Abstract
In this study we identified single nucleotide polymorphism (SNP) and sequence characteristic amplification region (SCAR) markers for specific identification of antler-shaped Ganoderma lucidum strains. When the partial mitochondrial SSU rDNA gene sequence of various antler- and kidney-shaped G. lucidum strains were analyzed and aligned, an SNP was found only in the antler-shaped G. lucidum strain at position 456 bp. In addition, this SNP of antler-shaped strains was digested by HinfI restriction enzyme. We further analyzed the polymorphism of various G. lucidum strains by random amplified polymorphic DNA (RAPD) analysis. In RAPD analysis, we isolated and sequenced a fragment, specific for antler-shaped G. lucidum strains. Based on this specific fragment sequence, two sets of specific primer pairs for antler-shaped G. lucidum strains were designed. PCR analysis revealed that two specific bands were observed only from antler-shaped strains. These two molecular markers will be helpful for identification of morphological characteristics of G. lucidum.
Keywords: antler-shape, Ganoderma lucidum, kidney-shape, mitochondrial SSU rDNA, SCAR marker, SNP marker
1. Introduction
Ganoderma lucidum (Curtis) P. Karst. was named by Petter Adolf Karsten in 1881 based on material from England [1,2]. The name G. lucidum has been applied to collections from various countries, including East Africa, Oceania, North America, South America, Asia (China, Japan, and Korea), and Europe [3]. G. lucidum has been used in traditional medicine for thousands of years in East Asian countries, such as China, Japan, and Korea. The bioactive compounds such as flavonoids, ganoderic acid, phenolics, and polysaccharides of G. lucidum have been reported to show immunomodulatory effect, antitumor activity, and inhibitory activity against histamine release and cholesterol synthesis [4,5,6,7]. Antler-shaped G. lucidum is a variant of G. lucidum that is rarely found in nature [8]. This rare variant is famous for its medicinal effect in China and Japan [9]. It is commonly called “Lu jiao Lingzhi” in China, “Rokkaku-Reishi” in Japan [10], and “Nokgak Yeongji” in Korea. Antler-shaped G. lucidum has been reported to contains much larger amounts of β-D-glucans and triterpenoids than kidney-shaped G. lucidum [11,12]. It is also known to be more effective in immunostimulatory and anti-tumor activities than kidney-shaped G. lucidum [12,13]. Therefore, antler-shaped G. lucidum is expected to show much stronger pharmacological activity. On phylogenetic analyses of the genus, G. lucidum from different parts of the world were reported to belong to several separated lineages [14,15,16,17,18]. Regarding research on the markers for Ganoderma species, there are several reports about taxonomic diversity by RAPD, RFLP, AFLP, and other methods [19,20,21,22]. However, there is no marker for specific identification of antler-shaped G. lucidum strain.
Molecular markers are developed by several techniques, such as amplified fragment length polymorphism (AFLP), polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), random amplified polymorphic DNA (RAPD), and sequence characterized amplification region (SCAR) [23]. Among these techniques, PCR-RFLP and RAPD are the quickest and relatively simplest methods and are particularly useful for genetic variation and mutation analyses [19,24]. Single nucleotide polymorphisms (SNPs) have an important role in biomedical and biological researches, including genetic variations, mutations, and investigation of complex genetic diseases [24,25]. SNPs are found most commonly between DNA sequences obtained from different individuals or the same individuals [26,27,28,29]. Therefore, SNP is the most abundant marker system in animal, plant, and microorganism genomes and has recently emerged as the new generation molecular marker for various applications. Furthermore, SNP based technique allows the successful detection and distinction of specific genetic variations even in a low diversity species [30]. The target SNPs are distinguished by digestion using specific restriction enzymes in a process called “PCR-RFLP” [24]. In addition, the combination of RAPD and SCAR markers is a simple and useful tool for molecular analysis or genetic characterization of different species [31].
Ganoderma species has different morphological characteristics due to environmental factors and genetic variations [32]. In addition, development of cultivation techniques has enabled the formation of antler-shaped pileus in China, Japan, and Korea [8,13]. Thus, genetic characterization and accurate identification of antler-shaped G. lucidum are important. In this study, we attempted to develop SNP and SCAR markers for specific identification of antler-shaped G. lucidum at the mycelial stage. These SNP and SCAR markers will be helpful in genetically identifying the morphological characteristics of G. lucidum.
