Abstract
Introduction
Although the mainstay of colorectal cancer treatment remains operative, a significant proportion of patients end up without surgery. This is because they are either deemed to have no oncological benefit from the resection (too much disease) or to be unfit for major surgery (too frail). The aim of this study was to assess the proportion and survival of these two groups among the totality of practice in a tertiary unit and to discuss the implications on the conceptual understanding of outcome measures.
Methods
Data was collected over two study periods with the total duration of four years. Patient demographics, comorbidities, cancer staging and management pathways were all recorded. The primary endpoint was all-cause mortality.
Results
The total of 909 patients were examined. In the 29% who did not undergo resectional surgery, 6.5% had too little disease, 13.8% had too much disease, while 8.7% were deemed too frail. The highest two-year mortality was observed in the too much (83.2%) and too frail (75.9%) groups, whereas in patients with too little cancer the rate was 5.1%, and in those undergoing a resection it was 19.2% (P < 0.001).
Conclusions
The study has expectedly shown poor survival in the too much and too frail groups. We believe that understanding the prognosis in these subgroups is vital, as it informs complex decisions on whether to operate. Moreover, an overall reporting taking into account the proportion of these groups in an multidisciplinary team practice (the non-surgical index) is proposed to render individual surgeon's mortality results meaningful as a comparative measure.
Keywords: Colorectal cancer, Survival, Treatment outcomes, Surgery
Introduction
Colorectal cancer is the second most common cancer in Europe and accounts for over 41,000 newly diagnosed cases every year in the UK.1,2 Although surgery is the mainstay of current potentially curative treatment, the 2017 National Bowel Cancer Audit (NBOCA) reported that 37% of patients diagnosed with colorectal cancer do not undergo major resection.3 NBOCA has categorised patients who do not undergo surgery into three main groups; ‘too much cancer’, ‘too little cancer’ and ‘too frail’, which reflects the rationale underlying the decision not to operate as either lack of perceived oncological benefit from surgery (for example too advanced disease or very early disease) or lack of fitness to withstand major resection (too frail).
Data regarding the long-term survival of colorectal cancer patients who do not undergo major resection are sparse and often limited to studies assessing patients with advanced age. Information about the survival and clinical outcomes of such patients would be beneficial in guiding often complex and difficult decisions about surgery. This is especially the case when studies have found poor quality of life after surgery, with one-tenth of patients over the age of 80 years needing residential care and two-thirds having difficulties with independence in daily activities.4,5 In addition, there is a wide variation across centres in the proportion of patients with colorectal cancer who are categorised into each of these non-operative groups; for example, the proportion of patients categorised as too frail in NBOCA varies from 0% to 33% across different centres.3
The reporting of individual surgeon outcomes is the currently established marker of performance. Since reporting commenced, the mortality from colorectal cancer surgery has reduced.3 Despite this measure being risk-adjusted, there remains the potential to improve mortality rates by avoiding high-risk cases. Both the proportion and the outcome of patients managed without major resection are therefore further vital indicators of multidisciplinary team performance, allowing surgeon outcomes to be compared on a level playing field.
This study was conducted to determine the frequency and survival outcomes of patients with colorectal cancer who did not undergo major resection, particularly for those categorised as being too frail or as having too much cancer.
Methods
Two study periods, each of which lasted two years, were combined to form an overall study cohort of patients over a total of four years. An initial retrospective study was performed assessing all patients with colorectal cancer at a UK teaching hospital who were discussed in the multidisciplinary team meeting over a two-year period from 1 January 2010 to 31 December 2011. This was performed to ensure a full five-year survival follow-up analysis. The study was repeated in an identical manner for a second two-year time period involving all patients with colorectal cancer discussed at the same multidisciplinary team over a two-year period from 1 April 2013 to 31 March 2015. This second time period coincided with the introduction of routine cardiopulmonary exercise testing for colorectal cancer patients and thus further information was available regarding the objective assessment of patients who did not undergo surgery.
Baseline demographic patient data including age, cancer staging (I–IV), serum albumin and haematocrit levels at the time of diagnosis was recorded. Staging for patients who were non-operative was pretreatment and observed radiologically by computed tomography and magnetic resonance imaging. Patients undergoing surgery had a final pathological stage recorded. Poor nutritional levels were defined by serum albumin less than 35 g/l and anaemia as a haematocrit less than 35%. After review of clinical records, each patient was categorised as either having a major resection or non-resectional management.
