared to the WT worms on day 10 of adulthood (Figure 6). In addition, Figure 7 shows that sir‐2.1(ok434) mutant worms had increased brood size (280.6 viable progeny)) compared to WT worms (259.1 viable progeny). It has been recently reported that initiation of germline apoptosis promotes gonad senescence in C. elegans (de la Guardia et al., 2016). SIR‐2.1 translocates from the nucleus into the cytoplasm and the translocation event is related to DNA damage‐induced apoptosis, known as an early event in germ cell apoptosis (Greiss, Hall, Ahmed, & Gartner, 2008). Taken together, loss of function of SIR‐2.1 may protect the C. elegans germline against DNA damage‐induced apoptosis as sir‐2.1(ok434) mutant worms seem to have improved germline status compared to WT worms. Although such an idea seems likely, much of this system remains unclear. Thus, further studies are required to clarify the genetic pathways between sir‐2.1 and germline aging during C. elegans aging process.
In summary, we novelly identified MPK‐1/ERK as a potent, critical RSV‐inducible factor, especially in terms of organismal longevity (Supporting Information Figure S5a) and GSC maintenance (Supporting Information Figure S5b). Given that MPK‐1 and SKN‐1 are highly conserved from C. elegans to mammals, these findings have important implications in utilizing RSV to improve the outcome of diseases associated with aging, diabetes, and cancer in mammals, including humans.
4. EXPERIMENTAL PROCEDURES
4.1. C. elegans strains
All C. elegans strains were maintained at 20°C as described previously (Brenner, 1974). We used the wild‐type Bristol strain N2 as well as the mutants and transgenic worms are listed in Supporting Information Table S1. sir‐2.1(ok434) mutants were outcrossed four times to wild‐type (N2) worms (Supporting Information Figure S1). For more details, please see Supporting Information Table S1.
4.2. Resveratrol preparation and lifespan assay
RSV (Sigma, St. Louis, MO, USA) preparation and lifespan assays were performed as described in the Supporting Information Appendix S1.
4.3. Western blot
Western blot analysis was performed as described in the Supporting Information Appendix S1.
4.4. RNA interference
RNA interference experiments were performed as described in the Supporting Information Appendix S1.
4.5. Real‐time quantitative polymerase chain reaction
Real‐time quantitative polymerase chain reaction (qRT–PCR) experiments were performed as described in the Supporting Information Appendix S1.
4.6. Immunohistochemistry
Immunohistochemistry experiments were performed as described in the Supporting Information Appendix S1.
4.7. Reproductive span analysis
Reproductive span‐related experiments were performed as described in the Supporting Information Appendix S1.
4.8. Statistics
Statistical significance from the lifespan and reproductive span assays was analyzed by logrank (Mantel–Cox) and analysis of variance (ANOVA) tests. Data are presented as mean ± standard deviation. Statistical significance of the phenotypes of C. elegans germline and brood size was calculated using the two‐tailed Student’s t test, and the error bars indicate standard deviation (SD).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
AUTHOR’S CONTRIBUTIONS
DSY and M‐HL performed study conception and design. DSY, DSC, YC, JWL, and M‐HL carried out experiments. DSY, DSC, YC, JWL, and M‐HL conducted analysis and interpretation of data. DSY drafted the manuscript, and M‐HL reviewed the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Professor T. Keith Blackwell (Harvard Medical School) for the LD1 (ldIs7 [skn‐1b/c::GFP + rol ‐6 (su1006)]) strain. This work was supported by NIH (AG060373) to M‐HL and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF‐2014R1A6A3A01053793) to DSY.
Yoon DS, Cha DS, Choi Y, Lee JW, Lee M‐H. MPK‐1/ERK is required for the full activity of resveratrol in extended lifespan and reproduction. Aging Cell. 2019;18:e12867 10.1111/acel.12867
REFERENCES
- An, J. H. , & Blackwell, T. K. (2003). SKN‐1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes and Development, 17, 1882–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov, V. N. , Berstein, L. M. , Egormin, P. A. , Piskunova, T. S. , Popovich, I. G. , Zabezhinski, M. A. , … Franceschi, C. (2005). Effect of metformin on life span and on the development of spontaneous mammary tumors in HER‐2/neu transgenic mice. Experimental Gerontology, 40, 685–693. 10.1016/j.exger.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Apfeld, J. , O'Connor, G. , McDonagh, T. , DiStefano, P. S. , & Curtis, R. (2004). The AMP‐activated protein kinase AAK‐2 links energy levels and insulin‐like signals to lifespan in C. elegans . Genes and Development, 18, 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, T. M. , Weinkove, D. , Houthoofd, K. , Gems, D. , & Partridge, L. (2007). Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans . Mechanisms of Ageing and Development, 128, 546–552. 10.1016/j.mad.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Baur, J. A. , Pearson, K. J. , Price, N. L. , Jamieson, H. A. , Lerin, C. , Kalra, A. , … Sinclair, D. A. (2006). Resveratrol improves health and survival of mice on a high‐calorie diet. Nature, 444, 337–342. 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky, A. , Viswanathan, M. , Horvitz, H. R. , & Guarente, L. (2006). C. elegans SIR‐2.1 interacts with 14‐3‐3 proteins to activate DAF‐16 and extend life span. Cell, 125, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Berman, J. R. , & Kenyon, C. (2006). Germ‐cell loss extends C. elegans life span through regulation of DAF‐16 by kri‐1 and lipophilic‐hormone signaling. Cell, 124, 1055–1068. [DOI] [PubMed] [Google Scholar]
- Bhullar, K. S. , & Hubbard, B. P. (2015). Lifespan and healthspan extension by resveratrol. Biochimica Et Biophysica Acta, 1852, 1209–1218. [DOI] [PubMed] [Google Scholar]
- Blackwell, T. K. , Steinbaugh, M. J. , Hourihan, J. M. , Ewald, C. Y. , & Isik, M. (2015). SKN‐1/Nrf, stress responses, and aging in Caenorhabditis elegans . Free Radical Biology and Medicine, 88, 290–301. 10.1016/j.freeradbiomed.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans . Genetics, 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrmann, C. , Shayan, P. , Popper, B. , Goel, A. , & Shakibaei, M. (2016). Sirt1 is required for resveratrol‐mediated chemopreventive effects in colorectal cancer cells. Nutrients, 8, 145 10.3390/nu8030145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y. , Yoon, D. S. , Lee, K.‐M. , Choi, S. M. , Lee, M.‐H. , Park, K. H. , … Lee, J. W. (2018). Enhancement of mesenchymal stem cell‐driven bone regeneration by resveratrol‐mediated SOX2 regulation. Aging and Disease, 10, 10.14336/AD.2018.0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, C. D. , Rasmussen, B. A. , Duca, F. A. , Zadeh‐Tahmasebi, M. , Baur, J. A. , Daljeet, M. , … Lam, T. K. (2015). Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. NatureMedicine, 21, 498–505. 10.1038/nm.3821 [DOI] [PubMed] [Google Scholar]
- Curtis, R. , O'Connor, G. , & DiStefano, P. S. (2006). Aging networks in Caenorhabditis elegans: AMP‐activated protein kinase (aak‐2) links multiple aging and metabolism pathways. AgingCell, 5, 119–126. [DOI] [PubMed] [Google Scholar]
- Dasgupta, B. , & Milbrandt, J. (2007). Resveratrol stimulates AMP kinase activity in neurons. Proceedings of the National Academy of Sciences of the United States of America, 104, 7217–7222. 10.1073/pnas.0610068104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Guardia, Y. , Gilliat, A. F. , Hellberg, J. , Rennert, P. , Cabreiro, F. , & Gems, D. (2016). Run‐on of germline apoptosis promotes gonad senescence in C. elegans . Oncotarget, 7, 39082–39096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling, R. J. , Zakikhani, M. , Fantus, I. G. , Pollak, M. , & Sonenberg, N. (2007). Metformin inhibits mammalian target of rapamycin‐dependent translation initiation in breast cancer cells. CancerResearch, 67, 10804–10812. 10.1158/0008-5472.CAN-07-2310 [DOI] [PubMed] [Google Scholar]
- Essers, M. A. , de Vries‐Smits, L. M. , Barker, N. , Polderman, P. E. , Burgering, B. M. , & Korswagen, H. C. (2005). Functional interaction between beta‐catenin and FOXO in oxidative stress signaling. Science, 308, 1181–1184. [DOI] [PubMed] [Google Scholar]