2. Materials and Methods
2.1. Strains and Culture Conditions
Five antler- and nineteen kidney-shaped G. lucidum strains were collected from the Mushroom Division of the Rural Development Administration (Eumseong, Korea), Incheon University (Incheon, Korea), the Korean Agricultural Culture Collection (KACC, Suwon, Korea), the Korean Collection for Type Culture (KTCT, Jeongeup, Korea) (Table 1). In addition, commercial kidney-shaped G. lucidum (Imsil, Korea) was purchased and used in this study. G. lucidum mycelia were cultured in potato dextrose broth (PDB; Difco, Detroit, MI, USA) at 28 °C for 2 weeks.
Table 1.
Ganoderma lucidum strains used in this study.
| No. | Species | Collection | Origin | Shape |
|---|---|---|---|---|
| 1 | Ganoderma lucidum | 1 ASI-7013 | Korea | antler |
| 2 | Ganoderma lucidum | ASI-7135 | Korea | antler |
| 3 | Ganoderma lucidum | ASI-7146 | Korea | antler |
| 4 | Ganoderma lucidum | ASI-7074 | Korea | antler |
| 5 | Ganoderma lucidum | ASI-7094 | Korea | antler |
| 6 | Ganoderma lucidum | ASI-7004 | Korea | kidney |
| 7 | Ganoderma lucidum | ASI-7071 | Korea | kidney |
| 8 | Ganoderma lucidum | ASI-7091 | Korea | kidney |
| 9 | Ganoderma lucidum | ASI-7117 | Korea | kidney |
| 10 | Ganoderma lucidum | 2 IUM-0047 | Korea | kidney |
| 11 | Ganoderma lucidum | IUM-0757 | Korea | kidney |
| 12 | Ganoderma lucidum | IUM-0938 | Korea | kidney |
| 13 | Ganoderma lucidum | IUM-3986 | Korea | kidney |
| 14 | Ganoderma lucidum | IUM-4002 | Korea | kidney |
| 15 | Ganoderma lucidum | IUM-4100 | Korea | kidney |
| 16 | Ganoderma lucidum | IUM-4304 | Bangladesh | kidney |
| 17 | Ganoderma lucidum | IUM-4310 | Bangladesh | kidney |
| 18 | Ganoderma lucidum | 3 KACC42232 | Japan | kidney |
| 19 | Ganoderma lucidum | KACC51689 | Japan | kidney |
| 20 | Ganoderma lucidum | KACC51690 | Japan | kidney |
| 21 | Ganoderma lucidum | ASI-7037 | Papuanewguinea | kidney |
| 22 | Ganoderma lucidum | 4 KCTC 16802 | Thailand | kidney |
| 23 | Ganoderma lucidum | ASI-7068 | USA | kidney |
| 24 | Ganoderma lucidum | ASI-7152 | Korea | kidney |
| 25 | Ganoderma lucidum | Commercial strain | Korea | Kideny |
1 Agricultural Science Institute, 2 Incheon University Mushroom, 3 Korean Agricultural Culture Collection, 4 Korean Collection for Type Cultures.
2.2. DNA Extraction and Amplification
Cultured mycelia [filtered through 2 layers of MiraCloth (Calbiochem, La Jolla, CA, USA)] and fruiting bodies were ground in liquid nitrogen, and genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB) method [33]. Samples (0.5 g) were mixed with 400 mL of extraction buffer (100 mM NaCl, 50 mM EDTA, 0.25 M Tris-HCl, 5% SDS) and 400 mL of 2 × CTAB buffer (2% CTAB, 100 mM Tris-Hcl pH 8.0, 20 mM EDTA pH 8.0, 1.4 M NaCl, 1% polyvinyl pyrrolidone). The extracted DNA was clarified with an extraction solution (phenol–chloroform–isoamyl alcohol, 25:24:1) and then was precipitated with 1/30 volume of 3 M sodium acetate and 1 volume of isopropanol. Purified DNA was sequentially washed with 70% ethanol and dried. The DNA pellet was dissolved in 60 μL of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and treated sequentially with 6 μL of RNase A (20 mg/mL).
