Abstract
Lack of verification is often cited as a root cause of medication errors; however, medication errors occur in spite of conventional verification practices and it appears that human factors engineering (HFE) can inform the design of a more effective method. To this end, an HFE-driven process was designed and implemented in an urban, Midwestern emergency medical service agency. Medication error data were collected over a 54-month period, 27 months before and after implementation. A decrease in the average monthly error rate was realized for all medications administered (49.0%) during the post-intervention time period. The average monthly error rate for fentanyl, a commonly administered analgesic, demonstrated a 71.1% error rate decrease. This study is the first to evaluate the effectiveness of a team-based cross-check process for medication verification to prevent errors in the prehospital setting.
Keywords: emergency medical services, verification, medication errors, cross-check
Introduction
Across healthcare, organizations are increasingly focused on improving patient safety. Yet in the prehospital setting, there is a paucity of information about the safety of care, in spite of the well-known and documented idiosyncrasies.1–6 One component of prehospital care that is particularly understudied, yet highly influential to patient safety is medication administration.2,3,5,6,7,8 This paper will discuss the issue of medication errors in emergency medical services (EMSs), describe a novel method developed to reduce their frequency, and analyze its effectiveness.
Like many other healthcare providers, paramedics are taught to verify medications prior to administration using the ‘five rights’.9,10 The five rights of medication administration require a provider to mentally confirm that their actions are performed on the right patient, using the right drug, with the right dose, via the right route, and at the right time. Emphasized in textbooks, the five rights are commonly taught and practiced as a one-person set of mental considerations.10,11–15 Yet, like many practices in EMSs, the efficacy of the five rights in preventing errors has never been evaluated or quantified in any setting.16 Several authors have questioned the ecological validity of the five rights as a medication verification process citing that they more accurately represent goals of safe medication administration and do not reflect the mental work, complexity, and context of performing the actual task.17–22 Interestingly, recommendations are commonly made for additional ‘rights’ to supplement the original five in an apparent attempt to improve error sensitivity and refine the process’ ergonomic application,20,22,23–25 but empirical support is lacking. In spite of these issues, a commonly cited ‘root cause’ of medication error is lack of verification, implicitly (and occasionally explicitly) assuming that proper execution of the verification process would have prevented them; relegating causal explanation of error to human failure.26 Although recommendations for validated features of verification processes have been in existence for some time,27,28 contemporary approaches to error reduction continue to focus on individual aptitude.29 These improvement efforts (which often take the form of remedial education or admonishing individuals to ‘be more careful’) often fail in part because they do not address sources of error embedded in processes amenable to sustained improvement3,30–32 and do not account for the sociotechnical nature of situated work, both cognitive and physical.33
Using a systems approach informed by human factors science and extant archival data, providers and managers at Sedgwick County Emergency Medical Service (SCEMS) developed and implemented a team-based communication protocol known as the medication administration cross-check (MACC) which serves as a standardized method of medication verification to reduce errors. It was hypothesized that by inserting another provider into the process of medication verification and designing error specific traps, the practice would capitalize on the core components (e.g. mutual performance monitoring and backup behavior) and coordinating mechanisms (i.e. closed-loop communication) of teamwork to create a process that is robust to the errors of a single provider.34
Methods
Sedgwick County EMS is a medium-sized municipal ambulance agency serving a population of nearly 500,000 within a 1008 square mile jurisdiction in the state of Kansas, USA. Because this investigation analyzed archival data collected by SCEMS to determine the effectiveness of a quality improvement intervention, a certificate of exemption was granted by the institutional review board.
Self-reporting of medication errors is a compulsory practice at SCEMS and was performed by electronic means using a centralized system, generally done when crews are at the station after an error has been identified. Reports are not anonymized and include a narrative description of the error in question as well as a patient care report number for reference. Beyond simply tabulating frequencies, SCEMS routinely categorizes medication errors according to the following four taxonomies: (1) errors by medication, (2) errors by type, (3) Reason’s generic error-modelling system (GEMS), and (4) the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) index.35 There are limitations with each and there is no universally accepted standard for the prehospital setting (for a review, see Hughes and colleagues),36 which is why SCEMS leverages all four to inform strategic improvement initiatives. Since each taxonomy offers unique information about a single error, all were considered important to the design and the evaluation of the MACC’s effectiveness and errors that might persist after implementation could inform future process refinements. As such, our analysis compared pre/post-intervention error rates within each taxonomy as they are operationalized by SCEMS:
‘Errors by medication’ refers to the tabulation of errors according to the corresponding medication that was administered. In the event of a wrong drug error, SCEMS tabulates the error according to that which the provider intended to give, not the medication that was inadvertently administered.
‘Error type’ consists of distinctions shown in Table 1. While some of these distinctions are common descriptions of active errors, they are by no means universal nor are they necessarily mutually exclusive. However, for this study no errors were tabulated as more than one type.
The GEMS taxonomy is a performance-based categorization of behavior as either skill-based, rule-based, or knowledge-based. This was first described by Rasmussen and later refined by Reason.31,33,37,38 Skill-based errors are designated as errors of action, such as a slip or lapse, in which the action executed (or failed to be executed in the case of a lapse) was not that which was intended. Rule and knowledge-based errors are considered errors of intent, essentially the execution of a bad plan.29,39 However, the distinction was made between knowledge-based and rule-based on the grounds that a provider who executed an appropriate treatment in the wrong way committed a rule-based error. An example of a rule-based error would be the administration of an appropriate medication without proper authorization either by medical protocol or online medical direction (i.e. direct verbal contact with a physician). A medication error was tabulated as a knowledge-based error when a medication administration was inappropriate, not indicated, or contraindicated. A dose error could be either skill, rule, or knowledge-based, and was determined by the information provided within the error report submitted by the provider.
