Abstract
Hospitalization for acute heart failure (HF) is associated with a substantial morbidity burden and with associated healthcare costs and an increased mortality risk. However, few if any major medical innovations have been witnessed in this area in recent times. Levosimendan is a first‐in‐class calcium sensitizer and potassium channel opener indicated for the management of acute HF. Experience in several clinical studies has indicated that administration of intravenous levosimendan in intermittent cycles may reduce hospitalization and mortality rates in patients with advanced HF; however, none of those trials were designed or powered to give conclusive insights into that possibility. This paper describes the rationale and protocol of LeoDOR (levosimendan infusions for patients with advanced chronic heart failure), a randomized, double‐blind, placebo‐controlled, international, multicentre trial that will explore the efficacy and safety of intermittent levosimendan therapy, in addition to optimized standard therapy, in patients following hospitalization for acute HF. Salient features of LeoDOR include the use of two treatment regimens, in order to evaluate the effects of different schedules and doses of levosimendan during a 12 week treatment phase, and the use of a global rank primary endpoint, in which all patients are ranked across three hierarchical groups ranging from time to death or urgent heart transplantation or implantation of a ventricular assist device to time to rehospitalization and, lastly, time‐averaged proportional change in N‐terminal pro‐brain natriuretic peptide. Secondary endpoints include changes in HF symptoms and functional status at 14 weeks.
Keywords: Levosimendan, Advanced heart failure, Randomized controlled trial, Hospitalization, N‐terminal pro‐brain natriuretic peptide, Global rank endpoint
Introduction
Heart failure (HF) is the leading cause of adult hospitalization in the industrialized world and imposes a substantial burden on public health. Healthcare costs for HF patients are high and rising,1 driven by an increase in hospitalizations related to HF.2 Over recent decades, adoption of evidence‐based therapies has reduced the event rate in patients with chronic HF.3 However, the therapeutic approach for hospitalized patients with acute HF has remained practically unchanged, and outcomes for these patients are still poor4, 5: hospitalization for acute HF is associated with higher mortality, recurrent hospitalization, and increased resource consumption.4, 6 In a European survey, 1 year mortality was 23.6% for individuals hospitalized with acute HF, compared with 6.4% for outpatients with chronic HF.4 The majority of rehospitalizations occur early after hospital discharge: about a quarter of patients are rehospitalized within the first month and two‐thirds within 1 year.2, 7
Strategies to prevent rehospitalizations during this vulnerable period have not been consistently successful so far, although adherence to well‐established medical therapies at optimal doses is considered essential.5 Recently, sacubitril/valsartan,8 ivabradine,9 and cardiac resynchronization therapy10 were found to reduce both hospitalizations and rehospitalizations. Intermittent or continuous inotropic therapy has also been tested, but, despite favourable haemodynamic and symptomatic improvement in pilot trials,11 no positive effect on hospitalizations has been observed, and, in fact, there has been a trend towards a higher mortality risk.12
Levosimendan, a calcium sensitizer and potassium channel opener, is indicated for acute HF. The compound has an active metabolite (OR1896) that prolongs its effects beyond the infusion period.13 The theoretical advantages of levosimendan in comparison with traditional inotropic agents include (i) a lack of increase in intracellular calcium concentration and thus no increase in myocardial oxygen demand; (ii) prolonged effects via the formation of an active metabolite; (iii) beneficial symptomatic, neurohormonal, and haemodynamic effects (e.g. decrease in pulmonary capillary wedge pressure and increase in cardiac output); (iv) no attenuation of effect in patients using beta‐blockers; and (v) beneficial effects on renal function and peripheral organ perfusion.14 Several clinical studies of the repetitive use of intravenous (i.v.) levosimendan have suggested that such a strategy may benefit patients with advanced HF by reducing hospitalization and mortality.15, 16, 17 However, some of these studies were open‐label, single‐centre studies, and even the largest, double‐blind trial performed to date—LevoRep (NCT01065194)17—was limited to 120 patients. Therefore, a larger study is needed to verify these favourable preliminary results.18, 19
In this paper, the design of the randomized, double‐blind, placebo‐controlled LeoDOR (levosimendan infusions for patients with advanced chronic heart failure) trial is presented. This study will assess the efficacy and safety of intermittent levosimendan therapy started during the vulnerable phase following a hospitalization for acute HF. The overarching hypothesis is that, compared with placebo, repetitive administration of levosimendan during the post‐acute phase will be associated with greater clinical stability over a follow‐up period of 14 weeks.