The extracted DNA was used as a template (adjusted to 100 ng/µL) for PCR amplification of the partial mitochondrial SSU rDNA gene and for RAPD analysis. All PCR reactions were performed with a premixed polymerase kit (Taq PreMix; TNT Research, Anyang, Korea) in a 20 μL reaction mixture containing 1 μL DNA (100 ng/μL) and 2.5 pmol of each primer. All PCR primer sequences used are shown in Table 2. Partial mitochondrial SSU rDNA gene was amplified using primer pairs of BMS 105 and BMS 173 [34]. Amplification conditions for the mitochondrial SSU rDNA were 3 min of initial denaturation at 94 °C, followed by 25 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 2 min, and final extension at 72 °C for 10 min using TaKaRa Thermal cycler (TaKaRa, Tokyo, Japan). RAPD-PCR amplification conditions were 10 min of initial denaturation at 94 °C, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 2 min, and final extension at 72 °C for 7 min using TaKaRa Thermal cycler (TaKaRa, Tokyo, Japan). PCR products were detected by electrophoresis on 1.2% agarose gel in 0.5 × TAE buffer (Tris-acetic acid-EDTA), stained with ethidium bromide (EtBr), and visualized on a UV transilluminator. The PCR product sizes were determined by comparison to 1 kb Plus Ladder Marker (TNT research, Anyang, Korea).
Table 2.
Primers used in this study.
| Primer | Sequences (5′—3′) | Target |
|---|---|---|
| BSM105_F | ATTAGTCGGTCTCGAAGCAAACG | Partial mitochondrial SSU rDNA gene |
| BSM173_R | TGCTATGACTTTTGAGATGTTAC | Partial mitochondrial SSU rDNA gene |
| URP 1 | ATCCAAGGTCCGAGACAACC | 1 RAPD |
| URP 5 | GGCAAGCTGGTGGGAGGTAC | RAPD |
| KAGL1_F | GGAGGCCGCTGGACTGAGG | Antler-specific |
| KAGL1_R | ATGGGACTGGATCTTGAGGAACA | Antler-specific |
| KAGL2_F | GGCGGCGGCAGAGGAGAG | Antler-specific |
| KAGL2_R | TCGCGACTTGAGAACTGGCATAGC | Antler-specific |
1 Random Amplified Polymorphic DNA.
2.3. Cloning, Sequencing and Sequence Analysis
The PCR products and specific DNA fragments were ligated into pGEM-T easy vector (Promega, Madison, WI, USA), according to the manufacturer’s instruction. After ligation, the plasmids were transformed into competent cell (E. coli DH5α; RBC, New Taipei City, Taiwan) by the heat-shock method [35]. Plasmid DNAs were extracted using FavorPrep™ Plasmid Extraction Kit (Favorgen Biotech Corporation, Pingtung, Taiwan). Insert DNAs of the recombinant plasmids were confirmed by restriction enzyme EcoRI. Sequences were determined by a commercial service (Genotech, Daejeon, Korea) and analyzed using the BioEdit program (http://www.mbio.ncsu.edu/bioedit/ bioedit.html).
2.4. SNP Detection and Validation
Restriction enzyme capable of cleaving antler-shaped G. lucidum specific SNP within partial mitochondrial SSU rDNA sequences was analyzed using SeqBuilder program (DNAStar, Inc., Madison, Wis., USA). PCR products of partial mitochondrial SSU rDNA gene were digested by the restriction enzyme FastDigest HinfI (Fermentas, Vilnius, Lithuania). The restriction enzyme digestion reaction was done by mixing 5 μL of PCR products in 0.5 μL of restriction enzyme and 1.2 μL of 10 × FastDigest buffer. The total volume was made up to 12 μL using autoclaved distilled water and was then incubated for 10 min at 37 °C. The digested product was visualized by electrophoresis in 1.2% agarose gel using 1 kb Plus Ladder Marker (TNT research, Anyang, Korea).
2.5. SCAR Primer Design and Validation
Specific DNA fragments were eluted using the Qiaquick Gel Extraction Kit (Qiagen INC., Chatsworth, CA, USA), according to the manufacturer’s instruction. Two sets of specific primer pairs for antler-shaped G. lucidum were designed for SCAR marker. PrimerSelect in Lasergene (DNAStar, Inc. Madison, WI, USA) was used for the primer design. Specific primers are shown in Table 2. PCR reactions of specific primer pairs for antler-shaped G. lucidum were performed in a total volume of 20 μL, containing 1 μL of DNA (100 ng/μL) and 2.5 pmol of each primers (KAGL 1F/1R and KAGL 2F/2R primer pairs). The PCR conditions were 5 min of initial denaturation 94 °C, followed by 27 cycles of denaturation at 94 °C for 15 s, annealing at 61 °C for 15 s, and extension at 72 °C for 30 s, and final extension at 72 °C for 10 min using TaKaRa Thermal cycler (TaKaRa, Tokyo, Japan). The PCR product was visualized by electrophoresis in 1.2% agarose gel using 1 kb Plus Radder Marker (TNT research, Anyang, Korea).