The NCC MERP index was applied to evaluate errors according to the algorithmically prescribed groups, which is essentially a severity-of-outcome index.35 NCC MERP taxonomy definitions are presented in Table 2. It should be noted however, that this index was not intended for the prehospital setting; nonetheless, there are no alternatives and the managers of SCEMS find the information useful for tabulating the outcomes of errors committed.
Table 1.
Medication error types and examples.
| Error type | Example |
|---|---|
| Incorrect dose (regardless of appropriateness) | Intended 50 mg, administered 75 mg |
| Inappropriate situation for administration/ not indicated | Administration of an antiarrhythmic to a patient in cardiac arrest with pulseless electrical activity |
| No order/ not authorized (regardless of appropriateness) | Appropriate administration of drug, without protocol or online authorization |
| Wrong drug | Intended one drug, administered another |
| Dilution/ preparation error (correct dosage) | Administered a drug at full concentration when it requires dilution for clinical reasons |
| Omission of an appropriate drug | Protocol prescribes a medication that was not given |
| Inappropriate route (correct dose) | Medication given by a route that is not authorized, for example, intramuscular versus intravenous |
| Contraindicated | Medication is explicitly prohibited given clinical presentation, for example, known allergy |
| Expired medication | Medication is beyond the labelled expiration date |
| Incorrect time | Medications that require specific timing which lend to errors, for example, repeat doses given too soon after initial dose |
Table 2.
National coordinating council for medication error reporting and prevention index category definitions.35
| Category | Definition |
|---|---|
| Errors that do not ‘reach’ the patient/ incomplete errors | |
| No error | |
| Category A | Circumstances or events that have the capacity to cause error (i.e. a safety concern). |
| Error, no harm | |
| Category B | An error occurred but the error did not reach the patient, an ‘error of omission’ does reach the patient (i.e. a near-miss). |
| Errors that ‘reach’ the patient/ Completed errors | |
| Error, no harm | |
| Category C | An error occurred that reached the patient, but did not cause patient harm. |
| Category D | An error occurred that reached the patient and required monitoring to confirm that it resulted in no harm to the patient and/or required intervention to preclude harm. |
| Error, harm | |
| Category E | An error occurred that may have contributed to or resulted in temporary harm to the patient and required intervention. |
| Category F | An error occurred that may have contributed to or resulted in temporary harm to the patient and required initial or prolonged hospitalization. |
| Category G | An error occurred that may have contributed to or resulted in permanent patient harm. |
| Category H | An error that occurred that required intervention to sustain life. |
| Error, death | |
| Category I | An error that occurred that may have contributed to or resulted in the patient’s death. |
Intervention
During the first months of 2012, SCEMS undertook the development of an intervention to reduce the frequency of medication errors committed by its paramedics. The MACC procedure was designed to meet the goals of safe medication administration and was refined through an iterative process of field evaluation (pilot testing) and feedback to assure practicality and usability. Because the nature of the intervention is novel to the prehospital setting and the information used for its development may be informative to other agencies seeking to address the issue of medication errors, a brief description of its development is provided in Appendix A. Figure 1 depicts the final implemented version of the MACC evaluated during the study period.
Figure 1.
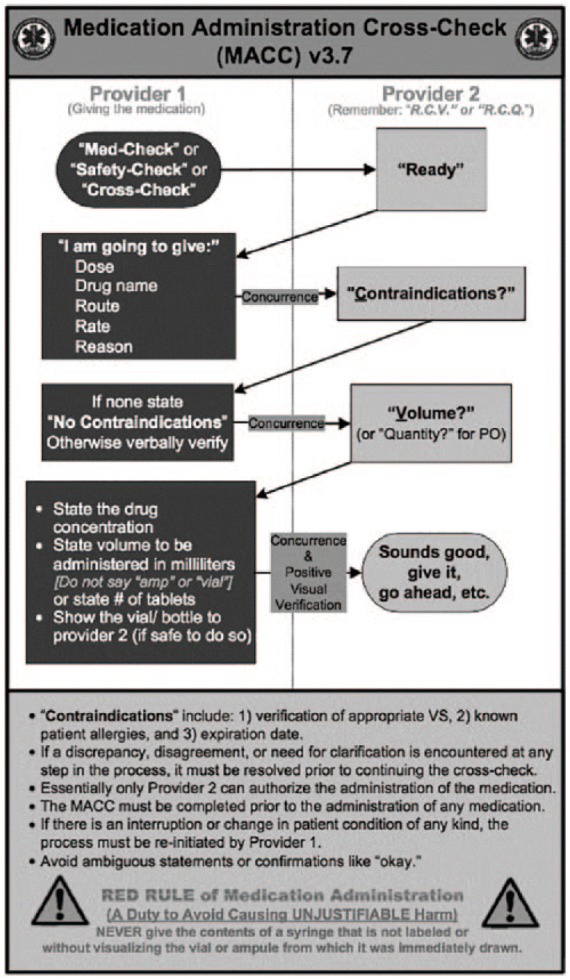
The medication administration cross-check© procedure. From The Medication Administration Cross-Check by P. Misasi, 2013, Wichita-Sedgwick County EMS System.40 Copyright 2013 by Paul Misasi. Reprinted with permission.
Over the course of a month (March 2012), all full-time field providers [n = 140; paramedics = 97%, emergency medical technicians (EMTs) = 3%] received an online introduction of the intent and rationale of the standardized procedure as well as didactic education explaining its execution, followed by lab practice, drawing medications, verifying, and plunging medications into a receptacle. Timed trials of the procedure in a lab setting averaged 24 s for completion of the verification process. The MACC was then implemented and codified into the medical protocols as the official procedure for verification, to be executed by all providers. Half-page sized MACC procedure cards were laminated and placed in the ambulance and medication box, and small, plastic identification badge size copies were given to all providers in the agency.
