Abstract
Insulin/IGF signaling (IIS) regulates essential processes including development, metabolism, and aging. The Drosophila genome encodes eight insulin/IGF‐like peptide (dilp) paralogs, including tandem‐encoded dilp1 and dilp2. Many reports show that longevity is increased by manipulations that decrease DILP2 levels. It has been shown that dilp1 is expressed primarily in pupal stages, but also during adult reproductive diapause. Here, we find that dilp1 is also highly expressed in adult dilp2 mutants under nondiapause conditions. The inverse expression of dilp1 and dilp2 suggests these genes interact to regulate aging. Here, we study dilp1 and dilp2 single and double mutants to describe epistatic and synergistic interactions affecting longevity, metabolism, and adipokinetic hormone (AKH), the functional homolog of glucagon. Mutants of dilp2 extend lifespan and increase Akh mRNA and protein in a dilp1‐dependent manner. Loss of dilp1 alone has no impact on these traits, whereas transgene expression of dilp1 increases lifespan in dilp1 − dilp2 double mutants. On the other hand, dilp1 and dilp2 redundantly or synergistically interact to control circulating sugar, starvation resistance, and compensatory dilp5 expression. These interactions do not correlate with patterns for how dilp1 and dilp2 affect longevity and AKH. Thus, repression or loss of dilp2 slows aging because its depletion induces dilp1, which acts as a pro‐longevity factor. Likewise, dilp2 regulates Akh through epistatic interaction with dilp1. Akh and glycogen affect aging in Caenorhabditis elegans and Drosophila. Our data suggest that dilp2 modulates lifespan in part by regulating Akh, and by repressing dilp1, which acts as a pro‐longevity insulin‐like peptide.
Keywords: aging, Akh, dilp1, dilp2, Drosophila, insulin, insulin/IGF signaling
1. INTRODUCTION
Insulin/IGF signaling (IIS) is a fundamental pathway that regulates aging, development, metabolism, growth, and reproduction. The Drosophila melanogaster genome encodes several insulin‐like peptide genes (dilps) that signal through a single insulin‐like receptor (InR) (Brogiolo et al., 2001; Colombani, Andersen, & Leopold, 2012; Garofalo, 2002; Grönke, Clarke, Broughton, Andrews, & Partridge, 2010). Among their physiological functions, dilps regulate aging: Mutation of dilp2 alone is sufficient to extend lifespan, whereas loss of other dilps does not (Grönke et al., 2010). How reduction of one specific dilp modulates aging is not understood. Here, we demonstrate that dilp1 is upregulated in the absence of dilp2, that dilp1 expression is required for loss of dilp2 to slow aging, and that exogenous expression of dilp1 in a dilp1‐2 mutant is sufficient to extend lifespan.
The dilp1 gene is encoded approximately 1.2 kb upstream of dilp2, potentially as a result of a tandem duplication event (Tatar, Bartke, & Antebi, 2003). These paralogs are expressed in different developmental and life history stages. Dilp2 is initially expressed in embryos and then throughout larval instar stages (Brogiolo et al., 2001; Slaidina, Delanoue, Gronke, Partridge, & Leopold, 2009). Pupae show decreased expression of dilp2, but the ligand is again highly expressed in adults. In contrast, during normal development, dilp1 is only expressed in the pupal stage (Slaidina et al., 2009). While their timing is distinct, dilp1 and dilp2 are both expressed in median neurosecretory cells of the Drosophila brain, the insulin‐producing cells (IPCs) analogous to mammalian pancreatic β cells (Brogiolo et al., 2001; Liu, Liao, Veenstra, & Nässel, 2016; Rulifson, Kim, & Nusse, 2002).
The function of dilps in aging has been best studied for dilp2, dilp3, dilp5, and dilp6 (Bai, Kang, & Tatar, 2012; Broughton et al., 2010; Grönke et al., 2010). Lifespan is extended by mutation of dilp2 alone, or dilp2, dilp3, and dilp5 together: The normal function of dilp2 appears to promote processes permissive to aging. On the other hand, induction of dilp6 in fat body promotes longevity, perhaps because this decreases DILP2 peptide secreted from the IPCs (Bai et al., 2012). Similarly, increased FOXO expression in head fat body and increased JNK activity in IPCs extend lifespan, perhaps again because these manipulations decrease dilp2 expression in the IPCs (Hwangbo, Gershman, Tu, Palmer, & Tatar, 2004; Wang, Bohmann, & Jasper, 2005). Across these studies, there has been no attention to dilp1. Yet notably, in contrast to normal laboratory conditions, DILP1 is produced in adult IPCs during reproductive diapause (Liu et al., 2016), which is a quiescent phase strongly associated with negligible aging (Tatar & Yin, 2001). The positive association between dilp1 and diapause survival suggests this enigmatic insulin hormone may possess unusual functions in the control of aging.
Understanding how insulin peptides of Drosophila regulate aging is complicated by the fact that genetic or RNAi reduction of any one dilp gene induces compensatory expression in other dilp genes. For instance, a dilp2 mutant increases expression of dilp3 and dilp5 (Grönke et al., 2010). Complex compensation and interaction is also known for Caenorhabditis elegans insulin‐like gene paralogs (Fernandes de Abreu et al., 2014). For instance, ins‐6 is upregulated in an ins‐23 mutant, and these paralogs appear to interact to regulate lifespan (Fernandes de Abreu et al., 2014). Notably, C. elegans ins‐18 and ins‐23 are proposed to function as insulin‐like receptor antagonists to regulate Dauer formation and favor longevity (Matsunaga, Matsukawa, Iwasaki, Nagata, & Kawano, 2018). To date, aside from the inverse regulation of aging by dilp6 and dilp2 (Bai et al., 2012), functional interactions among Drosophila insulin paralogs have not been described.
Here, we study the relationship between dilp1 and dilp2. We find that dilp1 is strongly upregulated in dilp2 mutants, consistent with dilp1 serving a role in diapause conditions where it might regulate metabolism and slow aging. To test this model, we generated a dilp1‐2 double mutant to complement revised dilp1 and dilp2 single mutants (Grönke et al., 2010). As previously reported, dilp2 mutants are long‐lived. We now see that dilp1 mutants have wild‐type longevity as do dilp1 − dilp2 double mutants; thus, loss of dilp1 fully rescues the extended longevity of dilp2. We find that dilp1 is also genetically downstream of dilp2 in the control of Drosophila adipokinetic hormone (AKH), the functional homolog of mammalian glucagon. We confirmed the positive role of dilp1 upon longevity and AKH by transgene dilp1 expression in a dilp1 − dilp2 double mutant. In contrast to longevity and AKH, dilp1 and dilp2 do not epistatically control other tested physiological traits (e.g., hemolymph glucose or trehalose, starvation resistance, and glycogen), suggesting these phenotypes are not regulated through the same mechanisms by which these insulin‐like peptides interact to modulate aging. Our data together reveal a novel pathway by which a unique insulin‐like ligand, DILP1, positively regulates longevity.