2.6. Cultivation of G. lucidum Fruit Body
Sawdust mixed with rice bran in 4:1 ratio was watered and placed into polypropylene bottles. The substrate was sterilized at 121 °C for 40 min in an autoclave and cooled at room temperature for 24 h. Then, the sawdust medium was inoculated with the cultured Ganoderma mycelium in potato dextrose agar (PDA; Difco, Detroit, MI, USA) medium. The inoculated sawdust media were incubated at 28 °C for approximately one month until mycelia spread all over the media. When the mycelium had colonized the substrate completely, it was transferred to a fruiting room at 26 °C. The substrate was wetted to increase the moisture content to approximately 60%–80%. The artificially induced formation of antler shape from kidney shape was achieved under dark and 0.1% CO2 conditions.
3. Results
3.1. SNP Analysis of Partial Mitochondrial SSU rDNA Gene
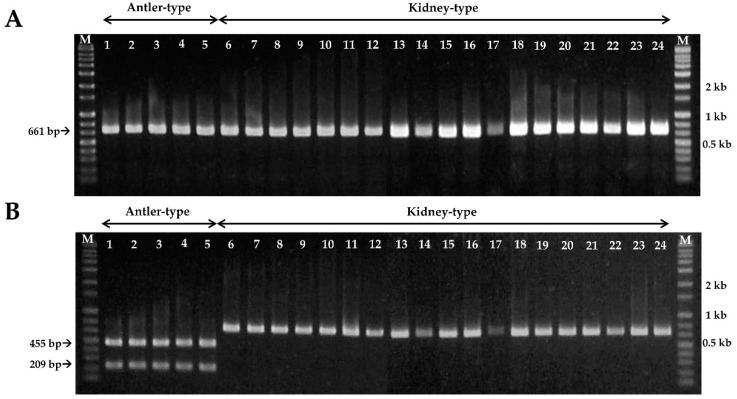
In PCR analysis, the amplified PCR product sizes from the partial mitochondrial SSU rDNA gene sequences of the antler- and kidney-shaped G. lucidum were of identical length of 661-bp (Figure 1, Figure 2A and Figure S1). Among them, SNPs were found only in the antler-shaped G. lucidum strains at location 456 bp (Figure 1). At 456 bp location, antler-shaped G. lucidum strains contain the nucleotide cytosine (C) but kidney-shaped counterparts contain the nucleotide adenine (A). This SNP region in antler-shaped G. lucidum is recognized by the HinfI restriction enzyme. Consequently, the PCR products of the antler-shaped G. lucidum strains were digested to 209-bp and 455-bp sizes by HinfI restriction enzyme. (Figure 2B). Thus, antler- and kidney-shaped G. lucidum strains could be distinguished by HinfI restriction enzyme.
Figure 1.
Alignment of partial mitochondrial SSU rDNA sequences of Ganoderma lucidum strains. Arrow indicates the single nucleotide polymorphism (SNP) found from antler-shaped G. lucidum strains.
Figure 2.
PCR-RFLP of antler- and kidney-shaped Ganoderma lucidum. (A) PCR amplification products in partial mitochondrial SSU rDNA gene containing the SNP location from G. lucidum; (B) The result of digestion with HinfI restriction enzyme from the PCR products. The arrow indicates the restriction fragment sizes. Lanes 1–5: antler–shaped G. lucidum. Lanes 6–24: kidney-shaped G. lucidum (numbers 1–24, respectively, in Table 1). M: size markers (1 kb ladder).