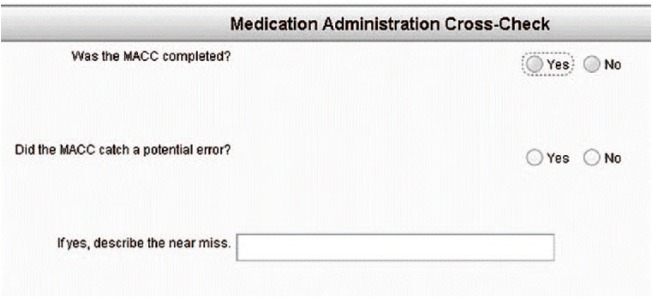
Modifications to the patient care reporting system were also made such that any entry of a medication in the flowchart would prompt the two questions depicted in Figure 2.
Figure 2.
Pop-up dialog box following the entry of a medication in the patient care report. Information of a near-miss is automatically forwarded to management.
After notification of a near-miss (an email generated automatically by the patient care reporting system), an investigation was conducted. Near-miss information was collected and evaluated by the same taxonomies used for medication errors to provide insight into the types and nature of errors prevented by the process, and to inform process modification or training at the system level. However, since near-miss data was only collected after implementation, it is not submitted here for analysis.
Statistical analysis
The absolute and relative frequency of medication errors (i.e. errors per opportunity) were tabulated for the entire 54 months study period so as to provide an overall picture of the medication error data, and then were analyzed separately for the 27 months pre/post-MACC periods (Tables 3 and 4). A timeframe of 27 months post-implementation was chosen as the endpoint of the evaluation period a priori since this would allow for equal periods of sound, consistent, and reliable medication error data before and after implementation. Two-tailed independent samples Student’s t tests were used to evaluate statistical significance. The change in error rates within each taxonomy were evaluated in the same manner. Additionally, the error rate for fentanyl was of particular interest because errors involving this medication were identified a priori as occurring with higher frequency; and because at the time the intervention was developed, fentanyl was the most commonly administered parenteral medication, it presented the greatest opportunity to demonstrate improvement.
Table 3.
Overall and pre/post-medication administration cross-check error analysis.
| Overall | Pre-MACC | Post-MACC | % change | p | |
|---|---|---|---|---|---|
| Month count | 54 | 27 | 27 | ||
| Responses | 250,416 | 120,503 | 129,913 | 7.8% | |
| Transports | 169,334 | 81,599 | 87,735 | 7.5% | |
| Medication doses | 73,522 | 34,531 | 38,991 | 12.9% | |
| Average monthly doses | 1361.5 | 1278.93 | 1444.11 | 12.9% | |
| Enteral medication doses | 42,609 | 20,449 | 22,160 | 8.4% | |
| Average monthly doses | 789.1 | 757.4 | 820.7 | 8.4% | |
| Parenteral medication doses | 30,913 | 14,082 | 16,831 | 19.5% | |
| Average monthly doses | 572.5 | 521.6 | 623.4 | 19.5% | |
| Fentanyl doses | 7421 | 2922 | 4499 | 54.0% | |
| Avg. monthly fentanyl doses | 137.4 | 108.2 | 166.6 | 54.0% | |
| Medication error count† | 91 | 58 | 33 | −43.1% | |
| Average number of errors (per month) | 1.7 | 2.2 | 1.2 | −45.5% | |
| Average monthly error rate (all medications) | 0.128% | 0.169% | 0.086% | −49.0% | .013 |
| Average monthly error rate (fentanyl only) | 0.407% | 0.632% | 0.182% | −71.1% | .004 |
| Average monthly error rate (all meds excluding fentanyl) | 0.100% | 0.127% | 0.073% | −42.1% | .065 |
| Medication error type rates | |||||
| Error count† | 83 | 50 | 33 | −34.0% | |
| Incorrect dose administered | 54.2% | 44.0% | 69.7% | 26.0% | |
| Average monthly errors | .83 | .81 | .85 | −4.5% | .552 |
| Inappropriate situation for administration/not indicated | 18.1% | 28.0% | 3.0% | −25.0% | |
| Average monthly errors | .28 | .52 | .04 | −92.9% | .026 |
| No order (unauthorized) | 8.4% | 10.0% | 6.1% | −3.9% | |
| Average monthly errors | .13 | .19 | .07 | −60.0% | .119 |
| Wrong drug | 7.2% | 8.0% | 6.1% | −1.9% | |
| Average monthly errors | .11 | .15 | .07 | −50.0% | .200 |
| Dilution/ preparation error (correct dose) | 6.0% | 6.0% | 6.1% | 0.1% | |
| Omission of appropriate drug | 3.61% | 4.0% | 3.0% | −1.0% | |
| Inappropriate route | 1.2% | 0.0% | 3.0% | 3.0% | |
| Contraindicated | 1.2% | 0.0% | 3.0% | 3.0% | |
| Expired medication | 0.0% | 0.0% | 0.0% | 0.0% | |
| Incorrect time of administration | 0.0% | 0.0% | 0.0% | 0.0% | |
| Error count† | 83 | 50 | 33 | −34.0% | |
| Skill-based error | 31.3% | 24.0% | 42.4% | 18.4% | |
| Average monthly errors | .48 | .44 | .52 | 16.7% | .635 |
| Rule-based error | 44.6% | 42.0% | 48.5% | 6.5% | |
| Average monthly errors | .69 | .78 | .59 | 23.8% | .193 |
| Knowledge-based error | 24.1% | 34.0% | 9.1% | −24.9% | |
| Average monthly errors | .37 | .63 | .11 | −82.4% | .030 |
| NCC MERP index rates †† | |||||
| Error count† | 83 | 50 | 33 | ||
| Category C | 88.0% | 92.0% | 81.8% | −10.2% | |
| Average monthly errors | 1.38 | 1.70 | 1.04 | −39.0 | .055 |
| Category D | 10.8% | 8.0% | 15.2% | 7.2% | |
| Average monthly errors | .17 | .15 | .19 | 25.0% | .639 |
| Category E | 1.2% | 0.0% | 3.0% | 3.0% | |
| Category F | 0.0% | 0.0% | 0.0% | 0.0% | |
| Category G | 0.0% | 0.0% | 0.0% | 0.0% | |
| Category H | 0.0% | 0.0% | 0.0% | 0.0% | |
| Category I | 0.0% | 0.0% | 0.0% | 0.0% |
Error rate = frequency/ # months; average monthly error rate = (# errors/ # opportunities to error)/ # months.
