Abstract
Aims
Impaired left ventricular diastolic function leading to elevated left atrial pressures, particularly during exertion, is a key driver of symptoms and outcomes in heart failure with preserved ejection fraction (HFpEF). Insertion of an interatrial shunt device (IASD) to reduce left atrial pressure in HFpEF has been shown to be associated with short‐term haemodynamic and symptomatic benefit. We aimed to investigate the potential effects of IASD placement on HFpEF survival and heart failure hospitalization (HFH).
Methods and results
Heart failure with preserved ejection fraction patients participating in the Reduce Elevated Left Atrial Pressure in Patients with Heart Failure study (Corvia Medical) of an IASD were followed for a median duration of 739 days. The theoretical impact of IASD implantation on HFpEF mortality was investigated by comparing the observed survival of the study cohort with the survival predicted from baseline data using the Meta‐analysis Global Group in Chronic Heart Failure heart failure risk survival score. Baseline and post‐IASD implant parameters associated with HFH were also investigated. Based upon the individual baseline demographic and cardiovascular profile of the study cohort, the Meta‐analysis Global Group in Chronic Heart Failure score‐predicted mortality was 10.2/100 pt years. The observed mortality rate of the IASD‐treated cohort was 3.4/100 pt years, representing a 33% lower rate (P = 0.02). By Kaplan–Meier analysis, the observed survival in IASD patients was greater than predicted (P = 0.014). Baseline parameters were not predictive of future HFH events; however, poorer exercise tolerance and a higher workload‐corrected exercise pulmonary capillary wedge pressure at the 6 months post‐IASD study were associated with HFH.
Conclusions
The current study suggests IASD implantation may be associated with a reduction in mortality in HFpEF. Large‐scale ongoing randomized studies are required to confirm the potential benefit of this therapy.
Keywords: Heart failure, HFpEF, Survival, Interatrial shunt, Medical device
Introduction
Heart failure with preserved ejection fraction (HFpEF) represents one of the most important contemporary challenges in cardiovascular medicine. Prevalent HFpEF already accounts for roughly half of the current heart failure (HF) burden, and the incidence of HFpEF is increasing in the context of an ageing population together with concomitant hypertension, diabetes, and obesity.1, 2, 3, 4 Patients with established HFpEF are just as symptomatic as those with heart failure with reduced ejection fraction (HFrEF).5, 6 All‐cause mortality in HFpEF is substantially greater than that in age‐matched healthy individuals,7, 8 approaching that observed in HFrEF patients.4 The relative contribution of death due to non‐cardiovascular events in HFpEF is somewhat higher than in HFrEF.9, 10
The pathophysiological basis of HFpEF is complex, including both central and peripheral cardiovascular components together with non‐cardiovascular elements. In particular, impaired left ventricular diastolic function leading to elevated left atrial (LA) pressure during physical activity is considered a key driver of symptoms in HFpEF.11, 12, 13, 14 Intrinsic LA mechanical dysfunction may also contribute significantly to symptoms.15, 16 To date, pharmacological interventions with proven effectiveness in HFrEF have failed to achieve their primary endpoints in HFpEF.17, 18, 19, 20 Additionally, other recent interventions directed at the nitric oxide–cyclic guanosine monophosphate pathway have also proven ineffective.21, 22, 23, 24
As further evidence of the central role of impaired diastolic performance in HFpEF, it has been shown that poorer exercise tolerance25 and survival 26 are associated with a more rapid rise in pulmonary capillary wedge pressure (PCWP) during exercise. Within this context, based on haemodynamic simulations,27 an interatrial shunt device (IASD®, Corvia Medical Inc., Tewksbury, MA, USA) was developed to reduce LA pressure by allowing a modest amount of left‐to‐right shunting in HFpEF patients.28, 29 Open‐label studies have suggested a symptomatic and haemodynamic benefit at 6 and 12 months,30, 31 and a recent randomized trial demonstrated haemodynamic benefit compared with a sham control.32 To date, however, the effect of this approach on heart failure hospitalization (HFH) or survival remains unknown. Accordingly, the objective of this study was to investigate the potential impact of IASD implantation on HFH and survival in the intermediate term.