Study design
The LeoDOR (Figure 1) is a multicentre, randomized, double‐blind, placebo‐controlled, three‐arm trial designed to evaluate the efficacy and safety of intermittent levosimendan therapy, administered in addition to standard therapy for a period of 12 weeks either as a 6 h continuous infusion at a rate of 0.2 μg/kg/min every 2 weeks or as a 24 h continuous infusion at a rate of 0.1 μg/kg/min every 3 weeks. The primary endpoint will be evaluated after 14 weeks. Another follow‐up visit to obtain information on safety events is scheduled after 6 months. The study intends to include 264 patients in 28 centres in nine European countries.
Figure 1.
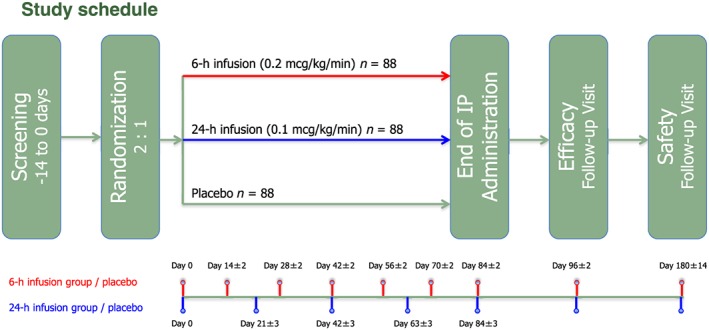
Schematic diagram of study design for LeoDOR trial. IP, investigational product.
Appraisal of levosimendan efficacy in LeoDOR is based on a hierarchical composite outcome. The primary efficacy assessment will be made using a global rank endpoint in which all participants are ranked across three hierarchical groups: (i) time to death or urgent heart transplantation or implantation of a ventricular assist device (VAD); (ii) time to non‐fatal HF requiring i.v. vasoactive therapy; and (iii) time‐averaged proportional change in N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) from baseline to Week 14 with (i) as the most important event. Secondary efficacy endpoints include individual components of the primary endpoint at short‐term (14 weeks) and intermediate‐term (26 weeks) follow‐up, as well as changes in functional status.
The LeoDOR Executive Committee ( Appendix ) designed the trial and wrote the study protocol. The protocol was approved by the Ethics Review Committee/Institutional Review Board affiliated to each centre, and the study will be conducted in accordance with Good Clinical Practice and the 2002 Declaration of Helsinki. All participants will provide written informed consent prior to any study‐related investigations. The trial has been registered at https://clinicaltrials.gov, NCT03437226.
Study population and inclusion/exclusion criteria
The study population will include male and female patients aged ≥18 years hospitalized for an acute HF event requiring i.v. diuretics, i.v. vasodilators, i.v. inotropic therapy, or any combination of those interventions. Patients must have had a diagnosis of chronic HF for ≥6 months prior to screening and must have been treated with individually optimized long‐term therapies for ≥1 month preceding the index hospitalization. Further inclusion criteria are left ventricular ejection fraction ≤30% assessed during index hospitalization, at least one previous hospitalization or visit to an outpatient clinic for acute decompensated HF within the 12 months before the index hospitalization and either NT‐proBNP levels (as measured by the local laboratory) after recompensation of ≥2500 ng/L (BNP ≥900 ng/L) or persistent New York Heart Association functional class III or IV at study entry. Patients are considered recompensated and ready for discharge when haemodynamically stable, euvolemic, established on evidence‐based oral medication, and with stable renal function for at least 24 h.20 Patients with severe obstruction of the ventricular outflow tract, severely impaired ventricular filling such as restrictive cardiomyopathy, predominantly right‐sided HF and/or severe tricuspid regurgitation, cardiac surgery, coronary angioplasty, or acute coronary syndromes within 30 days of initiation of study drug are not eligible. Further exclusion criteria include patients scheduled for cardiac surgery or angioplasty within the next 3 months; history of torsades de pointes; stroke or transient ischaemic attack within 3 months of initiation of study drug; systolic blood pressure ≤90 mmHg or heart rate ≥120 beats/min at baseline; serum potassium <3.5 mmol/L before initiation of study drug; estimated glomerular filtration rate <30 mL/min/1.73 m2; haemoglobin <10 g/dL; significant hepatic impairment; hypersensitivity to levosimendan; other serious diseases that considerably limit life expectancy; pregnancy or lactation; and administration of levosimendan within 14 days of initiation of study drug (Table 1).