Patients not undergoing resection were further classified according to NBOCA into four groups ‘too little cancer’, ‘too much cancer’, ‘too frail’ and ‘others’:
Too little cancer: those undergoing a local excision or polypectomy or those with rectal cancer with an apparent complete clinical response after long-course chemoradiotherapy.
Too much cancer: no major resection due to advanced stage and/or metastatic disease.
Too frail: no metastatic disease and a primary cancer suitable for major resection but patient fitness or comorbidities deem patient too frail for major resection.
Others: those not possible to classify into one of the four pathways.
Patient comorbidities were recorded (Charlson’s Comorbidity Index) for all patients and, in addition, the need for blood transfusion, colonic stenting and details regarding cardiopulmonary exercise testing and anaesthesia assessments were recorded for those deemed too frail.
The primary outcome measure was two-year all-cause mortality. The median survival time was calculated between all four groups of patients and Kaplan–Meier estimator plotted. Categorical data were evaluated using the Freeman–Halton’s extension of the Fisher’s exact test and analysis of continuous data using the analysis of variance two-tailed test. The P-value for significance was considered as less than 0.05.
Results
During the combined study period of four years there were 909 patients with colorectal cancer discussed at departmental colorectal multidisciplinary team meetings. These were equally distributed between the two separate two-year study periods (2010–2011 and 2013–2015) with 456 and 453 cases in each period respectively. The proportions of patients undergoing major resection were similar within study periods and overall accounted for 67.6% of cases (614 patients). The remaining 28.9% (263 patients) did not undergo major resection and 3.5% (32 patients) were excluded for incomplete records or inability to classify them into a treatment pathway.
Non-resectional management comprised 6.5% of patients with too little cancer, 13.8% of patients with too much cancer and 8.7% of patients who were too frail. The categorisation of all patients over the 4 year study period is presented in Table 1.
Table 1.
Categorisation of patients with colorectal cancer discussed at multidisciplinary team meetings.
| Category | Patients | Two-year mortality (%)a | Median survival (months) | |
| (n) | (%) | |||
| Total colorectal cancer cases | 909 | 100 | – | – |
| Major resection | 614 | 67.5 | 19.2 | – |
| Too little cancer | 59 | 6.5 | 5.1 | – |
| Too much cancer | 125 | 13.8 | 83.2 | 8.54 |
| Too frail | 79 | 8.7 | 75.9 | 10.73 |
| Incomplete records/othersb | 32 | 3.5 | 64.3 | – |
a Comparison of 2-year mortality between major resection, too little, too much and too frail; groups demonstrated a significant difference (P < 0.001 chi square test).
b ‘Others’ were patients who were not able to be categorised into one of the named pathways.
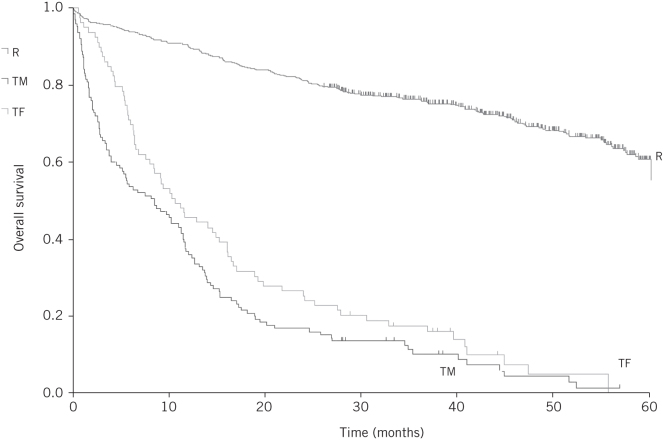
There was a significant difference between the groups in the two-year mortality. The highest mortality was observed in those with too much cancer (83.2%) and those who were too frail (75.9%). Patients with too little cancer had a two-year mortality of 5.1% and in those undergoing a major resection the mortality rate was 19.2% (P < 0.001; Table 1). Median survival from the time of initial multidisciplinary team discussion was limited in patients with too much cancer and those who were too frail to 8.5 months and 10.7 months respectively. Survival outcomes are represented on the Kaplan–Meier estimator (Fig 1).
Figure 1.