Not all medication errors were able to be appropriately typed due to varying amounts of error information collected in the first 27 months.
Information for Category A (safety concerns) and Category B (near-misses) were excluded due to lack of sufficient data.
MACC, medication administration cross-check; NCC MERP, National Coordinating Council for Medication Error Reporting and Prevention.
Table 4.
Medication errors by medication (entire study period, 54 months). Medications without errors are not listed.
| Medication | Error count | Doses | Overall relative error frequency |
|---|---|---|---|
| Adenosine | 1 | 396 | 0.25% |
| Albuterol | 1 | 7840 | 0.01% |
| Amiodarone | 1 | 253 | 0.40% |
| Aspirin | 1 | 8619 | 0.01% |
| Atropine | 12 | 2110 | 0.57% |
| Calcium chloride | 2 | 78 | 2.56% |
| Dextrose | 1 | 3714 | 0.03% |
| Epinephrine 1:1,000 | 2 | 179 | 1.12% |
| Epinephrine 1:10,000 | 2 | 7031 | 0.03% |
| Fentanyl | 27 | 7421 | 0.36% |
| Ketorolac | 2 | 63 | 3.17% |
| Labetalol | 0 | 34 | 0.00% |
| Lidocaine | 11 | 396 | 2.78% |
| Lidocaine drip 1G / 250D5W | 1 | 131 | 0.76% |
| Lorazepam | 11 | 796 | 1.38% |
| Magnesium sulfate | 3 | 109 | 2.75% |
| Midazolam | 9 | 685 | 1.31% |
| Narcan | 2 | 810 | 0.25% |
| NTG | 1 | 18,559 | 0.01% |
| Prednisone | 1 | 287 | 0.35% |
NTG = nitroglycerin.
Results
Sedgwick County EMS responded to 250,416 requests for service during the 54 months study period, an average of 4637 per month [standard deviation (SD) = 295]; transporting 169,334 patients for an average of 3136 per month (SD = 188). It should be noted however, that the general trend of call volume for the study period was slightly increasing; the average monthly call volume during the post-MACC period was 7.2% higher than the pre-MACC period. Further, the linear trend of the number of medication doses administered also increased throughout the study period. Providers administered a total of 73,522 medication doses, 42% of which were parenteral medications. Medications (both enteral and parenteral) were given on an average of 29.4% of total calls (SD = 2.8%) or 43.4% of transported patients (SD = 3.6%). Parenteral medications were given on an average of 12.4% of total calls (SD = 1.6%) or 18.2% of transported patients (SD = 2.2%).
Pre/Post-MACC results
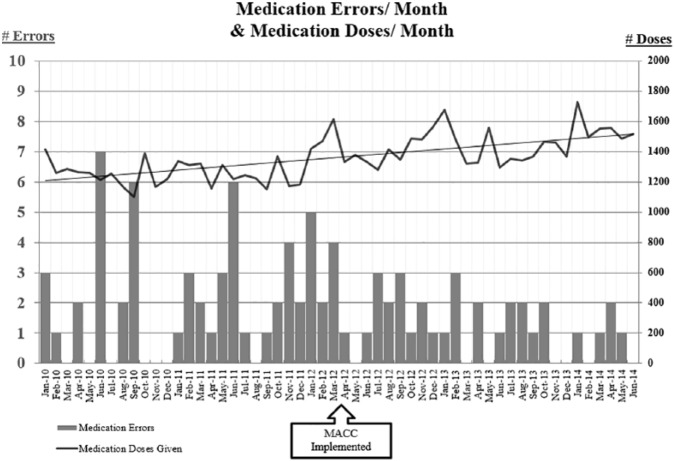
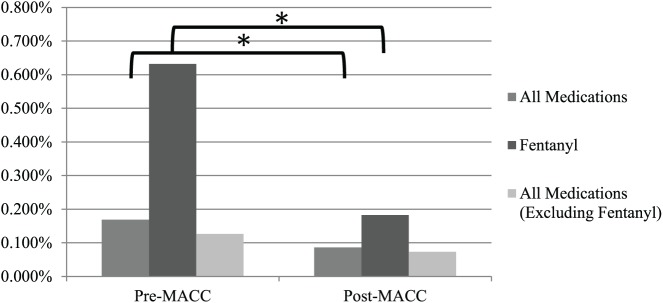
Error frequencies and medication dose counts (i.e. opportunities for error) for the course of the study period are juxtaposed in Figure 3 and average monthly error rates (errors per opportunity per month) before and after MACC implementation and are depicted in Figure 4.
Figure 3.
Frequency of medication errors (measured on the primary axis) per month during the study period compared against the opportunities to error (doses administered); measured on the secondary axis.
Figure 4.
Reduction in average monthly error rates.
*Indicates p < .025.
A total of 58 errors were recorded pre-MACC for an average monthly error rate of 0.17% (SD = 0.17%) and 33 errors were recorded post-MACC for an average monthly error rate of 0.09% (SD = 0.07%), a decrease of 49.0%, p = .01. The overall reduction of error frequency was realized in spite of a 12.9% increase in the number of medication doses during the post-MACC period.
Errors by medication
Results showed a 71.1% decrease in the average monthly fentanyl error rate (pre-MACC: mean = 0.63%, SD = 0.75%; post-MACC: mean = 0.18%, SD = 0.35%; p = .004) contrasted against a 35.1% increase in the number of fentanyl doses administered in the post-MACC period. If the number of fentanyl doses and errors are removed from the monthly totals and the pre/post-MACC monthly error rate change is analyzed in the same manner, the reduction is still substantial (42.1%) but not significant (p = .065).