3.2. Development of SCAR Marker for Antler-Shaped G. lucidum
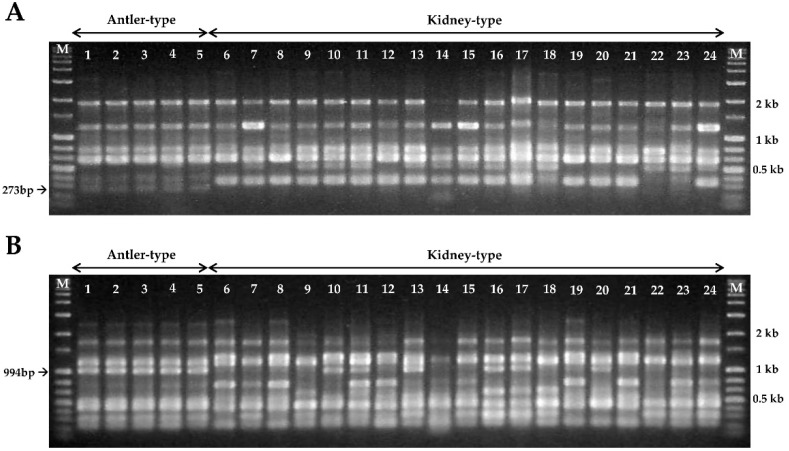
In this study, 12 URP primers were used to evaluate the specific polymorphism of G. lucidum strains. Among them, the URP1 and URP5 primers revealed a good polymorphic amplification pattern for antler- and kidney-shaped G. lucidum strains. In addition, amplification with URP1 and URP5 primers from all antler-shaped G. lucidum strains showed specific DNA bands of 273-bp and 994-bp, respectively (Figure 3). The two target DNA bands (273-bp and 994-bp) for the specific identification of antler-shaped G. lucidum were isolated and sequenced to design strain-specific primers.
Figure 3.
RAPD analysis of 24 Ganoderma lucidum strains using the (A) URP 1 and (B) URP 5 primers. The arrows indicate the specific fragment sizes of antler-shaped G. lucidum strains. Lanes 1–5: antler-shaped G. lucidum. Lanes 6–24: kidney-shaped G. lucidum (numbers 1–24, respectively, in Table 1). M: size markers (1 kb ladder).
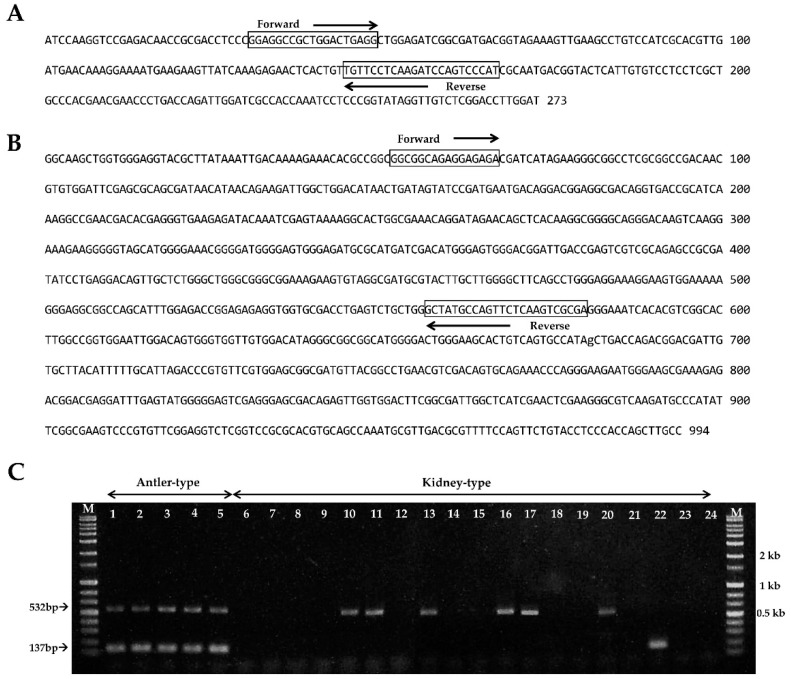
The DNA fragment sequences and specific primers of antler-shaped G. lucidum strain are shown in Figure 4A,B. As predicted, the PCR results from all of antler-shaped G. lucidum strains were found to have two specific DNA bands in 137-bp and 532-bp (Figure 4C). However, either one of the two specific bands (137-bp or 532-bp) or no band was observed from kidney-shaped G. lucidum strains (Figure 4C). This result indicates that two sets of specific primer pairs (KAGL 1F/1R and KAGL 2F/1R) are specific to antler-shaped G. lucidum and could be used to differentiate it from kidney-shaped G. lucidum.
Figure 4.
Primers for specific detection of antler-shaped Ganoderma lucidum and PCR amplification. The specific DNA fragments sequences amplified with (A) URP 1 and (B) URP 5 from the antler-shaped G. lucidum. Black boxes indicate the primer positions; (C) PCR amplification. Arrows indicate the antler-shaped G. lucidum-specific amplified fragments. Lanes 1–5: antler-shaped G. lucidum. Lanes 6–24: kidney-shaped G. lucidum (numbers 1–24, respectively, in Table 1). M: size markers (1 kb ladder).