2. RESULTS
Studies on the control of aging by IIS in Drosophila have measured dilp2, dilp3, and dilp5 mRNA or protein (Alic, Hoddinott, Vinti, & Partridge, 2011; Broughton et al., 2010; Hwangbo et al., 2004). While dilp1 of the adult IPC is not observed in nondiapause conditions, we sought to characterize its expression in dilp mutants known to extend lifespan. In wild‐type adult females, dilp1 mRNA is considerably lower than that of dilp2 (Figure 1a). Strikingly, dilp1 mRNA is elevated about 14‐fold in dilp2 mutants relative to its expression in wild‐type (Figure 1b), while there is little compensatory expression of dilp2 in dilp1 mutants (Figure 1c). Dilp2 appears to repress dilp1, and here, we test the proposition that dilp1 may function in the absence of dilp2 to regulate metabolism and aging.
Figure 1.
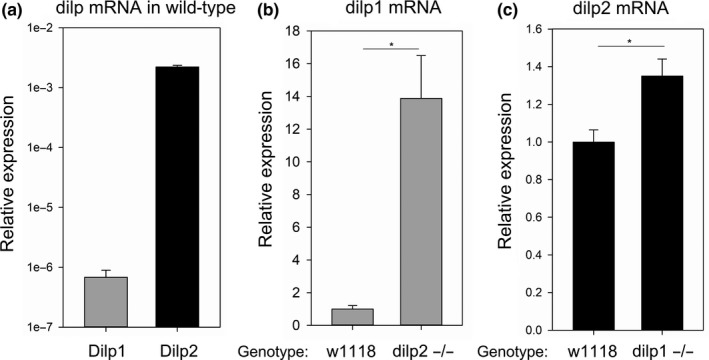
Dilp1 mRNA is induced by depletion of dilp2. RNA from 7‐ to 10‐day‐old female adult flies was assayed by q‐RT–PCR. n = 6 per genotype. (a) dilp1 mRNA expression is 100‐fold lower than dilp2 expression in wild‐type flies. (b) dilp1 mRNA expression increases 14‐fold in dilp2 mutant flies compared to wild‐type flies, t test p < 0.001. (c) dilp2 mRNA expression increases approximately 30% in dilp1 mutant flies compared to wild‐type, t test p = 0.005
2.1. Epistasis analysis of lifespan
Adult dilp2 mutants have elevated blood sugar and extended lifespan (Grönke et al., 2010). To test whether these phenotypes require the expression of dilp1, we generated a dilp1 − dilp2 null double mutant by homologous recombination (HR), in parallel with matching dilp1 and dilp2 null single mutant knockouts (Supporting Information Figure S1). Previous studies evaluated dilp HR null mutants retaining the white marker gene, but we found these lines to disrupt gene expression of the nearby gene Zasp67. Importantly, Zasp67 expression is not disrupted in our white marker excised lines (Supporting Information Figure S1). Similarly, dilp2 mutants of Grönke et al. (2010) that retain the white marker have increased expression of another gene near the dilp locus, CG32052, while this misexpression is absent from our dilp2 mutants where the white marker is excised. Thus, using mutant lines without the white marker, if the functions of dilp1 are redundant to those of dilp2, we expect the dilp1 − dilp2 double mutants to have greater longevity and higher blood sugar than either single mutant. Alternatively, if the functions of dilp1 are downstream of dilp2, we expect the dilp1 − dilp2 double mutants to have wild‐type lifespan and metabolism.
The marker‐free null allele of dilp2 increases lifespan by 20%–30% (Figure 2a, Supporting Information Figure S2d), confirming previous observations (Grönke et al., 2010). Null mutation of dilp1 has no effect on adult survival, again as previously reported (Grönke et al., 2010). Remarkably, survival of the dilp1 − dilp2 double null mutant is indistinguishable from wild‐type or the dilp1 mutant (Figure 2a,b), revealing a classic epistatic interaction between dilp1 and dilp2 in the control of longevity.
Figure 2.

dilp1 mutation suppresses aging and Akh phenotypes of mutant dilp2. (a) dilp2 mutants but not double mutants are long‐lived, Cox hazard analysis p < 0.0001, χ 2 = 201, n = 341–365 per genotype. (b) dilp2 mutants but not double mutants have decreased mortality. (c) dilp2 mutants but not double mutants have increased Akh mRNA expression, female adults at 7‐ to 10‐day‐old, n = 9 per genotype. Two‐way ANOVA dilp1 p ≤ 0.001, dilp2 p = 0.921, dilp1 × dilp2 interaction p < 0.001. (D) dilp2 mutants but not dilp1 or double mutants have increased AKH immune‐labeling in corpora cardiaca from 6‐ to 7‐day‐old female flies, representative images. (E) Quantification of AKH immune‐labeling, n = 9–14 samples from three replicates, ANOVA *p < 0.05
2.2. Epistasis analysis of adipokinetic hormone
Dilp1 is only normally expressed in adults during reproductive diapause, a slow‐aging stage associated with many metabolic changes, including elevated adipokinetic hormone (AKH), the functional homolog of mammalian glucagon (Kubrak, Kucerova, Theopold, & Nassel, 2014; Kucerova et al., 2016; Y. Liu et al., 2016). Accordingly, we studied how dilp1 and dilp2 affect AKH through genetic analysis of single and double mutants. Akh mRNA is increased in dilp2 mutants, is similar to wild‐type in dilp1 mutants, and is restored to wild‐type levels in the dilp1 − dilp2 double mutant (Figure 2c). We likewise examined AKH immunostaining in the adult corpora cardiaca (CC). AKH peptide in the CC is increased in dilp2 mutants and is similar to wild‐type in dilp1 and in dilp1 − dilp2 double mutants (Figure 2d,e). These data suggest that dilp1 is epistatically downstream of dilp2 such that dilp1 expression is required for dilp2 to modulate lifespan and AKH.
2.3. Epistasis analysis of developmental and metabolic traits
Drosophila insulins affect many traits including body weight and metabolism. Similar to the epistatic interactions observed for lifespan and AKH, body mass was decreased in dilp2 mutants (as previously reported (Grönke et al., 2010)), but similar to wild‐type in dilp1 mutants and in dilp1 − dilp2 double mutants (Figure 3a). In contrast, hemolymph (blood) glucose and trehalose concentrations in single mutants of dilp1 and dilp2 are similar to those seen in wild‐type, while the dilp1 − dilp2 double mutant has elevated hemolymph sugars (Figure 3b). For these traits, the insulin paralogs appear to have parallel, redundant functions. On the other hand, glycogen content is equally decreased by both single mutants and the double mutant, indicating that both dilp1 and dilp2 are required to maintain the pool of this energy storage molecule (Figure 3c).
Figure 3.