Errors by type
Analysis was conducted to assess any change between the top four pre-MACC error types: (1) incorrect dose, (2) inappropriate situation for administration/ not indicated, (3) no order/ unauthorized, and (4) wrong drug. Only errors designated as ‘inappropriate situation for administration/ not indicated’, approached significance at the p < .025 level with a decrease of 92.9% (pre-MACC: M = .52, SD = 1.22; post-MACC: M = .04, SD = .19; p = .027).
Errors by GEMS
In the same manner, analyses were conducted on changes in the average monthly errors based on the GEMS taxonomy. Of the three categories, only knowledge-based errors approached a statistically significant decrease of 82.4% at the p < .025 level (pre-MACC: M = .63, SD = 1.33; post-MACC: M = .11, SD = .32), p = .03.
Errors by NCC MERP
Lastly, the average monthly error rate change for errors categorized using the NCC MERP index was assessed, however none of the changes reached the level of statistical significance.
Discussion
In spite of the difficult and debatable nature of quantifying errors, let alone assessing the effect of an intervention on those errors, the MACC shows potential as an important component in the effort to reduce medication errors committed in the prehospital setting. Notwithstanding limitations described below, this study is the first of its kind. Additionally, the implications of these findings may well extend to other areas of healthcare whose providers utilize unproven methods for verifying medication accuracy or work in less controlled and less technologically sophisticated settings. A 49% monthly error rate decrease over a substantial time period was demonstrated, with particular success (71% monthly error rate reduction) in the administration of a commonly used analgesic in the prehospital setting. Thus, the MACC appears to be successful in achieving the purpose of medication error reduction.
Conventional methods of verification as commonly taught and practiced rely on the mind that produced an error to also prevent it, which is a brittle strategy to assure patient safety.10 Whereas the MACC was designed with the presumption of human fallibility, not the expectation of perfection. There is simply too much variability with mental verification processes to solely rely on the mental processes of a single provider for safe medication administration. The nature of achieving expertise in any field results in the reduced amount of consciously directed cognitive effort devoted to a task41,42; in other words, less ‘thinking’ is involved. Thus, admonishing providers to think really hard, be really careful, or double or triple-check, especially when they have performed the task hundreds, if not thousands of times, is diametrical to the nature of the cognitive task.29 Morrow, North and Wickens43 described that preventing the majority of medication errors does not require a re-doubled effort of concentration, but a carefully designed and guided interruption of automatized behavior.44 Although there are multiple scientifically sound recommendations in the literature that argue for reducing the load on working memory,28,43,45 the prehospital setting has yet to strategically and systematically adopt many of these practices, despite the vulnerabilities imposed by stressful situations, time compression, and the unique context in which care is delivered.36,46 Thus, the MACC likely achieves error sensitivity by requiring communication between providers. Not only does the cross-check serve to interrupt the automaticity of the process of giving a medication, it also slows the process enough for attention to focus on behavior because cross-checking is cognitively effortful, requiring a reconceptualization of the task.47
Another reason the MACC may have demonstrated a significant reduction in medication errors could also be due to the insertion of another provider as an additional barrier of defense, a ‘defenses-in-depth’ strategy,31 which adds redundancy and resilience into the process. In other words, part of the system can fail, but does not result in total system failure. The reduction of knowledge-based errors (82.4%) lends itself as a demonstration of this effect; by bringing the knowledge of the additional provider to bear, errors were likely corrected and averted, a phenomenon described in the teamwork literature.34 Given the design of the MACC, in order for a medication error to reach the patient it must penetrate the knowledge and abilities of two providers.
Although not all errors were prevented after implementation of the cross-check, analysis of these errors has proven quite informative. Investigations identified that in these cases, the MACC was not used as designed or at all, which was expected. Paramedicine has traditionally been practiced individualistically rather than team-based, so adapting to a team-based medication verification process may require a shift in providers’ mindset. More importantly, when errors occurred despite the MACC, they were identified as knowledge-based or rule-based errors committed by two providers simultaneously, which suggests system-level education or training may be necessary (as opposed to individual remediation) or the redesign of decision support systems such as protocols or the introduction of cognitive aids such as dosing references may be warranted.
The MACC also leverages the short and long-term benefits of collaborative cross-checking, teamwork, and communication, which have been realized in other domains such as aviation. Teamwork has been shown to improve performance by as much as 20%48 and Patterson and colleagues47 and Freund and colleagues49 detail many of the benefits of cross-checking. This could be of great benefit at a low cost; the MACC can be learned and utilized relatively quickly, and for the cost of printing off a card for each provider within the organization. Given the data presented, we believe that this could be a very beneficial intervention in regard to patient safety. The MACC was developed with good team practice and communication skills in mind and should enhance teamwork between providers while handling the complexity of administering medications.
Limitations
There are some limitations to bear in mind in review of these results. First, the study lacks a nonequivalent control group to compare respective amounts of change in prevailing and resultant error rates over time. Therefore, to improve the generalizability of the results and lend convergent validity, a multi-site or multi-group quasi-experimental design comparing the MACC with other methods of medication verification are recommended.50,51 Other variables such as the managerial efforts to develop a culture of safety as well as incorporate so called ‘just culture’ philosophies (whereby human error is explicitly managed in a nonpunitive fashion) may also have played a role across the latter half of the study period; although it could be argued that this would have increased the number of errors reported, in spite of which our results demonstrate a reduction. Inherent difficulties in assessing the true number of medication errors are well known,2,3,5,6,25 especially since most errors are self-reported and direct field observation is not practical.6 The most common errors (by medication) identified in this study were controlled substances, which require strict control and reconciliation processes not common to all medications administered; so it should not necessarily be inferred that administration of narcotics are particularly troublesome or error prone. Also, just like any cognitive aid, compliance and complacency can become a problem as individuals become used to using the tool over time. Lastly, the formulary of medications was not consistent across the entire study, some were added, and some were removed at various times. For example, midazolam was inserted into the formulary during the 32nd month; the last dose of lorazepam was administered during the 33rd month. It should be noted however that fentanyl was a part of the formulary for the entire 54-month duration of the study.