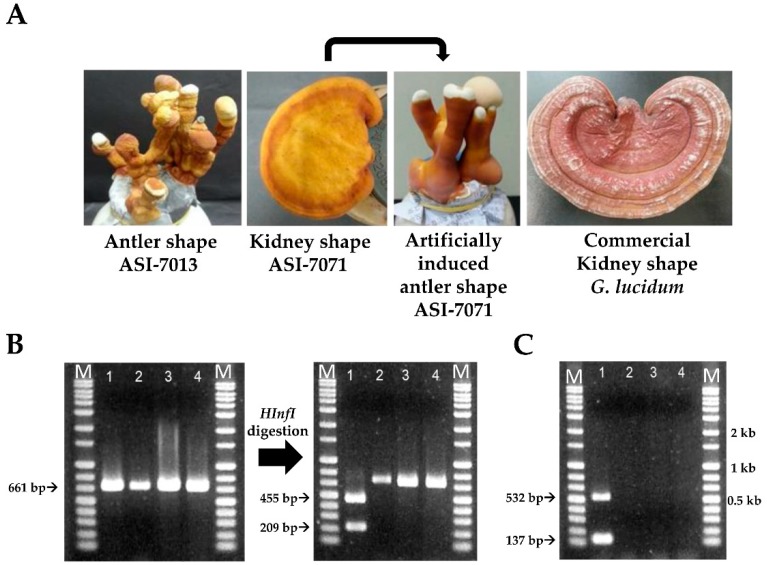
We checked SNP and SCAR markers for the specific identification of antler-shaped G. lucidum fruit body and artificial forming of antler shape (Figure 5). Consequently, SNP marker was confirmed to have two fragments of the sizes 209-bp and 455-bp by HinfI restriction enzyme digestion in the antler-shaped G. lucidum fruit body (ASI-7013), except for kidney-shaped G. lucidum fruit bodies (ASI-7071 and commercial G. lucidum) and artificial forming antler-shape from kidney-shape (ASI-7071) (Figure 5B). Furthermore, SCAR marker revealed that both of two specific bands were found only in the fruit body of antler-shaped G. lucidum strain (ASI-7013) (Figure 5C). These results showed that SNP and SCAR markers can help distinguish between the antler- or kidney-shaped G. lucidum fruit body.
Figure 5.
SNP and SCAR markers for specific identification of antler-shaped Ganoderma lucidum fruit body. (A) Morphology of antler- and kidney-shaped G. lucidum; (B) SNP marker validation for antler-shaped fruit bodies by HinfI digestion; (C) SCAR marker validation for antler-shaped fruit bodies. Lane 1: antler-shaped G. lucidum (ASI-7013). Lane 2: Kidney-shaped G. lucidum (ASI-7071). Lane 3: artificially induced antler-shape (ASI-7071). Lane 4: commercial G. lucidum of kidney-shape. M: size markers (1 kb ladder).
4. Discussion
This study aimed to develop the SNP and SCAR markers for specific identification of antler-shaped Ganoderma lucidum strains. G. lucidum has been widely used as a valuable medicinal agent because of its wide variety of anti-inflammatory, antitumor, antioxidant, and other biological activities [36,37]. It has been reported that antler-type G. lucidum produces higher levels of bioactive compounds such as flavonoids, ganoderic acid, and phenolics than kidney-shaped G. lucidum [11,12,38].
Mitochondrial DNA is one of the most important genetic resources and it is used as a marker for various phylogenetic classifications [39]. In addition, Hong et al. [34] reported that the information of valuable domains in mitochondrial SSU rDNA gene was useful in phylogenetic analysis of the Ganoderma species. In this study, we analyzed the partial mitochondrial small-subunit ribosomal DNA gene sequence of various antler- and kidney-shaped G. lucidum strains. We found that the antler-shaped G. lucidum has an SNP in the mitochondrial SSU rDNA gene sequence and can be digested by the HinfI restriction enzyme.