Table 1.
LeoDOR inclusion and selected exclusion criteria
| Inclusion criteria |
| Male and female patients aged >18 years |
| HF diagnosed at least 6 months before screening and treated with individually optimized long‐term oral and device treatment for the last month, unless not tolerated |
| LVEF ≤30% as assessed using echocardiography, radionuclide ventriculography, or contrast angiography during the index hospitalization |
| Currently hospitalized for decompensated HF requiring i.v. diuretics or i.v. vasodilators or i.v. inotropic therapy |
| Previous hospitalization or visit to an outpatient clinic requiring i.v. diuretics, i.v. vasodilators, or i.v. inotropic therapy for acute decompensated HF within 12 months prior to index hospitalization |
| NT‐proBNP level (as measured by the local laboratory) after compensation of ≥2500 ng/L and/or NYHA class III or IV at study entry. A compensated status is reached when the patient is haemodynamically stable, euvolemic, established on evidence‐based oral medication, and with stable renal function for at least 24 h |
| Exclusion criteria |
| Severe obstruction of ventricular outflow tracts, such as haemodynamically significant uncorrected primary valve disease or hypertrophic cardiomyopathy or impaired ventricular filling, such as restrictive cardiomyopathy |
| Predominantly right‐sided heart failure and/or severe tricuspid regurgitation |
| Cardiac surgery or coronary angioplasty within 30 days prior to study drug initiation |
| Acute coronary syndromes within 30 days prior to study drug initiation |
| Patients scheduled for cardiac surgery or angioplasty in the next 3 months |
| History of torsades de pointes |
| Systolic blood pressure <90 mmHg at baseline |
| Heart rate ≥120 beats/min at baseline |
| Serum potassium <3.5 mmol/L before study drug initiation |
| eGFR ≤30 mL/min/1.73 m2 |
| Administration of levosimendan within 14 days prior to study drug initiation (first study drug application to be postponed for at least 14 days after the end of this premedication) |
| Hypersensitivity to levosimendan |
| Other serious diseases that considerably limit life expectancy (e.g. end‐stage cancer, end‐stage renal disease, and end‐stage lung disease) |
eGFR, estimated glomerular filtration rate; HF, heart failure; i.v., intravenous; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Patients will be randomized after recompensation from the index episode of acute HF. Randomization can take place either at the end of the hospital stay or within 14 days after discharge for the index hospitalization.
Study treatment
Candidates for the study will be screened during hospitalization for acute decompensated HF. During the first treatment visit, eligibility for the trial will be confirmed, the patient will be randomized, and the first infusion of the study drug will be administered. The first treatment visit should preferentially be scheduled during the index hospitalization or, at the latest, within 14 days after discharge from hospital. If the baseline visit takes place during the index hospitalization, the patient's condition should be stabilized so as to facilitate administration of the study drug on the last day of the index hospitalization.
Levosimendan or matching placebo will be administered as an i.v. infusion. For the 6 h infusion group, the study drug will be administered at a continuous infusion rate of 0.2 μg/kg/min for 6 h. The scheduled study drug administrations will take place on Day 0, Day 14 (±2 days), Day 28 (±2 days), Day 42 (±2 days), Day 56 (±2 days), Day 70 (±2 days), and Day 84 (±2 days). For the 24 h infusion group, the study drug will be administered at a continuous infusion rate of 0.1 μg/kg/min for 24 h. The scheduled study drug administrations will take place on Day 0, Day 21 (±3 days), Day 42 (±3 days), Day 63 (±3 days), and Day 84 (±3 days) (Figure 1 ).
At Day 84, the study medication will be stopped for all patients; a follow‐up visit will be arranged for 14 days (±2 days) after cessation of treatment. A second follow‐up visit will be arranged at 180 days (±14 days) after initiation of study medication.
