Abstract
Background: Postural balance and fall efficacy (self-perceived confidence in performing daily physical activities) have been found to be risk factors associated with falls in older adults. Stretching is one intervention that has been investigated to improve balance and therefore reduce fall risk. Various forms of stretching have been evaluated with different outcomes, but there is a lack of knowledge about the effect of stretching (continuous and intermittent) on plantar pressures and balance. Therefore, the aim of the present study was to analyze the effects of stretching (continuous and intermittent) of the bilateral ankle plantar flexors on plantar pressures and static balance. Methods: A randomized clinical trial was carried out. Forty-eight healthy subjects (42 females and 6 males) were recruited in an outpatient clinic. Subjects were randomly assigned to an intermittent stretching group (five sets of 1 min; 15 s of rest) or a continuous stretching group (2 min of continuous stretching) of the plantar flexors. Plantar pressures and balance using stabilometry were measured before and after stretching. Results: There were significant differences between intermittent and continuous stretching in rearfoot maximum pressure, forefoot surface area, and center of pressure surface area with eyes open. Conclusions: Bilateral intermittent stretching of the ankle plantar flexors was found to be more effective than continuous stretching for the reduction of rearfoot maximum pressure and improved balance.
Keywords: muscle stretching exercise, postural balance, stabilometry, platform
1. Introduction
Worldwide, each year an estimated 646,000 individuals die from falls [1]. Fall efficacy (self-perceived confidence in performing daily physical activities) and postural balance have been identified as risk factors for falls in older adults [2]. Several forms of rehabilitation have previously been used to improve fall risk. Static stretching is one technique of rehabilitation that is commonly used and has been widely researched [3,4,5,6,7,8,9,10,11,12,13,14]. Nevertheless, each form of static stretching may provide different effects and their relationship with balance has not been accurately described.
Two of the more common forms of static stretching include continuous and intermittent modalities. Continuous static stretching has been shown to improve range of motion (ROM) more than intermittent stretching [4]. Specifically, a 2-min continuous stretch increased ROM [5]; however, 5 sets of 1 min stretching with 15 s of rest demonstrated no change in ROM. This type of stretching seems to be duration-dependent, with shorter durations reducing the ROM effects [5].
Static stretching has also been shown to cause a decrease in force production of the muscle. The loss of force with stretching occurs following a minimum of 2 min [5]. When comparing forms of static stretching, it has been found that intermittent stretching (5 sets of 1 min stretching with 15 s of rest) produced greater strength loss compared to continuous stretching (of 5 min) [4]. Both forms of stretching reduce central nervous system pathways conduction, which can reduce force production, but in the case of intermittent stretching there may be other factors that are responsible for prolonged force loss [5].
Postural control is maintained by continuous contraction of anti-gravitational musculature throughout the body. Alterations in the position of the body may lead to variations of load due to the acceleration of gravity and may influence the contraction of the lower back/pelvic (e.g., transversus abdominis) and lower limb flexor muscles. Posture has been deeply analyzed by means of electromyography (EMG), Romberg test analysis, pressure platform analysis, and thermography. The lower limb flexor muscles muscle activation pattern has demonstrated a relationship with static and dynamic balance. Therefore, stretching of the flexor muscles may help to improve the muscle functionality [15,16,17,18,19,20,21].
Currently, there is a lack of consensus on the relationship between lower limb stretching and balance. Several systems may be involved for individuals’ postural control, which may include the neuromuscular, vestibular, visual, and somatosensory systems [6,7]. Behm et al. [9] found a detrimental effect on balance due to an intermittent lower limb stretching protocol. In contrast, Costa et al. [10] found positive effects on dynamic balance due to static stretching. Nevertheless, static and proprioceptive neuromuscular facilitation stretching did not show balance effects, according to Lim et al. [11]. In addition, Chatzopoulos et al. [12] found that static stretching of limbs and arms had negative effects on balance, while dynamic stretching did not show balance effects [13]. Ankle plantar flexors may be considered as postural tonic muscles and can affect postural control by ankle stabilization strategies [14]. Lima et al. [22] also investigated the acute effects of unilateral ankle plantar flexors static- stretching on the center of pressure (COP) during a single-leg balance task, which showed negative balance effects.
Thus, there is a lack of knowledge about the effects of static stretching (continuous and intermittent) on plantar pressures and balance. We hypothesized that static intermittent stretching could improve balance effects. Therefore, the aim of the present study was to analyze the effects of stretching (continuous and intermittent) of the bilateral ankle plantar flexors on plantar pressures and static balance.