We also designed two sets of specific primer pairs to develop SCAR-marker for the antler-shaped G. lucidum strains based on RAPD analysis. PCR analysis with antler-shaped specific-primers revealed that the artificially induced antler-shaped G. lucidum could also be specifically discriminated. RAPD with random arbitrary primers has been widely used in genetic diversity studies of fungi [40,41,42]. In addition, genetic relationships can also be inferred even within fungal pathogen species using RAPD markers. Manulis et al. [43] reported that specific banding patterns from RAPD were subsequently used as probes to distinguish between races of the carnation wilt fungal pathogen Fusarium oxysporum f. sp. dianthi. Moreover, RAPD markers have been reported to be useful in diagnostic studies of fungal pathogens such as Alternaria species (the causal agent of brown spot of citrus) and Leptosphaeria maculans (the causal agent of blackleg of crucifers) [40,44]. However, RAPD analysis using random arbitrary primers tends to result in low reproducibility. Universal rice primers (URPs) developed from the repetitive sequences of rice genome can be used for PCR fingerprinting of various organisms including plants, animals, and microorganisms due to their high reproducibility [45]. In addition, RAPD analysis using URP primers is a useful tool for the characterization and grouping of fungal species at intraspecific and interspecific levels [46,47,48,49,50].
Antler-shaped G. lucidum is a valuable herbal medicine in China, Japan, and Korea. Antler-shaped G. lucidum can be cultivated naturally or artificially. A dark condition with poor ventilation does not expand the pileus. In addition, high levels of carbon dioxide (CO2) have been reported to support the production of antler-shaped fruiting bodies [9,38]. Thus, its morphology can be changed by artificially modulating the cultivation conditions. Antler-shaped G. lucidum strain has been mainly distinguished by morphological characteristics. However, this form is not easy to distinguish because of the various formulations and development of cultivation techniques. Therefore, the SNP and SCAR markers for the identification of antler-shaped G. lucidum strains will be useful for protection of the breed, breeding, time saving, and the cost-effective part.
Supplementary Materials
The following figures are available online at http://www.mdpi.com/2076-2607/7/1/12/s1. Figure S1: Alignment of partial mitochondrial SSU rDNA sequences of Ganoderma lucidum strains.
Author Contributions
Y.-J.P. and O.-C.K. conceived and designed the experiments; O.-C.K. performed the experiments; C.-S.L. and Y.-J.P. analyzed the data; O.-C.K. contributed to the drafting of the manuscript; Y.-J.P. wrote the paper.
Funding
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ010223112018)” Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Curtis W. Flora Londinensis: Plates and Descriptions of Such Plants as Grow Wild in the Environs of London. Printed by the Author; London, UK: 1781. p. 530. [DOI] [Google Scholar]
- 2.Fries E.M. Systema Mycologicum: Sistens Fungorum Ordines, Genera et Species, huc usque cognitas, quas ad Normam Methodi Naturalis Determinavit. Volume 1. Ex Officina Berlingiana; Lundae, Sweden: 1821–1832. [DOI] [Google Scholar]
- 3.Wang X.C., Xi R.J., Li Y., Wang D.M., Yao Y.J. The species identity of the widely cultivated Ganoderma, ‘G. lucidum’ (Ling-zhi), in China. PLoS ONE. 2012;7:e40857. doi: 10.1371/journal.pone.0040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao C.H.J., Jesuthasan A.C., Bishop K.S., Glucina M.P., Ferguson L.R. Anti-cancer activities of Ganoderma lucidum: Active ingredients and pathways. Funct. Foods Health Dis. 2013;3:48–65. doi: 10.31989/ffhd.v3i2.65. [DOI] [Google Scholar]
- 5.Komada Y., Shimizu M., Sonoda Y., Sato Y. Ganoderic acid and its derivatives as cholesterol synthesis inhibitors. Chem. Pharm. Bull. 1989;37:531–533. doi: 10.1248/cpb.37.531. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y.Y., Kho K.H., Chen S.T., Lin C.C., Wong C.H., Lin C.H. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg. Med. Chem. 2002;10:1057–1062. doi: 10.1016/S0968-0896(01)00377-7. [DOI] [PubMed] [Google Scholar]