Methods
The Reduce Elevated Left Atrial Pressure in Patients with Heart Failure (REDUCE LAP‐HF) study was a multi‐centre prospective, non‐randomized, open‐label, single‐arm study designed to investigate the safety and performance of a novel transcatheter IASD in 64 patients with symptoms of HF, haemodynamic evidence of raised LA pressure (measured as the PCWP) at rest or during supine bicycle exercise, and a left ventricular ejection fraction (LVEF) > 40%. To account, in part, for the potential effect differences in peak workload and weight on PCWP, the exercise PCWP is also presented as the ratio of PCWP to workload (W)/kg, that is, PCWP/(W/kg) as previously reported.11, 26 The study design, patient demographics, and primary results have been described in detail elsewhere.30, 33 In brief, the average age of the cohort was 70 years, and the majority (65%) were women. Co‐morbidities including hypertension, obesity, diabetes, chronic obstructive pulmonary disease, and atrial fibrillation were common. Full exercise invasive haemodynamic studies were conducted in 60 patients at 6 months post‐IASD implant.
The principle objective of the current study was to investigate hard clinical outcomes in the REDUCE LAP‐HF cohort over the intermediate term. Exercise capacity, quality of life (Minnesota Living with Heart Failure Score), and echocardiography were optional assessments at 2 years. Haemodynamic analyses were not conducted at this time point. All serious adverse events were reported per protocol and adjudicated by an independent clinical event committee. A second aim was to investigate the relationship between the post‐IASD haemodynamic profile (i.e. at 6 months) and HFH events.
Statistical methods
Normally distributed data are presented as mean ± standard error of the mean and non‐normal data as median and 25th–75th percentile range. Between‐group comparisons were performed using an unpaired Student's t‐test. Non‐parametric between‐group comparisons were performed using a Mann–Whitney U test. Repeated measures ANOVA analysis of New York Heart Association (NYHA) status was performed using Friedman's test, with post hoc testing performed using a Wilcoxon test.
To determine the theoretical impact of IASD implantation on survival, we calculated 1 and 3 year predicted cohort outcomes using the Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) prognostic model,34, 35 a risk prediction tool derived from a large (>40 000 patient group composed of mixed clinical trial and observational cohorts) and validated in a large real‐world cohort of HF patients.36 The predicted survival using the MAGGIC score was based on all relevant patient and disease factors in the IASD cohort at baseline. An exponential transformation was then applied to estimate predicted survival for each 3 month time point between baseline and 39 months. A comparison of predicted survival with that observed following IASD treatment was performed for the entire observation period balanced for key baseline confounders such as LVEF and all other baseline MAGGIC score variables. Observed and MAGGIC‐predicted Kaplan–Meier survival curves were compared over the total follow‐up period and at 12, 24, and 36 months using log‐rank test and Z‐tests. Similarly, validated scores for HFH in HFpEF patients are not presently available. Hazard proportionality was assessed via analysis of scaled Schoenfeld residuals. The null hypothesis was rejected at P < 0.05. Statistical analyses were conducted using SPSS version 22 (IBM, Armonk, NY, USA) or Stata version 15 (StataCorp, College Station, TX, USA). Statistical modelling of survival outcomes was conducted independently by Synergus (Synergus AB, Stockholm, Sweden).
Results
Clinical event rates
Over the 3 year follow‐up period, six study patients (9.4%) died during the follow‐up period, representing a mortality rate of 3.4 deaths per 100 person‐years (95% confidence interval 1.52–7.54) over a total observation period of 177.2 patient years. Of the six observed deaths, three were adjudicated as being HF related, two were assessed not being directly attributable to HF, and one occurred following a cerebrovascular accident. Over the follow‐up period, there were 42 HFH episodes occurring in 19 patients.
The observed all‐cause mortality rate in IASD‐treated patients was significantly lower than that predicted by the MAGGIC score. The mortality rate predicted by the MAGGIC score was 10.2/100 pt years (6.1–16.9). Taken together, this observation represents a 33% lower mortality rate (hazard ratio 0.67; 95% confidence interval 0.09–0.89) relative to the MAGGIC‐predicted mortality across the full observation period (P = 0.02). The corresponding Kaplan–Meier curves are demonstrated in Figure 1 , representing a greater survival in IASD patients compared with MAGGIC‐predicted outcomes, P (log‐rank) = 0.014. χ 2 and P values for the log‐rank tests of observed vs. predicted mortality at 12, 24, and 36 months were, respectively, 2.96 (P = 0.085), 2.15 (P = 0.143), and 21.43 (P < 0.01).
Figure 1.
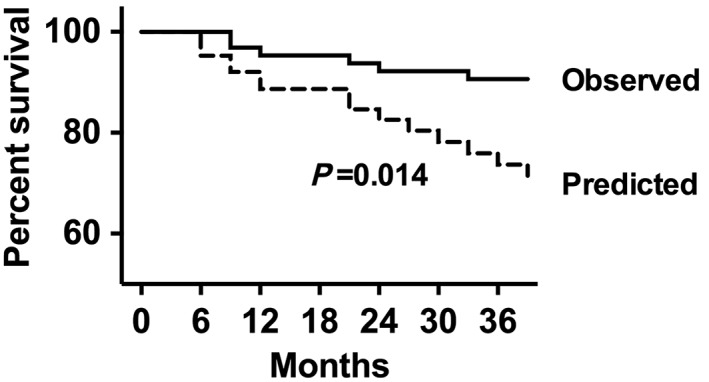
Kaplan–Meier survival curves representing the observed and predicted outcome for the heart failure with preserved ejection fraction cohort.
Heart failure hospitalization and New York Heart Association class
Over the follow‐up period, 19 patients experienced an HFH event, and the first hospitalization event occurred at a median of 182 days. Of those with HFH events, nine patients experienced two or more HFH events. Survival was similar in patients with an HFH event compared with those without (three deaths per group, P = 0.31) albeit with small numbers.
As shown in Table 1, there were no significant differences in key baseline (pre‐IASD) demographic, echocardiographic, and haemodynamic features of patients according to subsequent HFH. Similarly, as shown in Figure 2 , there were no significant differences in the baseline exercise intra‐cardiac pressures of patients categorized according to subsequent HFH. Prior to IASD implantation, the peak exercise cardiac index was similar in non‐HFH vs. HFH patients: 4.3 ± 0.2 vs. 4.3 ± 0.3 L/min/m2. Six months after IASD implantation, HFH patients had a lower 6 min walk distance and lower peak exercise work capacity as shown in Table 2. The resting echocardiographic and haemodynamic features were similar in patients who experienced an HFH event (Table 2). Comparison of intra‐cardiac pressures during exercise 6 months after IASD implantation showed (Figure 3 ) that patients with an HFH event had higher workload‐corrected exercise PCWP (94 ± 13 vs. 60 ± 5 mmHg/(W/kg), P = 0.021). The peak exercise cardiac index was similar in non‐HFH and HFH patients (5.2 ± 0.2 vs. 4.8 ± 0.3 L/min/m2).
Table 1.
Baseline features according to HFH status
| No HFH (n = 45) | HFH (n = 19) | P value | |
|---|---|---|---|
| Age (years) | 69 ± 1 | 71 ± 2 | 0.37 |
| Body mass index (kg/m2) | 32 ± 1 | 34 ± 2 | 0.27 |
| NT‐proBNP (pg/mL) | 332 (218–862) | 595 (222–1790) | 0.24 |
| Atrial fibrillation (%) | 36 | 37 | 0.92 |
| Hypertension (%) | 80 | 84 | 0.69 |
| IHD (%) | 31 | 32 | 0.97 |
| eGFR (mL/min/1.73 m2) | 65 ± 3 | 54 ± 4 | 0.08 |
| 6MWD (m) | 335 ± 16 | 316 ± 18 | 0.49 |
| Peak exercise workload (W) | 44 ± 3 | 40 ± 4 | 0.48 |
| Echocardiography | |||
| LVEF (%) | 47 ± 1 | 47 ± 1 | 0.66 |
| LAVI (mL/m2) | 34 ± 3 | 34 ± 3 | 0.88 |
| RAVI (mL/m2) | 35 ± 3 | 35 ± 3 | 0.95 |
| TAPSE (cm) | 2.0 ± 0.1 | 1.9 ± 0.1 | 0.52 |
| Resting haemodynamics | |||
| RA pressure (mmHg) | 9 ± 1 | 10 ± 1 | 0.15 |
| PAm pressure (mmHg) | 23 ± 1 | 25 ± 1 | 0.39 |
| PCWP (mmHg) | 17 ± 1 | 18 ± 1 | 0.40 |
| Cardiac index (L/min/m2) | 2.7 ± 0.1 | 2.7 ± 0.2 | 0.78 |
6MWD, 6 min walk distance; eGFR, estimated glomerular filtration rate; HFH, heart failure hospitalization; IHD, ischaemic heart disease; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PAm, mean pulmonary artery; PCWP, pulmonary capillary wedge pressure; RA, right atrial; RAVI, right atrial volume index; TAPSE, tricuspid annular plane systolic excursion.
Values are mean ± SEM or median (25th–75th percentile interquartile range).
Figure 2.
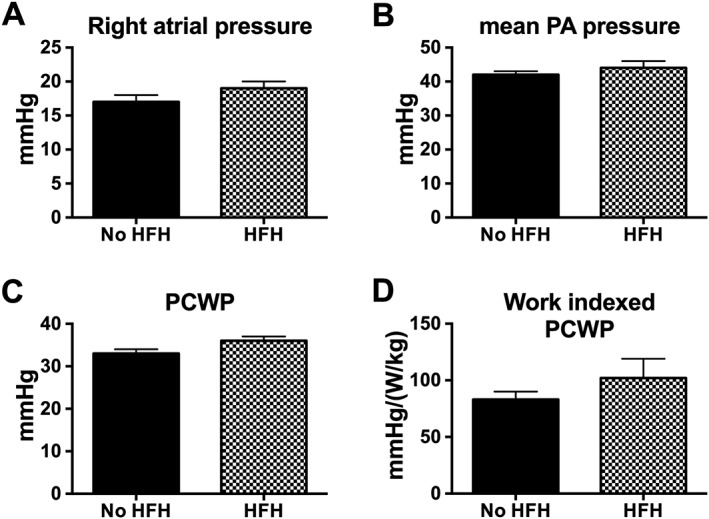
Bar graphs represent baseline (pre‐interatrial shunt device) peak exercise haemodynamic parameters in relation to subsequent heart failure hospitalization (HFH) events. PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure.
Table 2.
Six month post‐IASD features according to HFH status
| No HFH (n = 45) | HFH (n = 19) | P value | |
|---|---|---|---|
| Body mass index (kg/m2) | 32 ± 1 | 34 ± 2 | 0.28 |
| NT‐proBNP (pg/mL) | 332 (180–821) | 609 (304–2022) | 0.09 |
| eGFR (mL/min/1.73 m2) | 63 ± 3 | 53 ± 4 | 0.08 |
| 6MWD (m) | 375 ± 14 | 312 ± 23 | 0.03 |
| Peak exercise workload (W) | 52 ± 3 | 41 ± 5 | 0.048 |
| Echocardiography | |||
| LVEF (%) | 50 ± 1 | 47 ± 2 | 0.19 |
| LAVI (mL/m2) | 35 ± 4 | 35 ± 4 | 0.99 |
| RAVI (mL/m2) | 40 ± 4 | 40 ± 3 | 0.99 |
| TAPSE (cm) | 2.0 ± 0.1 | 1.9 ± 0.1 | 0.21 |
| Resting haemodynamics | |||
| RA pressure (mmHg) | 10 ± 1 | 12 ± 1 | 0.12 |
| PAm pressure (mmHg) | 24 ± 1 | 25 ± 1 | 0.49 |
| PCWP (mmHg) | 16 ± 1 | 18 ± 1 | 0.24 |
| Cardiac index (L/min/m2) | 3.4 ± 0.1 | 3.2 ± 0.2 | 0.37 |
6MWD, 6 min walk distance; eGFR, estimated glomerular filtration rate; HFH, heart failure hospitalization; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PAm, mean pulmonary artery; PCWP, pulmonary capillary wedge pressure; post‐IASD, post‐interatrial shunt device; RA, right atrial; RAVI, right atrial volume index; TAPSE, tricuspid annular plane systolic excursion.
Figure 3.
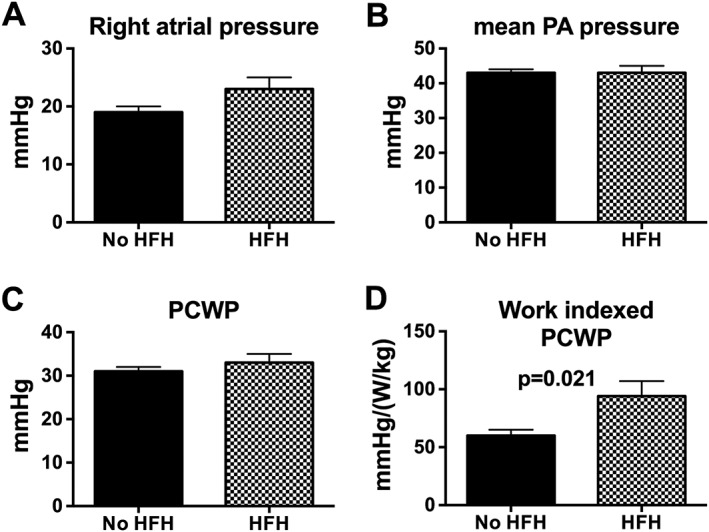
Bar graphs represent peak exercise haemodynamic parameters 6 months after interatrial shunt device (n = 60) implantation in relation to subsequent heart failure hospitalization (HFH) events. PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure.
At 2 years post‐IASD implantation, there was a sustained and significant improvement in NYHA class compared with baseline; however, there was a modest but significant diminution in NYHA class compared with the 12 month time point shown in Figure 4 .
Figure 4.
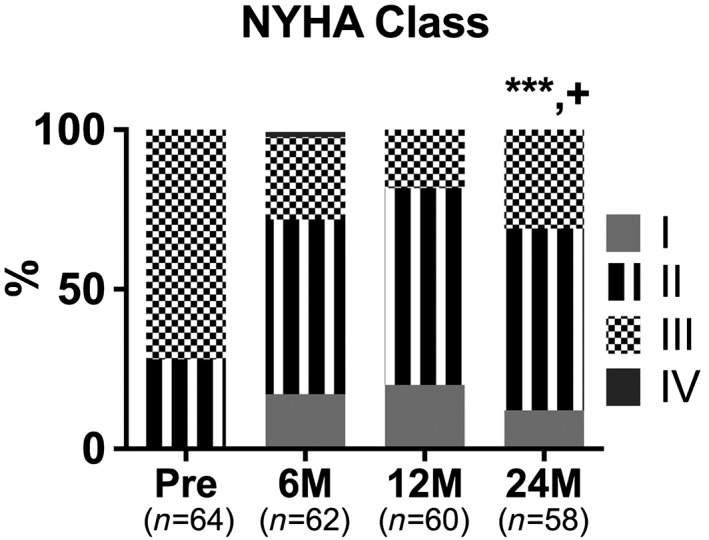
Bar graph represents the New York Heart Association (NYHA) class distribution of heart failure with preserved ejection fraction patients prior to and after interatrial shunt device implantation. *** P < 0.001 vs. baseline and + P < 0.05 vs. 12 months.
Discussion
Based upon evidence that impaired left ventricular diastolic function and abnormal LA mechanical function contribute substantially to the pathophysiology of HFpEF, our group and others have investigated devices aimed at reducing LA pressure by left‐to‐right atrial shunting through an iatrogenic atrial septal passage.30, 37 To date, sustained improvements in quality of life, functional capacity, and exercise haemodynamics have been observed in open‐label IASD studies extending out to 1 year post‐implant.31 The recent pilot double‐blind, randomized trial demonstrated a significant reduction in exercise PCWP in IASD‐treated HFpEF patients32 compared with sham control. The potential impact of IASD placement on HFpEF mortality and HFH remains unknown. Accordingly, the objective of this study was to examine the mortality and HFH rates in IASD‐treated HFpEF patients and to determine potential mechanisms by which IASD placement may alter clinical outcomes.
In patients treated with an IASD in the open‐label REDUCE LAP‐HF cohort, we observed a 33% lower mortality rate than that predicted by the MAGGIC risk prediction score over the entire observation period. The MAGGIC score algorithm was derived from a meta‐analysis of 30 clinical trials and observational studies of over 40 000 patients with HF across a range of LVEF, including those with HFpEF and HFrEF, from which a risk prediction tool was developed using 13 common parameters.35 A similar approach has previously been applied to assess the impact of other interventions in HFrEF.38 The MAGGIC score estimated a substantially higher event rate, consistent with that recently observed in the regional sub‐analysis of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist study.39 The ability of the MAGGIC score to reliably predict outcomes at 3 years was recently validated in a large Swedish registry,36 although the study suggested that the MAGGIC score may overestimate risk in lower risk patients. However, the validation study had several limitations, including multiple imputation procedures for missing data and the combination of patients with LVEF > 50% and those with an LVEF of 40–49%.36
Mortality events occurring in the present study were predominantly cardiovascular, albeit in a small population. The cause of death in patients with HFpEF has been somewhat unclear until recently. Meta‐analyses of randomized clinical trials together with observational studies and registries9, 10 have shown that although the majority of deaths are of a cardiovascular nature, the overall rate, mechanism, and their proportion vary substantially. Specifically, whilst progressive HF remains the commonest cause of cardiovascular death, the contribution of other cardiovascular events including sudden death, myocardial infarction, and stroke is more variable.
The present study raises the possibility that a device‐based approach to reducing LA pressure may favourably affect mortality in HFpEF patients. This observation is consistent with two bodies of prior data. First, in a retrospective analysis, Dorfs et al.26 showed that HFpEF patients with a more marked exercise‐mediated rise in PCWP had poorer survival than those with lesser elevations. In conjunction, Adamson et al.40 showed that HF therapy guided by an implanted haemodynamic monitor in HFpEF patients was associated with a lower HFH rate. Moreover, Zile et al.41 showed that a 3–5 mmHg reduction in estimated pulmonary artery diastolic pressure was associated with lower mortality during a trial of an implanted haemodynamic monitor, albeit in HFrEF patients. The mechanism by which a reduction in LA filling pressure might reduce mortality in HFpEF is unclear. Elevated PCWP has been previously shown to drive cardiac sympathetic outflow in HFrEF,42 which is implicated in the progression of HF and in the pathogenesis of arrhythmia.43
Elevated natriuretic peptide levels have also been associated with a poorer outcome in HFpEF,44 which extends the concept that elevated filling pressure per se may directly contribute to outcome in HFpEF. Interestingly, whilst angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists have not been shown to improve survival overall in HFpEF, post hoc analyses indicate that they may be effective in patients with relatively lower natriuretic peptide levels.45 Whilst this may appear counter‐intuitive, the effect of these pharmacological therapies on filling pressures in HFpEF has not been studied in detail. A prior study by Kitzman et al.46 showed that angiotensin‐converting enzyme inhibition was without effect on echocardiographic indices of diastolic function or left ventricular filling pressures.
Heart failure hospitalization events are an important contributor to the overall clinical burden associated with HFpEF. Recent data indicate that cardiovascular precipitants are the lead trigger for HFH in HFpEF patients.47 Specific causes include arrhythmia, myocardial ischaemia, and dietary or medication non‐adherence. Common non‐cardiovascular precipitants include infection and worsening renal function. From the present study, patients with subsequent HFH events tended to have worse renal function and higher N‐terminal pro‐brain natriuretic peptide levels at baseline, although these were not statistically significant. Heart failure hospitalization patients had similar haemodynamics prior to IASD implantation both at rest and during exercise. During follow‐up after IASD implantation, patients with a greater 6 month exercise capacity and a lower workload‐corrected peak PCWP had a lower likelihood of HFH. This observation is consistent with the importance of pulmonary congestion as a trigger for HFH.48 Importantly, we did not observe a relationship between haemodynamic or echocardiographic features of right heart volume overload, in the setting of the IASD, with HFH. In the present study, patients experiencing HFH events were not associated with subsequent increased mortality; however, the small sample size prevents a definitive conclusion about the potential relationship between these events.
Limitations
The current data should be interpreted in the context of several limitations. First, the study was an open‐label study. Second, although the predicted survival is consistent with other reports in HFpEF patients, the use of the MAGGIC score to derive a comparator survival curve may have generated an overestimation of the true survival in a contemporaneous group.39 Finally, whilst protocol‐driven safety outcome follow‐up was available at up to 3 years, complete NYHA class at 3 years was not available, and systematic echocardiography was not required after 12 months.
Conclusions
Taken together, the current study is consistent with recent data suggesting that both symptoms and outcomes are related to the magnitude of elevation of LA pressure in patients with HFpEF. Our study also suggests that mitigation of LA pressure elevation by deployment of an IASD may be associated with a reduction in mortality in HFpEF patients. Double‐blind randomized, sham procedure‐controlled studies are currently underway to further investigate the utility of this therapeutic approach in HFpEF.
Conflict of interest
None declared.
Funding
This study was funded by the Corvia Medical Incorporated.
Acknowledgements
The authors wish to thank the all REDUCE LAP‐HF coinvestigators and study coordinators for their technical expertise and diligence.
Kaye, D. M. , Petrie, M. C. , McKenzie, S. , Hasenfuβ, G. , Malek, F. , Post, M. , Doughty, R. N. , Trochu, J.‐N. , Gustafsson, F. , Lang, I. , Kolodziej, A. , Westenfeld, R. , Penicka, M. , Rosenberg, M. , Hausleiter, J. , Raake, P. , Jondeau, G. , Bergmann, M. W. , Spelman, T. , Aytug, H. , Ponikowski, P. , Hayward, C. , and on behalf of the REDUCE LAP‐HF study investigators (2019) Impact of an interatrial shunt device on survival and heart failure hospitalization in patients with preserved ejection fraction. ESC Heart Failure, 6: 62–69. 10.1002/ehf2.12350.
References
- 1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 2. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017; 14: 591–602. [DOI] [PubMed] [Google Scholar]
- 5. Kitzman DW. Exercise intolerance. Prog Cardiovasc Dis 2005; 47: 367–379. [DOI] [PubMed] [Google Scholar]
- 6. Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJ, Yusuf S, Swedberg K, Pfeffer MA, Investigators C . Characterization of health‐related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007; 9: 83–91. [DOI] [PubMed] [Google Scholar]
- 7. Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population‐based study. Eur Heart J 2008; 29: 339–347. [DOI] [PubMed] [Google Scholar]
- 8. Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation 2009; 119: 3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan MMY, Lam CSP. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail 2013; 15: 604–613. [DOI] [PubMed] [Google Scholar]
- 10. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 2017; 69: 556–569. [DOI] [PubMed] [Google Scholar]
- 11. Maeder MT, Thompson BR, Brunner‐La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 2010; 56: 855–863. [DOI] [PubMed] [Google Scholar]
- 12. Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3: 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 2011; 97: 964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc 2014; 3: e001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart 2012; 98: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Roeder M, Rommel KP, Kowallick JT, Blazek S, Besler C, Fengler K, Lotz J, Hasenfuss G, Lucke C, Gutberlet M, Schuler G, Schuster A, Lurz P. Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2017; 10. [DOI] [PubMed] [Google Scholar]
- 17. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 18. Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J 2006; 27: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 20. van Veldhuisen DJ, Cohen‐Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole‐Wilson PA, Flather MD, Investigators S. Beta‐blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure). J Am Coll Cardiol 2009; 53: 2150–2158. [DOI] [PubMed] [Google Scholar]
- 21. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E, Network NHFCR . Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373: 2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2015; 66: 1672–1682. [DOI] [PubMed] [Google Scholar]
- 24. Zamani P, Rawat D, Shiva‐Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 2015; 131: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, Lam CSP, Ponikowski P, Shah SJ, Gustafsson F. Resting and exercise haemodynamics in relation to six‐minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2018; 20: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long‐term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3103–3112. [DOI] [PubMed] [Google Scholar]
- 27. Kaye D, Shah SJ, Borlaug BA, Gustafsson F, Komtebedde J, Kubo S, Magnin C, Maurer MS, Feldman T, Burkhoff D. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail 2014; 20: 212–221. [DOI] [PubMed] [Google Scholar]
- 28. Malek F, Neuzil P, Gustafsson F, Kaye DM, Walton A, Mates M, Sondergaard L, Ihlemann N, Mariani JA, Reddy V. Clinical outcome of transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel implant. Int J Cardiol 2015; 187: 227–228. [DOI] [PubMed] [Google Scholar]
- 29. Sondergaard L, Reddy V, Kaye D, Malek F, Walton A, Mates M, Franzen O, Neuzil P, Ihlemann N, Gustafsson F. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail 2014; 16: 796–801. [DOI] [PubMed] [Google Scholar]
- 30. Hasenfuss G, Hayward C, Burkhoff D, Silvestry FE, McKenzie S, Gustafsson F, Malek F, Van der Heyden J, Lang I, Petrie MC, Cleland JG, Leon M, Kaye DM, Investigators RL‐Hs . A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP‐HF): a multicentre, open‐label, single‐arm, phase 1 trial. Lancet 2016; 387: 1298–1304. [DOI] [PubMed] [Google Scholar]