2. Materials and Methods
2.1. Subjects
Forty-eight healthy subjects (42 females and 6 males) were recruited for the study. The demographic data of the study participants were as follows: 32.12 ± 7.60 years old, 166.64 ± 8.13 cm height, and 62.72 ± 8.97 kg weight. All demographic data are shown in Table 1. An ethics committee approved the study, and all subjects gave their written informed consent before participating in this research. Ethical standards in human experimentation contained in the World Medical Association Declaration of Helsinki, the Council of Europe Convention on Human Rights and Biomedicine, the United Nations Educational, Scientific and Cultural Organization (UNESCO) Universal Declaration on the Human Genome and Human Rights, and those of the relevant national bodies and institutions were observed at all times. All subjects were randomly distributed in two different groups: continuous stretching group and intermittent stretching group. Inclusion criteria included: non-trained healthy individuals with no pain [22]. Exclusion criteria included: previous lower extremities surgery; history of lower extremities injury with residual symptoms (pain, “giving-away” sensations) within the last year; evidence of a leg-length discrepancy (difference in distance from the anterior superior iliac spine to the superior surface of the most prominent aspect of the medial malleolus) of more than 1 cm; and evidence of balance deficits (determined by oral questionnaire regarding falls) [22].
Table 1.
Socio-demographic characteristics of the sample population.
| Variable Total (n = 24) |
Total Group Mean ± SD (CI 95%) |
Continuous Group Mean ± SD (CI 95%) |
Intermittent Group Mean ± SD (CI 95%) |
p-Value * |
|---|---|---|---|---|
| Age (years) | 32.12 ± 7.60 (29.08–35.16) |
32.20 ± 8.08 (28.97–35.44) |
32.04 ± 7.28 (29.18–34.89) |
0.940 |
| Weight (kg) | 62.72 ± 8.97 (59.14–66.31) |
62.77 ± 9.52 (58.96–66.57) |
62.68 ± 8.58 (59.32–66.05) |
0.975 |
| Height (cm) | 166.64 ± 8.13 (163.39–169.90) |
166.20 ± 8.43 (162.83–169.58) |
167.08 ± 7.98 (163.95–170.21) |
0.714 |
| BMI (kg/m2) | 22.58 ± 2.75 (21.48–23.69) |
22.71 ± 2.90 (21.55–23.87) |
22.46 ± 2.66 (21.41–23.50) |
0.753 |
| Size of shoe | 38.87 ± 2.32 (37.94–39.80) |
38.81 ± 2.26 (37.90–39.72) |
38.93 ± 2.43 (37.98–39.89) |
0.855 |
Abbreviations: kg, kilograms; cm, centimeters; BMI, body mass index; SD, standard deviation; CI 95%, confidence interval 95%. In all the analyses, p < 0.05 (with a 95% confidence interval) was considered statistically significant. p-values are from Student’s t-test *.
2.2. Procedures
First, a clinician with ten years of experience performed a baseline balance evaluation to confirm the inclusion and exclusion criteria of each subject using the Balance Evaluation Systems test (BESTest) [23] in order to diagnose equinus foot. All subjects had at least 15 degrees of ankle dorsiflexion [24,25]. For the balance evaluation participants were instructed to remain in a relaxed standing posture with feet shoulder-width apart and positioned at 30° away from the midline on a digital portable pressure sensor platform (Medicapteurs, Balma, France) [26,27,28]. The technical specifications of the pressure platform are shown in the Table 2. Pressure sensor measurements from the platform were accurate to the nearest 0.001 kg/cm2. Before each use, auto-calibration was performed.
Table 2.
Technical specifications of the pressure platform.
| Specification | Description |
|---|---|
| Size (length × width × height) | 530 × 600 × 45 mm |
| Thickness | 4 mm |
| Active surface | 400 × 400 mm |
| Weight | 6.8 kg |
| Sensors | Calibrated resistive |
| Sensor | 8 × 8 mm |
| Sensor thickness | 0.15 mm |
| No. of sensors | 2304 (48 × 48) |
| Permissible temperature | −40 °C to 85 °C |
| Sensor pressure (minimum/maximum) | 0.4 N/m2 (0.0004 kPa)/100 N/m2 (0.1 kPa) |
| Type of PC interface/platform | Universal Serial Bus (USB) |
| Supply | USB cable |
| Data acquisition frequency | 200 images/s |
| Vertical force recording | 60 Hz |
| Operating system required | Windows XP, Vista, or 7 |
The protocol started with a pre-stretching evaluation. Participants were then randomly divided into two groups (intermittent stretching group or continuous stretching group) by a random number table provided by the clinician. Each group then performed the prescribed stretching protocol. In order to maintain consistency between groups, the stretching methods were standardized. The subjects climbed on a raised platform and placed the forefoot of both feet on the edge of the platform. They next dropped both heels off the platform to the ground without making contact with the ground [29,30] and held that position to perform a weight-bearing static stretch. The continuous stretching group performed one repetition that was held for two minutes [5]. The intermittent stretching group performed 5 repetitions of 1-min duration stretches with 15 s of rest [4]. During the rest period, the subjects descended from the platform and remained standing. The subjects were asked to quantify a feeling of discomfort during the stretch in both legs. Subjects were instructed to stretch to the point of discomfort [9,16], and this sensation was maintained for all of the stretching [29,31]. The desired point of discomfort (POD) [22] intensity range was 70–90%, considering 0 as “no stretch discomfort at all” and 100% as “the maximum imaginable stretch discomfort”. The POD intensity was recorded during all stretches [22]. Immediately following the completion of the stretching, the testing methods were repeated [22]. All measures were performed at the same hour of the day, between 09:00 and 11:00 h [22].