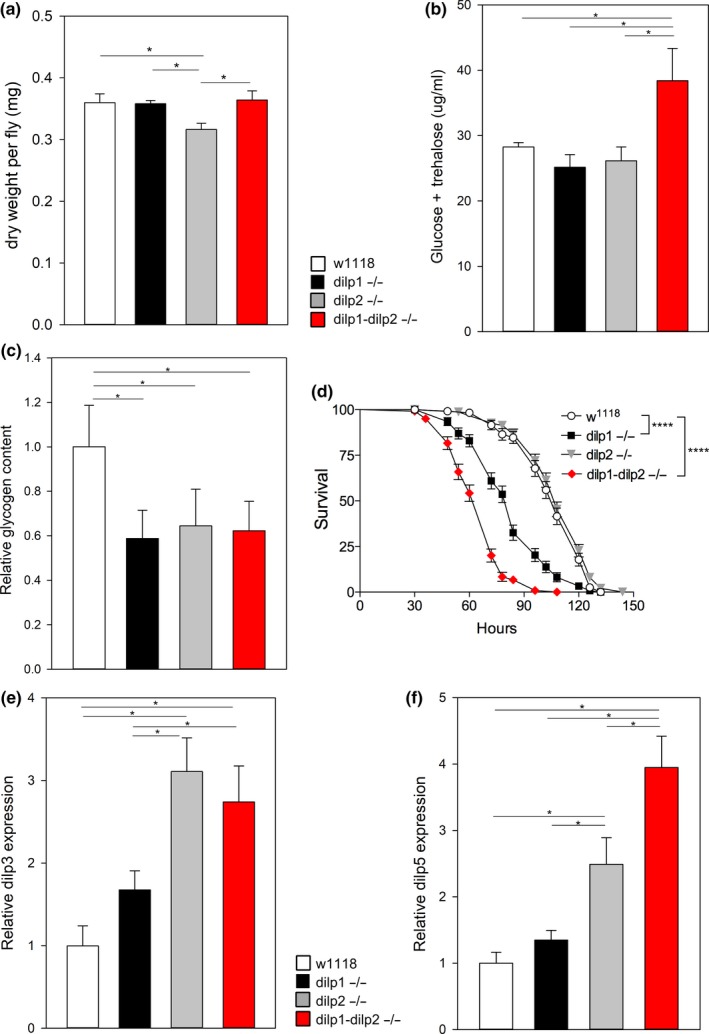
dilp1 and dilp2 interact to regulate metabolism, physiology, and compensatory dilp expression. Female flies aged 7–10 days old, 22–44 flies per replicate sample. Figure 3a–c,e,f show significance (*p < 0.05, **p < 0.01) from post hoc pairwise comparisons in two‐way ANOVA. (a) Adult mass reduced by dilp2 mutant; dilp1 × dilp2 interaction p = 0.03 (n = 5 samples per genotype). Hemolymph sugar (b) and glycogen (c) reduced by single dilp mutants, with significant dilp1 × dilp2 interaction, respectively, p < 0.001 and p = 0.005 (n = 5 samples per genotype). (d) Survival when fasted, each cohort with n = 118–150 flies, three replicate cohorts. Log‐rank tests relative to w1118, ****p < 0.0001. dilp3 (e) and dilp5 (f) mRNA are moderately induced by mutation of dilp2 but not of dilp1, with significant dilp1 × dilp2 interaction, respectively, p = 0.02 and p = 0,02 (n = 3–6 replicate samples per genotype)
Many longevity‐extending IIS manipulations increase resistance to fasting (Clancy et al., 2001; Grönke et al., 2010). In contrast to previous report (Grönke et al., 2010), survival during fasting for the long‐lived dilp2 null genotype is similar to wild‐type, while fasted dilp1 mutants and dilp1 − dilp2 double mutants are shorter lived (Figure 3d). These data suggest that dilp1, which is increased during nonfeeding developmental stages (Liu et al., 2016), may be required for starvation survival by inducing catabolism of nutrients.
Dilp3 mRNA is increased in dilp2 mutants and in dilp1 − dilp2 double mutants to a similar extent, but not significantly increased in dilp1 mutants (Figure 3e). Dilp5 mRNA is increased to a greater extent in dilp1 − dilp2 double mutants relative to its increase in either single mutant, representing synergistic genetic interaction between dilp1 and dilp2 (Figure 3f). We observed no induction or repression of mRNA for dilp6 in single and double mutants of dilp1 and dilp2, but slight increases in expression of dilp7 and dilp8 mRNA in dilp1 and double mutants (Supporting Information Figure S2a–c).
Fecundity is not significantly different among wild‐type, single and double dilp1 and dilp2 mutants for adult females at one or three weeks old, although at two weeks old, dilp2 mutants lay slightly more eggs per day than other genotypes (Supporting Information Figure S2e). Finally, egg‐to‐pupa viability was 50% less in dilp1 − dilp2 double mutants relative to wild‐type and to single mutants, suggesting that these insulin loci have redundant functions in larval survival (Supporting Information Figure S2f).
In sum, lifespan, body size, and AKH are mediated by genetic epistasis between dilp1 and dilp2, where dilp1 is inferred to function downstream of dilp2. Other measured phenotypes are jointly or independently regulated by dilp1 and dilp2, in some cases by redundant functions of the ligands and in other cases through synergistic interactions.
2.4. Epistatic analysis of insulin/IGF and juvenile hormone signaling
To understand how dilp1 is required to extend longevity, we evaluated insulin/IGF signal (IIS) transduction and juvenile hormone (JH) signaling in single and double dilp1 and dilp2 mutants. Insulin ligands in Drosophila induce phosphorylation of Akt and ERK, which in turn regulate activity of transcription factors including FOXO. Here, we measured Akt and ERK phosphorylation from thorax tissue, which primarily consists of flight muscle (Figure 4a–c, Supporting Information Figure S3g). While loss of dilp1 had no impact on Akt phosphorylation, loss of dilp2 increased Akt phosphorylation in single and double mutants, suggesting that compensatory expression of other dilps (dilp3 and dilp5) is sufficient to maintain and even elevate this branch of IIS in the absence of dilp2. In contrast, ERK phosphorylation in thorax is reduced in dilp2 mutants, is unaffected in dilp1 mutants, and is restored to wild‐type levels in the dilp1 − dilp2 double mutant. Dilp1 and dilp2 interact epistatically to control ERK phosphorylation. This pattern correlates with the epistatic interaction we observe for dilp1 and dilp2 in the control of longevity, and we note that ERK has been implicated in how IIS controls aging downstream of the insulin receptor substrate chico (Slack et al., 2015). In contrast, pAkt and pERK signaling measured from whole flies when fasted or fed was unaltered in dilp1 and dilp2 single and double mutants (Supporting Information Figure S3a–f). There is surprisingly little association among genotypes for longevity and systemically altered Akt or ERK activation.
Figure 4.
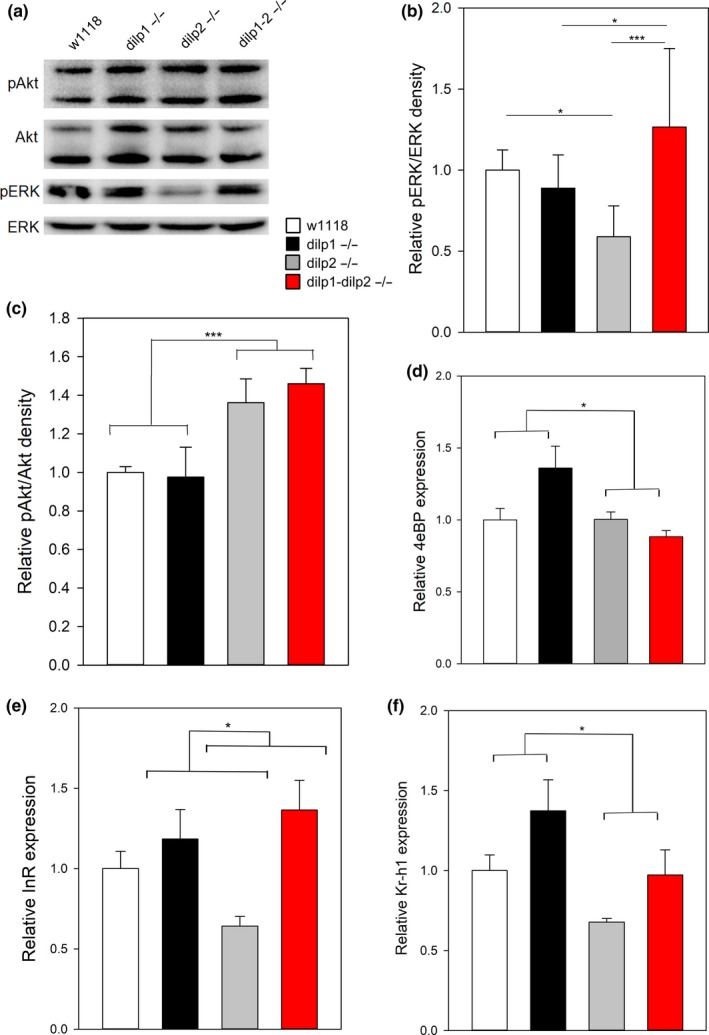
Components of insulin/IGF and JH signaling regulated by dilp1 and dilp2. (a) pAkt in dissected thorax tissue is increased in dilp2 and double mutants; pERK is decreased in dilp2 mutants in a dilp1‐dependent manner, representative blot. Figures B‐F show significance (*p < 0.05, **p < 0.01) from post hoc pairwise comparisons in two‐way ANOVA. (b) Quantification of thorax pERK/ERK phospho‐westerns, dilp1 × dilp2 interaction p = 0.003, n = 6 per genotype. (c) Quantification of thorax pAkt/Akt phospho‐westerns, dilp1 × dilp2 interaction: not significant, n = 6 per genotype. (d) 4eBP mRNA expression is not elevated in dilp2 mutants but interacts with dilp1, dilp1 × dilp2 interaction p = 0.01, n = 7–9 per genotype. (e) InR mRNA expression is reduced in dilp2 mutants relative to dilp1; dilp2 double mutant p < 0.05, n = 7–9 per genotype. (f) Kr‐h1 mRNA expression is decreased by dilp2 mutation, without significant dilp1 × dilp2 interaction, n = 8–9 per genotype
Reduced IIS extends Drosophila lifespan in part through activating the FOXO transcription factor (Bai, Kang, Hernandez, & Tatar, 2013; Hwangbo et al., 2004; Giannakou, Goss, & Partridge, 2008; Min, Yamamoto, Buch, Pankratz, & Tatar, 2008) which subsequently induces target genes including 4eBP and InR. Accordingly, we measured transcriptional targets of activated FOXO in dilp1 and dilp2 single and double mutants. Measured from whole animals, message from neither gene was elevated in the long‐lived dilp2 single mutant, although some increase was seen in dilp1 and dilp1‐2 mutants with normal lifespan (Figure 4d,e). Thus, in this mutant series, we find no association between longevity and elevated 4eBP or InR expression, suggesting that activated FOXO may not be responsible for how reduced dilp2 slows aging.
Juvenile hormone (JH) is an insect terpenoid hormone produced by the corpora allata that is documented to modulate how IIS impacts aging. The exceptional longevity of insulin receptor mutants is restored to wild‐type by treating adults with JH, while in flies with wild‐type IIS, eliminating adult JH production is sufficient to extend lifespan (Tatar et al., 2001; Yamamoto, Bai, Dolezal, Amdam, & Tatar, 2013). JH controls transcriptional programs by regulating expression and activation of the transcription factor Kruppel homolog 1 (Kr‐h1) (Liu et al., 2018; Minakuchi, Zhou, & Riddiford, 2008). Unlike for FOXO, dilp1 and dilp2 interact epistatically to control Kr‐h1 mRNA, consistent with the epistatic interaction between dilp1 and dilp2 seen for longevity: Kr‐h1 is reduced in dilp2 mutants and restored to wild‐type expression in the dilp1 − dilp2 double mutant, while dilp1 single mutants tend to have greater Kr‐h1 mRNA than wild‐type (Figure 4f). Dilp1 appears to normally repress JH activity, and we hypothesize this inhibition may be how longevity is extended by elevated dilp1 when induced in dilp2 mutants.
2.5. Overexpression of dilp1 rescues phenotypes in dilp1 − dilp2 double mutant
We corroborate inferences on epistasis by exogenously expressing dilp1. We generated and validated a UAS‐dilp1 stock capable of expressing this insulin protein using GAL4 drivers (Supporting Information Figure S4a). To verify whether loss of dilp2 requires expression of dilp1 to slow aging and increase Akh expression, we induced UAS‐dilp1 in the double dilp1 − dilp2 null mutant background. Expressing exogenous dilp1 in IPCs via dilp2‐GAL4 (Figure 5a,b, Supporting Information Figure S5a) or in all neurons with the RU486‐inducible GeneSwitch elav‐GSGal4 (Figure 5c,d) significantly extended lifespan by consistently decreasing age‐specific mortality. In one qualification, we note that elav‐GSGal4>dilp1 flies not treated with RU486 (RU control cohort) nonetheless presented somewhat elevated dilp1 expression and extended longevity relative to elav‐GSGal4/+ (genetic control cohort). Yet as required, survival of genetic controls was unaltered by RU486 treatment (Supporting Information Figure S5b). Likewise, Akh mRNA was elevated by dilp1 transgene expression when driven by dilp2‐GAL4 in the double mutant background (Figure 5e). Confirmative outcomes were also seen when UAS‐dilp1 was expressed in otherwise dilp wild‐type backgrounds: dilp1 transgene expression in IPCs with dilp2‐GAL4 extended lifespan (Supporting Information Figure S6a,b).
Figure 5.
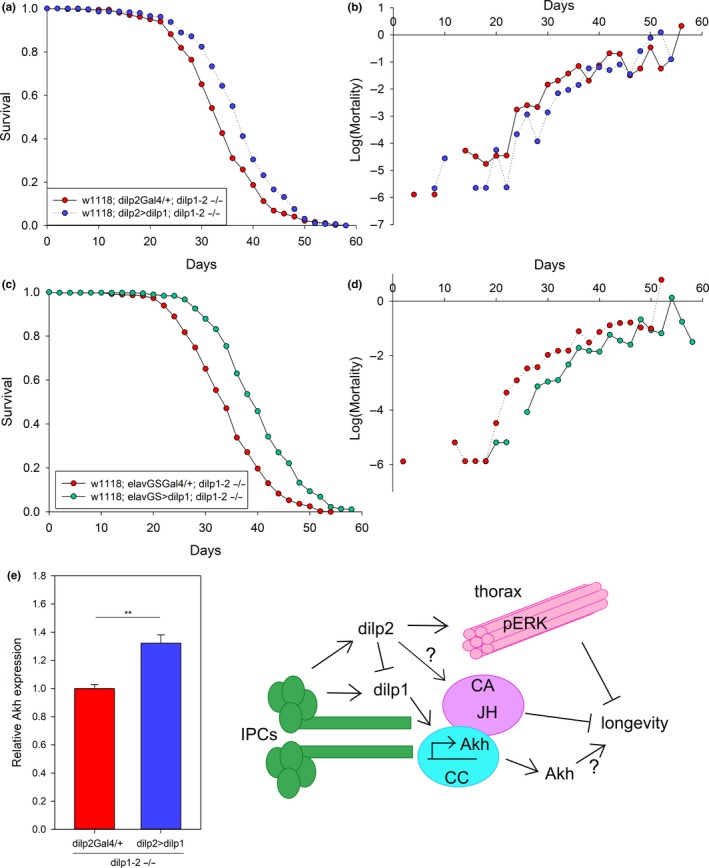
Dilp1 expression in double mutants rescues longevity and AKH. (a) Lifespan is extended by dilp2‐ GAL4>UAS‐dilp1 overexpression rescue in the dilp1 − dilp2 double mutant background compared to dilp2‐GAL4/+ controls, Cox hazard analysis p < 0.0001, χ 2 = 25.8, n = 289–364 per genotype. (b) Mortality is decreased when dilp2‐ GAL4>UAS‐dilp1 is overexpressed in the dilp1 − dilp2 double mutants compared to dilp2‐GAL4/+ controls. (c) Lifespan is extended by elav‐GS>dilp1 overexpression in the dilp1 − dilp2 double mutant background treated with RU486 in adults relative to elav‐GS/+ controls, Cox hazard analysis p < 0.0001, χ 2 = 89.7, n = 361–377 per genotype. (d) Mortality is decreased by elav‐GS>UAS‐dilp1 overexpression in the dilp1 − dilp2 double mutant background treated with RU486 in adults compared to elav‐GS/+ controls. (e) Dilp2>dilp1 rescue in the dilp1 − dilp2 double mutant background increases Akh expression compared to dilp2‐Gal4/+ controls, t test p < 0.001, n = 5–6 per genotype. (f) Model for interaction between dilp1 and dilp2 in regulating lifespan. IPCs, insulin‐producing cells; CC, corpora cardiaca; CA, corpora allata; JH, juvenile hormone
DILP1 functions as a pro‐longevity factor, and it is necessary and sufficient for mutants of dilp2 to extend longevity. This result could be explained if DILP1 peptide inhibits insulin receptor tyrosine kinase activity. DILP1 might then slow aging by decreasing insulin/IGF signaling, indicated by reduced pAkt and pErk, induced FOXO target genes, and small body size. However, our current observations are inconsistent with this hypothesis. In long‐lived dilp1‐2 double mutants with the dilp1 transgene expressed in IPCs, pAkt and pERK in peripheral tissues were similar to levels seen in wild‐type (Supporting Information Figure S5e–g); among FOXO targets, 4eBp and InR mRNA were slightly increased (Supporting Information Figure S5c); body mass was similar to wild‐type (Supporting Information Figure S5d). Likewise, in the long‐lived dilp wild‐type background with dilp1 transgene expressed in IPCs, pAKT and pERK were not significantly reduced, 4eBP mRNA was constant and InR mRNA was slightly repressed (Supporting Information Figure S6c–e). Overall, these data are contrary to expectations if DILP1 acts as an insulin receptor antagonist. Finally, we find few compensatory impacts on the expression of other dilp loci when dilp1 is expressed in either mutant or wild‐type backgrounds (Supporting Information Figures S5h, Figure S6f), suggesting that feedback to the other dilp paralogs is not involved in this lifespan extension.
3. DISCUSSION
Based on mutational analyses of the insulin receptor (daf‐2, InR) and its associated adaptor proteins and signaling elements, numerous studies in C. elegans and Drosophila established that decreased insulin/IGF signaling (IIS) extends lifespan (Clancy et al., 2001; Kenyon, Chang, Gensch, Rudner, & Tabtiang, 1993; Tatar et al., 2001). Studies on how reduced IIS in Drosophila systemically slows aging also reveal systems of feedback where repressed IIS in peripheral tissue decreases DILP2 production in brain insulin‐producing cells (IPC), which may then reinforce a stable state of longevity assurance (Bai et al., 2012; Hwangbo et al., 2004; Wang et al., 2005). Here, we find that expression of dilp1 is required for loss of dilp2 to extend longevity (Figure 5f). This novel observation contrasts with conventional interpretations where reduced insulin ligand is required to slow aging: Elevated dilp1 is associated with longevity in dilp2 mutants, and transgene expression of dilp1 increases longevity.
Dilp1 and dilp2 are encoded in tandem, likely having arisen from a duplication event (Tatar et al., 2003). Perhaps as a result, some aspects of dilp1 and dilp2 are regulated in common: Both are expressed in IPCs (Liu et al., 2016; Rulifson et al., 2002), are regulated by sNPF (Lee et al., 2008), and have strongly correlated responses to dietary composition (Post & Tatar, 2016). Nonetheless, the paralogs are differentially expressed throughout development (Brogiolo et al., 2001). While dilp2 is expressed in larvae, dilp1 expression is elevated in the pupal stage when dilp2 expression is minimal (Slaidina et al., 2009). In reproductive adults, dilp1 expression decreases substantially after eclosion and dilp2 expression increases (Slaidina et al., 2009).
Furthermore, DILP1 production is associated with adult reproductive diapause (Liu et al., 2016). IIS regulates adult reproductive diapause in Drosophila, a somatic state that prolongs survival during inclement seasons (Tatar & Yin, 2001). DILP1 may stimulate these diapause pro‐longevity pathways, while expression in nondiapause adults is sufficient to extend survival even in optimal environments.
Our data suggest a hypothesis whereby dilp1 extends longevity in part through induction of adipokinetic hormone (AKH), which is also increased during reproductive diapause (Kucerova et al., 2016) and acts as a functional homolog of mammalian glucagon (Bednarova, Kodrik, & Krishnan, 2013). Critically, AKH secretion has been shown to increase Drosophila lifespan and to induce triacylglycerides and free fatty acid catabolism (Waterson et al., 2014). Here, we note that dilp1 mutants were more sensitive to starvation than wild‐type and dilp2 mutants, as might occur if DILP1 and AKH help mobilize nutrients during fasting and diapause (Liu et al., 2016). Mammalian insulin and glucagon inversely regulate glucose storage and glycogen breakdown, while insulin decreases glucagon mRNA expression (Petersen, Vatner, & Shulman, 2017). We propose that DILP2 in Drosophila indirectly regulates AKH by repressing dilp1 expression, while DILP1 otherwise induces AKH (Figure 5f).
A further connection between dilp1 and diapause involves juvenile hormone (JH). In many insects, adult reproductive diapause and its accompanied longevity are maintained by the absence of JH (Tatar & Yin, 2001). Furthermore, ablation of JH‐producing cells in adult Drosophila is sufficient to extend lifespan, and JH is greatly reduced in long‐lived Drosophila insulin receptor mutants (Tatar et al., 2001; Yamamoto et al., 2013). In each case, exogenous treatment of long‐lived flies with a JH analog (methoprene) restores survival to the level of wild‐type or nondiapause controls. JH is a terpenoid hormone that interacts with a transcriptional complex consisting of Met (methoprene tolerant), Taimen, and Kruppel homolog 1 (Kr‐h1) (Jindra, Bellés, & Shinoda, 2015). As well, JH induces expression of kr‐h1 mRNA, and this serves as a reliable proxy for functionally active JH. Here, we find that dilp2 mutants have reduced kr‐h1 mRNA, while the titer of this message is similar to that of wild‐type in dilp1 − dilp2 double mutants. DILP1 may normally repress JH activity, as would occur in diapause when DILP1 is highly expressed. Such JH repression may contribute to longevity assurance during diapause as well as in dilp2 mutant flies maintained in laboratory conditions.
Does DILP1 act as an insulin receptor agonist or inhibitor? Inhibitory DILP1 could directly interact with the insulin receptor to suppress IIS, potentially even in the presence of other insulin peptides. Such action could induce programs for longevity assurance that are associated with activated FOXO. Alternatively, DILP1 may act as a typical insulin receptor agonist that induces autophosphorylation and represses FOXO. In this case, to extend lifespan, DILP1 should stimulate cellular responses distinct from those produced by other insulin peptides such as DILP2 or DILP5 (Post et al., 2018). Through a third potential mechanism, DILP1 may interact with binding proteins such as IMPL2 or dALS to indirectly inhibit IIS output (Alic et al., 2011; Okamoto et al., 2013). We anticipate resolving these distinctions in a future study using synthetic DILP1 applied to cells in culture.
A precedent exists from C. elegans where some insulin‐like peptides are thought to function as antagonists (Matsunaga et al., 2018; Pierce et al., 2001). In genetic analyses, ins‐23 and ins‐18 stimulate larval diapause and longevity (Matsunaga et al., 2018), while ins‐1 promotes Dauer formation during development and longevity in adulthood (Pierce et al., 2001). Moreover, C. elegans ins‐6 acts through DAF‐2 to suppress ins‐7 expression in neuronal circuits to affect olfactory learning, where ins‐7 expression inhibits DAF‐2 signaling. These studies propose that additional amino acid residues of specific insulin peptides contribute to their distinct functions, and notably, the B‐chain of DILP1 has an extended N‐terminus relative to other DILP sequences (Brogiolo et al., 2001).
While dFOXO and DAF‐16 are intimately associated with how reduced IIS regulates aging in Drosophila and C. elegans (Martins, Lithgow, & Link, 2016), in our current work, the behavior of FOXO does not correspond with how longevity is controlled epistatically by dilp1 and dilp2. Mutation of dilp2 did not impact FOXO activity, as measured by expression of target genes InR and 4eBP, and interactions with dilp1 did not modify this result. Some precedence suggests only a limited role for dfoxo as the mediator of reduced IIS in aging, as dfoxo only partially rescues longevity benefits of chico mutants, revealing that IIS extends lifespan through some FOXO‐independent pathways (Yamamoto & Tatar, 2011). On the other hand, dilp1 expression from a transgene in the dilp1–2 double mutant background did induce FOXO targets. Differences among these results might arise if whole animal analysis of dFOXO targets obscures its role when IIS regulates aging through actions in specific tissues (Tain et al., 2017; Wolkow, Kimura, Lee, & Ruvkun, 2000). In this vein, we find that dilp2 controls thorax ERK signaling but not AKT, suggesting that dilp2 mutants may activate muscle‐specific ERK/MAPK anti‐aging programs.
Dilp1 and dilp2 redundantly regulate glycogen levels and blood sugar, while these dilp loci interact synergistically to modulate dilp5 expression and starvation sensitivity. In contrast, dilp1 and dilp2 interact in a classic epistatic fashion to modulate longevity and AKH. Such distinct types of genetic interactions may reflect unique ways DILP1 and DILP2 stimulate different outcomes from their common tyrosine kinase insulin‐like receptor, along with outcomes based on cell‐specific responses. Understanding how and what is stimulated by DILP1 in the absence of dilp2 will likely reveal critical outputs that specify longevity assurance.
4. EXPERIMENTAL PROCEDURES
4.1. Fly husbandry
Flies were reared and maintained at 25°C, 40% relative humidity, and 12‐hr light/dark. Adults were maintained upon agar‐based diet with cornmeal (0.8%), sugar (10%), and yeast (2.5%). Fly stocks from Bloomington Stock Center include w1118, dilp1 (#30,880) and w1118, dilp2 (#30,881) mutants. dilp2‐Gal4 stock was originally obtained from Ernst Hafen, and elav‐GSGal4 stock was obtained from Steven Helfand (Brown University). All stocks were backcrossed to w1118 for at least five generations.
4.2. Homologous Recombination
Homologous recombination (HR) of dilp1 and dilp2 in tandem was conducted as previously performed (Grönke et al., 2010; Staber, Gell, Jepson, & Reenan, 2011). See Supplemental Methods in Supporting Information Data S1.
4.3. Production of UAS‐dilp1
See Supporting Information Data S1, for detailed cloning procedures. Embryos were injected with UAS‐dilp1 by BestGene Inc. (thebestgene.com) yielding five independent transformants for dilp1. For this study, we selected one transformant for dilp1 that produced the strongest DILP1 immunolabeling when testing various Gal4 lines in larval and adult flies (see Supporting Information Figure S3).
4.4. Lifespan assays
Two‐ to three‐day‐old female adult flies, reared in density‐controlled bottles and mated after eclosion, were collected with light CO2 anesthesia and pooled in 1 L demography cages at a density of 100–125 flies per cage. Three independent cages were used per genotype. Food vials were changed every day for the first three weeks and then every two days for the remainder of each experiment. Dead flies were removed and recorded every other day. Cox proportional hazard analysis was conducted in R using the “surv” package and “survdiff” function.
4.5. RNA purification and quantitative RT–PCR
Total RNA was extracted from 20 whole mated female flies (8–10 days old) in TRIzol (Invitrogen, Grand Island, NY, USA) and treated with Turbo DNase (Invitrogen). RNA was quantified with a NanoDrop ND‐1000 (Thermo Fisher Scientific Inc., Wilmington, DE, USA) and reverse‐transcribed with iScript cDNA synthesis (Bio‐Rad Laboratories, Inc., Hercules, CA, USA). Quantitative RT–PCR was conducted with SYBR Green PCR master mix (Applied Biosystems, Carlsbad, CA, USA) and measured on an ABI PRISM 7,300 Sequence Detection System (Applied Biosystems). mRNA abundance was calculated by comparative CT relative to ribosomal protein 49 (RP49). Primer sequences are listed in Supporting Information Table S1.