Study assessments
Components of the primary endpoint, which comprises death, urgent heart transplantation or VAD implantation, as well as non‐fatal acute HF events, will be reported from receipt of completed patient informed consent forms until Week 26. All reported events will be adjudicated by the Endpoint Adjudication Committee ( Appendix ).
A ‘non‐fatal acute HF event’ is defined as presentation of the patient for an urgent, unscheduled visit at a clinic, office, emergency department, or hospital with a primary diagnosis of HF, i.e. where the patient exhibits new or worsening symptoms of HF on presentation, has objective evidence of new or worsening HF, and receives i.v. vasoactive therapy, defined according to Hicks et al.21 as i.v. diuretics, i.v. vasodilators, i.v. inotropes, or any combination of those interventions.
N‐terminal pro‐brain natriuretic peptide will be assessed at baseline and at Weeks 6, 12, and 14. Biomaterials will be shipped to and stored and analysed at the Interdisciplinary Bank of Biomaterials and Data, University of Würzburg.
Symptoms and functional status will be assessed at baseline and at Week 14 using the following instruments: (i) the Kansas City Cardiomyopathy Questionnaire, a self‐administered, 23‐item questionnaire that quantifies physical limitation and quality of life in patients with HF.22 Changes in clinical summary score will be used for analyses; (ii) the 6 min walk test conducted according to the guidelines for the six‐minute walk test published by the American Thoracic Society23; and (iii) New York Heart Association HF classification recorded by the investigator.
The seven‐item patient's global assessment (PGA) is scheduled to take place at Week 14 after beginning administration of the study drug and will be conducted before any other assessment, interview, or study drug administration, so that the answers will not be influenced by the study procedures.24 For patients hospitalized for any reason by the planned date of the PGA, every effort will be made to conduct it. If the PGA cannot be performed in a hospital, the patient will be categorized as ‘hospitalized’ and ranked under the category ‘much worse’. Patients who die on or before the planned date of PGA will be categorized as ‘died’ and ranked under the category ‘hospitalized’.
Cost‐effectiveness will be assessed using the five‐level version of the EuroQol EQ‐5D questionnaire, which will be completed by the patient at baseline and Week 14.25
Endpoint selection
Primary endpoint
The primary efficacy objective of the study is to compare the effects of pulsed application of levosimendan versus placebo in patients with advanced chronic heart failure during a vulnerable period of 14 weeks following a recent hospitalisation on a global rank endpoint, in which all participants are ranked across three hierarchical groups (in ascending order):
time to death or urgent heart transplantation or implantation of a VAD;
time to a non‐fatal HF event requiring i.v. vasoactive therapy (i.v. diuretics, i.v. vasodilators, or i.v. inotropes); and
time‐averaged proportional change in NT‐proBNP from baseline to 14 weeks.
Secondary endpoints
The secondary efficacy objectives are to determine the effects of pulsed application of levosimendan on the individual components of the primary endpoint at 14 and 26 weeks (time‐averaged proportional change in NT‐proBNP will be determined from baseline to 14 weeks only), plus changes in symptoms and functional status at 14 weeks and the cumulative number of acute HF events and cumulative days alive and out of hospital at 14 and 26 weeks.
Additional study objectives are to determine the effects of pulsed application of levosimendan on changes in background medication and biomarkers, as well as cost‐effectiveness.
Statistical considerations and randomization
The statistical hypothesis is that, compared with placebo, levosimendan in patients with advanced HF will be associated with greater clinical stability as assessed using a global rank endpoint, which is a composite of (i) time to death or urgent heart transplantation or VAD implantation, (ii) time to a non‐fatal HF event requiring i.v. vasoactive therapy, and (iii) time‐averaged proportional change in NT‐proBNP from baseline to 14 weeks. Patients will be ranked so that those who meet component (i) will get the lowest rank starting with Rank 1 for the patient with the earliest endpoint. Thereafter, patients meeting component (ii) will be ranked similarly, where the patient with the earliest endpoint is ranked the number of deaths plus 1. Finally, patients who do not meet components (i) or (ii) will be ranked so that those who respond best (i.e. who have the largest decline in NT‐proBNP) will get the highest rank (equal to sample size).