Stabilometry assessment was used and subjects were instructed to stand barefoot on the force platform [26]. The feet were placed at equal distance from the midline [27] and 30 degrees from midline [28]. During all the examinations, the upper limbs were placed in a relaxed position along the body [27]. The subjects were instructed to stand as still as possible for 30 s, with their eyes open (EO), while concentrating on a point at eye level 2-m away [22]. The subjects then repeated the methods with their eyes closed (EC) [26]. Two trials were recorded for each condition [22,26,27] and the order of the conditions was randomized across subjects [26]. Foot plantar pressure and surface area of static footprints were measured during bipodal standing. The foot surface area was then divided into three areas: the rearfoot, midfoot, and forefoot. Two trials were recorded and the average was used for analysis data.
3. Measures
3.1. Variables
Stabilometry was measured by displacement of the center of pressures in X and Y with eyes open and closed [26], center of pressure (COP) with eyes open and closed, COP area with eyes open and closed, COP antero-posterior (a-p) and medio-lateral (m-lat) directions with eyes open and closed, and COP speed [22].
Static plantar pressure was evaluated by means of maximum pressure, medium pressure and surface area of each aspect of the foot (rearfoot, midfoot, and forefoot).
3.2. Statistical Analysis
All data were explored for normality using the Shapiro Wilks test, and data were considered normally distributed if p > 0.05. Descriptive statistical analysis was performed using mean ± SD and a 95% confidence interval.
The Mann–Whitney U test was performed to examine differences in non-parametric variables. Student’s t-test was used for parametric variables. A p-value < 0.05 with a confidence interval of 95% was considered statistically significant for all tests (SPSS for Windows, version 20.0; SPSS Inc., Chicago, Illinois, USA).
4. Results
All variables were not normally distributed and therefore non-parametric statistics were used (p < 0.05), except for socio-demographic characteristics of the sample population. There were no significant differences between groups at baseline (Table 3). There were significant differences between groups after stretching. Specifically, reduced rearfoot maximum pressure, increased forefoot surface area, and reduced surface area of COP with eyes open were shown in the intermittent stretching group compared to the continuous stretching group (Table 4).
Table 3.
Stabilometry and static footprints variables before bilateral intermittent and continuous stretching.
| Variable | Intermittent Group Pretest Values (n = 20) Mean ± SD (CI 95%) |
Continuous Group Pretest Values (n = 20) Mean ± SD (CI 95%) |
p-value * |
|---|---|---|---|
| Rearfoot maximum pressure (kPa) | 106.24 ± 21.36 (97.00–115.48) |
105.52 ± 24.08 (95.02–105.93) |
0.918 |
| Rearfoot medium pressure (kPa) | 39.61 ± 6.51 (36.79–42.43) |
41.59 ± 8.96 (37.71–45.46) |
0.483 |
| Rearfoot surface (cm2) | 85.84 ± 10.51 (81.30–90.39) |
84.71 ± 10.33 (80.25–89.18) |
0.828 |
| Midfoot maximum pressure (kPa) | 13.05 ± 14.82 (6.64–19.46) |
11.18 ± 11.69 (6.12–16.23) |
0.865 |
| Midfoot medium pressure (kPa) | 5.72 ± 6.10 (3.08–8.36) |
5.63 ± 5.68 (3.17–8.09) |
0.966 |
| Midfoot surface (cm2) | 16.71 ± 19.08 (8.46–24.96) |
17.93 ± 20.07 (9.25–26.61) |
0.787 |
| Forefoot maximum pressure (kPa) | 69.41 ± 13.19 (63.71–75.12) |
70.16 ± 10.36 (65.68–74.64) |
0.606 |
| Forefoot medium pressure (kPa) | 25.54 ± 6.07 (22.91–28.16) |
25.23 ± 2.77 (24.03–26.43) |
0.338 |
| Forefoot surface(cm2) | 94.15 ± 17.75 (86.47–101.82) |
90.02 ± 12.02 (84.81–95.22) |
0.496 |
| X displacement eyes open (mm) | 6.91 ± 6.14 (4.26–9.57) |
8.34 ± 9.01 (6.49–10.19) |
0.083 |
| Y displacement eyes open (mm) | 16.68 ± 9.89 (12.40–20.95) |
19.32 ± 9.01 (15.43–23.22) |
0.359 |
| Surface Eyes Open (mm2) | 13.35 ± 9.58 (9.21–17.50) |
9.83 ± 7.12 (6.75–12.92) |
0.180 |
| Medium speed of the laterolateral displacement. Eyes open (mm/s) | 1.20 ± 0.27 (1.08–1.32) |
1.16 ± 0.28 (1.03–1.28) |
0.657 |
| Medium speed of the anteroposterior displacement. Eyes open (mm/s) | 0.98 ± 0.26 (0.87–1.10) |
1.04 ± 0.39 (0.87–1.21) |
0.542 |
| X displacement eyes closed (mm) | 7.74 ± 5.05 (5.55–9.92) |
7.61 ± 4.40 (5.71–9.52) |
0.926 |
| Y displacement eyes closed (mm) | 17.08 ± 9.93 (12.78–21.37) |
21.34 ± 9.46 (17.24–25.43) |
0.164 |
| Surface eyes closed (mm2) | 25.96 ± 14.77 (19.57–32.34) |
32.44 ± 52.54 (9.71–55.16) |
0.599 |
| Medium speed of the laterolateral displacement. Eyes closed (mm/s) | 1.39 ± 0.36 (1.23–1.55) |
1.37 ± 0.42 (1.19–1.56) |
0.788 |
| Medium speed of the anteroposterior displacement. Eyes closed (mm/s) | 1.48 ± 0.58 (1.22–1.73) |
1.41 ± 0.70 (1.10–1.72) |
0.332 |
Abbreviation: kg (kilograms); cm (centimeters), cm2 (centimeters2), SD (standard deviation), CI 95% (confidence interval 95%). In all the analyses, p < 0.05 (with a 95% confidence interval) was considered statistically significant. p-values are from Mann–Whitney U test *.
