Abstract
Background: The number of total hip arthroplasties (THA) performed across the world is growing rapidly. We performed this meta-analysis to evaluate the incidence of acute kidney injury (AKI) in patients undergoing THA. Methods: A literature search was performed using MEDLINE, EMBASE and Cochrane Database from inception until July 2018 to identify studies assessing the incidence of AKI (using standard AKI definitions of RIFLE, AKIN, and KDIGO classifications) in patients undergoing THA. We applied a random-effects model to estimate the incidence of AKI. The protocol for this meta-analysis is registered with PROSPERO (no. CRD42018101928). Results: Seventeen cohort studies with a total of 24,158 patients undergoing THA were enrolled. Overall, the pooled estimated incidence rates of AKI and severe AKI requiring dialysis following THA were 6.3% (95% CI: 3.8%–10.2%) and 0.5% (95% CI: 0.1%–2.3%). Subgroup analysis based on the countries by continent was performed and demonstrated the pooled estimated incidence of AKI following THA of 9.2% (95% CI: 5.6%–14.8%) in Asia, 8.1% (95% CI: 4.9%–13.2%) in Australia, 7.4% (95% CI: 3.2%–16.3%) in Europe, and 2.8% (95% CI: 1.2%–17.0%) in North America. Meta-regression of all included studies showed significant negative correlation between incidence of AKI following THA and study year (slope = −0.37, p <0.001). There was no publication bias as assessed by the funnel plot and Egger’s regression asymmetry test with p = 0.13 for the incidence of AKI in patients undergoing THA. Conclusion: The overall estimated incidence rates of AKI and severe AKI requiring dialysis in patients undergoing THA are 6.3% and 0.5%, respectively. There has been potential improvement in AKI incidence for patients undergoing THA over time.
Keywords: acute kidney injury, acute renal failure, hip arthroplasty, hip Surgery, postoperative acute kidney injury, incidence, epidemiology, systematic reviews, meta-analysis
1. Introduction
Acute kidney injury (AKI) is a complex clinical syndrome, characterized by an abrupt decrease in glomerular filtration, associated with various etiologies and pathophysiological pathways [1,2,3,4,5,6]. Globally, AKI is a common condition, affecting 13.3 million patients a year [3,4]. This complex syndrome is associated with significant morbidity, considerable mortality resulting in 1.7 million deaths a year, and increased hospital costs and subsequent burden on national health care budgets across the world [7,8].
Total hip arthroplasty (THA) is one of the most consistently successful and cost-effective orthopedic procedures performed today. It is indicated in patients with severe hip pain from a variety of conditions and ultimately provides significant improved pain relief, functionality and quality of life [9,10,11,12,13,14,15]. The total number of THA performed across the world is growing rapidly with close to 522,800 surgeries in the United States alone in 2014, making it the fourth most common operative procedure [16,17].
Previous studies have demonstrated different incidence of AKI following total joint arthroplasties using standard AKI criteria ranging from 0.5% to 22% based on types of joint arthroplasties [5,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. The clinical and economic landscape of joint replacement surgeries including both general orthopedic and more specifically THA is rapidly changing, thus making it imperative for clinicians and administrators to understand the various risk factors and post-operative complications in order to better provide excellence in clinical and financial outcomes. However, despite progress in perioperative medicine, the incidence, incidence trend, and risk factors for AKI in patients following THA remain unclear [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. In addition, complication rates such as reoperation and readmission among patients undergoing THA remain high [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Thus, we performed this systematic review to summarize the incidence and associated risk factors for AKI in patients undergoing THA.
2. Methods
2.1. Search Strategy and Literature Review
The protocol for this meta-analysis is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42018101928). A systematic literature search of EMBASE (1988 to July 2018), MEDLINE (1946 to July 2018), and the Cochrane Database of Systematic Reviews (database inception to July 2018) was performed to evaluate the incidence of AKI in patients undergoing THA. The systematic literature review was undertaken independently by two investigators (C.T. and W.C) using the search strategy that combined the terms of “acute kidney injury” or “renal failure” and “hip arthroplasty” or “hip surgery” which is provided in Online Supplementary Data 1. No language limitation was applied. A manual search for conceivably relevant studies using references of the included articles was also performed. This study was conducted by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [41] and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [42].
2.2. Selection Criteria
Eligible studies must be clinical trials or observational studies (cohort, case-control, or cross-sectional studies) that reported incidence of AKI in patients undergoing THA. Included studies must provide the data to estimate incidence of AKI with 95% confidence intervals (CI). Retrieved articles were individually reviewed for eligibility by the two investigators (C.T. and W.C.). Discrepancies were addressed and solved by mutual consensus. Inclusion was not limited by the size of the study.
2.3. Data Abstraction
A structured data collecting form was used to obtain the following information from each study: title, name of the first author, year of the study, publication year, country where the study was conducted, definition of THA, AKI definition, incidence of AKI, and risk factors for AKI.
2.4. Statistical Analysis
Analyses were performed utilizing the Comprehensive Meta-Analysis 3.3 software (Biostat Inc, Englewood, NJ, USA). Adjusted point estimates from each study were consolidated by the generic inverse variance approach of DerSimonian and Laird, which designated the weight of each study based on its variance [43]. Given the possibility of between-study variance, we used a random-effect model rather than a fixed-effect model. Cochran’s Q test and I2 statistic were applied to determine the between-study heterogeneity. A value of I2 of 0% to 25% represents insignificant heterogeneity, 26% to 50% low heterogeneity, 51% to 75% moderate heterogeneity and 76%–100% high heterogeneity [44]. The presence of publication bias was assessed by the Egger test [45].