Kaplan–Meier curve for overall survival for patients undergoing major resection (R), those with too much disease (TM) and those who were too frail (TF)
Patient demographics, staging and comorbidities are presented in Table 2. Patients with too little cancer were significantly younger compared with those in the too much and too frail groups (P < 0.001). The percentage of rectal compared with colon cancer was higher in the too little group, which reflects the choice of patients for no resection following an apparent complete response to long-course chemoradiotherapy (P < 0.001). As expected, advanced stage was significantly more common in the too much group compared with too little and too frail groups (P < 0.001). The too much and too frail groups had significantly more comorbidities as reflected by high Charlson comorbidity indices (P < 0.001). Over 50% of the patients in each of these groups suffered from anaemia at presentation and over 25% with hypoalbuminaemia.
Table 2.
Patient demographics, tumour characteristics and comorbidities.
| Too little | Too much | Too frail | P-values | ||||
| (n) | (%) | (n) | (%) | (n) | (%) | ||
| Patients | 59 | 6.5a | 125 | 13.8a | 79 | 8.7a | |
| Patient demographics: | |||||||
| Age (mean, years) | 65.2 | 72.2 | 79.4 | < 0.001b | |||
| Sex (male : female) | 36 : 23 | 73 : 52 | 40 : 39 | 0.406c | |||
| Tumour site: | |||||||
| Colon | 22 | 37.3 | 80 | 64.0% | 51 | 64.6 | < 0.001c |
| Rectum | 37 | 62.7 | 45 | 36.0% | 28 | 35.4 | < 0.001c |
| Radiological stage: | |||||||
| 1 | 36 | 61.0 | 0 | 0.0 | 13 | 16.5 | |
| 2 | 4 | 6.8 | 1 | 0.8 | 14 | 17.7 | |
| 3 | 15 | 25.4 | 14 | 11.2 | 31 | 39.2 | < 0.001c |
| 4 | 4 | 6.8 | 110 | 88.0 | 17 | 21.5 | < 0.001c |
| Comorbidities: | |||||||
| CCI score (mean) | 2.67 | 8.46 | 5.86 | < 0.001b | |||
| Haematocrit < 35% | 5 | 8.5 | 64 | 51.2 | 50 | 63.3 | < 0.001c |
| Albumin < 35 g/l | 2 | 3.3 | 48 | 38.4 | 20 | 25.3 | < 0.001c |
CCI, Charlson’s Comorbidity Index.
a Percentage of all colorectal cancer.
b Analysis of variance two-tailed test.
c Freeman–Halton extension of Fisher’s exact test.
The frequency of cardiopulmonary exercise testing, blood transfusions and colonic stenting is presented in Table 3. The too much and too frail groups required intervention with blood transfusion (in 37.6% and 43% respectively) and colonic stenting (in 18.4% and 25.3% respectively).
Table 3.
World Health Organization performance scores, cardiopulmonary exercise test results and interventions with stenting and blood transfusion for the study patients.
| Too little | Too much | Too frail | P-valuesa | ||||
| (n) | (%) | (n) | (%) | (n) | (%) | ||
| Patients | 59 | 6.5 | 125 | 13.8 | 79 | 8.7 | |
| WHO performance score: | |||||||
| 0 | 36 | 61.0 | 40 | 32 | 8 | 10.1 | < 0.05 |
| 1 | 5 | 8.5 | 37 | 29.6 | 9 | 11.4 | < 0.05 |
| 2 | 1 | 1.7 | 16 | 12.8 | 25 | 31.7 | < 0.05 |
| 3 | 2 | 3.4 | 16 | 12.8 | 23 | 29.1 | < 0.05 |
| 4 | 0 | 0 | 0 | 0 | 5 | 6.3 | n/a |
| Missing | 15 | 25.4 | 16 | 12.8 | 9 | 11.4 | < 0.05 |
| CPET:b | |||||||
| Patients | 38 | 81 | 41 | ||||
| Yes | 1 | 2.6 | 0 | 02 | 6 | 14.6 | n/a |
| No | 37 | 97.4 | 81 | 100 | 35 | 85.4 | < 0.05 |
| Colonic stenting | 1 | 1.7 | 23 | 18.4 | 20 | 25.3 | < 0.05 |
| Blood transfusion | 4 | 6.8 | 47 | 37.6 | 34 | 43 | < 0.05 |
CPET, cardiopulmonary exercise test; WHO, World Health Organization.
a Freeman–Halton extension of Fisher’s exact test.
b CPET was only available during the second two-year study period.
