Abstract
Aims
Bromocriptine is thought to facilitate left ventricular (LV) recovery in peripartum cardiomyopathy (PPCM) through inhibition of prolactin secretion. However, this potential therapeutic effect remains controversial and was incompletely studied in diverse populations.
Methods and results
Consecutive women with new‐onset PPCM (n = 76) between 1994 and 2015 in Quebec, Canada, were classified according to treatment (n = 8, 11%) vs. no treatment (n = 68, 89%) with bromocriptine. We assessed LV functional recovery at mid‐term (6 months) and long‐term (last follow‐up) and compared outcomes among groups. Women treated with bromocriptine experienced better mid‐term left ventricular ejection fraction (LVEF) recovery from 23 ± 10% at baseline to 55 ± 12% at 6 months, compared with a change from 30 ± 12% at baseline to 45 ± 13% at 6 months in women treated with standard medical therapy (P interaction < 0.01). At long‐term, a similar positive association was found with bromocriptine (9% greater LVEF variation, P interaction < 0.01). In linear regressions adjusted for obstetrical, clinical, echocardiographic, and pharmacological variables, treatment with bromocriptine was associated with a greater improvement in LVEF [β coefficient (standard error), 14.1 (4.4); P = 0.03]. However, there was no significant association between bromocriptine use and the combined occurrence of all‐cause death and heart failure events (hazard ratio, 1.18; 95% confidence interval, 0.15 to 9.31), using univariable Cox regressions based over a cumulative follow‐up period of 285 patient‐years.
Conclusions
In women newly diagnosed with PPCM, treatment with bromocriptine was independently associated with greater LV functional recovery.
Keywords: Peripartum cardiomyopathy, Bromocriptine, Heart failure, Pregnancy
Introduction
Peripartum cardiomyopathy (PPCM) is a potentially disabling cardiomyopathy affecting women in the puerperal period. It is distinctively triggered by 16 kDa prolactin, an antiangiogenic fragment resulting from the cleavage of the nursing hormone prolactin.1, 2 Bromocriptine is a central dopamin‐D2‐receptor agonist that counteracts the inflammatory cascade triggered by the secretion and cleavage of prolactin.3 In a pilot trial from South Africa, bromocriptine for 8 weeks substantially reduced mortality compared with placebo in new‐onset PPCM.4 While encouraging, results from this trial were offset by a small sample size (n = 20) and concern over external generalizability, notably because of the high mortality rate (40%) recorded in the control group.4 To this day, the adoption of bromocriptine as a legitimate option in the therapeutic armamentarium for PPCM has varied greatly across the world, ranging from 67% in Germany to 1% in the USA.5, 6, 7
More recently, Hilfiker‐Kleiner et al. found no difference in left ventricular ejection fraction (LVEF) recovery at 6 months when comparing a short (2.5 mg for 7 days) and long (5 mg for 2 weeks and then 2.5 mg for 6 weeks) course of bromocriptine (n = 63).8 This trial was informative for favourable outcomes and the relative safety of bromocriptine in PPCM, but because of the lack of a control group without bromocriptine, the efficacy conundrum was left incompletely solved. The cumulative body of evidence from clinical trials and observational studies assessing the effect of bromocriptine on outcomes remains scarce, and to our knowledge, no data from America have been published on this specific topic.4, 6, 7, 8, 9 In the absence of more evidence, many women with PPCM are not currently receiving this affordable, easily accessible, and possibly life‐saving treatment. Thus, the present study sought to assemble a population‐based cohort of women with PPCM across Quebec in order to assess the effect of adding bromocriptine to standard medical therapy on left ventricular (LV) functional recovery and clinical outcomes.
Methods
Study population
We performed a retrospective cohort study by screening the administrative records of 17 independent medical centres, which integrally covered the hospital networks of all four Faculties of Medicine in the Province of Quebec, Canada (Université de Montréal, Université de Sherbrooke, Université Laval, and McGill University; see the Appendix for details). Methodological details have been published previously.10 Briefly, consecutive women hospitalized between 1 January 1994 and 31 December 2015 who displayed the International Classification of Disease 9 and 10 codes for [peripartum cardiomyopathy (674.5 − O90.3)] or [diseases of the circulatory system (390‐459 − I00‐I99) + pregnancy, childbirth, and the puerperium (630‐679 − O00‐O9A)] in their discharge abstract summary were screened as possible cases of PPCM.
Women were included if they fulfilled the PPCM diagnostic criteria by the National Heart, Lung and Blood Institute and National Institutes of Health, defined as (i) development of cardiac failure in the last month of pregnancy or within 5 months of delivery, (ii) absence of an identifiable cause for cardiac failure, (iii) absence of recognizable heart disease prior to the last month of pregnancy, and (iv) LV systolic dysfunction.11 Although a more recent definition by the European Society of Cardiology states that LVEF is nearly always reduced <45% in PPCM, any below‐normal value of LVEF (<54%) satisfied inclusion criteria in this study.3, 12 In the event of recurrence of PPCM in subsequent pregnancies during the study interval, patient characteristics were derived from the first pregnancy associated with PPCM.
A multicentric approval was granted by the Montreal Heart Institute Ethics Review Board. As this was a retrospective analysis conducted in conformity with institutional guidelines for data security and privacy, a waiver of consent was granted. The study was initiated, designed, and conducted by cardiology fellows in compliance with the published Collectively Operated Fellow‐initiated Research principles.10
Data collection and follow‐up
Baseline characteristics and study‐specific information at index hospitalization and during follow‐up were abstracted from medical records by trained investigators in a dedicated database. Data collected included obstetrical, clinical, biochemical, pharmacological, and echocardiographic variables. Duplicate and parallel medical records (in the case of a transfer between institutions) were chronologically reconciled and harmonized using a unique health identification number.
Values of LVEF were abstracted from transthoracic echocardiography reports at baseline, 6 months after diagnosis, and up until the last available echocardiograms thereafter. As per the American Society of Echocardiography guidelines adapted for the epoch,12 LVEF was calculated using the modified Simpson's discs summation rule or the wall motion score index (normal range for women, 54–74%). Other echocardiography parameters including LV end‐diastolic diameter, mitral and tricuspid regurgitation, estimated pulmonary artery systolic pressure, and presence of pericardial effusion were recorded at baseline.
Patient classification
Guideline‐directed medical therapy for heart failure was defined according to the national guidelines prevailing at the time of diagnosis.13 Advanced therapies included implantable cardioverter defibrillator (International Classification of Disease), cardiac resynchronization, mechanical circulatory support, and heart transplantation. The decision to treat patients with bromocriptine as an adjunct to guideline‐directed medical therapy was left at the discretion of the treating physician. Patients were classified as bromocriptine (as opposed to no bromocriptine) if treated with bromocriptine during their index hospitalization, at the intended dosage of 2.5 mg twice daily for 2 weeks followed by 2.5 mg daily for 6 weeks.4 Patients classified as no bromocriptine did not receive any bromocriptine during their disease course.
Endpoints and definitions
The primary endpoint was the variation of LVEF from baseline to 6 months (mid‐term) and to longest available follow‐up (long‐term) between patients treated and not treated with bromocriptine. The secondary endpoint was the combined occurrence of all‐cause death and heart failure events. Heart failure events were defined as per the 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials.14 Among heart failure events, the following death surrogates resulting from end‐stage heart failure were included: heart transplantation, extracorporeal membrane oxygenation, ventricular assist device implantation, and resuscitated cardiac arrest.
Statistical analyses
Continuous variables are presented using mean ± standard deviation or median (interquartile range) and were compared using the t‐test or Wilcoxon rank‐sum test according to the normality of distribution. Categorical variables are presented as frequencies (percentages) and compared using either χ2 or Fisher's exact tests, as appropriate.
To compare the effect of bromocriptine on LVEF variation from baseline to 6 months and from baseline to last available follow‐up, a random growth model was chosen (instead of a repeated measures analysis of variance) because of its ability to use time as a continuous variable and thus to account for irregular time frame between baseline and follow‐up visits among subjects. An interaction term between the time effect and the treatment group effect was added to the model in order to test the possible difference in LVEF recovery between subjects treated and not treated with bromocriptine. As an exploratory analysis, we thereafter conducted an analysis of covariance (ANCOVA) on the change in LVEF from baseline to 6 months adjusted for LVEF at baseline to assess whether it modulated response to bromocriptine.
To determine the predictors of LVEF recovery from baseline to 6 months, univariable and multivariable linear regression models were produced. All variables presented in Table 1, including treatment with bromocriptine, were pre‐specified candidates. They included maternal age; multiparity; single vs. multiple gestation; timing of PPCM diagnosis in relation to delivery; co‐morbidities; previous resolved myocarditis; complications of pregnancy such as hypertensive disease and pre‐eclampsia; clinical, biochemical, and echocardiography parameters at diagnosis; and heart failure medication at discharge. Ethnicity was recorded as a baseline characteristic but not considered in multivariable models because of incomplete reporting in medical records. Variables significant at the 0.2 level in univariable analyses were included in the stepwise selection process of a multivariable model.
Table 1.
Baseline characteristics by bromocriptine use
| Bromocriptine | No bromocriptine | P‐value | |
|---|---|---|---|
| Characteristics | (n = 8) | (n = 68) | |
| Age, years | 32 ± 7 | 31 ± 5 | 0.77 |
| Multiparous women, n (%) | 4 (50%) | 36 (64%) | 0.41 |
| Multiple gestation pregnancy, n (%) | 1 (13%) | 7 (14%) | 0.89 |
| Diagnosis in the post‐partum period, n (%) | 8 (100%) | 60 (90%) | 0.33 |
| Time post‐partum at diagnosis,a weeks, median (IQR) | 0.5 (0.95) | 1.4 (4.4) | 0.03 |
| Past medical history, n (%) | |||
| Essential hypertension | 1 (13%) | 10 (15%) | 0.87 |
| History of myocarditisb | 0 (0%) | 1 (1%) | 0.73 |
| Coronary artery disease | 0 (0%) | 0 (0%) | — |
| Peripheral vascular disease | 0 (0%) | 0 (0%) | — |
| Diabetes mellitus | 0 (0%) | 10 (15%) | 0.25 |
| Dyslipidaemia | 0 (0%) | 2 (3%) | 0.62 |
| Tobacco use | 3 (38%) | 15 (23%) | 0.37 |
| Alcohol abuse | 0 (0%) | 1 (2%) | 0.74 |
| Substance abusec | 1 (13%) | 3 (5%) | 0.36 |
| Pregnancy‐related complications, n (%) | |||
| Gestational hypertension | 1 (13%) | 9 (13%) | 0.95 |
| Pre‐eclampsia | 2 (25%) | 18 (26%) | 0.92 |
| Eclampsia | 0 (0%) | 1 (1%) | 0.72 |
| Pulmonary embolism | 0 (0%) | 6 (9%) | 0.38 |
| Peripartum haemorrhage | 0 (0%) | 2 (3%) | 0.62 |
| Infection | 2 (25%) | 5 (7%) | 0.10 |
| Clinical parameters at diagnosis | |||
| NYHA class ≥ 3, n (%) | 6 (100%) | 46 (77%) | 0.18 |
| Systolic blood pressure, mmHg | 107 ± 24 | 130 ± 28 | 0.04 |
| Diastolic blood pressure, mmHg | 69 ± 19 | 84 ± 19 | 0.045 |
| Heart rate, beats/min | 136 ± 33 | 112 ± 23 | 0.01 |
| Haemoglobin, g/L | 121 ± 14 | 112 ± 20 | 0.24 |
| Creatinine, μmol/L | 82 ± 20 | 71 ± 23 | 0.22 |
| Lactates, mmol/L | 3.8 ± 2.5 | 2.3 ± 2.8 | 0.08 |
| Elevated troponins,d n (%) | 4 (50%) | 16 (30%) | 0.27 |
| Echocardiographic parameters at diagnosis | |||
| Left ventricular ejection fraction, % | 21 ± 10 | 30 ± 12 | 0.048 |
| Left ventricular end‐diastolic diameter, mm | 52 ± 9 | 59 ± 8 | 0.02 |
| Severe mitral regurgitation, n (%) | 2 (25%) | 9 (14%) | 0.42 |
| Severe tricuspid regurgitation, n (%) | 0 (0%) | 4 (7%) | 0.46 |
| Systolic pulmonary artery pressure, mmHg | 35 ± 8 | 42 ± 12 | 0.18 |
| Pericardial effusion ≥ moderate, n (%) | 1 (13%) | 1 (2%) | 0.01 |
IQR, interquartile range; NYHA, New York Heart Association.
Continuous variables are presented as mean ± standard deviation unless otherwise specified.
Applies only to women with a post‐partum diagnosis.
Remote and resolved history of viral myocarditis without left ventricular dysfunction.
Illicit use of cannabis, cocaine, amphetamine, or intravenous drugs.
Cardiac troponin I or troponin T above the 99th percentile reference limit.
To verify the robustness of our findings, a series of pre‐specified sensitivity analyses were conducted to assess the impact of missing LVEF measures on results. We conducted a worse‐case scenario analysis where women who experienced death or a death surrogate were imputed an LVEF follow‐up value of 0%. In those for whom no echocardiographic follow‐up was available but who did not die, we factored no improvement (change of 0%) and thus carried forward the LVEF measurement at baseline. This worse‐case scenario cohort was used as sensitivity analyses to corroborate results from the random growth analysis, ANCOVA, and univariable and multivariable linear regression models, as described above.
Kaplan–Meier curves and the log‐rank test were used to illustrate and compare freedom from the combined endpoint of all‐cause death or heart failure events in those treated and not treated with bromocriptine. Univariable predictors of all‐cause death or heart failure events were assessed using Cox regression models, after verifying proportionality assumptions. Because of the small number of events, multivariable models were not attempted. The list of univariable candidate predictors is the same as above.
Two‐tailed P‐values < 0.05 were considered statistically significant, and no correction or adjustment was used or done for multiple testing. Data analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC, USA).
Results
Population characteristics
A total of 76 consecutive women diagnosed with PPCM were included in this study, of whom eight (11%) had bromocriptine initiated during their index hospitalization. Baseline characteristics were similar between patients treated and not treated with bromocriptine, with noticeable exceptions (Tables 1 and 2). While the rates of pregnancy‐related complications were similar, women treated with bromocriptine were sicker with lower blood pressure, lower LVEF, but smaller left ventricular end diastolic diameter at baseline. At discharge, >80% of patients of both groups were treated with beta‐blockers and inhibitors of the renin–angiotensin system. Ethnicity was specified only if not Caucasian in medical records. Assuming this general rule, there were 1 (13%) and 17 (25%) women of African heritage in those treated and not treated with bromocriptine, respectively (P = 0.43).
Table 2.
Medication at discharge
| Bromocriptine | No bromocriptine | P‐value | |
|---|---|---|---|
| (n = 8) | (n = 68) | ||
| Medication at discharge, n (%) | |||
| Beta‐blocker | 7 (88%) | 54 (82%) | 0.69 |
| ACE‐inhibitor or angiotensin II receptor blocker | 7 (88%) | 56 (85%) | 0.84 |
| Mineralocorticoid antagonist | 1 (13%) | 9 (14%) | 0.93 |
| Hydralazine | 2 (25%) | 3 (5%) | 0.03 |
| Nitrates | 0 (0%) | 1 (2%) | 0.73 |
| Digitalis | 3 (38%) | 11 (17%) | 0.16 |
| Furosemide | 4 (50%) | 43 (65%) | 0.40 |
| Oral anticoagulant | 2 (25%) | 19 (29%) | 0.82 |
ACE, angiotensin‐converting enzyme.
Effect of bromocriptine on left ventricular functional recovery
Women treated with bromocriptine experienced a better recovery of their LV function compared with those not treated with bromocriptine, expressed as a change in LVEF from 23 ± 10% at baseline to 55 ± 12% at 6 months in women treated with bromocriptine compared with a change from 30 ± 12% at baseline to 45 ± 13% at 6 months in controls (P interaction < 0.01) (Figure 1). Similarly, last available LVEF values were higher in women treated with bromocriptine vs. those not treated with bromocriptine (56 ± 4% vs. 47 ± 14%, respectively; P interaction < 0.01). In the sensitivity analyses, LVEF was imputed in 9 (12%) and 22 (29%) women at 6 months and at long‐term follow‐up, respectively. In this analysis, the LV functional recovery remained better in women treated with bromocriptine, with P interaction <0.01 both at 6 months and at longest available follow‐up.
Figure 1.
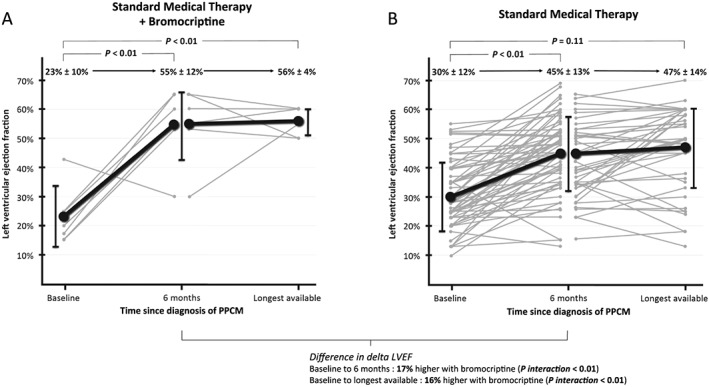
Variation in LVEF over time in women treated and not treated with bromocriptine. Shown is the variation in LVEF over time for each woman (grey lines) and the mean ± standard deviation (black lines) in women treated (panel A) and not treated (panel B) with bromocriptine. For the whole cohort, the median time from baseline to 6 months and from baseline to longest available follow‐up was 6 (3) months and 25 (61) months, respectively. P‐values were derived from the random growth model performed on the complete‐case cohort, representing the slope of the variation in LVEF over time. Women for whom both LVEF follow‐up values were missing (n = 9) were excluded from this plot, among whom two women had death surrogates before the 6 month follow‐up (heart transplant and left ventricular assist device implantation). The total number of LVEF values available in patients free of death, heart transplant, or left ventricular assist device was 76 at baseline, 66 at 6 months, and 61 at last follow‐up. LVEF, left ventricular ejection fraction; PPCM, peripartum cardiomyopathy.
Further, the determinants for LV functional recovery in PPCM were investigated (Figure 2). After multivariable adjustment, bromocriptine remained associated with LVEF improvement at 6 months [β coefficient (standard error), 14.1 (4.4); P = 0.03]. The worse‐case scenario analysis yielded stable findings, although bromocriptine use was associated with only a trend towards better LVEF recovery at 6 months [β coefficient (standard error), 10.3 (6.0); P = 0.09].
Figure 2.
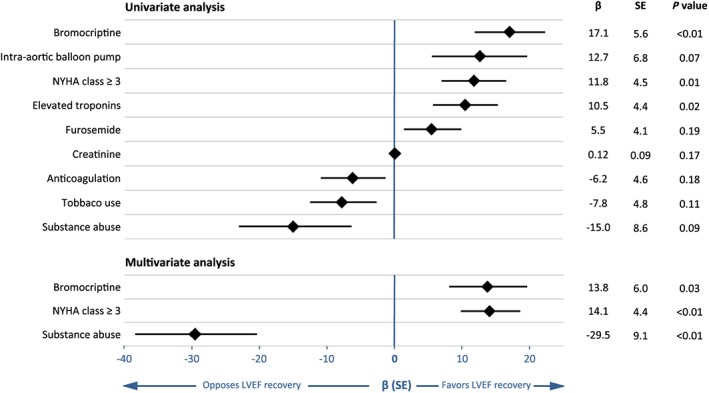
Univariable and multivariable predictors of LVEF recovery at 6 months. Shown are variables significant at the 0.2 alpha level in univariable linear regressions and variables significant at the 0.05 alpha level in multivariable linear regressions. A positive β coefficient indicates that the variable is associated with greater improvement of LVEF. The final multivariable model was built on 47 patients, because of missing baseline variables. An additional multivariable model was performed excluding the patient with history of myocarditis, yielding unchanged results. LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SE, standard error.
Effect of bromocriptine on outcomes
Patients were followed up for a median duration of 25 (61) months, providing a total of 285 patient‐years of information available for the survival analysis. Follow‐up information beyond 1 year was available for 54 (71%) patients. Crude and time‐specific Kaplan–Meier estimates of occurrence of the clinical endpoint are shown in Table 3. Outcomes occurred in a similar proportion of patients between groups. The survival analysis showed no significant association between freedom from the combined clinical endpoint and bromocriptine use (Figure 3).
Table 3.
Outcomes by bromocriptine use
| Outcomes | Bromocriptine (n = 8) | No bromocriptine (n = 68) | Univariate HR (95% CI) for bromocriptine use | P‐valuea , b |
|---|---|---|---|---|
| Combined clinical endpoint | 1.18 (0.15–9.31) | 0.88 | ||
| Number of event (crude event rate, %) | 1 (13%) | 10 (15%) | ||
| KM estimate of 1 year event rate, % | 5% | 13% | ||
| KM estimate of 2 year event rate, % | 8% | 13% | ||
| KM estimate of 3 year event rate, % | 14% | 13% | ||
| Components of the clinical endpoint (crude event rate, %) | ||||
| All‐cause deathc | 1 (13%)b | 2 (3%)d | 0.29 | |
| Hospitalization for heart failure | 0 (0%) | 6 (9%) | 1.0 | |
| Heart transplantation | 0 (0%) | 4 (6%) | 1.0 | |
| Extracorporeal membrane oxygenation | 0 (0%) | 0 (0%) | — | |
| VAD implantation | 1 (13%) | 0 (0%) | 0.11 | |
| Resuscitated cardiac arrest | 0 (0%) | 2 (3%) | 1.0 |
CI, confidence interval; HR, hazard ratio; ICD, implantable cardioverter defibrillator; KM, Kaplan–Meier; VAD, ventricular assist device.
Peripartum cardiomyopathy diagnosis: second death due to cardiac allograft vasculopathy 14 years after heart transplant and 16 years after PPCM diagnosis.
The P‐value for the univariate HR was derived from Cox regression.
The P‐values for crude event rates were derived from Fisher's exact test.
Death due to heart failure, 30 days after left VAD implantation, 46 days after peripartum cardiomyopathy diagnosis.
First death due to heart failure 6 months after.
Figure 3.
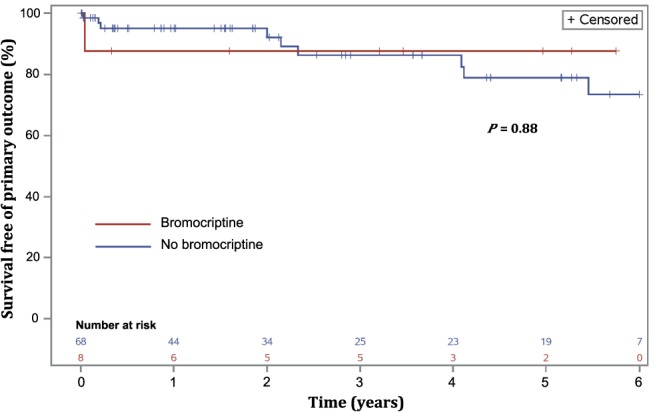
Kaplan–Meier estimates: cumulative incidence of all‐cause death and heart failure events in women treated and not treated with bromocriptine. The cumulative incidence of the combined clinical endpoint was similar in women treated and not treated with bromocriptine (P = 0.88).
The univariable predictors of the combined occurrence of clinical endpoints are presented in Table 4. Bromocriptine was not associated with the combined occurrence of all‐cause death and heart failure events (univariate hazard ratio [95% confidence interval], 1.18 [0.15–9.31]; P = 0.88).
Table 4.
Hazard ratios for the combined occurrence of all‐cause death, heart failure event, and surrogate markers for end‐stage heart failure
| Variable | Univariate HR (95% CI) | P‐value |
|---|---|---|
| Tobacco use | 4.61 (1.30–16.39) | 0.02 |
| Baseline LVEF (5% increment) | 0.58 (0.39–0.87) | <0.01 |
| Severe mitral regurgitation | 4.58 (1.39–15.08) | 0.01 |
| Severe tricuspid regurgitation | 4.96 (1.02–24.03) | 0.047 |
| ACE‐inhibitor or angiotensin II receptor blocker | 0.23 (0.06–0.90) | 0.03 |
| Digitalis | 5.21 (1.52–17.86) | <0.01 |
| Oral anticoagulant | 4.37 (1.27–14.93) | 0.02 |
| Bromocriptine | 1.18 (0.15–9.31) | 0.88 |
ACE, angiotensin‐converting enzyme; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
All variables significant at the 0.05 alpha level are presented. Bromocriptine is also presented as it was the main exposition variable.
The variable history of myocarditis was associated with an HR (95% CI) of 33.33 (3.06–333.33) based on a single patient and was therefore not included in the table.
Discussion
In this analysis, bromocriptine was found to be associated with a significantly greater LV functional recovery both at 6 months and at long‐term follow‐up in women with new‐onset PPCM. Women not treated with bromocriptine exhibited a higher burden of LV dysfunction at long‐term. In this series, the use of bromocriptine was not associated with improved clinical outcomes, such as all‐cause death and heart failure events. Our findings have important clinical implications because this study is the first to report an association between bromocriptine and LV functional recovery in North America.
In their pilot randomized trial, Sliwa et al. were the first to show the therapeutic potential of bromocriptine in South African women with PPCM by observing a mortality benefit with the molecule.4 A subsequent observational PPCM cohort from Germany found a greater proportion of women exposed to bromocriptine among those who improved their LVEF by 10% or more (59/82; 72%) compared with non‐improvers (5/14; 36%), but these analyses were unadjusted.6 The IPAC (Investigations of Pregnancy‐associated Cardiomyopathy) cohort, which prospectively observed 100 women with PPCM, reported no data on bromocriptine as the drug is seldom used for this indication in the USA. Interestingly, they found similar rates of LV functional recovery among women who did and did not breastfeed, suggesting that sustained prolactin secretion may in fact not always be deleterious in PPCM.
More recently, Hilfiker‐Kleiner et al.8 published a randomized trial that found no significant difference in LVEF recovery between shorter and longer courses of bromocriptine. The authors postulated that the high magnitude of LV functional recovery in both groups was attributable to bromocriptine. This postulate is concordant with the observation that the mean variation in LVEF from baseline to 6 months (+32%) observed by both the Hilfiker‐Kleiner et al. study and our study numerically exceeds the variation observed by the IPAC investigators (+16%) and by us (+15%) among control women not exposed to bromocriptine.
In this investigation, missing follow‐up data on LVEF required validation with worse‐case scenario sensitivity analyses, which revealed consistency in the associations found with complete‐case analyses. However, as an independent predictor of LVEF recovery, bromocriptine yielded only a trend (P = 0.09) in the worse‐case scenario cohort, as opposed to a significant effect (P = 0.03) in the complete‐case analysis, emphasizing that the interpretation of our results must remain hypothesis generating. Nevertheless, our study substantiates prior observations by showing a persistent association between bromocriptine and LV functional recovery after multivariable adjustment for guideline‐directed medications such as beta‐blockers, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and mineralocorticoid antagonists. If one accepts the relative safety of bromocriptine when proper thromboprophylaxis is used,8 and in particular cases where breastfeeding is neither essential to the well‐being of the offspring nor desired by the family unit, then the potential benefits of bromocriptine in PPCM deserve consideration in dedicated controlled trials. Different initiatives in these regards are currently underway, including a multicentre, randomized, and controlled (bromocriptine vs. no bromocriptine with breastfeeding allowed) trial in Canada (ClinicalTrials.gov, study number: NCT02590601).
In our series, bromocriptine was more frequently used in sicker women with lower LVEF, lower blood pressure, higher heart rate, and higher lactates at baseline. As low LVEF at baseline allows for a numerically greater improvement during follow‐up, our results may have been confounded by a regression to the mean bias. To minimize the scale of this possible bias, we performed a series of multivariable and sensitivity analyses, including an ANCOVA where the change in LVEF from baseline to 6 months was adjusted for the LVEF at baseline. In the latter analysis, a lower LVEF at baseline was associated with a significantly greater effect of bromocriptine (P interaction < 0.01), but this association remains uncertain as the interaction was no longer significant with the worse‐case sensitivity analysis (P = 0.53). The complex interaction between bromocriptine and LV function recovery needs to be interpreted in the light of previous reports consistently showing lower recovery rates among women with severely depressed LVEF at baseline.5, 6, 15 Potential reasons for this impediment to recovery include a greater burden of myocardial fibrosis, as suggested by cardiac magnetic resonance studies that linked myocardial late gadolinium enhancement to persistent LV dysfunction in PPCM women.16
Rather than waiting until severe LV dysfunction has occurred before initiating bromocriptine, its use early in the disease process could limit the extent of persistent damage, if one conceives that prompt inhibition of prolactin release is foremost. However, while cleaved 16 kDa prolactin likely reunites all subsets of PPCM phenotypes, molecular studies suggest that further hits are needed to develop heart failure in the peripartum period.2, 17 Proposed additional hits include pre‐existing genetic mutations conferring myocardial susceptibility and other modulators of angiogenic balance such as pre‐eclampsia‐related sFLT‐1 secretion, microchimerism, immune activation, and stress‐activated cytokines.1, 2, 17, 18 Therefore, solely addressing prolactin secretion may not be sufficient to restore cardiac homeostasis in all patients and may explain why some women exhibited mild residual LV dysfunction despite treatment with bromocriptine in our cohort.
Limitations
The present study is a retrospective analysis of observational data, and unknown confounders and biases may have affected the complex interplay between bromocriptine, LV functional recovery, and outcomes. To minimize this, we adjusted for key variables known to affect LV function and performed sensitivity analyses. PPCM is a rare disease, and the field invariably remains limited by low numbers; despite having >75 patients, our sample size remains modest. Of note, because of the rarity of series reported in the world, proper external validation of our findings in an independent dataset remains difficult at this time. Because of incomplete reporting in source documentation, the ethnic background could not be adjusted for in multivariable analyses. For these reasons, extrapolation of our results to other populations should be made with caution, as ethnicity is known to be related to prognosis.19, 20 Other variables such as atrial dimension, diastolic function, use of heparin at prophylactic doses, presence of LV thrombus, and incidence of stroke were not recorded. Additionally, the dose and compliance to bromocriptine outside of hospital was not monitored, and we could not ascertain the actual duration of treatment beyond what was intended and prescribed at hospital discharge. Likewise, the breastfeeding status, which could potentially influence endpoints, was not recorded. Because of confidentiality issues, the health of newborn babies could not be investigated. Finally, as our series covers 20 years of experience with PPCM, heart failure management has changed significantly throughout this period. However, drugs and intervention known to improve survival were reasonably well distributed at baseline between groups and were adjusted for in multivariable analyses.13 The rate of mineralocorticoid antagonist use was <15% in both groups at discharge, which can presumably be explained by initiation of this medication as an outpatient basis, although this was not specifically assessed.
Conclusions
In this multicentre, retrospective study encompassing 76 women with newly diagnosed PPCM, treatment with bromocriptine was independently associated with better LV functional recovery, compared with treatment with standard medical treatment alone. Recognizing limitations related to the non‐randomized design, this study may influence clinical management of PPCM by increasing the role of bromocriptine in specific circumstances where the potential to bolster mid‐term and long‐term LV functional recovery would outweigh breastfeeding benefits. Additional controlled trials will help determine the precise role of bromocriptine in PPCM therapeutics.
Conflict of interest
None declared.
Funding
This work was supported by the Canadian Cardiovascular Society and Bayer Resident Vascular Award and the Montreal Heart Institute Research Center. Dr Maxime Tremblay‐Gravel and Dr Guillaume Marquis‐Gravel received a bursary from the Montreal Heart Institute Foundation. Dr Robert Avram received a bursary from the Fond de Recherche en Santé du Québec.
Acknowledgements
The authors would like to acknowledge the expert input of Dr Stéphane Quenneville, Dr Marie‐Claude Parent, Dr Simon Kouz, Dr Valérie Gaudreault, and Dr Eric Sabbah. The authors would also like to thank Monique Masse for the technical support throughout the study.
| Number of patients recruited per site | ||||
|---|---|---|---|---|
| List of hospitals | Affiliated university | City | Province | Number of patients |
| Montreal Heart Institute | Université de Montreal | Montreal | Quebec | 19 |
| Centre Hospitalier de l'Université de Montréal (CHUM) | Université de Montreal | Montreal | Quebec | 5 |
| Hôpital du Sacré‐Cœur de Montréal | Université de Montreal | Montreal | Quebec | 0 |
| Hôpital Ste‐Justine | Université de Montreal | Montreal | Quebec | 3 |
| Hôpital Maisonneuve‐Rosemont | Université de Montreal | Montreal | Quebec | 9 |
| Hôpital de la Cité‐de‐la‐Santé | Université de Montreal | Laval | Quebec | 4 |
| Hôpital Pierre‐Le Gardeur | Université de Montreal | Terrebonne | Quebec | 0 |
| Hôpital Pierre‐Boucher | Université de Montreal | Longueuil | Quebec | 0 |
| Centre Hospitalier Affilié Universitaire Régional de Trois‐Rivières | Université de Montreal | Trois‐Rivières | Quebec | 5 |
| St‐Mary's Hospital | McGill University | Montreal | Quebec | 3 |
| Jewish General Hospital | McGill University | Montreal | Quebec | 4 |
| McGill University Health Center (MUHC) | McGill University | Montreal | Quebec | 5 |
| Hôpital LaSalle | McGill University | Montreal | Quebec | 1 |
| Hôpital de Gatineau | McGill University | Gatineau | Quebec | 1 |
| Centre Hospitalier Universitaire de Sherbrooke | Université de Sherbrooke | Sherbrooke | Quebec | 11 |
| Hôpital de Chicoutimi | Université de Sherbrooke | Saguenay | Quebec | 4 |
| Institut Universitaire de Cardiologie et Pneumologie de Québec | Université Laval | Quebec City | Quebec | 5 |
| Hôpital Saint‐François d'Assise | Université Laval | Quebec City | Quebec | 4 |
| Centre Hospitalier de l'Université Laval | Université Laval | Quebec City | Quebec | 1 |
| 19 Hospitals | 4 Faculties of Medicine | 9 Cities | 84 patientsa | |
Number of patients after duplicates was reconciled: 76 patients.
Tremblay‐Gravel, M. , Marquis‐Gravel, G. , Avram, R. , Desplantie, O. , Ducharme, A. , Bibas, L. , Pacheco, C. , Couture, E. , Simard, F. , Poulin, A. , Malhamé, I. , Tran, D. , Rey, E. , Tournoux, F. , Harvey, L. , Sénéchal, M. , Bélisle, P. , Descarries, L. , Farand, P. , Pranno, N. , Diaz, A. , Afilalo, J. , Ly, H. Q. , Fortier, A. , Jolicoeur, E. M. , and on behalf of the BRO‐HF investigators (2019) The effect of bromocriptine on left ventricular functional recovery in peripartum cardiomyopathy: insights from the BRO‐HF retrospective cohort study. ESC Heart Failure, 6: 27–36. 10.1002/ehf2.12376.
References
- 1. Hilfiker‐Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D‐cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007; 128: 589–600. [DOI] [PubMed] [Google Scholar]
- 2. Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker‐Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012; 485: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sliwa K, Hilfiker‐Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz‐Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Watkins H, Shah AJ, Seferovic PM, Elkayam U, Pankuweit S, Papp Z, Mouquet F, McMurray JJ. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 2010; 12: 767–778. [DOI] [PubMed] [Google Scholar]
- 4. Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, McMurray J, Yamac H, Labidi S, Struman I, Hilfiker‐Kleiner D. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof‐of‐concept pilot study. Circulation 2010; 121: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 5. McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J 3rd, Wu WC, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD, Investigators I. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy‐associated Cardiomyopathy). J Am Coll Cardiol 2015; 66: 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, Tsikas D, Jordan J, Lichtinghagen R, von Kaisenberg CS, Struman I, Bovy N, Sliwa K, Bauersachs J, Hilfiker‐Kleiner D. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013; 108: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sliwa K, Mebazaa A, Hilfiker‐Kleiner D, Petrie MC, Maggioni AP, Laroche C, Regitz‐Zagrosek V, Schaufelberger M, Tavazzi L, van der Meer P, Roos‐Hesselink JW, Seferovic P, van Spandonck‐Zwarts K, Mbakwem A, Bohm M, Mouquet F, Pieske B, Hall R, Ponikowski P, Bauersachs J. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail 2017; 19: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 8. Hilfiker‐Kleiner D, Haghikia A, Berliner D, Vogel‐Claussen J, Schwab J, Franke A, Schwarzkopf M, Ehlermann P, Pfister R, Michels G, Westenfeld R, Stangl V, Kindermann I, Kühl U, Angermann CE, Schlitt A, Fischer D, Podewski E, Böhm M, Sliwa K, Bauersachs J. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J 2017; 38: 2671–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desplantie O, Tremblay‐Gravel M, Avram R, Marquis‐Gravel G, Ducharme A, Jolicoeur EM. The medical treatment of new‐onset peripartum cardiomyopathy: a systematic review of prospective studies. Can J Cardiol 2015; 31: 1421–1426. [DOI] [PubMed] [Google Scholar]
- 10. Marquis‐Gravel G, Avram R, Tremblay‐Gravel M, Desplantie O, Ly HQ, Ducharme A, Jolicoeur EM. Collectively Operated Fellow‐initiated Research as a novel teaching model to bolster interest and increase proficiency in academic research. Can J Cardiol 2017; 33: 685–687. [DOI] [PubMed] [Google Scholar]
- 11. Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, Ansari A, Baughman KL. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 2000; 283: 1183–1188. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 13. McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, Ducharme A, Estrella‐Holder E, Giannetti N, Grzeslo A, Harkness K, Howlett JG, Kouz S, Leblanc K, Mann E, Nigam A, O'Meara E, Rajda M, Steinhart B, Swiggum E, Le VV, Zieroth S, Arnold JM, Ashton T, D'Astous M, Dorian P, Haddad H, Isaac DL, Leblanc MH, Liu P, Rao V, Ross HJ, Sussex B. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol 2013; 29: 168–181. [DOI] [PubMed] [Google Scholar]
- 14. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015; 66: 403–469. [DOI] [PubMed] [Google Scholar]
- 15. Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, Staniloae C. Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the Internet. Int J Cardiol 2012; 154: 27–31. [DOI] [PubMed] [Google Scholar]
- 16. Arora NP, Mohamad T, Mahajan N, Danrad R, Kottam A, Li T, Afonso LC. Cardiac magnetic resonance imaging in peripartum cardiomyopathy. Am J Med Sci 2014; 347: 112–117. [DOI] [PubMed] [Google Scholar]
- 17. Hilfiker‐Kleiner D, Sliwa K. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 2014; 11: 364–370. [DOI] [PubMed] [Google Scholar]
- 18. Ware JS, Seidman JG, Arany Z. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med 2016; 374: 2601–2602. [DOI] [PubMed] [Google Scholar]
- 19. Goland S, Modi K, Hatamizadeh P, Elkayam U. Differences in clinical profile of African‐American women with peripartum cardiomyopathy in the United States. J Card Fail 2013; 19: 214–218. [DOI] [PubMed] [Google Scholar]
- 20. Kao DP, Hsich E, Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail 2013; 1: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]