- 31. Kaye DM, Hasenfuss G, Neuzil P, Post MC, Doughty R, Trochu JN, Kolodziej A, Westenfeld R, Penicka M, Rosenberg M, Walton A, Muller D, Walters D, Hausleiter J, Raake P, Petrie MC, Bergmann M, Jondeau G, Feldman T, Veldhuisen DJ, Ponikowski P, Silvestry FE, Burkhoff D, Hayward C. One‐year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail 2016; 9: e003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feldman T, Mauri L, Kahwash R, Litwin S, Ricciardi MJ, van der Harst P, Penicka M, Fail PS, Kaye DM, Petrie MC, Basuray A, Hummel SL, Forde‐McLean R, Nielsen CD, Lilly S, Massaro JM, Burkhoff D, Shah SJ, Investigators RL‐HI, Study C . Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP‐HF I [Reduce Elevated Left Atrial Pressure in Patients with Heart Failure]): a phase 2, randomized, sham‐controlled trial. Circulation 2018; 137: 364–375. [DOI] [PubMed] [Google Scholar]
- 33. Hasenfuss G, Gustafsson F, Kaye D, Shah SJ, Burkhoff D, Reymond MC, Komtebedde J, Hunlich M, Reduce LAPHFTI . Rationale and design of the Reduce Elevated Left Atrial Pressure in Patients with Heart Failure (Reduce LAP‐HF) trial. J Card Fail 2015; 21: 594–600. [DOI] [PubMed] [Google Scholar]
- 34. Meta‐analysis Global Group in Chronic Heart Failure . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 35. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta‐Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 36. Sartipy U, Dahlstrom U, Edner M, Lund LH. Predicting survival in heart failure: validation of the MAGGIC heart failure risk score in 51,043 patients from the Swedish heart failure registry. Eur J Heart Fail 2014; 16: 173–179. [DOI] [PubMed] [Google Scholar]
- 37. Del Trigo M, Bergeron S, Bernier M, Amat‐Santos IJ, Puri R, Campelo‐Parada F, Altisent OA, Regueiro A, Eigler N, Rozenfeld E, Pibarot P, Abraham WT, Rodes‐Cabau J. Unidirectional left‐to‐right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof‐of‐principle cohort study. Lancet 2016; 387: 1290–1297. [DOI] [PubMed] [Google Scholar]
- 38. Schau T, Isotani A, Neuss M, Schopp M, Seifert M, Hopfner C, Burkhoff D, Butter C. Long‐term survival after MitraClip® therapy in patients with severe mitral regurgitation and severe congestive heart failure: a comparison among survivals predicted by heart failure models. J Cardiol 2016; 67: 287–294. [DOI] [PubMed] [Google Scholar]
- 39. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O'Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34–42. [DOI] [PubMed] [Google Scholar]
- 40. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014; 7: 935–944. [DOI] [PubMed] [Google Scholar]
- 41. Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, Bourge RC, Warner Stevenson L. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail 2017; 10: e003594. [DOI] [PubMed] [Google Scholar]
- 42. Kaye DM, Jennings GL, Dart AM, Esler MD. Differential effect of acute baroreceptor unloading on cardiac and systemic sympathetic tone in congestive heart failure. J Am Coll Cardiol 1998; 31: 583–587. [DOI] [PubMed] [Google Scholar]
- 43. Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 1995; 26: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 44. Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, Carson PE. Prognostic value of baseline plasma amino‐terminal pro‐brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I‐PRESERVE trial. Circ Heart Fail 2011; 4: 569–577. [DOI] [PubMed] [Google Scholar]
- 45. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O'Meara E, Fleg JL, Pfeffer MA, Pitt B, Solomon SD. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Failure 2017; 5: 241–252. [DOI] [PubMed] [Google Scholar]
- 46. Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, Little WC. A randomized double‐blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail 2010; 3: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Platz E, Jhund PS, Claggett BL, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Solomon SD, McMurray JJ. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: recurrent hospitalizations and mortality. Eur J Heart Fail 2018; 20: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tromp J, Khan MAF, Mentz RJ, O'Connor CM, Metra M, Dittrich HC, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JGF, Givertz MM, Bloomfield DM, Van Veldhuisen DJ, Hillege HL, Voors AA, van der Meer P. Biomarker profiles of acute heart failure patients with a mid‐range ejection fraction. JACC Heart Failure 2017; 5: 507–517. [DOI] [PubMed] [Google Scholar]