Statistical assumptions of rates of death and acute HF events in the placebo group (10% and 30%, respectively) are based on data from Gheorghiade et al.,26 Cowie et al.,27 and OPTIME‐CHF.28 Effect‐size assumptions for repetitive levosimendan treatment (20% relative risk reduction for death and acute HF) are derived from experience in the LevoRep,17 LionHeart,29 and LAICA (NCT00988806; unpublished data, from personal communication with Martín Jesús García‐Gonzáles) trials.
Assuming a total of 264 patients (6 h infusion group: levosimendan, n = 88; placebo, n = 44; 24 h infusion group: levosimendan, n = 88; placebo, n = 44), LeoDOR has ≈90% simulated power to detect a difference between the pooled levosimendan and placebo groups at a two‐sided significance level of 5% using the Wilcoxon–Mann–Whitney test. Using the same simulation model, the individual components of the global rank endpoint (death, acute HF, and ΔNT‐proBNP) have ≈10%, ≈20%, and ≈99% power, respectively, to detect a difference between groups.
The intention‐to‐treat data set including all randomized patients will be used for the primary evaluation. Sensitivity analyses using the per‐protocol data set will be considered and will be described in the statistical analysis plan should a meaningful number of patients exhibit major protocol violations. All patients who receive any study treatment will be included in the safety evaluation.
Patients will be randomized to receive either levosimendan or matching placebo in a ratio of 2:1. Clinical effect is assumed to be similar between the two infusion schedules, and the statistical hypothesis will compare the pooled levosimendan contingent with the pooled placebo group. Of note, the infusion schedule (6 or 24 h) is not part of the randomization or stratification scheme; the infusion group will be selected based on centre preference or convenience for the patient. Randomization will be balanced at each centre using randomly permutated blocks specific for each centre and infusion group.
Study schedule
The first patients will be enrolled in LeoDOR in February 2018. The recruitment period will last for 16 months until June 2019. Study completion is anticipated in February 2020.
Discussion
The aim of the LeoDOR trial is to investigate efficacy and safety of intermittent levosimendan therapy during the vulnerable phase after a recent hospitalization for acute HF in advanced HFrEF patients.
Hospitalization for the management of acute decompensation is a sentinel event in the trajectory of HF and one that has important prognostic implications.27 Readmission and mortality rates are particularly high during the 3 month period after discharge. Rehospitalizations during this early discharge period, which is also known as the ‘vulnerable phase’, account for a disproportionate amount of the extensive overall costs of HF care. Although up to 75% of early readmissions may be preventable,30 effective measures to prevent rehospitalizations during this vulnerable period remain ill defined.
Recent meta‐analyses suggest that repetitive levosimendan may reduce hospitalization and mortality in patients with advanced HF.15, 16 Individual studies, including the LevoREP trial,17 were, however, underpowered to provide definite conclusions regarding these endpoints. Accordingly, the LeoDOR trial is powered to compare the effects of repetitive administration of levosimendan vs. placebo during the vulnerable post‐discharge period following hospital admission for acute HF using a hierarchical composite endpoint including death and rehospitalization. Efficacy will be tested 14 weeks after the first infusion; a safety endpoint including death and hospitalization is scheduled for 12 weeks after the first infusion.
Rationale for study design and endpoint selection
Outpatient therapy and management is highly desirable for patients with advanced HF and compromised quality of life, which is often related to repeat HF‐related hospitalizations. In this context, repetitive infusions of levosimendan in an ambulatory setting appear promising. Such a strategy is safe, as evidenced by the LevoRep17 and LionHEART.29 The LeoDOR trial will follow this concept. A second active‐treatment study arm with a 24 h continuous infusion of levosimendan is included to meet the infrastructural and economic requirements of countries and centres that currently do not support ambulatory therapy in advanced HF. To achieve comparable cumulative drug doses in both treatment arms, the dosage per hour (0.2 vs. 0.1 μg/kg/min) and the number of infusions (seven vs. five repetitions) will be higher in the 6 h arm compared with the 24 h arm. Patients will be allocated to levosimendan or placebo in a ratio of 2:1.