- Gems, D. , & Riddle, D. L. (2000). Defining wild‐type life span in Caenorhabditis elegans . The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 55, B215–B219. 10.1093/gerona/55.5.B215 [DOI] [PubMed] [Google Scholar]
- Greer, E. L. , Maures, T. J. , Hauswirth, A. G. , Green, E. M. , Leeman, D. S. , Maro, G. S. , … Brunet, A. (2010). Members of the H3K4 trimethylation complex regulate lifespan in a germline‐dependent manner in C. elegans . Nature, 466, 383–387. 10.1038/nature09195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiss, S. , Hall, J. , Ahmed, S. , & Gartner, A. (2008). C. elegans SIR‐2.1 translocation is linked to a proapoptotic pathway parallel to cep‐1/p53 during DNA damage‐induced apoptosis. Genes and Development, 22, 2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, D. E. , Strong, R. , Sharp, Z. D. , Nelson, J. F. , Astle, C. M. , Flurkey, K. , … Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460, 392–395. 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz, D. , Munoz‐Martin, M. , Canamero, M. , Mulero, F. , Martinez‐Pastor, B. , Fernandez‐Capetillo, O. , & Serrano, M. (2010). Sirt1 improves healthy ageing and protects from metabolic syndrome‐associated cancer. NatureCommunications, 1, 3 10.1038/ncomms1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne, M. D. , & Ricciardo, J. L. (1988). Hierarchy of response to handicaps. Psychological Reports, 62, 83–86. [DOI] [PubMed] [Google Scholar]
- Hsu, A. L. , Murphy, C. T. , & Kenyon, C. (2003). Regulation of aging and age‐related disease by DAF‐16 and heat‐shock factor. Science, 300, 1142–1145. 10.1126/science.1083701 [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Liu, J. , Wang, J. , & Liu, Q. (2011). The controversial links among calorie restriction, SIRT1, and resveratrol. Free Radical Biology and Medicine, 51, 250–256. 10.1016/j.freeradbiomed.2011.04.034 [DOI] [PubMed] [Google Scholar]
- Hubbard, E. J. (2007). Caenorhabditis elegans germ line: A model for stem cell biology. Developmental Dynamics: an Official Publication of the American Association of Anatomists, 236, 3343–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, B. P. , & Sinclair, D. A. (2014). Small molecule SIRT1 activators for the treatment of aging and age‐related diseases. Trends in Pharmacological Sciences, 35, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, B. K. , & Lamming, D. W. (2016). The mechanistic target of rapamycin: The grand ConducTOR of metabolism and aging. CellMetabolism, 23, 990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming, D. W. , Sabatini, D. M. , & Baur, J. A. (2012). Pharmacologic means of extending lifespan. Journal of Clinical and Experimental Pathology, XX(Suppl 4), 10.4172/2161-0681.S4-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leacock, S. W. , & Reinke, V. (2006). Expression profiling of MAP kinase‐mediated meiotic progression in Caenorhabditis elegans . PLoS Genetics, 2, e174 10.1371/journal.pgen.0020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. H. , Ohmachi, M. , Arur, S. , Nayak, S. , Francis, R. , Church, D. , … Schedl, T. (2007). Multiple functions and dynamic activation of MPK‐1 extracellular signal‐regulated kinase signaling in Caenorhabditis elegans germline development. Genetics, 177, 2039–2062. 10.1534/genetics.107.081356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G. D. , Wilson, M. A. , Zhu, M. , Wolkow, C. A. , de Cabo, R. , Ingram, D. K. , & Zou, S. (2006). Dietary deprivation extends lifespan in Caenorhabditis elegans . AgingCell, 5, 515–524. 10.1111/j.1474-9726.2006.00241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, K. , Hsin, H. , Libina, N. , & Kenyon, C. (2001). Regulation of the Caenorhabditis elegans longevity protein DAF‐16 by insulin/IGF‐1 and germline signaling. NatureGenetics, 28, 139–145. 10.1038/88850 [DOI] [PubMed] [Google Scholar]
- Lin, H. Y. , Lansing, L. , Merillon, J. M. , Davis, F. B. , Tang, H. Y. , Shih, A. , … Davis, P. J. (2006). Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 20, 1742–1744. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Ghosh, S. , Yang, X. , Zheng, H. , Liu, X. , Wang, Z. , … Zhou, Z. (2012). Resveratrol rescues SIRT1‐dependent adult stem cell decline and alleviates progeroid features in laminopathy‐based progeria. CellMetabolism, 16, 738–750. 10.1016/j.cmet.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Liu, T. , Qi, H. , Ma, L. , Liu, Z. , Fu, H. , Zhu, W. , … Li, G. (2015). Resveratrol attenuates oxidative stress and extends life span in the annual fish Nothobranchius guentheri . Rejuvenation Research, 18, 225–233. [DOI] [PubMed] [Google Scholar]