Discussion
This is the first UK study to assess mortality outcomes for individual groups of patients not treated with major resection. In line with the findings of the 2017 NBOCA report, in our study 67.6% of patients were managed with major resection with the remaining 29% managed without major resection (3.5% excluded for incomplete records or inability to classify). Two-year survival in the 614 patients who did undergo major resection was 80.8%, which is analogous to the most recent NBOCA report.3
Our study showed that a consistent proportion of patients were classified as having too much disease and/or deemed to be too frail for major resection, among patients evaluated and treated for colorectal cancer by the colorectal cancer multidisciplinary team in a UK tertiary centre. The proportion in the too much group was in the order of 14%, while those who were deemed unfit for a major resection was around 9% of all patients. Not surprisingly, these two groups, totalling 204 patients managed without major surgical resection had significantly worse survival outcomes, with over 75% of patients dying within the first year of presentation (two-year all-cause mortality was 83% in the too much group and 76% in the too frail group).
Previous reports have not assessed patient survival within these separate groups. The NBOCA 2017 reported that two-year survival for those not undergoing major resection was 29% but data regarding different patient groups were not specified.3 In contrast to the NBOCA report, our study has reported the individual survival for these two very different patient groups and has demonstrated poor survival for both the too much and too frail categories.
Management of the asymptomatic or minimally symptomatic colorectal primary in the presence of incurable metastatic disease remains controversial. Some studies have demonstrated survival benefit associated with the elective surgical resection of the primary tumour in those situations,6,7 while others have shown no advantage.8 A 2010 meta-analysis of eight retrospective studies showed median improved survival of six months in the group of patients managed with palliative resection.9 However, the case series included were old and are likely to have included patients with liver disease, which would currently be deemed resectable considering the significant advances made in liver surgery over the past ten years. On the other hand, systemic chemotherapy can downstage unresectable to resectable disease and does provide survival benefit with no doubt.10,11 The rate of complications related to the non-resected primary tumour appear to be low with the use of chemotherapy.12 In our cohort, the too much group who did not undergo major resection were discussed in two multidisciplinary team settings (the colorectal and the hepatobiliary teams) and, in principle, surgery was always considered in those for whom downstaging to resectable disease was achieved with chemotherapy.
The second group of patients who were not treated surgically is the too frail group. A significant proportion of the patients diagnosed with colorectal cancer are above the age of 65 years.13 Ageing is associated with higher postoperative mortality,14,15 prolonged postoperative hospital stay3 and deteriorated postoperative overall functional status.4 This represents an increasing challenge for the practising surgeon in this era of an increasingly aged population. The survival of this group of patients within our study was poor, with a two-year mortality rate of 76% and median survival of only 10.7 months post multidisciplinary team discussion. In a study by Bethune et al,16 the multidisciplinary team cases of a single centre were reviewed to assess the outcome of patients aged over 80 years who did not undergo major resection. The average survival of the 29 patients was one year and 176 days. In contrast, our study which was not limited by patient age and included a much larger patient cohort, demonstrated a poorer survival outcome for those deemed too frail for major resection.
With the pressures of attempting to meet patients’ high expectations of active treatment and the intention to avoid ageism, and with the wider availability of increasingly minimally invasive operative techniques, the surgeon often faces a real dilemma when assessing the suitability of an aged patient for a major surgical resection. The increased recognition of the concept of ‘frailty’ as a measure used for preoperative assessment of suitability of an individual for major surgery has emerged.17,18
Frailty does not automatically equate to advanced age. One widely used frailty definition is that of a multidimensional syndrome of reduced physiological reserves associated with increased susceptibility to disability and vulnerability towards stressors.19–22 However, there is not yet a single generally acceptable clinical tool for measuring frailty, and the identification of the ‘too frail’ patient remains a subtle, multifaceted task for the clinician. Measures used include the evaluation of comorbid conditions, physical function, nutrition, depression, cognitive function, medication and the level of social support. Rockwood et al devised a tool and validated it on a large cohort of patients reporting that higher scores on the scale successfully predicted patient death and need for institutional care.22 The scale ranges from 1 (robust health) to 7 (complete functional dependence on others) and includes an assessment of patients’ general and psychological condition, their ability in the activities of daily living, physical and neurological signs from clinical examination and the assessment of both the presence and severity of current diseases. This broader concept of frailty extends beyond the mere consideration of comorbidities or age alone. The Charlson comorbidity scores for our cohort of patients demonstrate that an age comorbidity index alone can underscore the frail patient (Table 2).