Endpoint selection for high‐risk HF populations is critical and is often the subject of debate. The main clinical targets are to relieve symptoms, to reduce the number and length of hospitalizations, and, ultimately, to reduce mortality. Symptomatic improvement has turned out to be a problematic endpoint in HF trials as confounding factors may influence its reliable measurement. A composite endpoint comprising mortality and rehospitalizations would potentially have wide acceptance.31 However, this approach might create substantial problems in interpreting trial results as both components of the composite would be treated equally, despite the fact that clinicians and patients may attach different values to the specific components. Furthermore, thresholds for hospitalization vary substantially between institutions and countries, and hospitalizations tend to occur more frequently than mortality events and therefore have a greater effect on the endpoint. This applies particularly when a ‘time‐to‐event’ method is used, where patients are followed up until the first event of the composite endpoint. Furthermore, the components of the composite may ‘move’ in different directions, which can further complicate coherent interpretation of the study results.32 Finally, restricting the combined endpoint to mortality and hospitalization requires a substantially large sample size and/or a considerably long follow‐up period.
An approach that assigns proper weight to different outcomes and also enables every patient to contribute to the endpoint would optimize the feasibility of a study, without compromising the clinical impact that may be derived from trial results.32
A study in advanced HF with a primary endpoint combining death, rehospitalization, and positive neurohormonal response has been published recently.33 Each patient was assigned a global rank score based on their outcomes during follow‐up. The components were analysed in a hierarchical manner so that deaths were the most important events, followed by rehospitalizations and, finally, effect on NT‐proBNP. No statistically resilient treatment effect was demonstrated in that study, but the credibility of the outcome‐ranking methodology was demonstrated.
The LeoDOR will apply a similar endpoint methodology for the assessment of repetitive cycles of levosimendan in the management of patients who have experienced a recent hospitalization for acutely decompensated HF. Adding changes in NT‐proBNP over time as an additional component also allows a more comprehensive interpretation of the effects of the study drug on the complex trajectory after discharge. As shown recently, follow‐up BNP level and per cent change in BNP within 3 months after discharge were predictors of long‐term mortality but were also superior to BNP levels on hospital admission.34
In summary, the LeoDOR trial will test efficacy and safety of intermittent levosimendan therapy in patients with ‘very’ advanced but not acute HF with the highest short‐term and long‐term mortality and rehospitalization rates.3, 19, 20
Perspective
The concept of pulsed administration of levosimendan for patients with advanced HF during the vulnerable phase after a recent hospitalization for acute HF, as will be tested in the LeoDOR trial, potentially represents an important contribution to the therapeutic armamentarium for such patients. In particular, the study is configured to examine evidence of efficacy of an intensified therapy using clinically relevant endpoints for severely ill patients managed on an outpatient basis.
Conflict of interest
G.P., J.C.‐C., J.F.D., F.G., J.M., Z.P., G.W., and J.A. have received speakers fees from Orion Pharma. G.P., M.J.G.‐G., and J.A. have received unrestricted grants from Orion Pharma.
Funding
The LeoDOR study is supported by an unrestricted grant from Orion Pharma, the manufacturer of levosimendan. Orion Pharma is not involved in data management, assessment of endpoints, and analysis or publication of the LeoDOR trial.
Appendix 1.
Sponsor: Medical University of Innsbruck.
Principal investigator: G. Pölzl, Department of Internal Medicine III, Medical University Innsbruck, Innsbruck, Austria.
Steering committee: J. Comin‐Colet, Spain; J.F. Delgado, Spain; F. Fedele, Italy; M.J. Gonzales, Spain; F. Gustafsson, Denmark; J. Masip, Spain; Z. Papp, Hungary; G. Pölzl, Austria; S. Störk, Germany; G. Wikström, Sweden; B. Vrtovec, Slovenia.
Executive committee: J. Altenberger, Austria; M. Kivikko, Finland; G. Pölzl, Austria; P. Pollesello, Finland; T. Sarapohja, Finland; S. Störk, Germany; H. Ulmer, Austria.
Data monitoring committee: C. Adelbrecht, Austria; J. Bauersachs, Germany; F. Hintringer, Austria; R. Matteucci Gothe, Austria; P. Mohacsi, Switzerland.
Endpoint adjudication committee: H.P. Brunner‐La Rocca, the Netherlands; S. Frantz, Germany; T. Suter, Switzerland.