- Martin‐Montalvo, A. , Mercken, E. M. , Mitchell, S. J. , Palacios, H. H. , Mote, P. L. , Scheibye‐Knudsen, M. , … de Cabo, R. (2013). Metformin improves healthspan and lifespan in mice. NatureCommunications, 4, 2192 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, S. J. , Martin‐Montalvo, A. , Mercken, E. M. , Palacios, H. H. , Ward, T. M. , Abulwerdi, G. , … de Cabo, R. (2014). The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. CellReports, 6, 836–843. 10.1016/j.celrep.2014.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud, L. , Houtkooper, R. H. , Moullan, N. , Katsyuba, E. , Ryu, D. , Canto, C. , … Auwerx, J. (2013). The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell, 154, 430–441. 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, J. , Lee, Y. D. , & Wagers, A. J. (2014). Stem cell aging: Mechanisms, regulators and therapeutic opportunities. NatureMedicine, 20, 870–880. 10.1038/nm.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama, T. , Inoue, H. , Ookuma, S. , Satoh, T. , Kano, K. , Honjoh, S. , … Nishida, E. (2010). The ERK‐MAPK pathway regulates longevity through SKN‐1 and insulin‐like signaling in Caenorhabditis elegans . Journal of Biological Chemistry, 285, 30274–30281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallauf, K. , Rimbach, G. , Rupp, P. M. , Chin, D. , & Wolf, I. M. (2016). Resveratrol and lifespan in model organisms. Current Medicinal Chemistry, 23, 4639–4680. 10.2174/0929867323666161024151233 [DOI] [PubMed] [Google Scholar]
- Pirola, L. , & Frojdo, S. (2008). Resveratrol: One molecule, many targets. IUBMB Life, 60, 323–332. 10.1002/iub.47 [DOI] [PubMed] [Google Scholar]
- Qin, Z. , & Hubbard, E. J. (2015). Non‐autonomous DAF‐16/FOXO activity antagonizes age‐related loss of C. elegans germline stem/progenitor cells. Nature Communications, 6, 7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum, H. A. , & Guarente, L. (2001). Increased dosage of a sir‐2 gene extends lifespan in Caenorhabditis elegans . Nature, 410, 227–230. [DOI] [PubMed] [Google Scholar]
- Um, J. H. , Park, S. J. , Kang, H. , Yang, S. , Foretz, M. , McBurney, M. W. , … Chung, J. H. (2010). AMP‐activated protein kinase‐deficient mice are resistant to the metabolic effects of resveratrol. Diabetes, 59, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan, M. , Kim, S. K. , Berdichevsky, A. , & Guarente, L. (2005). A role for SIR‐2.1 regulation of ER stress response genes in determining C. elegans life span. Developmental Cell, 9, 605–615. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Oh, S. W. , Deplancke, B. , Luo, J. , Walhout, A. J. , & Tissenbaum, H. A. (2006). C. elegans 14‐3‐3 proteins regulate life span and interact with SIR‐2.1 and DAF‐16/FOXO. Mechanisms of Ageing and Development, 127, 741–747. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , & Tissenbaum, H. A. (2006). Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF‐16/FOXO. Mechanisms of Ageing and Development, 127, 48–56. 10.1016/j.mad.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Zhang, J. , Lu, L. , & Zhou, L. (2015). The longevity effect of echinacoside in Caenorhabditis elegans mediated through daf‐16. BioScienceBiotechnology, and Biochemistry, 79, 1676–1683. [DOI] [PubMed] [Google Scholar]
- Wood, J. G. , Rogina, B. , Lavu, S. , Howitz, K. , Helfand, S. L. , Tatar, M. , & Sinclair, D. (2004). Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature, 430, 686–689. 10.1038/nature02789 [DOI] [PubMed] [Google Scholar]
- Ye, K. , Ji, C. B. , Lu, X. W. , Ni, Y. H. , Gao, C. L. , Chen, X. H. , … Guo, X. R. (2010). Resveratrol attenuates radiation damage in Caenorhabditis elegans by preventing oxidative stress. Journal of Radiation Research, 51, 473–479. 10.1269/jrr.10009 [DOI] [PubMed] [Google Scholar]
- Yoon, D. S. , Choi, Y. , Choi, S. M. , Park, K. H. , & Lee, J. W. (2015). Different effects of resveratrol on early and late passage mesenchymal stem cells through beta‐catenin regulation. Biochemical and Biophysical Research Communications, 467, 1026–1032. [DOI] [PubMed] [Google Scholar]
- Zarse, K. , Schmeisser, S. , Birringer, M. , Falk, E. , Schmoll, D. , & Ristow, M. (2010). Differential effects of resveratrol and SRT1720 on lifespan of adult Caenorhabditis elegans . Hormone and Metabolic Research = Hormon‐ Und Stoffwechselforschung = Hormones Et Metabolisme, 42, 837–839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