Table 4.
Stabilometry and plantar pressure variables after bilateral intermittent and continuous stretching.
| Variable | Intermittent Group Posttest Values (n = 20) Mean ± SD (CI 95%) |
Continuous Group Posttest Values (n = 20) Mean ± SD (CI 95%) |
p-Value * |
|---|---|---|---|
| Rearfoot maximum pressure (kPa) | 87.56 ± 22.77 (77.71–97.41) |
99.39 ± 18.76 (91.28–107.51) |
0.019 |
| Rearfoot medium pressure (kPa) | 33.84 ± 7.44 (30.62–37.06) |
37.19 ± 5.38 (34.86–39.51) |
0.105 |
| Rearfoot surface (cm2) | 81.86 ± 12.11 (76.62–87.10) |
83.54 ± 13.11 (77.87–89.21) |
0.703 |
| Midfoot maximum pressure (kPa) | 14.73 ± 13.98 (8.68–20.78) |
15.58 ± 14.85 (9.16–22.01) |
0.983 |
| Midfoot medium pressure (kPa) | 7.05 ± 6.55 (4.21–9.88) |
7.54 ± 6.22 (4.85–10.23) |
0.761 |
| Midfoot surface (cm2) | 20.78 ± 18.67 (12.70–28.85) |
20.56 ± 19.08 (12.31–28.81) |
0.957 |
| Forefoot maximum pressure (kPa) | 74.16 ± 15.62 (67.41–80.92) |
73.14 ± 15.38 (66.48–79.79) |
0.332 |
| Forefoot medium pressure (kPa) | 26.98 ± 4.29 (25.12–28.84) |
26.72 ± 6.73 (23.81–29.63) |
0.322 |
| Forefoot surface (cm2) | 105.23 ± 18.32) (97.31–113.16) |
97.36 ± 12.98 (91.75–102.98) |
0.038 |
| X displacement eyes open (mm) | 8.48 ± 5.23 (6.22–10.75) |
7.32 ± 4.89 (5.20–9.44) |
0.353 |
| Y displacement eyes open (mm) | 15.89 ± 8.39 (12.26–19.51) |
18.29 ± 10.84 (13.60–22.98) |
0.409 |
| Surface eyes open (mm2) | 6.34 ± 4.08 (4.57–8.10) |
11.02 ± 8.58 (7.31–14.74) |
0.031 |
| Medium speed of the laterolateral displacement. Eyes open (mm/s) | 1.19 ± 0.31 (1.05–1.32) |
1.35 ± 0.71 (1.04–1.66) |
0.910 |
| Medium speed of the anteroposterior displacement. Eyes open (mm/s) | 1.15 ± 0.65 (0.87–1.43) |
1.02 ± 0.57 (0.77–1.27) |
0.474 |
| X displacement eyes closed (mm) | 7.97 ± 5.86 (5.43–10.50) |
6.51 ± 4.09 (4.74–8.29) |
0.557 |
| Y displacement eyes closed (mm) | 15.54 ± 9.53 (11.42–19.66) |
16.90 ± 9.62 (12.74–21.06) |
0.640 |
| Surface eyes closed (mm2) | 18.24 ± 12.29 (12.93–23.56) |
23.17 ± 16.36 (16.09–30.24) |
0.167 |
| Medium speed of the laterolateral displacement. Eyes closed (mm/s) | 1.54 ± 0.47 (1.34–1.75) |
1.43 ± 0.85 (1.07–1.80) |
0.051 |
| Medium speed of the anteroposterior displacement. Eyes closed (mm/s) | 1.79 ± 0.87 (1.42–2.17) |
1.89 ± 2.31 (0.89–2.89) |
0.083 |
Abbreviation: kg (kilograms); cm (centimeters), cm2 (centimeters2); SD (standard deviation), CI 95% (confidence interval 95%). In all the analyses, p < 0.05 (with a 95% confidence interval) was considered statistically significant. p-values are from Mann–Whitney U test *.