3. Results
A total of 410 potentially eligible articles were identified using our search strategy. After the exclusion of 162 articles based on title and abstract for clearly not fulfilling inclusion criteria on the basis of type of article, study design, population or outcome of interest, and 209 due to being duplicates, 39 articles were left for full-length review. 11 of them were excluded from the full-length review as they did not report the outcome of interest while 5 articles were excluded because they were not observational studies. Six studies [9,10,11,12,13,14] were subsequently excluded because they did not use standard AKI definitions of Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE), Acute Kidney Injury Network (AKIN), and Kidney Disease: Improving Global Outcomes (KDIGO) classifications. Thus, 17 cohort studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,37] comprising 24,158 patients undergoing THA were included into the meta-analysis of associated AKI incidence. The literature retrieval, review, and selection process are demonstrated in Figure 1. The characteristics of the included studies are presented in Table 1.
Figure 1.
Outline of our search methodology.
Table 1.
Main characteristic of studies included in meta-analysis of AKI incidence in patients undergoing THA [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33].
| Study | Year | Country | Study Design | Procedures/Patients | Number of Patients | AKI Definition | Incidence |
|---|---|---|---|---|---|---|---|
| Ulucay et al. [18] | 2012 | Turkey | Cohort | Cemented bipolar hip arthroplasty for femur neck fracture; aged ≥65 years | 163 | Acute kidney injury; AKIN criteria | 25/163 (15.3%) Dialysis 3/163 (1.8%) |
| Choi et al. [1,19] | 2013 | South Korea | Cohort | Total hip replacement for avascular necrosis | 489 | Acute kidney injury; AKIN criteria | 47/489 (9.6%) |
| Challagunda et al. [20] | 2013 | UK | Cohort | Elective hip surgery | 112 | Acute kidney injury; RIFLE criteria | 33/112 (29.5%) |
| Craxford et al. [21] | 2014 | UK | Cohort | Total hip replacement | 200 | Acute kidney injury; RIFLE criteria | 7/200 (3.5%) |
| Li et al. [22] | 2014 | China | Cohort | Total hip replacement | 900 | Acute kidney injury; AKIN criteria | 93/900 (10.3%) |
| Kimmel et al. [23] | 2014 | Australia | Cohort | Primary elective total hip arthroplasty | 173 | Acute kidney injury; RIFLE criteria | 14/173 (8.1%) |
| Hassan et al. [37] | 2015 | Denmark | Cohort | Primary hip replacement | 586 | Acute kidney injury; RIFLE criteria | 81/586 (13.8%) |
| Warth et al. [24] | 2016 | USA | Cohort | Total hip arthroplasty | 488 | Acute kidney injury; AKIN criteria | 22/488 (4.5%) Dialysis 0/488 (0%) |
| Nowicka et al. [25] | 2016 | UK | Cohort | Elective hip arthroplasty | 156 | Acute kidney injury; AKIN criteria | 11/156 (7.1%) Dialysis 0/156 (0%) |
| Choi et al. [2,26] | 2016 | South Korea | Cohort | Total hip replacement | 2467 | Acute kidney injury; AKIN criteria | 119/2467 (4.8%) |
| Johansson et al. [27] | 2016 | Denmark | Cohort | Elective total hip replacement | 136 | Acute kidney injury; KDIGO criteria | 27/136 (19.9%) |
| Perregaard et al. [28] | 2016 | Denmark | Cohort | Primary elective total hip replacement | 3416 | Acute kidney injury; KDIGO criteria | 75/3416 (2.2%) |
| Ferguson et al. [29] | 2017 | UK | Cohort | Primary hip arthroplasty | 187 | Acute kidney injury; KDIGO criteria | 20/187 (10.7%) Dialysis 0/187 (0%) |
| Jiang et al. [30] | 2017 | USA | Cohort | Total hip arthroplasty | 411 | Acute kidney injury; RIFLE criteria | 30/411 (7.3%) |
| Gharaibeh et al. [31] | 2017 | USA | Cohort | Total hip arthroplasty | 10323 | Acute kidney injury; KDIGO criteria | 114/10323 (1.1%) |
| Weinstein et al. [32] | 2018 | USA | Cohort | Unilateral total hip arthroplasty with intraoperative controlled hypotension under neuraxial anesthesia | 2431 | Acute kidney injury; RIFLE or AKIN criteria | 45/2431 (1.9%) |
| Tucker et al. [33] | 2018 | UK | Cohort | Primary hip arthroplasty | 1420 | Acute kidney injury; AKIN criteria | 19/1420 (1.3%) |
Abbreviations: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network;KDIGO, Kidney Disease Improving Global Outcomes; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; THA, total hip arthroplasties; UK, United Kingdom; USA, United States of America.
3.1. Incidence of AKI in Patients Undergoing THA
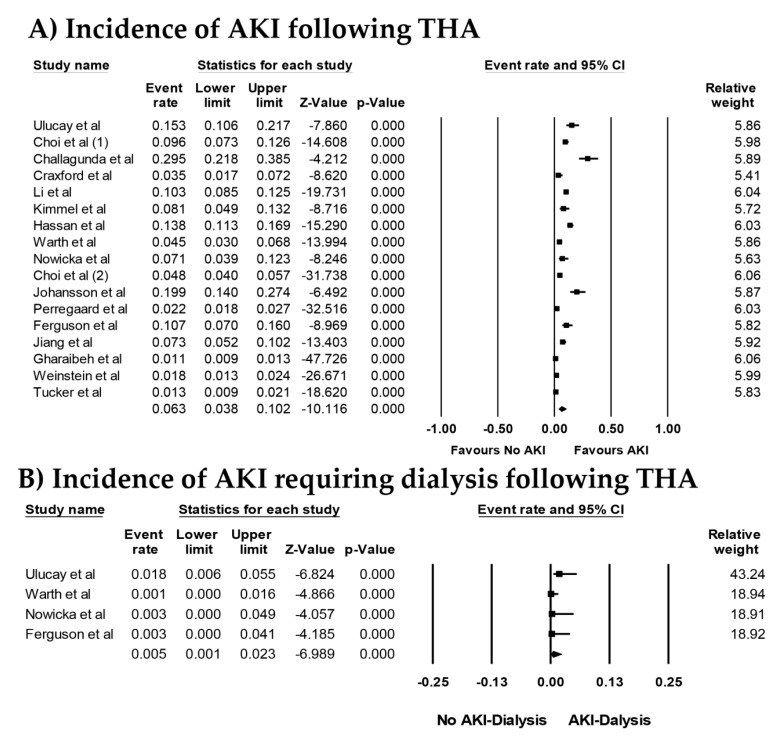
Overall, the pooled estimated incidence rates of AKI and severe AKI requiring dialysis following THA were 6.3% (95% CI: 3.8%–10.2%, I2 = 97%, Figure 2A) and 0.5% (95% CI: 0.1%–2.3%, I2 = 42%, Figure 2B), respectively. Subgroup analysis based on AKI definitions was performed and showed the pooled estimated incidence rates of AKI of 5.5% (95% CI: 1.3%–21.0%, I2 = 98%) by RIFLE criteria, 8.4% (95% CI: 5.2%–13.3%, I2 = 95%) by AKIN criteria, and 4.2% (95% CI: 1.9%–9.0%, I2 = 96%) by KDIGO criteria, respectively. We conducted a sensitivity analysis by excluding with patients undergoing THA for hip fractures. The pooled estimated incidence rates of AKI following THA for non-fracture indications was 5.6% (95% CI: 3.3%–9.3%, I2 = 97%). The pooled estimated incidence rates of AKI following THA among patients with fractures was 14.2% (95% CI: 11.8%–16.9%, I2 = 0%).
Figure 2.
Forest plots of the included studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] assessing (A) incidence rates of AKI and (B) incidence rates of AKI requiring dialysis following THA. A diamond data marker represents the overall rate from each included study (square data marker) and 95% confidence interval (CI).
Subgroup analysis based on the countries by continent was performed and demonstrated pooled estimated incidence of AKI following THA of 9.2% (95% CI: 5.6%–14.8%) in Asia, 8.1% (95% CI: 4.9%–13.2%) in Australia, 7.4% (95% CI: 3.2%–16.3%) in Europe, and 2.8% (95% CI: 1.2%–17.0%) in North America.
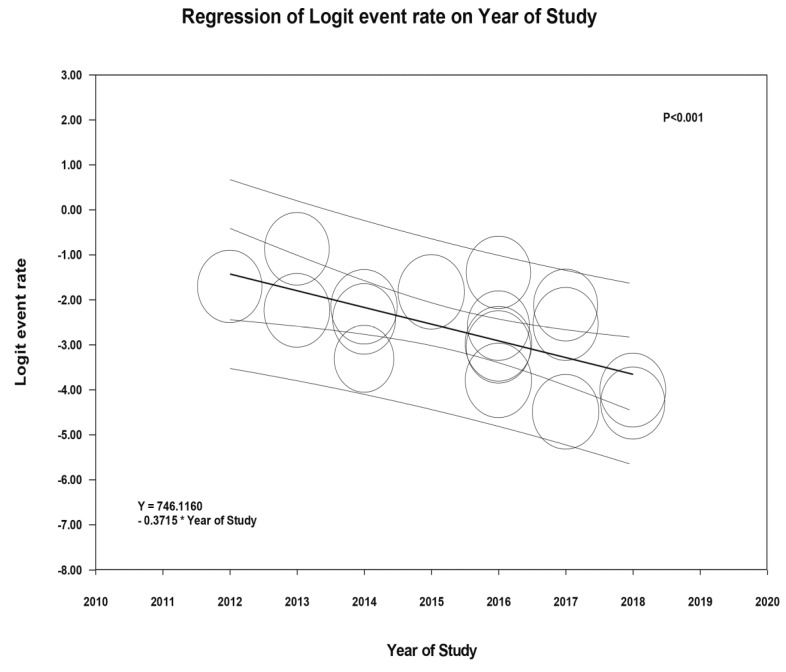
Meta-regression of all included studies showed significant negative correlation between incidence of AKI following THA and the study year (slope = −0.37, p <0.001), as shown in Figure 3.
Figure 3.
Meta-regression analyses showed significant negative correlation between incidence of AKI following THA and the study year (slope = −0.37, p <0.001). The solid black line represents the weighted regression line based on variance-weighted least squares. The inner and outer lines show the 95% confidence interval and prediction interval around the regression line. The circles indicate log event rates in each study.
3.2. Risk Factors for AKI in Patients Undergoing THA
Reported risk factors for AKI in patients undergoing THA are demonstrated in Table 2 [18,19,20,22,23,24,26,27,29,30,31,32,33,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. Older age [23,24,26,29,31,55,60], higher body mass index (BMI) [19,22,23,24,26,30,31,46,47,48,49,56,57,59], reduced baseline estimated glomerular filtration rate (eGFR)/chronic kidney disease (CKD) [31,54,55,57,60], diabetes mellitus (DM) [24,26,31,34,47,55,57], nonsteroidal anti-inflammatory drug (NSAID) [23,32,53] use, and perioperative blood transfusion [23,47,53,55] were consistently identified as important risk factors for AKI in patients undergoing THA.
Table 2.
Reported Risk Factors for AKI in patients undergoing THA [18,19,20,22,23,24,26,27,29,30,31,32,33,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62].
| Study | Risk Factors for AKI |
|---|---|
| Jafari et al. [46] | Elevated BMI, elevated baseline creatinine, history of COPD, liver disease, CHF, hypertension, underlying heart disease |
| Weingarten et al. [47] | Elevated BMI, DM, the number of baseline antihypertensive medications, cerebral or peripheral vascular disease, the use of general anesthesia, perioperative blood transfusion |
| Ulucay et al. [18] | Lower baseline eGFR |
| Choi et al. [1,19] | Transplantation, increased weight |
| Li et al. [22] | Transplantation, increased weight |
| Challagundla et al. [20] | Male, ACEI/ARB |
| Nielson et al. [48] | Preoperative ACEI/ARB, BMI, CAD, intra-operative hypotension |
| Kimmel et al. [23] | Older age, increased BMI, lower baseline eGFR, NSAID use, ACEI/ARB use, blood transfusion |
| Ward et al. [49] | Increased BMI |
| Marty et al. [50] | Postoperative resistive index |
| Courtney et al. [51] | Dual antibiotics prophylaxis (cefazolin + vancomycin vs. cefazolin), ASA classification, preoperative kidney disease |
| Opperer et al. [52] | Perioperative fluid resuscitation with 6% HES or 5% albumin |
| Aeng et al. [53] | Gentamicin in premanufactured bone cement, intraoperative blood transfusion, postoperative NSAID use |
| Warth et al. [24] | Older age, elevated BMI, DM, smoking |
| Tan et al. [54] | CKD |
| Nadkarni et al. [55] | Older age, male sex, black race, CKD, CHF, chronic liver disease, hypertension, DM, atrial fibrillation, HCV infection, postoperative sepsis, acute MI, blood transfusion, urban hospital, small hospital size |
| Meller et al. [56] | Morbid obesity |
| Choi et al. [2,26] | Postoperative anemia, older age, male sex, BMI <22 or ≥25, DM, beta-blocker, ARB use |
| Ferguson et al. [29] | Older age, the use of ≥1 L of postoperative fluid |
| Johansson et al. [27] | Gentamicin, female sex |
| Geller et al. [57] | Higher BMI, lower baseline hemoglobin, history of comorbid condition (DM, CKD, CVD, hypertension) |
| Jiang et al. [30] | Perioperative ACEI/ARB use, vancomycin use, increased BMI |
| Zainudheen et al. [58] | Use of renin-angiotensin antagonists |
| Jamsa et al. [59] | Lower preoperative eGFR, ASA classification, BMI, duration of operation |
| Ghareibeh et al. [31] | Entire cohort: older age, male, CKD, heart failure, diabetes, hypertension Nested case control: elevated BMI, heart failure, DM, hypertension, lower GFR, transfusion |
| Yadav et al. [60] | Older age, surgery for periprosthetic joint infection, CKD, total number of surgeries |
| Weinstein et al. [32] | Lower baseline GFR, lower baseline hemoglobin, previous NSAID use |
| Tucker et al. [33] | Gentamicin use |
| Klement et al. [61] | Co-occurrence of a mental illness and a substance abuse disorder |
| Abar et al. [62] | Elevated preoperative creatinine, larger postoperative drop in hemoglobin, and higher ASA classification |
| Hassan et al. [37] | Older age, hypertension, general anesthesia, high ASA score, low baseline systolic and diastolic blood pressure, hip fracture |
| Dubrovskaya et al. [34] | Hospital stay >1 day prior to surgery, knee or hip surgery, DM |
| Bailey et al. [38] | Prophylactic use of flucloxacillin and gentamicin versus cefuroxime alone |
Abbreviations: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers; AKI, acute kidney injury; ASA, American Society of Anesthesiologists; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; NSAID, nonsteroidal anti-inflammatory drug; THA, total hip arthroplasties.
3.3. Evaluation for Publication Bias
Funnel plot (Supplementary Figure S1) and Egger’s regression asymmetry test were performed to evaluate for publication bias in analysis evaluating incidence of AKI in patients undergoing THA. There was no significant publication bias in meta-analysis assessing incidence of AKI in patients undergoing THA, p = 0.20.
4. Discussion
In this systematic review and meta-analysis, we found that patients who underwent THA had incidences of AKI (using a standard AKI definition) and AKI requiring dialysis of 6.3% and 0.5%, respectively. In addition, our findings showed a statistically significant negative correlation between incidence of AKI following THA and the study year, representing potential improvement in the AKI incidence for patients undergoing THA over time.
Similar to other types of perioperative AKI, the pathogenesis of THA-related AKI is multifactorial including intraoperative hypotension, perioperative anemia and blood transfusion, antibiotic-related AKI, and nephrotoxic agents such as NSAID use [18,19,20,22,23,24,26,27,29,30,31,32,33,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. Our study demonstrated an overall low incidence of AKI following THA of 6.3%, and comparatively much lower than AKI incidence following other types of major surgeries such as coronary artery bypass grafting (AKI incidence approximately 20%) [63,64], vascular surgery (AKI incidence approximately 25%) [35,65], or heart valve replacement surgery (AKI incidence approximately 35% to 47%) [66]. In addition, the majority of AKI following THA is mild in severity [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33] and only 0.5% of patients undergoing THA developed severe AKI requiring dialysis, as demonstrated by our meta-analysis. Despite the low incidence of AKI among patients undergoing THA, those who develop AKI following THA still carry significant increased mortality and worse outcomes including prolonged hospital stay [39,46,67,68] and hospital readmissions after THA [69,70]. In addition, it is now evident that even mild AKI is still associated with poor long-term outcomes including development of cardiovascular diseases and CKD [71,72]. Thus, perioperative medicine remains critically important in patients undergoing THA in order to prevent perioperative AKI [73].
Our study also showed a potential improvement in the AKI incidence following THA over time. This is likely explained by advances in perioperative medicine care [73]. As described earlier in this systematic review, the pathogenesis of AKI in patients undergoing THA is multifactorial. Risk factors for AKI following THA are summarized in Table 2. Since older age, higher BMI, CKD, DM were consistently identified as important risk factors for AKI in patients undergoing THA [18,19,20,22,23,24,26,27,29,30,31,32,33,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62], clinicians and nephrologists should pay close attention to particular populations of patients. Limitation of nephrotoxic agents such as NSAIDs and a judicious use of perioperative blood transfusion should be considered [23,47,53,55] for preventive measures to reduce AKI following THA. In patients undergoing THA, recent studies have demonstrated significant avoidance of NSAID use in those possessing higher AKI risk, such as patients with CKD and congestive heart failure (CHF) [29,31]. In addition, in recent years, the use of hydroxyethyl starch (HES) solutions, unnecessary blood transfusions, chloride-rich intravenous fluids, and aminoglycosides have been discouraged [29,52,73]. Furthermore, although the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB) and perioperative AKI risk remains controversial [2,74,75], ACEIs/ARBs have been commonly discontinued before THA to prevent intraoperative hypotension [29]. Future studies are required to assess if discontinuation of ACEIs/ARBs before THA may affect the incidence of THA-associated AKI, and whether it should be included in AKI preventative strategies and care optimization among patients undergoing THA [37,40].