Other potential threats to internal validity such as history, maturation, statistical regression to the mean, and the Hawthorne effect should be considered. History threats could have been other incidents or interventions taking place throughout the MACC training period that led to the change in the outcome, although this seems unlikely because no other interventions at the time were focused on medication errors. Maturation effects could be due to overall safety culture growth in the organization. Regression to the mean could have led to the decrease in medication errors across time, but the significant drop in errors after the intervention provides evidence against this. Finally, the nature of the study was salient to the participants, which could affect their performance through the Hawthorne effect, although the long time span of this study makes Hawthorne effects unlikely.
In addition to addressing the issues enumerated above with additional research, some interesting and organic developments to the process should be evaluated. Since the end of the study period, SCEMS field providers developed a pocket-sized ‘contraindications card’ as a cognitive aide and to improve EMT’s engagement in the verification process. Another avenue of research that should be explored is the addition of dosing references (e.g. paper or electronic).
Also, an assessment of ‘near-misses’ was not conducted until after MACC implementation and are therefore not reported here. Thus, an additional site investigation into the effectiveness of the MACC would do well to include this variable into the analysis before implementation.
Lastly, to date there is no universally accepted definition of a medication error in the prehospital setting nor a taxonomy for their classification, and those that do exist are an extension from the hospital setting and have limitations in terms of applicability. For example, this study considered a dose error to be any dose other than that which was prescribed (i.e. exact measurement), while other studies offer as much as a 20% margin.52 Furthermore, the taxonomies utilized in this study describe active errors and provide insight into the technical nature of the error, but do not consider the antecedents or latent factors that may contribute to error production.31,36 Hughes and colleagues have proposed a taxonomy adapting the human factors analysis and classification system (HFACS) following a systematic review of medication error in EMS literature.36 The usefulness of this taxonomy has yet to be demonstrated with field data but is worth exploring since this would likely assist in the targeting of intervention efforts that do not simply focus on individual competence.
Conclusion
The MACC was designed to capitalize on the benefits of teamwork and collaborative cross-checking. Notwithstanding the limitations of this study, it is the first step toward an empirically validated method of medication verification to reduce errors.
Acknowledgments
Paul Misasi declares that he is the copyright claimant of the Medication Administration Cross-Check. Joseph R. Keebler reports no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The authors wish to thank Brady Patzer for his review of this manuscript.
Appendix A
Development of the MACC
Overall, four sources of information were used to support development and design elements of the MACC: (1) archival agency data on medication error frequency and analysis, (2) observational data recorded from a credentialing evaluation process (i.e. simulated patient scenarios) of paramedics, (3) data from an internal agency survey that assessed prevailing methods of verification, and (4) tenets and practices established in human factors science, such as teamwork (particularly closed-loop communication), collaborative cross-checking, and the use of cognitive artifacts; additional information regarding the last three is provided below.
A.1. Observations from the credentialing process
In 2011, the Wichita-Sedgwick County EMS System’s Office of the Medical Director instituted a provider credentialing process to assure continued competency. In addition to a written exam, case review, and interview with the medical director, providers were required to complete one randomly selected, medical call simulation in the lab and one cardiac arrest simulation (both video recorded for post hoc analysis). Of those evaluated, 31% committed a medication error that they were not aware of during the cardiac arrest simulation; most which were identified as dosing/skill-based errors whereby the provider intended to administer the correct dose (i.e. verbalized the correct dose) but delivered an incorrect dose. The information gleaned from this process suggested that a verification process that relies on one person may be a brittle strategy.
A.2. Agency medication administration methods survey
Information about the prevailing methods of medication verification and self-reported errors were collected by way of internal agency survey. A total of 107 of the 140 full-time field paramedics completed the survey in early 2012 for a response rate of 71.3%. One-hundred percent of respondents indicated that they perform a verification process prior to medication administration; 77% of whom stated they use the five rights method and the remaining indicated they verify by other means. Of these providers, 60% stated that they had committed a medication error in their career. This information reinforced the conclusions made by Grissinger9 and others that healthcare providers believe that they do verify appropriately using the five rights and yet still commit medication errors. Combined with the findings from error data and credentialing observation, the question of how to prevent someone from making a medication error, changed to one of whether it was possible for someone to prevent themselves from doing something they believed to be correct, as the common practice of the five rights would suggest.
A.3. MACC design considerations derived from human factors
The MACC was designed to ‘catch’ errors in production, including errors of dose, route, rate, contraindications, preparation (i.e. dilution/ concentration) and wrong drug administration. It was developed assuming providers are vulnerable to errors of action, i.e. a slip or lapse, and assumed local rationality.53 Given the prevalence of errors identified as dosing slips unbeknownst to those who committed them, and despite their belief of positive, correct verification, the MACC designers concluded that any effort to prevent errors would require another provider. Freund and colleagues27 identified that one of only two key factors associated with reduced adverse events in the emergency department was the involvement of an additional physician.27 In the prehospital setting, this is feasible because care is typically delivered in teams of dyads.6
Steps to assure closed-loop communication and visual verification were also designed into the process. Further, the MACC requires the provider administering the medication to verbalize why a drug is being given, thereby explicitly communicating intent and rationale, which serves to make the provider’s mental model explicit (i.e. the patient’s condition and need for a particular therapy). This was thought to improve the quality of the team’s shared mental model that has been shown to result in higher quality decision making in teams.34 The cross-check sequence requires concurrence from a verifier to proceed. In the event that disagreement occurs, or a reason that should preclude administration becomes known, the process must either be discontinued or corrections made; thereafter the process is repeated from the beginning.
Ultimately, the MACC creates a situation where the verifier must authorize the administration of a medication; and although designers anticipated the cross-check would not prevent all medication errors, the arrangement of a second provider’s concurrence creates two conditions worthy of consideration: (1) a situation whereby errors are informative beyond the deficiencies of a single provider (suggesting that a higher system-level issue may need addressed), and (2) team-level accountability for the safe and correct administration of medications to patients.
Lastly, designers understood that the process would be utilized by and with team members of lower-level certification than paramedics, for example, basic-level EMTs. The designers theorized that although this situation may jeopardize the sensitivity of the process, many of the engineered benefits of the process would be retained: (1) an EMT can still visually inspect that the drug prepared is the drug intended, (2) an EMT can confirm that the volume or quantity prepared corresponds with what was articulated, (3) the brief time it takes to execute the process serves as a ‘pause point,’ and (4) by requiring a provider to vocalize their intent, they are more likely to prevent their own errors, even when alone (e.g. during transport), similar to that of a proofreading process.
Footnotes
Funding: This research was funded in part by the Wichita State University President Bardo undergraduate research grant.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Paul Misasi  https://orcid.org/0000-0002-5437-5698
https://orcid.org/0000-0002-5437-5698
Contributor Information
Paul Misasi, Wichita State University, 1845 N. Fairmount, Wichita, KS, 67260, USA.
Joseph R. Keebler, Associate Professor, Embry-Riddle Aeronautical University, Daytona Beach, FL, USA
References
- 1. Croskerry P, Sinclair D. Emergency medicine: a practice prone to error? Can J Emerg Med 2001; 3: 271–276. [DOI] [PubMed] [Google Scholar]
- 2. Hobgood C, Weiner B, Tamayo-Sarver JH. Medical error identification, disclosure, and reporting: do emergency medicine provider groups differ? Acad Emerg Med 2006; 13: 443–451. [DOI] [PubMed] [Google Scholar]
- 3. Meisel ZF, Hargarten S, Vernick J. Addressing prehospital patient safety using the science of injury prevention and control. Prehosp Emerg Care 2008; 12: 411–416. DOI: 10.1080/10903120802290851. [DOI] [PubMed] [Google Scholar]
- 4. Misasi P. Dusting for fingerprints: how to reinforce the foundation of an EMS safety culture. EMS World 2013; 46: 63–66. [PubMed] [Google Scholar]
- 5. O’Connor EO, Slovis CM, Hunt RC, et al. Eliminating errors in emergency medical services: realities and recommendations. Prehosp Emerg Care 2006; 6: 107–113. [DOI] [PubMed] [Google Scholar]
- 6. Patterson PD, Lave JR, Weaver MD, et al. A comparative assessment of adverse event classification in the out-of-hospital setting. Prehosp Emerg Care 2014; 18: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cushman JT, Fairbanks RJ, O’Gara KG, et al. Ambulance personnel perceptions of near misses and adverse events in pediatric patients. Prehosp Emerg Care 2010; 14: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hobgood C, Bowen JB, Brice JH, et al. Do EMS personnel identify, report, and disclose medical errors? Prehosp Emerg Care 2006; 10: 21–27. [DOI] [PubMed] [Google Scholar]
- 9. Grissinger M. The five rights: A destination without a map. Pharmacy and Therapeutics 2010; 35: 542. [Google Scholar]
- 10. Misasi P, Braithwaite S, Keebler JR. Paramedics believe verbal verification with a team mate reduces medication errors more than mental verification alone [Abstract]. Ann Emerg Med 2013; 62: S47–S48. [Google Scholar]
- 11. Bledsoe BE, Clayden DE, Papa FJ. (eds). Prehospital emergency pharmacology. 5th ed. Upper Saddle River, NJ: Prentice-Hall, Inc, 2001. [Google Scholar]
- 12. Bledsoe BE, Porter RS, Cherry RA. (eds). Paramedic care: principals and practice. Vol. 1 Upper Saddle River, NJ: Prentice-Hall, Inc, 2000. [Google Scholar]
- 13. Bledsoe BE, Porter RS, Cherry RA. (eds). Essentials of paramedic care. Upper Saddle River, NJ: Pearson Education, 2003. [Google Scholar]
- 14. Bledsoe BE, Porter RS, Shade BR. (eds). Paramedic emergency care. (3 ed Upper Saddle River, NJ: Prentice-Hall, Inc., 1997. [Google Scholar]
- 15. Gonsoulin SM, Raynovich W. (eds). Prehospital drug therapy. St. Louis, MO: Mosby-Year Book, Inc, 1994. [Google Scholar]
- 16. Institute of Medicine. Emergency medical services: At the crossroads. Washington, DC: The National Academies Press, 2007. [Google Scholar]
- 17. Eisenhauer LA, Hurley AC, Dolan N. Nurses reported thinking during medication administration. J Nurs Scholarsh 2007; 39: 82–87. [DOI] [PubMed] [Google Scholar]
- 18. Grayson S, Gandy WE. Fatal mistakes in prehospital medicine [Press release], http://www.emsworld.com/article/10773595/errors-in-ems?print=true (2012, accessed 5 March 2014). [PubMed]
- 19. Institute for Safe Medication Practices (ISMP). The “five rights”. ISMP Safety Alert! Acute Care 1999; 4: 2. [Google Scholar]
- 20. Institute for Safe Medication Practices (ISMP). The five rights; a destination without a map. ISMP Safety Alert! Acute Care 2007; 12: 1. [Google Scholar]
- 21. Kim J, Bates DW. Medication administration errors by nurses: adherence to guidelines. J Clin Nurs 2012; 22: 590–598. DOI: 10.1111/j.1365-2702.2012.04344.x. [DOI] [PubMed] [Google Scholar]
- 22. Nguyen A. Preventing drug errors in the prehospital setting. J Emerg Med Serv 2008; 33, http://www.jems.com/article/patient-care/bad-medicine-preventing-drug-e (accessed 31 July 2013). [DOI] [PubMed] [Google Scholar]
- 23. Brown M-M. Managing medication errors by design. Crit Care Nurs Q 2001; 24: 77–99. [DOI] [PubMed] [Google Scholar]
- 24. Elliot M, Liu Y. The nine rights of medication administration. Br J Nurs 2010; 19: 301–305. [DOI] [PubMed] [Google Scholar]
- 25. Vilke GM, Tornabene SV, Stepanski B, et al. Paramedic self-reported medication errors. Prehosp Emerg Care 2006; 10: 457–462. [DOI] [PubMed] [Google Scholar]
- 26. Federico F. The five rights of medication administration. Institute for Healthcare Improvement, http://www.ihi.org/resources/Pages/ImprovementStories/FiveRightsofMedicationAdministration.aspx (2015, accessed 8 July 2015).