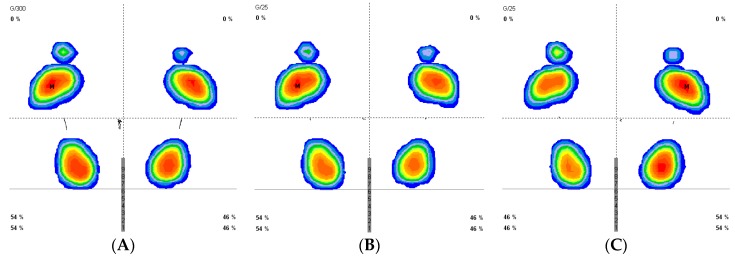
Figure 1 demonstrates the results for the stabilometry before and after continuous and intermittent stretching.
Figure 1.
Stabilometry pattern before and after stretching (Previous stabilometry (A) post continuous stretch (B) and post intermittent stretch(C)).
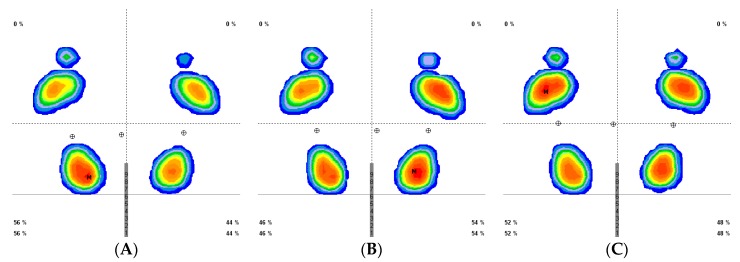
Figure 2 demonstrates the pattern of the results for plantar pressures prior to stretching and following continuous and intermittent stretching.
Figure 2.
Plantar pressures pattern before and after stretching (prior (A), post continuous stretch (B), and post intermittent stretch(C)).
5. Discussion
This study sought to analyze the effects of stretching (continuous and intermittent) of the bilateral ankle plantar flexors on plantar pressures and static balance.
Both stretching groups (intermittent and continuous) did not demonstrate significant differences during pre-intervention testing. Intermittent stretching demonstrated a reduced maximum pressure in the rearfoot compared to the continuous stretching group. There was also an increase of forefoot surface area in the intermittent group compared to the continuous group. Thus, these results suggest that a greater surface area in the forefoot can reduce the maximum pressure on the rearfoot.
Ankle equinus generates a large deforming force to the foot and may be considered as a related factor to several foot and ankle conditions, including plantar fasciitis [32], pes planus, hallux abduction valgus, Achilles tendinosis, Charcot’s midfoot collapse, and diabetic ulcerations [33]. Continuous stretching has been used to increase range of movement [4,34,35]. On the other hand, intermittent stretching has been shown to be an effective therapeutic tool for the reduction of muscle stiffness [4,36,37].
Stretching exercise leads biomechanical and physiological adaptations related to motor unit excitability improvements, body temperature increases, kinesthetic awareness improvements, and active ROM increases. These biomechanical and neuromuscular modifications may be deeply related to the changes of viscoelastic components of the muscle-tendon units and depressed reflex activity of neural outputs. This phenomenon is called viscoelastic stress relaxation and demonstrates that the muscle–tendon unit is affected during stretching activity [3].
Continuous stretching effects may be also related to a muscle compliance increase, which increases the time and decreases the force for myofibril-unit contraction during muscular elements activation. Continuous stretching may affect the maximal force production and reduce muscle stiffness and muscle activation, as well as generating an acute increase of ROM. These modifications have been related to an increased tolerance to the imposed stretching activity [9,29]. Nevertheless, intermittent stretching may enhance muscular condition by preserving muscle-tendon unit stiffness and may improve the recovery of the vascular, nervous, muscle–tendon unit, and metabolic systems, as well as stretching tolerance.
Both continuous and intermittent stretching interventions have been related to dynamic balance and postural control. Balance and joint position sense are proprioceptive parameters that depend on contributions from visual, vestibular and peripheral receptors that are found in skin, joints, muscles and ligaments [12].
Proprioception may provide conscious and subconscious responses for body posture and motion, being essential for lower limb joints functioning in order to maintain optimal balance control during daily physical activities. Joint position sense may be considered as an aspect of proprioception that plays an important role in functional dynamic stability of joints through the action of the muscles and ligaments around them, which seems to be influenced by ROM modifications [10].
Therefore, intermittent stretching use has been recommended for pathologies that present an increased heel pressure such as heel pain (Sever’s disease [20,21], fasciitis [32]) as well as those with increased pressure in the forefoot (fasciitis [32], diabetic foot ulcers [33,38], metatarsalgia [39], Achilles tendinopathy [40]). Gajdosik et al. [34] found an increased ROM after ten static wall intermittent stretches held for 15 s in each repetition, five times per week for 6 weeks. Nevertheless, more studies are necessary to examine the effect of intermittent stretching on equine feet and find the most appropriate protocol to reduce plantar pressures.
Morrin and Redding in 2013 [41] found that continuous static stretching on the hamstrings in dancers did not show detrimental effects on balance. A study by Behm et al. in 2004 [9] found significant decreases in static balance scores following an intermittent stretching protocol (5-min cycle warm-up, three stretches to the point of discomfort, 45 s each with 15-s rest periods for each muscle group of the lower limb). Depending on the muscle studied, different acute effects of static stretching may occur [42]. It is important to consider that the results obtained in calf muscles may not be generalized to other muscle groups.
This fact may explain the results of our study, which found intermittent stretching reduced COP surface area with the eyes open, while changes were not observed after continuous stretching.
Most authors suggest that changes in balance after stretching might be related to changes in both proprioception and mechanical outputs (influencing the musculotendinous unit stiffness and affecting the ability to adapt adequately to the stability challenges), [9,10,12,41]. The findings for COP surface area with the eyes open following intermittent stretching may be related to an increase in surface area of the forefoot following stretching, leading to an increase in proprioceptive stimulus from the skin receptors. This can also suggest that intermittent stretching may reduce force and inhibit central nervous system pathways conduction compared to continuous stretching [42] due to the fact that the modifications in osteotendinous and Golgi reflex in plantar flexors have been found to produce less static balance influence than other muscles, such as the tibialis anterior which has been highly related to center of pressure movement compared to the plantar flexor muscles in bipodal standing [43].
6. Conclusions
The force platform assessment demonstrated that bilateral intermittent stretching of the ankle plantar flexor muscles seems to be more effective than continuous stretching for the reduction of rearfoot maximum pressure and balance improvement.
Acknowledgments
No presentation of this material has been carried out previously. No financial support was provided.
Author Contributions
Conceptualization: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; data curation: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; investigation: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; methodology: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; resources: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; software: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; supervision: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; validation: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; visualization: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.; writing—original draft: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L..; writing—review and editing: E.M.M.-J., J.I.D.-V., C.C.-L., D.R.-S., R.B.-d.-B.-V., M.E.L.-I., P.P.-L. and D.L.-L.
Funding
This research received no external funding.
Conflicts of Interest
No conflicts of interest.
References
- 1.©Falls. [(accessed on 24 May 2018)]; Available online: http://www.who.int/news-room/fact-sheets/detail/falls.
- 2.Pua Y.-H., Ong P.-H., Clark R.A., Matcher D.B., Lim E.C.W. Falls efficacy, postural balance, and risk for falls in older adults with falls-related emergency department visits: Prospective cohort study. BMC Geriatr. 2017;17:291. doi: 10.1186/s12877-017-0682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avela J., Finni T., Liikavainio T., Niemelä E., Komi P.V. Neural and mechanical responses of the triceps surae muscle group after 1 h of repeated fast passive stretches. J. Appl. Physiol. 2004;96:2325–2332. doi: 10.1152/japplphysiol.01010.2003. [DOI] [PubMed] [Google Scholar]
- 4.Trajano G.S., Nosaka K.B., Seitz L., Blazevich A.J. Intermittent Stretch Reduces Force and Central Drive more than Continuous Stretch. Med. Sci. Sport Exerc. 2014;46:902–910. doi: 10.1249/MSS.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 5.Reid J.C., Greene R., Young J.D., Hodgson D.D., Blazevich A.J., Behm D.G. The effects of different durations of static stretching within a comprehensive warm-up on voluntary and evoked contractile properties. Eur. J. Appl. Physiol. 2018;118:1427–1445. doi: 10.1007/s00421-018-3874-3. [DOI] [PubMed] [Google Scholar]
- 6.Horak F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl. 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 7.Jancová J. Measuring the balance control system-review. Acta Med. 2008;51:129–137. doi: 10.14712/18059694.2017.14. [DOI] [PubMed] [Google Scholar]
- 8.Muehlbauer T., Gollhofer A., Granacher U. Relationship Between Measures of Balance and Strength in Middle-Aged Adults. J. Strength Cond. Res. 2012;26:2401–2407. doi: 10.1519/JSC.0b013e31823f8c41. [DOI] [PubMed] [Google Scholar]
- 9.Behm D.G., Bambury A., Cahill F., Power K. Effect of acute static stretching on force, balance, reaction time, and movement time. Med. Sci. Sports Exerc. 2004;36:1397–1402. doi: 10.1249/01.MSS.0000135788.23012.5F. [DOI] [PubMed] [Google Scholar]
- 10.Costa P.B.P., Graves B.B.S., Whitehurst M., Jacobs P.L. The Acute Effects of Different Durations of Static Stretching on Dynamic Balance Performance. Strength Cond. 2009;23:141–147. doi: 10.1519/JSC.0b013e31818eb052. [DOI] [PubMed] [Google Scholar]
- 11.Lim K.-I., Nam H.-C., Jung K.-S. Effects on Hamstring Muscle Extensibility, Muscle Activity, and Balance of Different Stretching Techniques. J. Phys. Ther. Sci. 2014;26:209–213. doi: 10.1589/jpts.26.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatzopoulos D., Galazoulas C., Patikas D., Kotzamanidis C. Acute effects of static and dynamic stretching on balance, agility, reaction time and movement time. J. Sports Sci. Med. 2014;13:403–409. [PMC free article] [PubMed] [Google Scholar]
- 13.Leblebici H., Yarar H., Aydın E.M., Zorlu Z., Ertaş U., Kıngır M.E. The Acute Effects of Different Stretching on Dynamic Balance Performance. Int. J. Sport Stud. 2017;7:2251–7502. [Google Scholar]
- 14.Gribble P.A., Hertel J. Effect of hip and ankle muscle fatigue on unipedal postural control. J. Electromyogr. Kinesiol. 2004;14:641–646. doi: 10.1016/j.jelekin.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Sanz D., Becerro-de-Bengoa-Vallejo R., Losa-Iglesias M.E., Martínez-Jiménez E.M., Muñoz-García D., Pérez-Boal E., Calvo-Lobo C., López-López D. Effects of Compressive Stockings and Standard Stockings in Skin Temperature and Pressure Pain Threshold in Runners with Functional Ankle Equinus Condition. J. Clin. Med. 2018;7:454. doi: 10.3390/jcm7110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Sanz D., Becerro-de-Bengoa-Vallejo R., López-López D., Calvo-Lobo C., Martínez Jiménez E.M., Perez-Boal E., Losa-Iglesias M.E., Palomo-López P. Slow velocity of the center of pressure and high heel pressures may increase the risk of Sever’s disease: A case-control study. BMC Pediatr. 2018;18:357. doi: 10.1186/s12887-018-1318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Sanz D., Losa-Iglesias M.E., Becerro de Bengoa-Vallejo R., Palomo-Lopez P., Beltran-Alacreu H., Calvo-Lobo C., Navarro-Flores E., Lopez-Lopez D. Skin temperature in youth soccer players with functional equinus and non-equinus condition after running. J. Eur. Acad. Dermatol. Venereol. 2018;32:2020–2024. doi: 10.1111/jdv.14966. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Sanz D., Losa-Iglesias M.E., López-López D., Calvo-Lobo C., Palomo-López P., Becerro-de-Bengoa-Vallejo R. Infrared thermography applied to lower limb muscles in elite soccer players with functional ankle equinus and non-equinus condition. PeerJ. 2017;5:e3388. doi: 10.7717/peerj.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero Morales C., Calvo Lobo C., Rodríguez Sanz D., Sanz Corbalán I., Ruiz Ruiz B., López López D. The concurrent validity and reliability of the Leg Motion system for measuring ankle dorsiflexion range of motion in older adults. PeerJ. 2017;5:e2820. doi: 10.7717/peerj.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becerro-de-Bengoa-Vallejo R., Losa-Iglesias M.E., Rodriguez-Sanz D. Static and dynamic plantar pressures in children with and without sever disease: A case-control study. Phys. Ther. 2014;94:818–826. doi: 10.2522/ptj.20120164. [DOI] [PubMed] [Google Scholar]
- 21.Becerro de Bengoa Vallejo R., Losa Iglesias M.E., Rodríguez Sanz D., Prados Frutos J.C., Salvadores Fuentes P., Chicharro J.L. Plantar pressures in children with and without sever’s disease. J. Am. Podiatr. Med. Assoc. 2011;101:17–24. doi: 10.7547/1010017. [DOI] [PubMed] [Google Scholar]
- 22.Lima B.N., Lucareli P.R.G., Gomes W.A., Silva J.J., Bley A.S., Hartigan E.H., Marchetti P.H. The Acute Effects of Unilateral Ankle Plantar Flexors Static-Stretching on Postural Sway and Gastrocnemius Muscle Activity during Single-Leg Balance Tasks. J. Sport Sci. Med. 2014;13:564–570. [PMC free article] [PubMed] [Google Scholar]
- 23.Padgett P.K., Jacobs J.V., Kasser S.L. Is the BESTest at Its Best? A Suggested Brief Version Based on Interrater Reliability, Validity, Internal Consistency, and Theoretical Construct. Phys. Ther. 2012;92:1197–1207. doi: 10.2522/ptj.20120056. [DOI] [PubMed] [Google Scholar]
- 24.Scharfbillig R., Scutter S.D. Measurement of foot dorsiflexion: A modified Lidcombe template. J. Am. Podiatr. Med. Assoc. 2004;94:573–577. doi: 10.7547/0940573. [DOI] [PubMed] [Google Scholar]