Several limitations in our meta-analysis are worth mentioning. First, there are statistical heterogeneities in our study. Possible sources for heterogeneities were the differences in patient characteristics between the individual studies. Studies involving large sample size observed lower AKI incidence compared to those with small sample size. Studies outside USA observed higher AKI incidence following THA, suggesting potential differences in operative surgery, antibiotic and blood transfusion, and perioperative care. Subgroup analysis based on the countries by continent demonstrated significantly different AKI incidences in each continent. Furthermore, the meta-regression analysis that demonstrated a significant negative correlation of post-THA AKI incidence with time adds the contribution of study year as a source of heterogeneity in our study. Second, there was limited data on the AKI incidence following THA from countries in South America and Africa. Third, there is a lack of data from the included studies on novel AKI biomarkers and AKI diagnosis based on urine output criteria. Lastly, this is a systematic review and meta-analysis of cohort studies and the data from population based studies were limited. Thus, future population based studies evaluating the incidence of AKI following THA are required.
In summary, there is an overall low incidence of AKI of 6.3% among patients undergoing THA. There has also been potential improvement in AKI incidence for patients undergoing THA over time.
Acknowledgments
None. All authors had access to the data and played essential roles in writing of the manuscript.
Supplementary Materials
The following are available online at http://www.mdpi.com/2077-0383/8/1/66/s1, Online Supplementary Data 1. Search terms for systematic review, Supplementary Figure S1. Funnel plot evaluating for publication bias evaluating incidence of AKI in patients undergoing THA.
Author Contributions
Conceptualization, C.T., W.K., N.T., W.C.; Data curation, C.T., W.K., W.C.; Formal analysis, C.T., W.C.; Funding acquisition, C.T. and K.W.; Investigation, C.T., W.K., W.C.; Methodology, C.T., W.K., N.T., W.C.; Project administration, K.W., P.L., S.A.S., P.U., A.T.-O., N.R.A.; Resources, K.W.; Software, K.W.; Supervision, M.A.M. and W.C.; Validation, C.T. and W.C.; Writing—original draft, C.T.; Writing—review & editing, C.T., W.K., N.T., T.B., K.W., P.L., S.A.S., P.U., N.R.A., A.T.-O., M.A.M., W.C.
Conflicts of Interest
The authors deny any conflict of interest.
References
- 1.Thongprayoon C., Cheungpasitporn W., Harrison A.M., Kittanamongkolchai W., Ungprasert P., Srivali N., Akhoundi A., Kashani K.B. The comparison of the commonly used surrogates for baseline renal function in acute kidney injury diagnosis and staging. BMC Nephrol. 2016;17:6. doi: 10.1186/s12882-016-0220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheungpasitporn W., Thongprayoon C., Srivali N., O’corragain O.A., Edmonds P.J., Ungprasert P., Kittanamongkolchai W., Erickson S.B. Preoperative renin-angiotensin system inhibitors use linked to reduced acute kidney injury: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2015;30:978–988. doi: 10.1093/ndt/gfv023. [DOI] [PubMed] [Google Scholar]
- 3.Hoste E.A., Kellum J.A., Selby N.M., Zarbock A., Palevsky P.M., Bagshaw S.M., Goldstein S.L., Cerdá J., Chawla L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018;14:607–625. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 4.Gameiro J., Agapito Fonseca J., Jorge S., Lopes J.A. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J. Clin. Med. 2018;7:307. doi: 10.3390/jcm7100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehgal V., Bajwa S.J., Sehgal R., Eagan J., Reddy P., Lesko S.M. Predictors of acute kidney injury in geriatric patients undergoing total knee replacement surgery. Int. J. Endocrinol. Metab. 2014;12:e16713. doi: 10.5812/ijem.16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H.J., Koh W.U., Kim S.G., Park H.S., Song J.G., Ro Y.J., Yang H.S. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: A retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine. 2016;95:e4489. doi: 10.1097/MD.0000000000004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta R.L., Burdmann E.A., Cerdá J., Feehally J., Finkelstein F., García-García G., Godin M., Jha V., Lameire N.H., Levin N.W., et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: A multinational cross-sectional study. Lancet. 2016;387:2017–2025. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 8.Mehta R.L., Cerdá J., Burdmann E.A., Tonelli M., García-García G., Jha V., Susantitaphong P., Rocco M., Vanholder R., Sever M.S., et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 9.Coventry M.B., Beckenbaugh R.D., Nolan D.R., Ilstrup D.M. 2012 total hip arthroplasties. A study of postoperative course and early complications. J. Bone Joint Surg. Am. 1974;56:273–284. doi: 10.2106/00004623-197456020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sharrock N.E., Beksac B., Flynn E., Go G., Della Valle A.G. Hypotensive epidural anaesthesia in patients with preoperative renal dysfunction undergoing total hip replacement. Br. J. Anaesth. 2006;96:207–212. doi: 10.1093/bja/aei308. [DOI] [PubMed] [Google Scholar]
- 11.Jameson S.S., Khan S.K., Baker P., James P., Gray A., Reed M.R., Deehan D.J. A national analysis of complications following hemiarthroplasty for hip fracture in older patients. QJM. 2012;105:455–460. doi: 10.1093/qjmed/hcs004. [DOI] [PubMed] [Google Scholar]