Frailty measurements can predict postoperative complications in elderly patients undergoing elective surgical resections.17,23,24 In a prospective study of 178 colorectal cancer patients, being 'frail' as determined by a geriatric assessment preoperatively, significantly predicted survival, with 'frailty' being found as an independent prognostic factor for survival in a multivariable analysis adjusting for TNM stage, age and sex.25 We believe that such frail patients require a comprehensive assessment, which includes, in addition to the tests focused on cardiopulmonary function, a more global assessment by an experienced geriatric anaesthetist or a geriatrician with specialist interest. Interestingly, within this study, even with the availability of cardiopulmonary exercise testing very few too frail patients underwent this test (Table 3).
Although our study represents only data from a single centre, which is an inherent weakness, it includes a large number of patients and has been conducted over two separate time periods spanning in total a duration of four years. The collection of data over two study periods is thought to be one of the strengths of this study, as it would be more likely to represent the totality of practice and the spectrum of cases faced by clinicians more generally. Moreover, it means that the data are less likely to be affected by clinician-specific practices or biases. Although the study was retrospective, data collection was completed through individual case note review, recording relevant clinical, radiological and demographic details for each case. Mortality outcomes were defined as all-cause mortality rather than cancer-specific mortality, as the interest of the research is to establish an understanding of the overall prognosis of this group of patients, which would expectedly be more helpful in the process of making a decision regarding a major surgical intervention. One of the weaknesses resulting from the retrospective nature of the study is the fact that the process by which the multidisciplinary team and the lead clinician had arrived at the conclusion that a patient is ‘too frail’ cannot be elicited. This also relates to the fact that there are no agreed set criteria for objectively defining frailty.
We believe that this study has a number of important implications. Understanding the survival outcomes of patients with confirmed colorectal cancer who do not undergo major resections (too much disease or too frail patients) is conducive to better counselling and more informed decision making when dealing with such patients, who are likely to represent an increasing proportion of the totality of our practice considering the ageing of the population.
The individual surgeon’s outcomes is currently used as one of the measures of the quality of services within a particular unit; however, as this study demonstrates, such an approach excludes a significant proportion of patients (that is, those who are deemed too frail or who have too much disease). The proportion of patients who are classified as either too much or too frail varies significantly between different UK units, as demonstrated by the NBOCA report,3 suggesting that an overall assessment of the totality of patient outcomes within a multidisciplinary team is a more accurate reflection of overall patient outcomes than measuring an individual surgeon’s outcomes alone. We therefore believe that the combined measurement and outcomes of the proportion of patients deemed ‘too frail’, in conjunction with individual consultant’s outcomes, represents a more accurate assessment of the services provided by a specific multidisciplinary team. Denying patients the option of an operative intervention could be a reflex mechanism by surgeons seeking to avoid the high-risk cases in the era of an ever-increasing scrutiny and the public reporting of results of individual practitioners, as shown in other areas of medical practice.26–28 We would therefore propose that future NBOCA analysis should include, in addition to surgeons’ mortality outcomes, a ‘non-surgical index’ for each multidisciplinary team. This index should be developed to include:
the proportion of all cases that were discussed but were not resected
the proportion that were excluded for too much cancer and their survival outcome
the proportion that were excluded as being too frail and their survival outcome.
We would suggest that surgeons’ mortality outcomes cannot be meaningfully compared between multidisciplinary teams unless and until the non-surgical index of each team is shown to be matched.
Conclusion
In conclusion, this study examined the overall survival and all-cause mortality of patients with colorectal cancer treated in a tertiary UK setting without major surgical resection. Poor survival outcomes have been demonstrated for patients classified as too much cancer and too frail. We believe that this analysis is of significant importance, especially when the proportion of patients labelled as ‘too frail’ and therefore not treated surgically varied markedly between different centres as reported by the UK national audit.3
Understanding the prognosis in this group of patients is vital, as it is important for the focus of management to take into account the quality of life achieved for the patient and their holistic needs, rather than the mere achievement of a disease-free status. This study shows the need for future similar research examining survival outcomes in patients with non-operated colorectal cancer, in addition to further developing the analysis of these non-operated cases within NBOCA. A prospective large multicentre study including defined objective measures to identify and label patients with ‘frailty’ and to address the decision making that occurs will surely expand our understanding of survival from colorectal cancer when major resection is not performed.
References
- 1.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol 2005; (3): 481–488. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK What is Bowel Cancer. 2018. www.cancerresearchuk.org/about-cancer/bowel-cancer/about-bowel-cancer (cited September 2018). [Google Scholar]
- 3.Boyle J, Braun M, Eaves E et al. . National Bowel Cancer Audit Annual Report 2017 Version 2: An Audit of the Care Received by People with Bowel Cancer in England and Wales. London: Healthcare Quality Improvement Partnership; 2017. [Google Scholar]
- 4.Ronning B, Wyller TB, Jordhoy MS et al. . Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol 2014; (1): 26–32. [DOI] [PubMed] [Google Scholar]
- 5.Clark AJ, Stockton D, Elder A et al. . Assessment of outcomes after colorectal cancer resection in the elderly as a rationale for screening and early detection. Br J Surg 2004; (10): 1,345–1,351. [DOI] [PubMed] [Google Scholar]
- 6.Liu SK, Church JM, Lavery IC, Fazio VW. Operation in patients with incurable colon cancer: is it worthwhile? Dis Colon Rectum 1997; (1): 11–14. [DOI] [PubMed] [Google Scholar]
- 7.Ruo L, Gougoutas C, Paty PB et al. . Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 2003; (5): 722–728. [DOI] [PubMed] [Google Scholar]
- 8.Scoggins CR, Meszoely IM, Blanke CD et al. . Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol 1999; (7): 651–657. [DOI] [PubMed] [Google Scholar]
- 9.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 2010; (4): 797–807. [DOI] [PubMed] [Google Scholar]
- 10.Giacchetti S, Itzhaki M, Gruia G et al. . Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol 1999; (6): 663–669. [DOI] [PubMed] [Google Scholar]
- 11.Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 2000; (7260): 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muratore A, Zorzi D, Bouzari H et al. . Asymptomatic colorectal cancer with un-resectable liver metastases: immediate colorectal resection or up-front systemic chemotherapy? Ann Surg Oncol 2007; (2): 766–770. [DOI] [PubMed] [Google Scholar]
- 13.Yancik R, Wesley MN, Ries LA et al. . Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer 1998; (11): 2,123–2,134. [PubMed] [Google Scholar]
- 14.Pearse RM, Moreno RP, Bauer P et al. . Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012; (9847): 1,059–1,065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heriot AG, Tekkis PP, Smith JJ et al. . Prediction of postoperative mortality in elderly patients with colorectal cancer. Dis Colon Rectum 2006; (6): 816–824. [DOI] [PubMed] [Google Scholar]
- 16.Bethune R, Sbaih M, Brosnan C, Arulampalam T. What happens when we do not operate? Survival following conservative bowel cancer management. Ann R Coll Surg Engl 2016; (6): 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson TN, Eiseman B, Wallace JI et al. . Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 2009; (3): 449–455. [DOI] [PubMed] [Google Scholar]
- 18.Hewitt J, Moug SJ, Middleton M et al. . Prevalence of frailty and its association with mortality in general surgery. Am J Surg 2015; (2): 254–259. [DOI] [PubMed] [Google Scholar]
- 19.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med 1992; (1): 1–17. [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; (3): M146–M156. [DOI] [PubMed] [Google Scholar]
- 21.Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev 2002; (11): 1,457–1,460. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K, Song X, MacKnight C et al. . A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; (5): 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristjansson SR, Nesbakken A, Jordhoy MS et al. . Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study Crit Rev Oncol Hematol 2010; (3): 208–217. [DOI] [PubMed] [Google Scholar]
- 24.Ronning B, Wyller TB, Seljeflot I et al. . Frailty measures, inflammatory biomarkers and post-operative complications in older surgical patients Age Ageing 2010; (6): 758–761. [DOI] [PubMed] [Google Scholar]
- 25.Ommundsen N, Wyller TB, Nesbakken A et al. . Frailty is an independent predictor of survival in older patients with colorectal cancer Oncologist 2014; (12): 1,268–1,275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narins CR, Dozier AM, Ling FS, Zareba W. The influence of public reporting of outcome data on medical decision making by physicians. Arch Intern Med 2005; (1): 83–187. [DOI] [PubMed] [Google Scholar]
- 27.Moscucci M, Eagle KA, Share D et al. . Public reporting and case selection for percutaneous coronary interventions: an analysis from two large multicenter percutaneous coronary intervention databases. J Am Coll Cardiol 2005; (11): 1,759–1,765. [DOI] [PubMed] [Google Scholar]
- 28.Werner RM, Asch DA. The unintended consequences of publicly reporting quality information. JAMA 2005; (10): 1,239–1,244. [DOI] [PubMed] [Google Scholar]