4.6. Body Mass
Two females and two males in each vial were allowed to lay eggs for 24–36 hr or until proper density was attained (about 60–80 eggs). Eclosed flies were mated for two days, and females were sorted to separate vials. Food was changed every other day, and at 8–10 days old, flies were counted, briefly anesthetized on CO2, and collected in a preweighed microcentrifuge tube. Tubes were weighed, and mass per fly was calculated.
4.7. Western Blots
Antibodies from Cell Signaling Technology: Drosophila phospho‐Akt Ser505 (#4054S), Pan‐Akt (#4691S), Pan‐phospho‐ERK (#4370S), Pan‐ERK (#9102S). See Supplemental Methods in Supporting Information Data S1.
4.8. Antisera and immunocytochemistry
Tissues from larvae or 7‐day‐old female adults were dissected in 0.1 M PBS, then fixed for 4 hr in ice‐cold 4% paraformaldehyde (PFA), and rinsed in PBS three times for 1 hr. Incubation with primary antiserum was performed for 48 hr at 4°C. After rinsing in PBS with 0.25% Triton X‐100 (PBS‐Tx) four times, tissues were incubated with secondary antibody for 48 hr at 4°C. After washing in PBS‐Tx, tissues were mounted in 80% glycerol with 0.1 M PBS. Primary antisera used were as follows: rabbit antisera to DILP1 C‐peptide (Liu et al., 2016) at a dilution of 1:10,000, rabbit antisera to DILP2 and DILP3 A‐chains (Veenstra, Agricola, & Sellami, 2008) at a dilution of 1:2,000, rabbit antisera to AKH was kindly donated by M. Brown (Athens, GA) used at 1:1,000, and rabbit anti‐GFP at 1:000 (Invitrogen, Carlsbad, CA). Secondary antisera used were as follows: goat anti‐rabbit Alexa 546 antiserum and goat anti‐rabbit Alexa 488 antiserum (Invitrogen, Carlsbad, CA) at 1:1,000.
4.9. Image analysis
Confocal images were captured with a Zeiss LSM 780 confocal microscope (Jena, Germany) using a 40× oil immersion objective. The projection of z‐stacks was processed using Fiji (https://imagej.nih.gov/ij/). The cell body outlines were extracted manually, and the staining intensity was determined using Fiji. The background intensity for all samples was recorded by randomly selecting three small regions near the cell body of interest. The final intensity value of the cell bodies was determined by subtracting the background intensity.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
SP and MT designed experiments and interpreted results. SP executed experiments. SF and RY executed experiments and contributed to experimental design. DN contributed to experimental design and interpretation of results. JV contributed to developing reagents. SP and MT wrote the manuscript and all authors edited the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Barry Pfeiffer for sharing the vector used to initiate production of the UAS‐dilp1 transgenic fly. Funding for SP, RY, and MT was supported by NIH R37 AG024360. SP was additionally supported by NIH T32 AG 41688‐3 and by AFAR GR5290420. SL and DRN were supported by The Swedish Research Council (Vetenskapsrådet 2015‐04626). JAV was supported by institutional funds from the CNRS.
Post S, Liao S, Yamamoto R, Veenstra JA, Nässel DR, Tatar M. Drosophila insulin‐like peptide dilp1 increases lifespan and glucagon‐like Akh expression epistatic to dilp2 . Aging Cell. 2019;18:e12863 10.1111/acel.12863
Contributor Information
Stephanie Post, Email: stephanie_post@brown.edu.
Marc Tatar, Email: marc_tatar@brown.edu.
REFERENCES
- Alic, N. , Hoddinott, M. P. , Vinti, G. , & Partridge, L. (2011). Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell, 10(1), 137–147. 10.1111/j.1474-9726.2010.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, H. , Kang, P. , Hernandez, A. M. , & Tatar, M. (2013). Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genetics, 9(11), e1003941 10.1371/journal.pgen.1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, H. , Kang, P. , & Tatar, M. (2012). Drosophila insulin‐like peptide‐6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin‐like peptide‐2 from the brain. Aging Cell, 11(6), 978–985. 10.1111/acel.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarova, A. , Kodrik, D. , & Krishnan, N. (2013). Unique roles of glucagon and glucagon‐like peptides: Parallels in understanding the functions of adipokinetic hormones in stress responses in insects. Comparative Biochemistry and Physiology Part A Molecular Integrative Physiology, 164(1), 91–100. 10.1016/j.cbpa.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Brogiolo, W. , Stocker, H. , Ikeya, T. , Rintelen, F. , Fernandez, R. , & Hafen, E. (2001). An evolutionarily conserved function of the Drosophila insulin receptor and insulin‐like peptides in growth control. Current Biology, 11(4), 213–221. 10.1016/S0960-9822(01)00068-9 [DOI] [PubMed] [Google Scholar]
- Broughton, S. J. , Slack, C. , Alic, N. , Metaxakis, A. , Bass, T. M. , Driege, Y. , & Partridge, L. (2010). DILP‐producing median neurosecretory cells in the Drosophila brain mediate the response of lifespan to nutrition. Aging Cell, 9(3), 336–346. 10.1111/j.1474-9726.2010.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy, D. J. , Gems, D. , Harshman, L. G. , Oldham, S. , Stocker, H. , Hafen, E. , Partridge, L. (2001). Extension of life‐span by loss of CHICO, a drosophila insulin receptor substrate protein. Science, 292(5514), 104–106. 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Colombani, J. , Andersen, D. S. , & Leopold, P. (2012). Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science, 336(6081), 582–585. 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- Fernandes de Abreu, D. A. , Caballero, A. , Fardel, P. , Stroustrup, N. , Chen, Z. , Lee, K. , Ch'ng, Q. (2014). An insulin‐to‐insulin regulatory network orchestrates phenotypic specificity in development and physiology. PLoS Genetics, 10(3), e1004225 10.1371/journal.pgen.1004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo, R. S. (2002). Genetic analysis of insulin signaling in Drosophila. Trends in Endocrinology and Metabolism, 13(4), 156–162. 10.1016/S1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- Giannakou, M. E. , Goss, M. , & Partridge, L. (2008). Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: Not required, but its activity modulates the response. Aging Cell, 7(2), 187–198. 10.1111/j.1474-9726.2007.00362.x [DOI] [PubMed] [Google Scholar]
- Grönke, S. , Clarke, D. F. , Broughton, S. , Andrews, T. D. , & Partridge, L. (2010). Molecular evolution and functional characterization of Drosophila insulin‐like peptides. PLoS Genetics, 6(2), e1000857 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo, D. S. , Gershman, B. , Tu, M. P. , Palmer, M. , & Tatar, M. (2004). Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature, 429(6991), 562–566. [DOI] [PubMed] [Google Scholar]