- 12.Horstmann W.G., Swierstra M.J., Ohanis D., Castelein R.M., Kollen B.J., Verheyen C.C. Reduction of blood loss with the use of a new combined intra-operative and post-operative autologous blood transfusion system compared with no drainage in primary total hip replacement. Bone Joint J. 2013;95-B:616–622. doi: 10.1302/0301-620X.95B5.30472. [DOI] [PubMed] [Google Scholar]
- 13.Regenbogen S.E., Cain-Nielsen A.H., Norton E.C., Chen L.M., Birkmeyer J.D., Skinner J.S. Costs and Consequences of Early Hospital Discharge After Major Inpatient Surgery in Older Adults. JAMA Surg. 2017;152:e170123. doi: 10.1001/jamasurg.2017.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenkel Rutenberg T., Daglan E., Heller S., Velkes S. A comparison of treatment setting for elderly patients with hip fracture, is the geriatric ward superior to conventional orthopedic hospitalization? Injury. 2017;48:1584–1588. doi: 10.1016/j.injury.2017.04.049. [DOI] [PubMed] [Google Scholar]
- 15.Pulido L., Parvizi J., Macgibeny M., Sharkey P.F., Purtill J.J., Rothman R.H., Hozack W.J. In hospital complications after total joint arthroplasty. J. Arthroplast. 2008;23(Suppl. 1):139–145. doi: 10.1016/j.arth.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 16.McDermott K.W., Freeman W.J., Elixhauser A. Overview of Operating Room Procedures During Inpatient Stays in U.S. Hospitals, 2014: Statistical Brief #233. [(accessed on 9 January 2019)];2006 Available online: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb233-Operating-Room-Procedures-United-States-2014.pdf. [PubMed]
- 17.Abdel M.P., Watts C.D., Houdek M.T., Lewallen D.G., Berry D.J. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: A 40-year experience. Bone Joint J. 2016;98-B:461–467. doi: 10.1302/0301-620X.98B4.37201. [DOI] [PubMed] [Google Scholar]
- 18.Ulucay C., Eren Z., Kaspar E.C., Ozler T., Yuksel K., Kantarci G., Altintas F. Risk factors for acute kidney injury after hip fracture surgery in the elderly individuals. Geriatr. Orthop. Surg. Rehabil. 2012;3:150–156. doi: 10.1177/2151458512473827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y.J., Lee E.H., Hahm K.D., Kwon K., Ro Y.J. Transplantation is a risk factor for acute kidney injury in patients undergoing total hip replacement arthroplasty for avascular necrosis: An observational study. Transplant. Proc. 2013;45:2220–2225. doi: 10.1016/j.transproceed.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Challagundla S.R., Knox D., Hawkins A., Hamilton D., WVFlynn R., Robertson S., Isles C. Renal impairment after high-dose flucloxacillin and single-dose gentamicin prophylaxis in patients undergoing elective hip and knee replacement. Nephrol. Dial. Transplant. 2013;28:612–619. doi: 10.1093/ndt/gfs458. [DOI] [PubMed] [Google Scholar]
- 21.Craxford S., Bayley E., Needoff M. Antibiotic-associated complications following lower limb arthroplasty: A comparison of two prophylactic regimes. Eur. J. Orthop. Surg. Traumatol. 2014;24:539–543. doi: 10.1007/s00590-013-1348-1. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Guo D., Shi G., Li R., Li X., Shen R., Wang H., Li Y., Yuan F., Han G. Role of total hip replacement arthroplasty between transplantation and acute kidney injury. Ren. Fail. 2014;36:899–903. doi: 10.3109/0886022X.2014.900387. [DOI] [PubMed] [Google Scholar]
- 23.Kimmel L.A., Wilson S., Janardan J.D., Liew S.M., Walker R.G. Incidence of acute kidney injury following total joint arthroplasty: A retrospective review by RIFLE criteria. Clin. Kidney J. 2014;7:546–551. doi: 10.1093/ckj/sfu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warth L.C., Noiseux N.O., Hogue M.H., Klaassen A.L., Liu S.S., Callaghan J.J. Risk of Acute Kidney Injury After Primary and Revision Total Hip Arthroplasty and Total Knee Arthroplasty Using a Multimodal Approach to Perioperative Pain Control Including Ketorolac and Celecoxib. J. Arthroplast. 2016;31:253–255. doi: 10.1016/j.arth.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Nowicka A., Selvaraj T. Incidence of acute kidney injury after elective lower limb arthroplasty. J. Clin. Anesth. 2016;34:520–523. doi: 10.1016/j.jclinane.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y.J., Kim S.O., Sim J.H., Hahm K.D. Postoperative Anemia Is Associated with Acute Kidney Injury in Patients Undergoing Total Hip Replacement Arthroplasty: A Retrospective Study. Anesth. Analg. 2016;122:1923–1928. doi: 10.1213/ANE.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 27.Johansson S., Christensen O.M., Thorsmark A.H. A retrospective study of acute kidney injury in hip arthroplasty patients receiving gentamicin and dicloxacillin. Acta Orthop. 2016;87:589–591. doi: 10.1080/17453674.2016.1231008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perregaard H., Damholt M.B., Solgaard S., Petersen M.B. Renal function after elective total hip replacement. Acta Orthop. 2016;87:235–238. doi: 10.3109/17453674.2016.1155130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson K.B., Winter A., Russo L., Khan A., Hair M., MacGregor M.S., Holt G. Acute kidney injury following primary hip and knee arthroplasty surgery. Ann. R. Coll. Surg. Engl. 2017;99:307–312. doi: 10.1308/rcsann.2016.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang E.X., Gogineni H.C., Mayerson J.L., Glassman A.H., Magnussen R.A., Scharschmidt T.J. Acute Kidney Disease After Total Hip and Knee Arthroplasty: Incidence and Associated Factors. J. Arthroplast. 