- 25.Martin R.L., McPoil T.G. Reliability of Ankle Goniometric Measurements. J. Am. Podiatr. Med. Assoc. 2005;95:564–572. doi: 10.7547/0950564. [DOI] [PubMed] [Google Scholar]
- 26.Ganesan M., Lee Y.J., Aruin A.S. The effect of lateral or medial wedges on control of postural sway in standing. Gait Posture. 2014;39:899–903. doi: 10.1016/j.gaitpost.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Dudek K., Drużbicki M., Przysada G., Śpiewak D. Assessment of standing balance in patients after ankle fractures. Acta Bioeng. Biomech. 2014;16:59–65. [PubMed] [Google Scholar]
- 28.Shim J.-M., Jung J.-H., Kim H.-H. The effects of plantar flexor static stretching and dynamic stretching using an aero-step on foot pressure during gait in healthy adults: A preliminary study. J. Phys. Ther. Sci. 2015;27:2155–2157. doi: 10.1589/jpts.27.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babault N., Kouassi B.Y.L., Desbrosses K. Acute effects of 15 min static or contract-relax stretching modalities on plantar flexors neuromuscular properties. J. Sci. Med. Sport. 2010;13:247–252. doi: 10.1016/j.jsams.2008.12.633. [DOI] [PubMed] [Google Scholar]
- 30.Zito M., Driver D., Parker C., Bohannon R. Lasting effects of one bout of two 15 s passive stretches on ankle dorsiflexion range of motion. J. Orthop. Sports Phys. Ther. 1997;26:214–221. doi: 10.2519/jospt.1997.26.4.214. [DOI] [PubMed] [Google Scholar]
- 31.Behm D.G., Kibele A. Effects of differing intensities of static stretching on jump performance. Eur. J. Appl. Physiol. 2007;101:587–594. doi: 10.1007/s00421-007-0533-5. [DOI] [PubMed] [Google Scholar]
- 32.Yoo S.D., Kim H.S., Lee J.H., Yun D.H., Kim D.H., Chon J., Lee S.A., Han Y.J., Soh Y.S., Kim Y., et al. Biomechanical Parameters in Plantar Fasciitis Measured by Gait Analysis System With Pressure Sensor. Ann. Rehabil. Med. 2017;41:979–989. doi: 10.5535/arm.2017.41.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Downey M., Banks A. Gastrocnemius recession in the treatment of nonspastic ankle equinus. A retrospective study. J. Am. Podiatr. Med. Assoc. 1989;79:159–174. doi: 10.7547/87507315-79-4-159. [DOI] [PubMed] [Google Scholar]
- 34.Gajdosik R.L., Vander Linden D.W., McNair P.J., Williams A.K., Riggin T.J. Effects of an eight-week stretching program on the passive-elastic properties and function of the calf muscles of older women. Clin. Biomech. 2005;20:973–983. doi: 10.1016/j.clinbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Yeh C.-Y., Tsai K.-H., Chen J.-J. Effects of Prolonged Muscle Stretching With Constant Torque or Constant Angle on Hypertonic Calf Muscles. Arch. Phys. Med. Rehabil. 2005;86:235–241. doi: 10.1016/j.apmr.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Nordez A., McNair P., Casari P., Cornu C. Acute Changes in Hamstrings Musculo-Articular Dissipative Properties Induced by Cyclic and Static Stretching. Int. J. Sports Med. 2008;29:414–418. doi: 10.1055/s-2007-964980. [DOI] [PubMed] [Google Scholar]
- 37.McNair P.J., Dombroski E.W., Hewson D.J., Stanley S.N. Stretching at the ankle joint: Viscoelastic responses to holds and continuous passive motion. Med. Sci. Sports Exerc. 2001;33:354–358. doi: 10.1097/00005768-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Fernando M.E., Crowther R.G., Lazzarini P.A., Sangla K.S., Wearing S., Buttner P., Golledge J. Plantar pressures are higher in cases with diabetic foot ulcers compared to controls despite a longer stance phase duration. BMC Endocr. Disord. 2016;16:51. doi: 10.1186/s12902-016-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortina R.E., Morris B.L., Vopat B.G. Gastrocnemius Recession for Metatarsalgia. Foot Ankle Clin. 2018;23:57–68. doi: 10.1016/j.fcl.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Munteanu S.E., Barton C.J. Lower limb biomechanics during running in individuals with achilles tendinopathy: A systematic review. J. Foot Ankle Res. 2011;4:15. doi: 10.1186/1757-1146-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrin N., Redding E. Acute effects of warm-up stretch protocols on balance, vertical jump height, and range of motion in dancers. J. Dance Med. Sci. 2013;17:34–40. doi: 10.12678/1089-313X.17.1.34. [DOI] [PubMed] [Google Scholar]
- 42.Bouvier T., Opplert J., Cometti C., Babault N. Acute effects of static stretching on muscle–tendon mechanics of quadriceps and plantar flexor muscles. Eur. J. Appl. Physiol. 2017;117:1309–1315. doi: 10.1007/s00421-017-3618-9. [DOI] [PubMed] [Google Scholar]
- 43.Han J., Anson J., Waddington G., Adams R., Liu Y. The Role of Ankle Proprioception for Balance Control in relation to Sports Performance and Injury. Biomed. Res. Int. 2015;2015:1–8. doi: 10.1155/2015/842804. [DOI] [PMC free article] [PubMed] [Google Scholar]