- Jindra, M. , Bellés, X. , & Shinoda, T. (2015). Molecular basis of juvenile hormone signaling. Current Opinion in Insect Science, 11, 39–46. 10.1016/j.cois.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Kenyon, C. , Chang, J. , Gensch, E. , Rudner, A. , & Tabtiang, R. (1993). A C. elegans mutant that lives twice as long as wild type. Nature, 366(6454), 461–464. 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kubrak, O. I. , Kucerova, L. , Theopold, U. , & Nassel, D. R. (2014). The sleeping beauty: How reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS ONE, 9(11), e113051 10.1371/journal.pone.0113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova, L. , Kubrak, O. I. , Bengtsson, J. M. , Strnad, H. , Nylin, S. , Theopold, U. , & Nässel, D. R. (2016). Slowed aging during reproductive dormancy is reflected in genome‐wide transcriptome changes in Drosophila melanogaster. BMC Genomics, 17, 50 10.1186/s12864-016-2383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. S. , Kwon, O. Y. , Lee, J. H. , Kwon, K. , Min, K. J. , Jung, S. A. , Yu, K. (2008). Drosophila short neuropeptide F signalling regulates growth by ERK‐mediated insulin signalling. Nature Cell Biology, 10(4), 468–475. 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Liu, S. , Li, K. , Gao, Y. , Liu, X. , Chen, W. , Ge, W. , Li, S. (2018). Antagonistic actions of juvenile hormone and 20‐hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proceedings of the National Academy of Sciences United States of America, 115(1), 139–144. 10.1073/pnas.1716897115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Liao, S. , Veenstra, J. A. , & Nässel, D. R. (2016). Drosophila insulin‐like peptide 1 (DILP1) is transiently expressed during non‐feeding stages and reproductive dormancy. Scientific Reports, 6, 26620 10.1038/srep26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, R. , Lithgow, G. J. , & Link, W. (2016). Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell, 15, 196–207. 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga, Y. , Matsukawa, T. , Iwasaki, T. , Nagata, K. , & Kawano, T. (2018). Comparison of physiological functions of antagonistic insulin‐like peptides, INS‐23 and INS‐18. Caenorhabditis Elegans. Biosci Biotechnol Biochem, 82(1), 90–96. 10.1080/09168451.2017.1415749. [DOI] [PubMed] [Google Scholar]
- Min, K. J. , Yamamoto, R. , Buch, S. , Pankratz, M. , & Tatar, M. (2008). Drosophila lifespan control by dietary restriction independent of insulin‐like signaling. Aging Cell, 7(2), 199–206. 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi, C. , Zhou, X. , & Riddiford, L. M. (2008). Kruppel homolog 1 (Kr‐h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mechanisms of Development, 125(1–2), 91–105. 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, N. , Nakamori, R. , Murai, T. , Yamauchi, Y. , Masuda, A. , & Nishimura, T. (2013). A secreted decoy of InR antagonizes insulin/IGF signaling to restrict body growth in Drosophila. Genes & Development, 27(1), 87–97. 10.1101/gad.204479.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, M. C. , Vatner, D. F. , & Shulman, G. I. (2017). Regulation of hepatic glucose metabolism in health and disease. Nature Reviews Endocrinology, 13(10), 572–587. 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, S. B. , Costa, M. , Wisotzkey, R. , Devadhar, S. , Homburger, S. A. , Buchman, A. R. , Ruvkun, G. (2001). Regulation of DAF‐2 receptor signaling by human insulin and ins‐1, a member of the unusually large and diverse C. elegans insulin gene family. Genes & Development, 15(6), 672–686. 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, S. , Karashchuk, G. , Wade, J. D. , Sajid, W. , De Meyts, P. , & Tatar, M. (2018). Drosophila insulin‐like peptides DILP2 and DILP5 differentially stimulate cell signaling and glycogen phosphorylase to regulate longevity. Frontiers in Endocrinology, 10.3389/fendo.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, S. , & Tatar, M. (2016). Nutritional geometric profiles of insulin/IGF expression in Drosophila melanogaster. PLoS One, 11(5), e0155628 10.1371/journal.pone.0155628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson, E. J. , Kim, S. K. , &Nusse, R., (2002). Ablation of Insulin‐producing neurons in flies: Growth and diabetic phenotypes. Science, 296(5570), 1118–1120. 10;296(5570):1118-20. [DOI] [PubMed] [Google Scholar]
- Slack, C. , Alic, N. , Foley, A. , Cabecinha, M. , Hoddinott, M. P. , & Partridge, L. (2015). The Ras‐Erk‐ETS‐signaling pathway is a drug target for longevity. Cell, 162(1), 72–83. 10.1016/j.cell.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina, M. , Delanoue, R. , Gronke, S. , Partridge, L. , & Leopold, P. (2009). A Drosophila insulin‐like peptide promotes growth during nonfeeding states. Developmental Cell, 17(6), 874–884. 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staber, C. J. , Gell, S. , Jepson, J. E. , & Reenan, R. A. (2011). Perturbing A‐to‐I RNA editing using genetics and homologous recombination. Methods Mol Bio, 718, 41–73. 10.1007/978-1-61779-018-8_3. [DOI] [PubMed] [Google Scholar]
- Tain, L. S. , Sehlke, R. , Jain, C. , Chokkalingam, M. , Nagaraj, N. , Essers, P. , Partridge, L. (2017). A proteomic atlas of insulin signalling reveals tissue‐specific mechanisms of longevity assurance. Molecular Systems Biology, 13(9), 939 10.15252/msb.20177663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar, M. , Bartke, A. , & Antebi, A. (2003). The endocrine regulation of aging by insulin‐like signals. Science, 299(5611), 1346–1351. 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tatar, M. , Kopelman, A. , Epstein, D. , Tu, M. P. , Yin, C. M. , & Garofalo, R. S. (2001). A mutant Drosophila insulin receptor homolog that extends life‐span and impairs neuroendocrine function. Science, 292(5514), 107–110. [DOI] [PubMed] [Google Scholar]
- Tatar, M. , & Yin, C. M. (2001). Slow aging during insect reproductive diapause: Why butterflies, grasshoppers and flies are like worms. Experimental Gerontology, 36(4–6), 723–738. 10.1016/S0531-5565(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Veenstra, J. A. , Agricola, H. J. , & Sellami, A. (2008). Regulatory peptides in fruit fly midgut. Cell and Tissue Research, 334(3), 499–516. 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- Wang, M. C. , Bohmann, D. , & Jasper, H. (2005). JNK extends life span and limits growth by antagonizing cellular and organism‐wide responses to insulin signaling. Cell, 121(1), 115–125. 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Waterson, M. J. , Chung, B. Y. , Harvanek, Z. M. , Ostojic, I. , Alcedo, J. , & Pletcher, S. D. (2014). Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. Proceedings of the National Academy of Sciences United States of America, 111(22), 8137–8142. 10.1073/pnas.1315461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow, C. A. , Kimura, K. D. , Lee, M. , & Ruvkun, G. (2000). Regulation of C. elegans life‐span by insulin like signaling in the nervous system. Science, 290(5489), 147–150. 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Yamamoto, R. , Bai, H. , Dolezal, A. G. , Amdam, G. , & Tatar, M. (2013). Juvenile hormone regulation of Drosophila aging. BMC Biology, 11, 85. doi:10.1186/1741‐7007‐11‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, R. , & Tatar, M. (2011). Insulin receptor substrate chico acts with the transcription factor FOXO to extend Drosophila longevity. Aging Cell, 10(4), 729–732. 10.1111/j.1474-9726.2011.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