2017;32:2381–2385. doi: 10.1016/j.arth.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Gharaibeh K.A., Hamadah A.M., Sierra R.J., Leung N., Kremers W.K., El-Zoghby Z.M. The Rate of Acute Kidney Injury After Total Hip Arthroplasty Is Low but Increases Significantly in Patients with Specific Comorbidities. J. Bone Joint Surg. Am. 2017;99:1819–1826. doi: 10.2106/JBJS.16.01027. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein S.M., YaDeau J.T., Memtsoudis S.G. Lack of Association Between Levels and Length of Intraoperative Controlled Hypotension and Acute Kidney Injury in Total Hip Arthroplasty Patients Receiving Neuraxial Anesthesia. Reg. Anesth. Pain Med. 2018;43:725–731. doi: 10.1097/AAP.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 33.Tucker A., Hegarty P., Magill P.J., Blaney J., Armstrong L.V., McCaffrey J.E., Beverland D.E. Acute Kidney Injury After Prophylactic Cefuroxime and Gentamicin in Patients Undergoing Primary Hip and Knee Arthroplasty-A Propensity Score-Matched Study. J. Arthroplast. 2018;33:3009–3015. doi: 10.1016/j.arth.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Dubrovskaya Y., Tejada R., Bosco I.I.I.J., Stachel A., Chen D., Feng M., Rosenberg A., Phillips M. Single high dose gentamicin for perioperative prophylaxis in orthopedic surgery: Evaluation of nephrotoxicity. SAGE Open Med. 2015;3 doi: 10.1177/2050312115612803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell S., Davey P., Nathwani D., Marwick C., Vadiveloo T., Sneddon J., Patton A., Bennie M., Fleming S., Donnan P.T. Risk of AKI with gentamicin as surgical prophylaxis. J. Am. Soc. Nephrol. 2014;25:2625–2632. doi: 10.1681/ASN.2014010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross A.D., Boscainos P.J., Malhas A., Wigderowitz C. Peri-operative renal morbidity secondary to gentamicin and flucloxacillin chemoprophylaxis for hip and knee arthroplasty. Scott. Med. J. 2013;58:209–212. doi: 10.1177/0036933013507850. [DOI] [PubMed] [Google Scholar]
- 37.Hassan B.K., Sahlstrom A., Dessau R.B. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J. Orthop. Surg. Res. 2015;10:158. doi: 10.1186/s13018-015-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey O., Torkington M.S., Anthony I., Wells J., Blyth M., Jones B. Antibiotic-related acute kidney injury in patients undergoing elective joint replacement. Bone Joint J. 2014;96-B:395–398. doi: 10.1302/0301-620X.96B3.32745. [DOI] [PubMed] [Google Scholar]
- 39.Bennet S.J., Berry O.M., Goddard J., Keating J.F. Acute renal dysfunction following hip fracture. Injury. 2010;41:335–338. doi: 10.1016/j.injury.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Bjerregaard L.S., Jorgensen C.C., Kehlet H. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol. 2016;82:757–776. [PubMed] [Google Scholar]
- 41.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 44.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 46.Jafari S.M., Huang R., Joshi A., Parvizi J., Hozack W.J. Renal impairment following total joint arthroplasty: Who is at risk? J. Arthroplast. 2010;25:49–53. doi: 10.1016/j.arth.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Weingarten T.N., Gurrieri C., Jarett P.D., Brown D.R., Berntson N.J., Calaro R.D., Jr., Kor D.J., Berry D.J., Garovic V.D., Nicholson W.T., et al. Acute kidney injury following total joint arthroplasty: Retrospective analysis. Can. J. Anaesth. 2012;59:1111–1118. doi: 10.1007/s12630-012-9797-2. [DOI] [PubMed] [Google Scholar]
- 48.Nielson E., Hennrikus E., Lehman E., Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J. Hosp. Med. 2014;9:283–288. doi: 10.1002/jhm.2155. [DOI] [PubMed] [Google Scholar]
- 49.Ward D.T., Metz L.N., Horst P.K., Kim H.T., Kuo A.C. Complications of Morbid Obesity in Total Joint Arthroplasty: Risk Stratification Based on BMI. J. Arthroplast. 2015;30:42–46. doi: 10.1016/j.arth.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 50.Marty P., Szatjnic S., Ferre F., Conil J.M., Mayeur N., Fourcade O., Silva S., Minville V. Doppler renal resistive index for early detection of acute kidney injury after major orthopaedic surgery: A prospective observational study. Eur. J. Anaesthesiol. 2015;32:37–43. doi: 10.1097/EJA.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 51.Courtney P.M., Melnic C.M., Zimmer Z., Anari J., Lee G.C. Addition of Vancomycin to Cefazolin Prophylaxis Is Associated With Acute Kidney Injury After Primary Joint Arthroplasty. Clin. Orthop. Relat. Res. 2015;473:2197–2203. doi: 10.1007/s11999-014-4062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opperer M., Poeran J., Rasul R., Mazumdar M., Memtsoudis S.G. Use of perioperative hydroxyethyl starch 6% and albumin 5% in elective joint arthroplasty and association with adverse outcomes: A retrospective population based analysis. BMJ. 2015;350:h1567. doi: 10.1136/bmj.h1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aeng E.S., Shalansky K.F., Lau T.T., Zalunardo N., Li G., Bowie W.R., Duncan C.P. Acute Kidney Injury With Tobramycin-Impregnated Bone Cement Spacers in Prosthetic Joint Infections. Ann. Pharmacother. 2015;49:1207–1213. doi: 10.1177/1060028015600176. [DOI] [PubMed] [Google Scholar]
- 54.Tan T.L., Kheir M.M., Tan D.D., Filippone E.J., Tischler E.H., Chen A.F. Chronic Kidney Disease Linearly Predicts Outcomes After Elective Total Joint Arthroplasty. J. Arthroplast. 2016;31:175–179. doi: 10.1016/j.arth.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Nadkarni G.N., Patel A.A., Ahuja Y., Annapureddy N., Agarwal S.K., Simoes P.K., Konstantinidis I., Kamat S., Archdeacon M., Thakar C.V. Incidence, Risk Factors, and Outcome Trends of Acute Kidney Injury in Elective Total Hip and Knee Arthroplasty. Am. J. Orthop. 2016;45:E12–E19. [PubMed] [Google Scholar]
- 56.Meller M.M., Toossi N., Gonzalez M.H., Son M.S., Lau E.C., Johanson N. Surgical Risks and Costs of Care are Greater in Patients Who Are Super Obese and Undergoing THA. Clin. Orthop. Relat. Res. 2016;474:2472–2481. doi: 10.1007/s11999-016-5039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geller J.A., Cunn G., Herschmiller T., Murtaugh T., Chen A. Acute Kidney Injury After First-Stage Joint Revision for Infection: Risk Factors and the Impact of Antibiotic Dosing. J. Arthroplast. 2017;32:3120–3125. doi: 10.1016/j.arth.2017.04.054. [DOI] [PubMed] [Google Scholar]
- 58.Zainudheen A., Scott I.A., Caney X. Association of renin angiotensin antagonists with adverse perioperative events in patients undergoing elective orthopaedic surgery: A case-control study. Intern. Med. J. 2017;47:999–1005. doi: 10.1111/imj.13487. [DOI] [PubMed] [Google Scholar]
- 59.Jamsa P., Jamsen E., Lyytikainen L.P., Kalliovalkama J., Eskelinen A., Oksala N. Risk factors associated with acute kidney injury in a cohort of 20,575 arthroplasty patients. Acta Orthop. 2017;88:370–376. doi: 10.1080/17453674.2017.1301743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yadav A., Alijanipour P., Ackerman C.T., Karanth S., Hozack W.J., Filippone E.J. Acute Kidney Injury Following Failed Total Hip and Knee Arthroplasty. J. Arthroplast. 2018;33:3297–3303. doi: 10.1016/j.arth.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Klement M.R., Nickel B.T., Bala A., Penrose C.T., Green C.L., Wellman S.S., Bolognesi M.P., Seyler T.M. Dual Diagnosis and Total Hip Arthroplasty. Orthopedics. 2018;41:e321–e327. doi: 10.3928/01477447-20180213-09. [DOI] [PubMed] [Google Scholar]
- 62.Abar O., Toossi N., Johanson N. Cost and determinants of acute kidney injury after elective primary total joint arthroplasty. Arthroplast. Today. 2018;4:335–339. doi: 10.1016/j.artd.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheungpasitporn W., Thongprayoon C., Kittanamongkolchai W., Srivali N., O’Corragain O.A., Edmonds P.J., Ratanapo S., Spanuchart I., Erickson S.B. Comparison of renal outcomes in off-pump versus on-pump coronary artery bypass grafting: A systematic review and meta-analysis of randomized controlled trials. Nephrology. 2015;20:727–735. doi: 10.1111/nep.12506. [DOI] [PubMed] [Google Scholar]
- 64.Sakhuja A., Kashani K., Schold J., Cheungpasitporn W., Soltesz E., Demirjian S. Hospital procedure volume does not predict acute kidney injury after coronary artery bypass grafting-a nationwide study. Clin. Kidney J. 2017;10:769–775. doi: 10.1093/ckj/sfx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheungpasitporn W., Thongprayoon C., Kashani K. Transcatheter Aortic Valve Replacement: A Kidney’s Perspective. J. Ren. Inj. Prev. 2016;5:1–7. doi: 10.15171/jrip.2016.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thongprayoon C., Cheungpasitporn W., Lin J., Mao M.A., Qian Q. Acute kidney injury in octogenarians after heart valve replacement surgery: A study of two periods over the last decade. Clin. Kidney J. 2017;10:648–654. doi: 10.1093/ckj/sfx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Napier R.J., Spence D., Diamond O., O’Brien S., Walsh T., Beverland D.E. Modifiable factors delaying early discharge following primary joint arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2013;23:665–669. doi: 10.1007/s00590-012-1053-5. [DOI] [PubMed] [Google Scholar]
- 68.Panteli M., Habeeb S., McRoberts J., Porteous M.J. Enhanced care for primary hip arthroplasty: Factors affecting length of hospital stay. Eur. J. Orthop. Surg. Traumatol. 2014;24:353–358. doi: 10.1007/s00590-013-1188-z. [DOI] [PubMed] [Google Scholar]
- 69.Schairer W.W., Vail T.P., Bozic K.J. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin. Orthop. Relat. Res. 2014;472:181–187. doi: 10.1007/s11999-013-3030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pugely A.J., Callaghan J.J., Martin C.T., Cram P., Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: Analysis from the ACS-NSQIP. J. Arthroplast. 2013;28:1499–1504. doi: 10.1016/j.arth.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 71.Coca S.G., Yusuf B., Shlipak M.G., Garg A.X., Parikh C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murugan R., Kellum J.A. Acute kidney injury: what’s the prognosis? Nat. Rev. Nephrol. 2011;7:209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goren O., Matot I. Perioperative acute kidney injury. Br. J. Anaesth. 2015;115(Suppl. 2):ii3–ii14. doi: 10.1093/bja/aev380. [DOI] [PubMed] [Google Scholar]
- 74.Xu N., Long Q., He T., Liu X., Dai H., Lu Y., Wen J., Wu Q., Yuan H. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin. Nephrol. 2018;89:403–414. doi: 10.5414/CN109319. [DOI] [PubMed] [Google Scholar]
- 75.Tagawa M., Ogata A., Hamano T. Pre- and/or Intra-Operative Prescription of Diuretics, but Not Renin-Angiotensin-System Inhibitors, Is Significantly Associated with Acute Kidney Injury after Non-Cardiac Surgery: A Retrospective Cohort Study. PLoS ONE. 2015;10:e0132507. doi: 10.1371/journal.pone.0132507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.