Pölzl, G. , Allipour Birgani, S. , Comín‐Colet, J. , Delgado, J. F. , Fedele, F. , García‐Gonzáles, M. J. , Gustafsson, F. , Masip, J. , Papp, Z. , Störk, S. , Ulmer, H. , Vrtovec, B. , Wikström, G. , and Altenberger, J. (2019) Repetitive levosimendan infusions for patients with advanced chronic heart failure in the vulnerable post‐discharge period. ESC Heart Failure, 6: 174–181. 10.1002/ehf2.12366.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlstrom U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A. European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 5. Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, Erglis A, Fazlibegovic E, Fonseca C, Fruhwald F, Gatzov P, Goncalvesova E, Hassanein M, Hradec J, Kavoliuniene A, Lainscak M, Logeart D, Merkely B, Metra M, Persson H, Seferovic P, Temizhan A, Tousoulis D, Tavazzi L. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2013; 15: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007; 116: 1482–1487. [DOI] [PubMed] [Google Scholar]
- 7. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013; 309: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Belohlavek J, Bohm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzalez‐Medina A, Hagege AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan O, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz‐Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva‐Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131: 54–61. [DOI] [PubMed] [Google Scholar]
- 9. Komajda M, Tavazzi L, Swedberg K, Bohm M, Borer JS, Moyne A, Ford I. Chronic exposure to ivabradine reduces readmissions in the vulnerable phase after hospitalization for worsening systolic heart failure: a post‐hoc analysis of SHIFT. Eur J Heart Fail 2016; 18: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 10. Gillis AM, Kerr CR, Philippon F, Newton G, Talajic M, Froeschl M, Froeschl S, Swiggum E, Yetisir E, Wells GA, Tang AS. Impact of cardiac resynchronization therapy on hospitalizations in the Resynchronization‐Defibrillation for Ambulatory Heart Failure trial. Circulation 2014; 129: 2021–2030. [DOI] [PubMed] [Google Scholar]
- 11. Young JB, Moen EK. Outpatient parenteral inotropic therapy for advanced heart failure. J Heart Lung Transplant 2000; 19(8 Suppl): S49–S57. [DOI] [PubMed] [Google Scholar]
- 12. Thackray S, Easthaugh J, Freemantle N, Cleland JG. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure—a meta‐regression analysis. Eur J Heart Fail 2002; 4: 515–529. [DOI] [PubMed] [Google Scholar]
- 13. Lilleberg J, Laine M, Palkama T, Kivikko M, Pohjanjousi P, Kupari M. Duration of the haemodynamic action of a 24‐h infusion of levosimendan in patients with congestive heart failure. Eur J Heart Fail 2007; 9: 75–82. [DOI] [PubMed] [Google Scholar]
- 14. Papp Z, Edes I, Fruhwald S, De Hert SG, Salmenpera M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikstrom BG, Jorgensen K, Filippatos G, Parissis JT, Gonzalez MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012; 159: 82–87. [DOI] [PubMed] [Google Scholar]
- 15. Silvetti S, Nieminen MS. Repeated or intermittent levosimendan treatment in advanced heart failure: an updated meta‐analysis. Int J Cardiol 2016; 202: 138–143. [DOI] [PubMed] [Google Scholar]
- 16. Silvetti S, Belletti A, Fontana A, Pollesello P. Rehospitalization after intermittent levosimendan treatment in advanced heart failure patients: a meta‐analysis of randomized trials. ESC Heart Fail 2017; 4: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altenberger J, Parissis JT, Costard‐Jaeckle A, Winter A, Ebner C, Karavidas A, Sihorsch K, Avgeropoulou E, Weber T, Dimopoulos L, Ulmer H, Poelzl G. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail 2014; 16: 898–906. [DOI] [PubMed] [Google Scholar]
- 18. Polzl G, Altenberger J, Baholli L, Beltran P, Borbely A, Comin‐Colet J, Delgado JF, Fedele F, Fontana A, Fruhwald F, Giamouzis G, Giannakoulas G, Garcia‐Gonzalez MJ, Gustafsson F, Kaikkonen K, Kivikko M, Kubica J, von Lewinski D, Lofman I, Malfatto G, Manito N, Martinez‐Selles M, Masip J, Merkely B, Morandi F, Molgaard H, Oliva F, Pantev E, Papp Z, Perna GP, Pfister R, Piazza V, Bover R, Rangel‐Sousa D, Recio‐Mayoral A, Reinecke A, Rieth A, Sarapohja T, Schmidt G, Seidel M, Stork S, Vrtovec B, Wikstrom G, Yerly P, Pollesello P. Repetitive use of levosimendan in advanced heart failure: need for stronger evidence in a field in dire need of a useful therapy. Int J Cardiol 2017; 243: 389–395. [DOI] [PubMed] [Google Scholar]