- 7.Shi L., Ren A., Mu D., Zhao M. Current progress in the study on biosynthesis and regulation of ganoderic acids. Appl. Microbiol. Biotechnol. 2010;88:1243–1251. doi: 10.1007/s00253-010-2871-1. [DOI] [PubMed] [Google Scholar]
- 8.Nonaka Y., Shibata H., Nakai M., Kurihara H., Ishibashi H., Kiso Y., Tanaka T., Yamaguchi H., Abe S. Anti-tumor activities of the antlered form of Ganoderma lucidum in allogeneic and syngeneic tumor-bearing mice. Biosci. Biotechnol. Biochem. 2006;70:2028–2034. doi: 10.1271/bbb.50509. [DOI] [PubMed] [Google Scholar]
- 9.Upton R. Reishi Mushroom Ganoderma Lucidum Standards of Analysis, Quality Control, and Therapeutics. American Herbal Pharmacopoeia; Santa Cruz, CA, USA: 2000. pp. 13–20. [Google Scholar]
- 10.Katagata Y., Sasaki F. Antiproliferative activity of extracts prepared from three species of Reishi on cultured human normal and tumor cell lines. Mol. Med. Rep. 2010;3:179–184. doi: 10.3892/mmr_00000237. [DOI] [PubMed] [Google Scholar]
- 11.Min B.S., Nakamura N., Miyashiro H., Bae K.W., Hattori M. Triterpenes from the spore of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998;46:1607–1612. doi: 10.1248/cpb.46.1607. [DOI] [PubMed] [Google Scholar]
- 12.Kohguchi M., Kunikata T., Watanabe H., Kudo N., Shibuya T., Ishihara T., Iwaki K., Ikeda M., Fukuda S., Kurimoto M. Immuno-potentiating effects of the antler-shaped fruiting body of Ganoderma lucidum (Rokkaku-Reishi) Biosci. Biotechnol. Biochem. 2004;68:881–887. doi: 10.1271/bbb.68.881. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K., Shuto T., Sato M., Onuki K., Mizunoe S., Suzuki S., Sato T., Koga T., Suico M.A., Kai H., Ikeda T. Lucidenic acids-rich extract from antlered form of Ganoderma lucidum enhances TNFα induction in THP-1 monocytic cells possibly via its modulation of MAP kinases p38 and JNK. Biochem. Biophys. Res. Commun. 2011;408:18–24. doi: 10.1016/j.bbrc.2011.03.108. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L.W., Nakasone K.K., Burdsall H.H., Ginns J., Vlasák J., Miettinen O., Spirin V., Niemela T., Yuan H.S., He S.H. Polypore diversity in North America with an annotated checklist. Mycol. Prog. 2016;15:771–790. doi: 10.1007/s11557-016-1207-7. [DOI] [Google Scholar]
- 15.Moncalvo J.M., Wang H.F., Hseu R.S. Gene phylogeny of the Ganoderma lucidum complex based on ribosomal DNA sequences: Comparison with traditional taxonomic characters. Mycol. Res. 1995;99:1489–1499. doi: 10.1016/S0953-7562(09)80798-3. [DOI] [Google Scholar]
- 16.Gottlieb A.M., Ferrer E., Wright J.E. rDNA analyses as an aid to the taxonomy of species of Ganoderma. Mycol. Res. 2000;104:1033–1045. doi: 10.1017/S095375620000304X. [DOI] [Google Scholar]
- 17.Smith B.J., Sivasithamparam K. Internal transcribed spacer ribosomal DNA sequence of five species of Ganoderma from Australia. Mycol. Res. 2000;104:943–951. doi: 10.1017/S0953756200002458. [DOI] [Google Scholar]
- 18.Hong S.G., Jung H.S. Phylogenetic analysis of Ganoderma based on nearly complete mitochondrial small-subunit ribosomal DNA sequences. Mycologia. 2004;96:742–755. doi: 10.1080/15572536.2005.11832922. [DOI] [PubMed] [Google Scholar]
- 19.Park Y.J., Kwon O.C., Son E.S., Yoon D.E., Han W., Nam J.Y., Yoo Y.B., Lee C.S. Genetic diversity analysis of Ganoderma species and development of a specific marker for identification of medicinal mushroom Ganoderma lucidum. Afr. J. Microbiol. Res. 2012;6:5417–5425. doi: 10.5897/AJMR12.846. [DOI] [Google Scholar]
- 20.Sun S.J., Gao W., Lin S.Q., Zhu J., Xie B.G., Lin Z.B. Analysis of genetic diversity in Ganoderma population with a novel molecular marker SRAP. Appl. Microbiol. Biotechnol. 2006;72:537–543. doi: 10.1007/s00253-005-0299-9. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L., Jia D., Fei X., Luo X., Yang Z. An assessment of the genetic diversity within Ganoderma strains with AFLP and ITS PCR-RFLP. Microbiol. Res. 2009;164:312–321. doi: 10.1016/j.micres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Rolim L.D.N., Cavalcante M.A.D.Q., Urben A.F., Buso G.S.C. Use of RAPD molecular markers on differentiation of Brazilian and Chinese Ganoderma lucidum strains. Braz. Arch. Biol. Technol. 2011;54:273–281. doi: 10.1590/S1516-89132011000200008. [DOI] [Google Scholar]
- 23.Jones N., Ougham H., Thomas H., