Abstract
Aims
Despite attempts to improve the management of patients with acute heart failure (HF), virtually all therapeutic agents investigated in large clinical trials failed to show any consistent reduction in mortality and morbidity. Complexity of the clinical syndrome of acute HF seems to be likely an underlying explanation. Traditionally, clinical trials studied mixed patient populations with acute HF, and only recently, better clinical characterization of patients has been proposed. Dyspnoea is the most common presenting symptom related to hospital admission for acute HF. Whether in patients with acute HF, the pattern of symptoms onset preceding hospital admission is associated with clinical characteristics, and the outcomes have not yet been established.
Methods and results
We investigated 137 patients (mean age: 65 ± 13 years; 80% men) hospitalized due to acute HF with dyspnoea as major reported symptom, who were divided according to the time of its onset into those with acute (n = 98) vs. subacute (n = 39) onset (i.e. within 7 days vs. >7 days preceding hospital admission, respectively). On admission, the former group presented higher blood pressure (138 ± 33 vs. 121 ± 32 mmHg), more often moderate–severe pulmonary congestion (33 vs. 8%), and lower bilirubin level [1.07 (0.72–1.60) vs. 1.27 (0.87–2.06); P < 0.05 in all comparisons]. There were no other differences in baseline clinical characteristics and laboratory indices. Higher percentage of patients with an acute dyspnoea onset reported marked/moderate dyspnoea relief after 6 (18% vs. 7%), 24 (59% vs. 24%), and 48 h (80% vs. 46% assessed as an improvement of at least 5 points in self‐reported 10‐point Likert scale; P < 0.05 in all time points). In patients with an acute onset of dyspnoea after 48 h, a decrease of N‐terminal pro BNP was more frequently observed (83% vs. 65%), and the levels of endothelin‐1 were more reduced [−1.1 (−2.9–0.03) vs 0.4 (−2.2–1.4); all P < 0.05]. Patients with acute onset experienced less in‐hospital HF worsening (13% vs. 40%, P = 0.001), and 1 year cardiovascular mortality was significantly lower (20% vs. 41%, P = 0.01). On the multivariable analysis, subacute pattern of dyspnoea was independent predictor of 12 month cardiovascular mortality in patients with acute HF after adjusting for other prognostic factors: systolic blood pressure, urea, and HF de novo [hazard ratio (95% confidence interval): 2.32 (1.13–4.75), P = 0.02].
Conclusions
In patients with acute HF, the pattern of symptoms onset is associated with baseline differences in clinical characteristics, biomarker profile, response to standard treatment, and the long‐term outcomes. This is relevant information for planning future clinical trials.
Keywords: Acute heart failure, Symptoms, Dyspnoea, Clinical trials
Introduction
In the recent years, the number of cases of acute heart failure (HF) is constantly growing, representing one of the most common causes of hospital admission, particularly in the elderly.1, 2, 3, 4, 5 Regardless whether it initially appears as deterioration of chronic HF or acutely developing de novo HF, it is always related to poor outcomes.6, 7, 8 Despite numerous attempts to improve the management of these patients, virtually all therapeutic agents investigated in large clinical trials failed to show any consistent reduction in mortality and morbidity.9, 10, 11, 12 Complexity of the clinical syndrome of acute HF seems to be the most likely underlying explanation. Patients with acute HF represent a heterogeneous group comprising a spectrum of different clinical conditions with various pathophysiologies and different risk of subsequent complications, requiring an individualized therapeutic approach.10 However, randomized clinical trials in acute HF seemed to largely neglect this well‐recognized observation and typically studied mixed patient populations while implying a certain therapeutic regimen.13 Only recently, better characterization of patients entering clinical trials has been proposed mainly using the evaluation of clinical profiles on admission.1, 14 This, however, does not take into account a pattern of symptoms development preceding hospitalization. In clinical practice, some patients experience relatively fast deterioration, which typically results in a search for urgent medical care, whereas the others present gradual worsening (days–weeks) before hospital admission. Although intuitively one expects they are profoundly different, in clinical trials, they are grouped together, as they fulfil a standard definition of acute HF.
We therefore aimed to characterize these two groups of patients and assess their in‐hospital and long‐term outcomes. As shortness of breath is a cardinal, most common presenting symptom related to hospital admission for acute HF,15, 16 in this study, we focused on the pattern of dyspnoea development preceding hospital admission to categorize study population.
Methods
Study population
Patients were enrolled between September 2010 and July 2012 after being hospitalized at the Centre for Heart Diseases, 4th Military Hospital, Wroclaw, Poland.17 Inclusion criteria for this analysis were as follows: age ≥ 18 years; acute HF as the primary cause of hospitalization18, 19; dyspnoea at rest as the major symptom reported by patient during admission to the hospital; and patients' written agreement to participate. Exclusion criteria included clinical diagnosis of concurrent acute coronary syndrome (ACS); cardiogenic shock; known severe pulmonary, liver disease or end stage of renal disease requiring or with planned renal replacement therapy, and known malignant disease affecting an outcome. All patients, prior to being included in the study, had to sign an agreement to participate in the study and to the processing of their personal data to the extent necessary to carry out the research project, and they had the possibility to withdraw their participation at any time during the study. Patients were treated in accordance with the recommendations of the European Society of Cardiology guidelines.18, 19 The study protocol was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki.
The presented study is a secondary analysis of a prospectively collected data set for a research study on clinical variables and multidimensional biomarker model for the assessment of risk stratification and to predict short‐term and long‐term prognosis in the population of patients with acute HF (the statutory grant no. ST‐905 for Department of Heart Diseases, Wroclaw Medical University, Poland). Therefore, our study is retrospective and includes patients who reported dyspnoea at rest as the major symptom. Patients were subsequently divided into two groups according to the self‐reported time of the onset of dyspnoea: acute (within 7 days preceding hospital admission) and subacute onset (more than 7 days). In our studied population, the median time of self‐reported time of the onset of dyspnoea was 5 days (with lower and upper quartiles 2–10 days accordingly). We decided to subdivide the studied population into two groups according to the time frame of 7 days (1 week), based on the experience that many patients described the duration of their complaints as ‘a few days’, and usually, it causes difficulties to distinguish if it means, for example, 5 or 6 days. Because 1 week is commonly and culturally easily recognized time frame, we decided arbitrarily to divide patients according to this time period.
Study design and procedures
After inclusion into the study, information on demographics, clinical history, and co‐morbidities was collected together with a complete physical examination. Venous blood samples were obtained from all recruited patients within the first 4 h after admission and subsequently after 24 and 48 h. The following laboratory parameters were assessed at baseline samples using standard methods in our laboratory: (i) haematology: haemoglobin (g/dL), haematocrit (%), leukocytes, and platelets; (ii) electrolytes: sodium (mEq/L) and potassium (mEq/L); (iii) renal and liver function tests: serum creatinine (mg/dL), urea (mg/dL), and estimated glomerular filtration rate (mL/min/1.73 m2)20; (iv) bilirubin (mg/dL), aspartate transaminase (IU/IL), and alanine transaminase (IU/L); (v) plasma N‐terminal pro B‐type natriuretic peptide (NT‐proBNP, pg/mL) using an immunoenzymatic method (Siemens, Germany); (vi) C‐reactive protein (mg/L) using an immunoturbidimetric method (Konelab 30, Finland); and (vii) acid–base balance in the capillary blood sample. High‐sensitivity troponin I (hs‐TnI) and endothelin‐1 (ET‐1) levels were assessed on a new diagnostic platform from Singulex, Inc. (Alameda, CA, USA) with the Erenna immunoassay system using a microparticle immunoassay and single‐molecule counting in a capillary flow system.17, 21 Echocardiography was performed at the discretion of the treating physician, in vast majority of patients in the first 48 h from the admission to the hospital.
Definitions
On physical examination, pulmonary congestion and peripheral oedema were graded using 4‐point scale: none–mild–moderate–severe, where none means no rales heard, after clearing with cough anywhere in the lung fields or the complete absence of oedema, as determined by applying mild digital pressure in all dependent areas and failing to elicit any indentation of skin and subcutaneous tissues, respectively; mild rales heard in the lower 1/3 of either or both lung fields that persist after a cough in attempt to clear or detection of limited areas where mild digital pressure elicits an indentation of skin and subcutaneous tissues that resolves over approximately 10–15 s; moderate 1/3–2/3 rales heard throughout the lower half to 2/3 of either or both lung fields or detection of moderate surface area in one or both areas (sacrum and lower extremities) where indentations of skin and subcutaneous tissues are easily created with limited pressure and these indentations disappear slowly (15–30 s or more); and severe means >2/3 rales heard throughout both lung fields or large areas of lower extremities (and sacrum if subject has been recumbent), often to mid‐calf or higher, having easily produced and slowly resolving (more than 30 s) indentations.
Dyspnoea was measured with the use of a self‐reported 10‐point Likert scale at the time of admission to the hospital, after 6, 24, and 48 h and at discharge, where 0 means ‘lack of dyspnoea’ and 10 points means ‘dyspnoea of the worst severity/maximal dyspnoea’. Ten‐point Likert scale was chosen, because it is easy to use in the clinical settings. Changes in dyspnoea were assessed as the differences in self‐reported dyspnoea after 6, 24, and 48 h compared with the degree of dyspnoea present at the time of hospital admission. The marked/moderate dyspnoea relief was arbitrary defined as improvement of at least 5 points.15, 22
The study had the following endpoints:
in‐hospital HF worsening—the presence of deterioration or lack of relevant improvement in clinical status during the first 48 h or in‐hospital death. In our study, in‐hospital HF worsening was a subjective assessment by the treating physician when patient presented an exacerbation, a refractory, or unresponsive symptoms of HF despite applied hospital treatment and
cardiovascular death during the 365 day follow‐up.
Patients (all survivors) were regularly seen by the study investigators in the outpatient HF clinics. Information regarding survival and HF hospitalizations was obtained directly from the patients or their families, the HF clinic database, or from the hospital system. All cases of death were carefully evaluated to establish most likely cause of death; however, there was no central board to adjudicate the causes of death. Information regarding the survival was obtained from the hospital computer system and/or confirmed directly by patients or their relatives during follow‐up phone calls.
The length of follow‐up of the survivors and patients in whom an event occurred after 1 year was censored at 365 days. No patient was lost to follow‐up.
Results
Of 146 patients hospitalized with acute HF, 137 patients (94%) reported dyspnoea as major symptom and were included into this analysis. Baseline clinical characteristics and laboratory values of the study population are presented in Table 1. They were mostly men [110 (80%)] with decompensated chronic HF [108 (79%)] and mean age of 65 ± 13 years. The mean (±standard deviation) systolic blood pressure and median (upper and lower quartiles) plasma concentration of NT‐proBNP were 133 ± 33 mmHg and 5407 [2629, 9111] pg/mL, respectively. During hospitalization, all patients received intravenous (i.v.) loop diuretic, 55 (40%) vasodilator (nitroglycerine), and 15 (11%) inotropic agents (dobutamine and dopamine).
Table 1.
Baseline characteristics at admission according to the patterns of dyspnoea onset in patients with acute heart failure
| All patients | Rapid onset of dyspnoea (≤7 days) | Subacute onset of dyspnoea (>7 days) | P | |
|---|---|---|---|---|
| n = 137 | n = 98 | n = 39 | ||
| Demographics and clinical variables | ||||
| Age, years | 65 ± 13 | 66 ± 13 | 64 ± 14 | 0.48 |
| Male, n [% (95% CI)] | 110 [80 (72–86)] | 77 [79 (69–86)] | 33 [85 (69–94)] | 0.42 |
| BMI, kg/m2 | 28 ± 5 | 27 ± 4 | 29 ± 6 | 0.13 |
| LVEF, % | 30 [23–36] | 30 [24–36] | 27 [22–31] | 0.11 |
| Physical findings | ||||
| Systolic BP, mmHg | 133 ± 33 | 138 ± 33 | 121 ± 32 | 0.005 |
| Diastolic BP, mmHg | 78 ± 17 | 81 ± 18 | 72 ± 14 | 0.003 |
| Heart rate, b.p.m. | 90 [75–105] | 90 [78–110] | 90 [72–103] | 0.28 |
| Moderate–severe pulmonary congestion, n [% (95% CI)] | 35 [26 (19–34)] | 32 [33 (24–43)] | 3 [8 (2–22)] | 0.003 |
| Moderate–severe peripheral oedema, n [% (95% CI)] | 72 [53 (44–61)] | 48 [49 (39–59)] | 24 [62 (45–76)] | 0.18 |
| Ascites, n [% (95% CI)] | 19 [14 (9–21)] | 8 [8 (4–16)] | 11 [28 (16–45)] | 0.002 |
| Elevated JVD, n [% (95% CI)] | 80 [58 (50–67)] | 54 [55 (45–65)] | 26 [67 (50–80)] | 0.22 |
| Medical history | ||||
| HF de novo, n [% (95% CI)] | 29 [21 (15–28)] | 19 [19 (12–29)] | 10 [26 (14–42)] | 0.42 |
| Ischaemic HF aetiology, n [% (95% CI)] | 77 [56 (47–65)] | 54 [55 (45–65)] | 23 [59 (42–74)] | 0.68 |
| Hypertension, n [% (95% CI)] | 92 [67 (59–75)] | 63 [64 (54–74)] | 29 [74 (58–86)] | 0.26 |
| Atrial fibrillation/flutter, n [% (95% CI)] | 84 [61 (53–69)] | 65 [66 (56–75)] | 19 [49 (33–65)] | 0.06 |
| Previous CAD, n [% (95% CI)] | 81 [59 (50–67)] | 57 [58 (48–68)] | 24 [62 (45–76)] | 0.72 |
| Chronic kidney disease, n [% (95% CI)] | 70 [51 (43–60)] | 51 [52 (42–62)] | 19 [49 (33–65)] | 0.72 |
| Stroke/TIA, n [% (95% CI)] | 15 [11 (7–18)] | 7 [7 (3–15)] | 8 [21 (10–37)] | 0.02 |
| DM, n [% (95% CI)] | 57 [41 (33–50)] | 39 [39 (30–50)] | 18 [46 (30–63)] | 0.50 |
| COPD, n [% (95% CI)] | 21 [15 (10–23)] | 14 [14 (8–23)] | 7 [18 (8–34)] | 0.60 |
| Asthma, n [% (95% CI)] | 3 [2 (0.6–7)] | 2 [2 (0.4–8)] | 1 [3 (0.1–15)] | 0.85 |
| Laboratory data | ||||
| Creatinine, mg/dL | 1.2 [0.92–1.43] | 1.2 [0.9–1.4] | 1.11 [0.92–1.49] | 0.95 |
| eGFR, mL/min/1.73m2 | 58 [47–76] | 59 [47–76] | 56 [39–83] | 0.97 |
| Urea, mg/dL | 51 [39–72] | 51 [39–69] | 56 [38–78] | 0.31 |
| Sodium, mEq/L | 139 [135–141] | 139 [135–141] | 138 [134–141] | 0.51 |
| Glucose, mg/dL | 100 [89–153] | 123 [104–162] | 115 [100–147] | 0.17 |
| AST, IU/L | 26 [19–36] | 26 [18–36] | 27 [20–40] | 0.45 |
| ALT, IU/L | 26 [16–40] | 26 [17–37] | 25 [15–48] | 0.72 |
| Bilirubin, mg/dL | 1.12 [0.79–1.70] | 1.07 [0.72–1.60] | 1.27 [0.87–2.06] | 0.04 |
| WBC, G/L | 8.6 [6.7–10.1] | 8.6 [6.7–10.4] | 8.2 [6.4–10.1] | 0.70 |
| C‐reactive protein, mg/L | 7.4 [3.3–16.1] | 7.2 [2.9–14.8] | 7.5 [4.0–21.5] | 0.27 |
| Haemoglobin on admission, g/dL | 13.1 [11.7–14.3] | 13.3 [12.1–14.5] | 12.5 ± 2.0 | 0.06 |
| Haematocrite on admission, % | 39 ± 5 | 40 ± 5 | 38 ± 6 | 0.06 |
| Acid–base balance in the capillary blood | ||||
| pH | 7.45 [7.41–7.47] | 7.45 [7.41–7.47] | 7.45 [7.41–7.48] | 0.71 |
| sO2 | 94 [89–96] | 94 [90–96] | 93 [89–96] | 0.51 |
| pO2 | 65 [56–75] | 67 [58–75] | 63 [56–72] | 0.37 |
| pCO2 | 36 [32–41] | 36 [33–42] | 35 [32–39] | 0.35 |
| HCO3 − standard | 25 [23–27] | 25 [24–27] | 25 [23–27] | 0.65 |
| Lactates | 2.0 [1.5–2.6] | 2.0 [1.6–2.6] | 1.9 [1.4–2.4] | 0.26 |
| Baseline therapies (during hospitalization) | ||||
| Loop diuretics i.v., n (% [95% CI]) | 137 [100 (97–100)] | — | — | — |
| Total dose of i.v. diuretics during the first 48 h, mg (furosemide or equivalent) | 156 ± 103 | 146 ± 99 | 182 ± 110 | 0.08 |
| Vasodilator, n [% (95% CI)] | 55 [40 (31–49)] | 42 [43 (33–53)] | 14 [36 (22–53)] | 0.45 |
| Inotropes, n [% (95% CI)] | 15 [11 (7–18)] | 7 [7 (3–15)] | 8 [21 (10–37)] | 0.02 |
| Beta‐blokers, n [% (95% CI)] | 129 [96 (90–98)] | 92 [94 (87–98)] | 37 [100 (88–100)] | 0.12 |
| ACE inhibitor/ARB, n [% (95% CI)] | 126 [93 (87–97)] | 91 [94 (85–98)] | 35 [92 (78–98)] | 0.72 |
| MRA, n [% (95% CI)] | 70 [51 (43–60)] | 49 [50 (40–60)] | 21 [54 (37–70)] | 0.68 |
| Digoxin, n [% (95% CI)] | 56 [41 (33–50)] | 40 [41 (31–51)] | 16 [41 (26–58)] | 0.98 |
ACE, angiotensin‐converting enzyme; ALT, alanine transaminase; ARB, angiotensin II receptor blockers; AST, aspartate transaminase; BMI, body mass index; BP, blood pressure; b.p.m., beats per minute; CAD, coronary artery disease; CI, confidence intervals; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HF, heart failure; JVD, jugular vein distension; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; n, number of patients; TIA, transient ischaemic attack; WBC, white blood cells.
Results are presented as a number of patients (and percentage with the 95% confidence intervals), mean ± standard deviations, or median [with lower and upper quantile].
Patterns of dyspnoea onset and baseline clinical characteristics and laboratory findings
Ninety‐eight (72%) patients reported acute dyspnoea onset (within 7 days preceding hospital admission), whereas 39 (28%) reported subacute (more than 7 days) onset. Detailed comparisons of the clinical characteristics, laboratory findings, and therapies between these two groups are presented in Table 1.
On admission, patients with an acute onset of dyspnoea presented with higher systolic and diastolic blood pressure had more often moderate–severe pulmonary congestion and less often ascites once compared with those with a subacute symptoms onset. There were no differences in the history of chronic HF, presence of co‐morbidities, and left ventricular ejection fraction. Regarding laboratory findings, we have only detected higher serum bilirubin in patients with subacute onset, whereas remaining indices including serum NT‐proBNP, creatinine, hs‐TnI, ET‐1 levels, and serum blood gases did not differ between groups.
Patterns of dyspnoea onset and in‐hospital course
Patients with an acute onset:
received numerically lower total loop diuretic i.v. dose during the first 48 h (146 ± 99 vs. 182 ± 110 mg of i.v. furosemide or equivalent, P = 0.08) and required less often inotropic agents [7 (7%) vs. 8 (21%), P < 0.05]; there were no other differences in therapies applied during hospital stay (Table 1);
presented bigger reductions in systolic blood pressure and greater self‐reported dyspnoea relief after 6, 24, and 48 h after admission, whereas there were no differences in weight change after 24 and 48 h (Table 2);
more often experienced a reduction in the serum levels of NT‐proBNP and had bigger reduction in ET‐1 serum levels after 48 h (Table 3); and
had higher peak hs‐TnI level during the first 48 h of hospital stay; there were no significant difference in the change of hs‐TnI between baseline and 48 h (Table 3).
Table 2.
Changes in vital signs and symptoms during the first 48 h according to the patterns of dyspnoea onset in patients with acute heart failure
| All patients | Acute onset of dyspnoea (≤7 days) | Subacute onset of dyspnoea (> 7 days) | P | |
|---|---|---|---|---|
| n = 137 | n = 98 | n = 39 | ||
| Vital signs | ||||
| Systolic blood pressure on admission, mmHg | 133 ± 33 | 138 ± 33 | 121 ± 32 | 0.005 |
| Change at 6 h | −8 [−21 to 0] | −10 [−25 to 0] | −4 [−20 to 5] | 0.01 |
| Change at 24 h | −14 [−30 to 0] | −20 [−34 to 0] | −9 [−22 to 0] | 0.051 |
| Change at 48 h | −15 [−30 to 0] | −20 [−30 to −2] | −6 [−20 to 5] | 0.02 |
| Diastolic blood pressure on admission, mmHg | 78 ± 17 | 81 ± 18 | 72 ± 14 | 0.003 |
| Change at 6 h | −3 [−15 to 0] | −6 [−20 to 0] | 0 [−10 to 5] | 0.01 |
| Change at 24 h | −5 [−15 to 0] | −10 [−18 to 0] | 0 [−10 to 5] | 0.005 |
| Change at 48 h | −6 [−16 to 0] | −10 [−20 to 0] | 0 [−5 to 0] | 0.002 |
| Heart rate, b.p.m. on admission | 90 [75–105] | 90 [78–110] | 90 [72–103] | 0.28 |
| Change at 6 h | −9 [−10 to 0] | −9 [−26 to 0] | −8 [−20 to 0] | 0.57 |
| Change at 24 h | −10 [−30 to 0] | −10 [−30 to 0] | −8 [−20 to 0] | 0.50 |
| Change at 48 h | −10 [−30 to −1] | −15 [−30 to 0] | −10 [−20 to −2] | 0.21 |
| Weight change, kg | −1.0 [−1.6 to 0] | −1.0 [−1.6 to 0] | −1.0 [−1.8 to 0] | 0.66 |
| Change at 24 h | ||||
| Change at 48 h | −0.4 [−1.0 to 0] | −0.3 [−1 to 0] | −0.5 [−1.0 to 0] | 0.38 |
| Self‐reported dyspnoea at rest on admission (points) | 8 [7–9] | 8 [7–10] | 8 [5–9] | 0.08 |
| Self‐reported marked/moderate dyspnoea relief, n [% (95% CI)] | 21 [15 (10–23)] | 18 [18 (12–28)] | 3 [7 (2–22)] | 0.12 |
| At 6 h | ||||
| At 24 h | 67 [50 (40–58)] | 58 [59 (49–69)] | 9 [24 (12–40)] | 0.0003 |
| At 48 h | 96 [71 (62–77)] | 78 [80 (70–87)] | 18 [46 (30–63)] | 0.0004 |
| Self‐reported marked/moderate dyspnoea relief in all studied time points, n (% [95% CI]) | 20 [15 (9–22)] | 18 [18 (12–28)] | 2 [5 (0.9–19)] | 0.048 |
b.p.m., beats per minute; CI, confidence interval.
Table 3.
Changes in NT‐proBNP, hs‐TnI, and endothelin during the first 48 h according to the patterns of dyspnoea onset in patients with acute heart failure
| Acute onset of dyspnoea (≤7 days) | Subacute onset of dyspnoea (≤ 7 days) | P | |
|---|---|---|---|
| n = 98 | n = 39 | ||
| NT‐proBNP | |||
| On admission, pg/mL | 5422 [2741–8572] | 3023 [2502–11551] | 0.94 |
| Median of change in NT‐proBNP after 48 h, [IQR]—pg/mL | n = 82 | n = 31 | |
| −1385 [−3221 to −341] | −433 [−3801 to 587] | 0.19 | |
| Number of patients with decrease of NT‐proBNP after 48 h, n (% [95% CI]) | 67 [83 (71–89)] | 20 [65 (45–80)] | 0.04 |
| hs‐TnI | |||
| On admission, pg/mL | 16.4 [7.3–31.6] | 9.7 [5.9–22.1] | 0.17 |
| Peak hs‐troponin level (baseline, 24 h, or 48 h), pg/mL | 18.9 [9.1–42.3] | 10.1 [5.2–23.4] | 0.04 |
| Median ratio of troponin after 48 h to baseline level, [IQR]—pg/mL | n = 68 | n = 25 | |
| 0.9 [0.6–1.1] | 0.8 [0.7–1.0] | 0.71 | |
| Endothelin | |||
| On admission, pg/mL | 9 [7–12] | 10 [8–13] | 0.28 |
| Peak ET‐1 level (baseline, 24 h, or 48 h), pg/mL | 10.1 [7.6–13.8] | 11.2 [9.5–14.8] | 0.06 |
| Median of change in ET‐1 after 48 h, [IQR]—pg/mL | −1.1 [−2.9 to 0.03] | 0.4 [−2.2 to 1.4] | 0.03 |
CI, confidence interval; ET‐1, endothelin‐1; hs‐TnI, high‐sensitivity troponin I; IQR, interquartile range; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide.
Patterns of dyspnoea onset and outcomes
Patients with an acute onset of dyspnoea experienced less in‐hospital HF worsening (13% vs. 40%, P = 0.001).
There were 39 (28%) deaths during 1‐year follow‐up, of which 36 (26%) were classified as cardiovascular: 20 (20%) vs. 16 (41%) patients with an acute vs. subacute dyspnoea onset, respectively (P = 0.01). Detailed causes of cardiovascular deaths were as follows: progression of HF [18 (18%) and 11 (28%), P = 0.2], sudden cardiac death [2 (2%) and 4 (10%), P = 0.03], and stroke [0 vs. 1 (3%), P = 0.1] in groups with acute and subacute dyspnoea onset, respectively. Four cardiovascular deaths occurred during index hospitalization [2 (2%) vs. 2 (5%), P = 0.4]. Another three deaths occurred in patients with acute onset of dyspnoea and were due to the course of neoplastic diseases. On the univariate analysis, subacute pattern of dyspnoea predicted cardiovascular mortality [HR (95% CI): 2.28 (1.18–4.41), P = 0.01] (Figure 1 ). This association remained significant after adjusting for the other prognostic markers, which also significantly predicted cardiovascular mortality: systolic blood pressure, urea, and HF de novo [HR (95% CI): 2.32 (1.13–4.75), P = 0.02] (Table 4).
Figure 1.
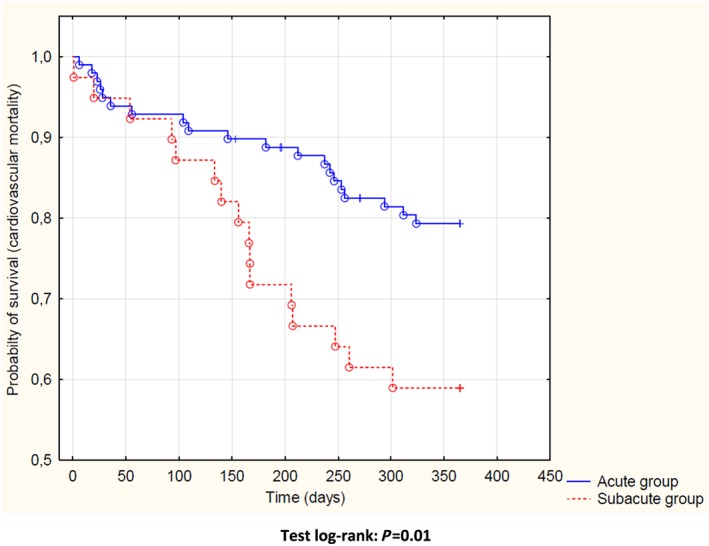
Kaplan–Meier curves for 1 year cardiovascular mortality according to the patterns of dyspnoea onset in patients with acute heart failure.
Table 4.
Predictors of 12 month CV mortality in patients with acute heart failure—univariable and multivariable models
| Univariable models | Multivariable model | |||
|---|---|---|---|---|
| Parameters | HR [95% CI] | P | HR [95% CI] | P |
| SBP/5, mmHg | 0.91 [0.86–0.97] | 0.003 | 0.99 [0.92–1.06] | 0.70 |
| HF de novo | 0.09 [0.01–0.67] | 0.02 | 0.11 [0.01–0.88] | 0.04 |
| Urea, mg/dL | 1.01 [1.01–1.02] | 0.0001 | 1.01 [1.00–1.02] | 0.006 |
| Subacute onset of dyspnoea symptoms | 2.28 [1.18–4.41] | 0.01 | 2.32 [1.13–4.75] | 0.02 |
CI, confidence intervals; CV, cardiovascular; HF, heart failure; HR, hazard ratio; SBP, systolic blood pressure. Statistically significant P values are shown in bold.
Discussion
We found that in patients with acute HF, the pattern of dyspnoea onset preceding hospital admission is associated with differences in clinical characteristics, biomarker profile, response to standard treatment, and the long‐term outcomes.
Currently, there is an ongoing debate concerning optimal endpoints in clinical trials in HF23, 24; however, our study has shown that careful evaluation of clinical characterization of patients with acute HF, including a pattern of symptoms development preceding hospitalization, might also play a key role in adequate selection of patients with unfavourable outcome and in improvement of clinical trial efficacy in this group of patients. This information may be useful for planning clinical trials.
The recent analysis from intercontinental Global Research on Acute Conditions Team (GREAT) registry on patients with acute HF from Europe and Asia has shown that acute HF precipitated by ACS or by infection is associated with increased 90 day risk of death, whereas atrial fibrillation as a precipitant leads to lower 90 day all‐cause mortality compared with acute HF without identified precipitants.25 In our study, ACS was an exclusion criteria; there was no difference in the presence of atrial fibrillation/flutter and hypertension, and also, there were similar levels of inflammatory parameters (C‐reactive protein and white blood cells) in studied groups; therefore, possible precipitants of worse or better outcomes in acute HF could not influence the results of our study. It is worth emphasizing that the clinical profile, the type of symptoms, their onset, and the triggering factors are important for long‐term outcome.
We have subdivided patients according to self‐reported time of the dyspnoea onset, and to the extent of our knowledge, it is the first paper looking for clinical characteristics and outcomes in patients with acute HF taking into account duration of symptoms occurrence before hospital admission. However, the concept of analysing different phenotypes of the patients with acute HF is well‐known and results from the wide heterogeneity of patients with HF. Fifteen years ago, Nohria et al. proved that simple clinical assessment of patients with acute HF during admission to the hospital and classifying them into four profiles according to the presence/absence of congestion and/or hypoperfusion (dry–warm, wet–warm, wet–cold, and dry–cold) predicted outcomes and should be used to adjust optimal therapy.14 This approach is now recommended to better characterize patients with acute HF.1, 26 Recently, the European Society of Cardiology Heart Failure Long‐Term Registry Investigators identified differences in outcomes among patients with acute HF stratified by clinical profile at admission (including acutely decompensated HF, cardiogenic shock, pulmonary oedema, HF in the setting of ACS, hypertensive HF, right HF, and classification based on the systolic blood pressure at initial presentation or even by geographical region.27, 28
Dyspnoea is the most common symptom leading to hospital admission in patients with acute HF. There are numerous papers evaluating predictors and consequences of an early dyspnoea relief16, 29, 30, 31, 32, 33, 34, 35, 36; however, to the best of our knowledge, this is the first study providing detailed analysis of the association between onset of dyspnoea before hospital admission and clinical status, laboratory findings, and prognosis.
Although an acute onset of dyspnoea intuitively seems to be more alarming and these patients appear to be most acutely ill, our study has shown that patients with longer deterioration in dyspnoea before hospital admission are at higher risk of poor response to standard therapy, in‐hospital HF worsening, and post‐discharge cardiovascular mortality.
In our study, a more acute symptomatic deterioration (i.e. within 7 days preceding hospital admission) was associated with a distinct clinical pattern—elevated blood pressure and apparent pulmonary congestion (moderate–severe in one‐third of these patients)—whereas peripheral congestion was less evident. It characterizes ‘vascular type’ of acute HF, where congestion is typically caused by a mismatch between increasing afterload and impaired left ventricle function with subsequent volume redistribution rather than fluid accumulation. Despite a greater severity of dyspnoea (both in a self‐reported and physician‐reported assessment) in this group, standard therapy was more efficient, as we observed moderate‐marked symptomatic improvement in dyspnoea after 24 h in 60% and after 48 h in 80% of patients (vs. only 24% and 40% in those with subacute onset, respectively). Importantly, there were no significant differences in the treatment algorithms in both groups—including diuretics in all patients and vasodilators in around 40% of them. These data seem to link a pattern of symptoms onset with the efficacy of therapy, and this information may well be applied for clinical trial planning. Further to this end, we have seen similar findings regarding in‐hospital HF worsening. This endpoint has only recently been considered in acute HF studies9, 35, 37 as it comprises lack of clinical improvement (including in‐hospital death) and typically manifests as either persistent or progressing congestion, which sometimes coincides with hypoperfusion, which require therapy escalation. It is been consistently shown as strong and independent predictor of poor post‐discharge outcomes.38, 39, 40, 41, 42 Importantly, patients with subacute dyspnoea onset experienced in‐hospital HF worsening twice as often vs. those with an acute onset. We believe that it cannot simply be explained on the basis of more severe/advanced disease status. Previous studies identified age, comorbidity status, elevated baseline levels of natriuretic peptides, and undertreatment with neurohormonal blockers as potential predictors of in‐hospital HF worsening.43 As we did not see any significant differences in these factors between groups, one may expect that slow clinical symptomatic deterioration preceding hospital admission itself carries a substantial risk of poor in‐hospital clinical course. It shows again that distinction of a pattern of symptoms onset is mandatory for defining a proper endpoint in clinical trials.
Interestingly, there were also distinct features of the biomarker changes characterizing study groups. Firstly, ET‐1 levels decreased in patients with an acute onset whereas remained unchanged in those with a subacute onset, which provides indirect evidence of more pronounced, persisting endothelin‐mediated vasoconstriction in the latter group with potential, resultant inadequate peripheral perfusion. In the recent analysis of the Double‐Blind, Placebo‐Controlled, Multicenter Acute Study of Clinical Effectiveness of Nesiritide in Subjects With Decompensated Heart Failure (ASCEND‐HF), the authors reported that ET‐1 is typically decreasing after stabilization with therapy, and persistently elevated ET‐1 levels after 48–72 h were independently associated with increased in‐hospital mortality and HF worsening as well as with 180 day mortality.44 Secondly, in those with an acute onset, haemodynamic changes (as evidenced by reductions in systolic blood pressure) were accompanied by a trend towards bigger decrease in NT‐proBNP levels, which seem to reflect more pronounced reduction in cardiac wall stress and decongestion achieved during the first 48 h of in‐hospital stay. It occurred despite similar treatments (including i.v. diuretics and vasodilators) received by both groups. It can be expected that these favourable changes would be paralleled by changes in hs‐TnI levels reflecting less myocardial damage in this group of patients. On the contrary, they demonstrated significantly higher peak hs‐TnI levels and no difference in the median ratio of hs‐TnI after 48 h vs. baseline levels. These findings seem to cast doubt on the theories linking an early reduction in cardiac wall stress achieved with vasodilating therapies with less myocardial necrosis and subsequent better outcomes. In fact, similar observations were reported in the Phase III, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Trial to Evaluate the Efficacy and Safety of Ularitide (Urodilatin) Intravenous Infusion in Patients Suffering From Acute Decompensated Heart Failure (TRUE‐AHF),9 where patients receiving ularitide presented better haemodynamic profile associated with less cardiac wall stress, which however did not translate in lower troponin levels.
Recent trials with vasoactive therapies9, 11, 12, 37, 45, 46 typically used normal or elevated blood pressure as major entry criterion to characterize patients for whom novel therapies could be safely tested but more importantly to identify those who could potentially present better in‐hospital symptomatic relief and longer term outcome effects. Our findings show that this approach needs to be revisited. Taking as an example systolic blood pressure ≥ 116 mmHg as the inclusion criterion for TRUE‐AHF trial9 and ≥125 mmHg for a Multicenter, Randomized, Double‐blind, Placebo‐controlled Phase III Study to Evaluate the Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in Acute Heart Failure Patients (RELAX‐AHF‐2)37 would result in 46% and 36% (respectively) of patients from the group with subacute onset of dyspnoea being candidates for the recruitment in these trials. It would end up with a heterogeneous combination of patients who respond differently for standard therapies and have different outcomes.
There are some limitations of our analysis, of which retrospective nature and relatively small number of patients seem to be most relevant, given the risk of type I error occurrence. We did not perform adjustment of statistical significance, due to an increase of the probability of type II error. However, in our registry, we obtained detailed information regarding pattern of symptoms preceding hospital admission, which can be reliably linked with detailed information regarding in‐hospital course, changes in biomarker profile, and 1 year outcomes. Another limitation of our study was that the division of studied population was based on an arbitrary determined time frame of 7 days.
In summary, in patients with acute HF, duration of dyspnoea onset preceding hospital admission is associated with a distinct pattern of clinical characteristics, biomarker profile, in‐hospital course, and long‐term outcomes. Besides a routine clinical assessment, information on a pattern of dyspnoea may help physicians to identify higher risk patients. All this information seems relevant for planning clinical trials in acute HF.
Statistical analysis
The assumption of normality was assessed for all studied parameters using Kolmogorov–Smirnov test. Normally distributed continuous variables were presented as means ± standard deviations. The inter‐group differences were tested using Student's t‐test and the Mann–Whitney U‐test. Variables with a skewed distribution were expressed as medians with lower and upper quartiles and were log transformed in order to normalize their distributions. The categorical variables were expressed as numbers and percentages with the 95% confidence intervals (CIs). The inter‐group differences were tested using the χ2 test. To establish the effect of patterns of dyspnoea on survival, we performed Cox proportional hazard regression models. Patterns of dyspnoea (and other clinical variables) were predictors (determining variables), whereas the endpoint was the cardiovascular mortality with the consideration that the follow‐up of patients who survived 365 days and longer was censored at 365 days. The associations between pattern of dyspnoea and survival were adjusted in multivariable models for subsequent variables, which appeared to be statistically significant predictors in univariable models. The number of analyzed determining variables in the multivariable models was limited by a low number of observations. Previously, examination of outliners and verification of correlation between variables were performed. The variables correlated were excluded from multivariable analysis. To estimate the effect of the dyspnoea pattern on 12‐month mortality rates, Kaplan–Meier curves for cumulative survival were constructed. Differences in survival rates were tested with the Cox–Mantel log‐rank test. Our study was a secondary analysis of a pre‐set database; therefore, a sample size calculation could not be executed a priori. A value of P < 0.05 was considered as statistically significant. Alpha was not corrected for multiple comparisons. Statistical analyses were performed using the STATISTICA 12 data analysis software system (StatSoft, Inc.).
Conflict of interest
J.T. is the employeer of Singulex Inc. and owns Singulex stock. All other authors declare no conflict of interest.
Funding
The research was financially supported from the statutory grant for Department of Heart Diseases, Wroclaw Medical University, Poland (ST‐905).
Sokolska, J. M. , Sokolski, M. , Zymliński, R. , Biegus, J. , Siwołowski, P. , Nawrocka‐Millward, S. , Jankowska, E. A. , Todd, J. , Banasiak, W. , and Ponikowski, P. (2019) Patterns of dyspnoea onset in patients with acute heart failure: clinical and prognostic implications. ESC Heart Failure, 6: 16–26. 10.1002/ehf2.12371.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Journal of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep 2014; 11: 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roger VL. The heart failure epidemic. Int J Environ Res Public Health 2010; 7: 1807–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kociol RD, Hammill BG, Fonarow GC, Klaskala W, Mills RM, Hernandez AF, Curtis LH. Generalizability and longitudinal outcomes of a national heart failure clinical registry: comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non‐ADHERE Medicare beneficiaries. Am Heart J 2010; 160: 885–892. [DOI] [PubMed] [Google Scholar]
- 5. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 6. Choi KH, Lee GY, Choi J‐O, Jeon E‐S, Lee H‐Y, Cho H‐J, Lee SE, Kim M‐S, Kim J‐J, Hwang K‐K, Chae SC, Baek SH, Kang S‐M, Choi D‐J, Yoo B‐S, Kim KH, Park H‐Y, Cho M‐C, Oh B‐H. Outcomes of de novo and acute decompensated heart failure patients according to ejection fraction. Heart 2018; 104: 525–532. [DOI] [PubMed] [Google Scholar]
- 7. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999; 353: 2001–2007. [PubMed] [Google Scholar]
- 8. Lassus JPE, Siirilä‐Waris K, Nieminen MS, Tolonen J, Tarvasmäki T, Peuhkurinen K, Melin J, Pulkki K, Harjola V‐P, FINN‐AKVA study group . Long‐term survival after hospitalization for acute heart failure—Differences in prognosis of acutely decompensated chronic and new‐onset acute heart failure. Int J Cardiol 2013; 168: 458–462. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, O'Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017; 376: 1956–1964. [DOI] [PubMed] [Google Scholar]
- 10. Krzysztofik J, Ponikowski P. Current and emerging pharmacologic options for the management of patients with chronic and acute decompensated heart failure. Expert Rev Clin Pharmacol 2017; 10: 517–534. [DOI] [PubMed] [Google Scholar]
- 11. JR. T . RELAX‐AHF‐2: serelaxin in acute heart failure, part 1. Heart Failure 2017 and the 4th World Congress on Acute Heart Failure. Late Breaking Trials I: Focus on acute heart failure. Paris, France; 2017.
- 12. Metra M. RELAX‐AHF‐2: serelaxin in acute heart failure, part 2. Heart Failure 2017 and the 4th World Congress on Acute Heart Failure. Late Breaking Trials I: Focus on acute heart failure. Paris, France; 2017.
- 13. Tamargo J, Rosano GMC, Delpón E, Ruilope L, López‐Sendón J. Pharmacological reasons that may explain why randomized clinical trials have failed in acute heart failure syndromes. Int J Cardiol 2017; 233: 1–11. [DOI] [PubMed] [Google Scholar]
- 14. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003; 41: 1797–1804. [DOI] [PubMed] [Google Scholar]
- 15. West RL, Hernandez AF, O'Connor CM, Starling RC, Califf RM. A review of dyspnea in acute heart failure syndromes. Am Heart J 160: 209–214. [DOI] [PubMed] [Google Scholar]
- 16. Mentz RJ, Mi X, Sharma PP, Qualls LG, DeVore AD, Johnson KW, Fonarow GC, Curtis LH, Hernandez AF. Relation of dyspnea severity on admission for acute heart failure with outcomes and costs. Am J Cardiol 2015; 115: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zymliński R, Sokolski M, Siwolowski P, Biegus J, Nawrocka S, Jankowska EA, Todd J, Yerramilli R, Estis J, Banasiak W, Ponikowski P. Elevated troponin I level assessed by a new high‐sensitive assay and the risk of poor outcomes in patients with acute heart failure. Int J Cardiol 2017; 230: 646–652. [DOI] [PubMed] [Google Scholar]
- 18. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, ESC Committee for Practice Guidelines (CPG) , Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Tendera M, Auricchio A, Bax J, Böhm M, Corrà U, della Bella P, Elliott PM, Follath F, Gheorghiade M, Hasin Y, Hernborg A, Jaarsma T, Komajda M, Kornowski R, Piepoli M, Prendergast B, Tavazzi L, Vachiery JL, Verheugt FW, Zamorano JL, Zannad F. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GYH, Pietro MA, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P, ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail 2012; 14: 803–869. [DOI] [PubMed] [Google Scholar]
- 20. Berman N, Hostetter TH. Comparing the Cockcroft–Gault and MDRD equations for calculation of GFR and drug doses in the elderly. Nat Clin Pract Nephrol 2007; 3: 644–645. [DOI] [PubMed] [Google Scholar]
- 21. Wu AHB, Lu QA, Todd J, Moecks J, Wians F. Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem 2009; 55: 52–58. [DOI] [PubMed] [Google Scholar]
- 22. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10: 407–415. [DOI] [PubMed] [Google Scholar]
- 23. Cowie MR, Filippatos GS, Alonso Garcia M d LA, Anker SD, Baczynska A, Bloomfield DM, Borentain M, Bruins Slot K, Cronin M, Doevendans PA, El‐Gazayerly A, Gimpelewicz C, Honarpour N, Janmohamed S, Janssen H, Kim AM, Lautsch D, Laws I, Lefkowitz M, Lopez‐Sendon J, Lyon AR, Malik FI, McMurray JJV, Metra M, Figueroa Perez S, Pfeffer MA, Pocock SJ, Ponikowski P, Prasad K, Richard‐Lordereau I, Roessig L, Rosano GMC, Sherman W, Stough WG, Swedberg K, Tyl B, Zannad F, Boulton C, De Graeff P, New medicinal products for chronic heart failure: advances in clinical trial design and efficacy assessment. Eur J Heart Fail 2017; 19: 718–727. [DOI] [PubMed] [Google Scholar]
- 24. Anker SD, Schroeder S, Atar D, Bax JJ, Ceconi C, Cowie MR, Crisp A, Dominjon F, Ford I, Ghofrani H‐A, Gropper S, Hindricks G, Hlatky MA, Holcomb R, Honarpour N, Jukema JW, Kim AM, Kunz M, Lefkowitz M, Le FC, Landmesser U, McDonagh TA, McMurray JJ, Merkely B, Packer M, Prasad K, Revkin J, Rosano GMC, Somaratne R, Stough WG, Voors AA, Ruschitzka F. Traditional and new composite endpoints in heart failure clinical trials: facilitating comprehensive efficacy assessments and improving trial efficiency. Eur J Heart Fail 2016; 18: 482–489. [DOI] [PubMed] [Google Scholar]
- 25. Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi D‐J, Park JJ, Alhabib KF, Sato N, Miro O, Maggioni AP, Zhang Y, Spinar J, Cohen‐Solal A, Iwashyna TJ, Mebazaa A. Precipitating factors and 90‐day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail 2017; 19: 201–208. [DOI] [PubMed] [Google Scholar]
- 26. Harjola V‐P, Mullens W, Banaszewski M, Bauersachs J, Brunner‐La Rocca H‐P, Chioncel O, Collins SP, Doehner W, Filippatos GS, Flammer AJ, Fuhrmann V, Lainscak M, Lassus J, Legrand M, Masip J, Mueller C, Papp Z, Parissis J, Platz E, Rudiger A, Ruschitzka F, Schäfer A, Seferovic PM, Skouri H, Yilmaz MB, Mebazaa A. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2017; 19: 821–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chioncel O, Mebazaa A, Harjola V‐P, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, Heart Failure Long‐Term Registry Investigators ESC. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 28. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A, Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 29. Solomonica A, Burger AJ, Aronson D. Hemodynamic determinants of dyspnea improvement in acute decompensated heart failureclinical perspective. Circ Heart Fail 2013; 6: 53–60. [DOI] [PubMed] [Google Scholar]
- 30. West RL, Hernandez AF, O'Connor CM, Starling RC, Califf RM. A review of dyspnea in acute heart failure syndromes. Am Heart J 2010; 160: 209–214. [DOI] [PubMed] [Google Scholar]
- 31. Metra M, O'Connor CM, Davison BA, Cleland JGF, Ponikowski P, Teerlink JR, Voors AA, Givertz MM, Mansoor GA, Bloomfield DM, Jia G, DeLucca P, Massie B, Dittrich H, Cotter G. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J 2011; 32: 1519–1534. [DOI] [PubMed] [Google Scholar]
- 32. O'Connor CM, Fiuzat M, Lombardi C, Fujita K, Jia G, Davison BA, Cleland J, Bloomfield D, Dittrich HC, DeLucca P, Givertz MM, Mansoor G, Ponikowski P, Teerlink JR, Voors AA, Massie BM, Cotter G, Metra M. Impact of serial troponin release on outcomes in patients with acute heart failure: analysis from the protect pilot study. Circ Heart Fail 2011; 4: 724–732. [DOI] [PubMed] [Google Scholar]
- 33. Metra M, Cleland JG, Weatherley BD, Dittrich HC, Givertz MM, Massie BM, O'Connor CM, Ponikowski P, Teerlink JR, Voors AA, Cotter G. Dyspnoea in patients with acute heart failure: an analysis of its clinical course, determinants, and relationship to 60‐day outcomes in the PROTECT pilot study. Eur J Heart Fail 2010; 12: 499–507. [DOI] [PubMed] [Google Scholar]
- 34. Metra M, Teerlink JR, Felker GM, Greenberg BH, Filippatos G, Ponikowski P, Teichman SL, Unemori E, Voors AA, Weatherley BD, Cotter G. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre‐RELAX‐AHF study. Eur J Heart Fail 2010; 12: 1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allen LA, Hernandez AF, O'Connor CM, Felker GM. End points for clinical trials in acute heart failure syndromes. J Am Coll Cardiol 2009; 53: 2248–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mentz RJ, Hernandez AF, Stebbins A, Ezekowitz JA, Felker GM, Heizer GM, Atar D, Teerlink JR, Califf RM, Massie BM, Hasselblad V, Starling RC, O'Connor CM, Ponikowski P. Predictors of early dyspnoea relief in acute heart failure and the association with 30‐day outcomes: findings from ASCEND‐HF. Eur J Heart Fail 2013; 15: 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Efficacy, safety and tolerability of serelaxin when added to standard therapy in AHF–full text view–ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01870778 (28 May 2017).
- 38. Kelly JP, Mentz RJ, Hasselblad V, Ezekowitz JA, Armstrong PW, Zannad F, Felker GM, Califf RM, O'Connor CM, Hernandez AF. Worsening heart failure during hospitalization for acute heart failure: insights from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND‐HF). Am Heart J 2015; 170: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carubelli V, Cotter G, Davison B, Gishe J, Senger S, Bonadei I, Gorga E, Lazzarini V, Lombardi C, Metra M. In‐hospital worsening heart failure in patients admitted for acute heart failure. Int J Cardiol 2016; 225: 353–361. [DOI] [PubMed] [Google Scholar]
- 40. Kelly JP, Cooper LB, Gallup D, Anstrom KJ, Chen HH, Redfield MM, O'Connor CM, Mentz RJ, Hernanadez AF, Felker GM. Implications of using different definitions on outcomes in worsening heart failure. Circ Heart Fail 2016; 9: e003048 10.1161/CIRCHEARTFAILURE.116.003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DeVore AD, Hammill BG, Sharma PP, Qualls LG, Mentz RJ, Waltman Johnson K, Fonarow GC, Curtis LH, Hernandez AF. In‐hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc 2014; 3 10.1161/JAHA.114.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. AlFaleh H, Elasfar AA, Ullah A, AlHabib KF, Hersi A, Mimish L, Almasood A, Al GS, Ghabashi A, Malik A, Hussein GA, Al‐Murayeh M, Abuosa A, Ali HW, Kashour T. Worsening heart failure in ‘real‐world’ clinical practice: predictors and prognostic impact. Eur J Heart Fail 2017; 19: 987–995. [DOI] [PubMed] [Google Scholar]
- 43. Butler J, Gheorghiade M, Kelkar A, Fonarow GC, Anker S, Greene SJ, Papadimitriou L, Collins S, Ruschitzka F, Yancy CW, Teerlink JR, Adams K, Cotter G, Ponikowski P, Felker GM, Metra M, Filippatos G. In‐hospital worsening heart failure. Eur J Heart Fail 2015; 17: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 44. Perez AL, Grodin JL, Wu Y, Hernandez AF, Butler J, Metra M, Felker GM, Voors AA, McMurray JJ, Armstrong PW, Starling RC, O'Connor CM, Tang WHW. Increased mortality with elevated plasma endothelin‐1 in acute heart failure: an ASCEND‐HF biomarker substudy. Eur J Heart Fail 2016; 18: 290–297. [DOI] [PubMed] [Google Scholar]
- 45. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M, RELAXin in Acute Heart Failure (RELAX‐AHF) Investigators . Serelaxin, recombinant human relaxin‐2, for treatment of acute heart failure (RELAX‐AHF): a randomised, placebo‐controlled trial. Lancet 2013; 381: 29–39. [DOI] [PubMed] [Google Scholar]
- 46. Anker SD, Ponikowski P, Mitrovic V, Peacock WF, Filippatos G. Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. Eur Heart J 2015; 36: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]