- 19. Nieminen MS, Altenberger J, Ben‐Gal T, Bohmer A, Comin‐Colet J, Dickstein K, Edes I, Fedele F, Fonseca C, Garcia‐Gonzalez MJ, Giannakoulas G, Iakobishvili Z, Jaaskelainen P, Karavidas A, Kettner J, Kivikko M, Lund LH, Matskeplishvili ST, Metra M, Morandi F, Oliva F, Parkhomenko A, Parissis J, Pollesello P, Polzl G, Schwinger RH, Segovia J, Seidel M, Vrtovec B, Wikstrom G. Repetitive use of levosimendan for treatment of chronic advanced heart failure: clinical evidence, practical considerations, and perspectives: an expert panel consensus. Int J Cardiol 2014; 174: 360–367. [DOI] [PubMed] [Google Scholar]
- 20. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray JJ, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine—short version. Eur Heart J 2015; 36: 1958–1966. [DOI] [PubMed] [Google Scholar]
- 21. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015; 66: 403–469. [DOI] [PubMed] [Google Scholar]
- 22. Dunlay SM, Gheorghiade M, Reid KJ, Allen LA, Chan PS, Hauptman PJ, Zannad F, Maggioni AP, Swedberg K, Konstam MA, Spertus JA. Critical elements of clinical follow‐up after hospital discharge for heart failure: insights from the EVEREST trial. Eur J Heart Fail 2010; 12: 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 24. Comin‐Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health‐related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR‐HF study. Eur Heart J 2013; 34: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Devlin NJ, Brooks R. EQ‐5D and the EuroQol Group: past, present and future. Appl Health Econ Health Policy 2017; 15: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013; 61: 391–403. [DOI] [PubMed] [Google Scholar]
- 27. Cowie MR, Anker SD, Cleland JGF, Felker GM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, Lopez‐Sendon J. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Fail 2014; 1: 110–145. [DOI] [PubMed] [Google Scholar]
- 28. Klein L, O'Connor CM, Leimberger JD, Gattis‐Stough W, Pina IL, Felker GM, Adams KF Jr, Califf RM, Gheorghiade M. Lower serum sodium is associated with increased short‐term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) study. Circulation 2005; 111: 2454–2460. [DOI] [PubMed] [Google Scholar]
- 29. Comín‐Colet J, Manito N, Segovia‐Cubero J, Delgado J, García Pinilla JM, Almenar L, Crespo‐Leiro MG, Sionis A, Blasco T, Pascual‐Figal D, Gonzalez‐Vilchez F, Lambert‐Rodríguez JL, Grau M, Bruguera J, LION‐HEART Study Investigators . Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION‐HEART multicentre randomised trial. Eur J Heart Fail 2018; 2018: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 30. van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011; 183: E391–E402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, Cohn JN, Dickstein K, Domanski MJ, Ekman I, Filippatos GS, Gheorghiade M, Hernandez AF, Jaarsma T, Koglin J, Konstam M, Kupfer S, Maggioni AP, Mebazaa A, Metra M, Nowack C, Pieske B, Pina IL, Pocock SJ, Ponikowski P, Rosano G, Ruilope LM, Ruschitzka F, Severin T, Solomon S, Stein K, Stockbridge NL, Stough WG, Swedberg K, Tavazzi L, Voors AA, Wasserman SM, Woehrle H, Zalewski A, McMurray JJ. Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail 2013; 15: 1082–1094. [DOI] [PubMed] [Google Scholar]
- 32. Felker GM, Anstrom KJ, Rogers JG. A global ranking approach to end points in trials of mechanical circulatory support devices. J Card Fail 2008; 14: 368–372. [DOI] [PubMed] [Google Scholar]
- 33. Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE, Anstrom KJ, Shah MR, Braunwald E, Cappola TP. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016; 316: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khanam SS, Son JW, Lee JW, Youn YJ, Yoon J, Lee SH, Kim JY, Ahn SG, Ahn MS, Yoo BS. Prognostic value of short‐term follow‐up BNP in hospitalized patients with heart failure. BMC Cardiovasc Disord 2017; 17: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]