- 27. Freund Y, Goulet H, Bokobza J, et al. Factors associated with adverse events resulting from medical errors in the emergency department: two work better than one. J Emerg Med 2013; 45: 157–162. [DOI] [PubMed] [Google Scholar]
- 28. Kohn LT, Corrigan JM, Donaldson MS. (eds). To err is human: building a safer health system. Washington, DC: National Academy Press, 1999. [PubMed] [Google Scholar]
- 29. Duthrie EA. Application of human error theory in case analysis of wrong procedures. J Patient Saf 2010; 6: 108–114. [DOI] [PubMed] [Google Scholar]
- 30. Huber C, Rebold B, Wallace C, et al. ECRI institute PSO deep dive analyzes medication events. Patient Saf Qual Health 2012; 9: 28-34. [Google Scholar]
- 31. Reason J. Human error: models and management. BMJ 2000; 320: 768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russ AL, Fairbanks RJ, Karsh B-T, et al. The science of human factors: separating faction from fiction. BMJ Qual Saf 2013; 22: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reason J. Managing the risks of organizational accidents. Burlington, VT: Ashgate Publishing Co, 1997. [Google Scholar]
- 34. Salas E, Rosen MA, Burke CS, et al. The wisdom of collectives in organizations: An update of the teamwork competencies. In: Salas E, Goodwin GF, Burke CS. (eds) Team effectiveness in complex organizations: Cross-disciplinary perspectives and approaches (pp. 624). New York: Taylor and Francis Group, 2009. [Google Scholar]
- 35. Hartwig SC, Denger SD, Schneider PJ. Severity-indexed, incident report-based medication error-reporting program. Am J Hosp Pharm 1991; 48: 2611–2616. [PubMed] [Google Scholar]
- 36. Hughes AM, Sonesh S, Zajac S, et al. Leveraging HFACS to understand medication error in emergency medical services (EMS): a systematic review. Proc Hum Factors Erg Soc 57th Annual Meeting, 2013; 57: 1688–1692. [Google Scholar]
- 37. Harwood K, Sanderson P. Skills, rules and knowledge: a discussion of Rasmussen’s classification. Proc Hum Factors Soc 1986; 30: 1002–1006. [Google Scholar]
- 38. Rasmussen J. Skills, rules, and knowledge; signals, signs, and symbols, and other distinctions in human performance models. IEEE Trans Syst Man Cybernet SMC 1983; 13: 257–266. [Google Scholar]
- 39. Reason J. Human error. New York, NY: Cambridge University Press, 1990. [Google Scholar]
- 40. Misasi P. The Medication Administration Cross-Check (MACC): User’s Manual. Wichita-Sedgwick County EMS System, 2012. [Google Scholar]
- 41. Ericsson KA, Krampe RT, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychological Review, (1993); 100(3): 363–406. [Google Scholar]
- 42. Ericsson KA, Lehmann AC. Expert and exceptional performance: evidence of maximal adaptation to task constraints. Annual Review of Psychology 1996; 47: 273–305. [DOI] [PubMed] [Google Scholar]
- 43. Morrow D, North R, Wickens CD. Reducing and mitigating human error in medicine. Rev Hum Factors Ergon 2005; 1: 254–296. [Google Scholar]
- 44. Kontogiannis T, Malakis S. A proactive approach to human error detection and identification in aviation and air traffic control. Saf Sci 2009; 47: 693–706. [Google Scholar]
- 45. Leape L. Error in medicine. JAMA 1994; 272: 1851–1857. [PubMed] [Google Scholar]
- 46. Misasi P, Lazzara EH, Keebler JR, et al. Understanding multi-team systems in emergency care, one case at a time. In: Salas E, Rico R, Shuffler M. (eds) Pushing the boundaries: multi-team systems in research and practice. Bradford, UK: Emerald Group, 2014. [Google Scholar]
- 47. Patterson ES, Woods DD, Cook RI, et al. Collaborative cross-checking to enhance resilience. Cogn Techn Work 2007; 9: 155–162. [Google Scholar]
- 48. Salas E, DiazGranados D, Klein C, et al. Does team training improve team performance? a meta-analysis. Hum Factors 2008; 50: 903–933. [DOI] [PubMed] [Google Scholar]
- 49. Freund Y, Rousseau A, Berard L, et al. Cross-checking to reduce adverse events resulting from medical errors in the emergency department: study protocol of the CHARMED cluster randomized study. BMC Emerg Med 2015; 15: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goodwin CJ. Research in psychology: Methods and design. 6th ed. Hoboken, NJ: John Wiley & Sons, Inc, 2010 [Google Scholar]
- 51. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. 2nd ed. Belmont, CA: Wadsworth Publishing, 2002. [Google Scholar]
- 52. Lifshitz AE, Goldstein LH, Sharist M, et al. Medication prescribing errors in the prehospital setting and in the ED. Am J Emerg Med 2012; 30: 726–731. [DOI] [PubMed] [Google Scholar]
- 53. Dekker SWA. What is rational about killing a patient with an overdose? enlightenment, continental philosophy and the role of the human subject in system failure. Ergonomics 2011; 54: 679–683. [DOI] [PubMed] [Google Scholar]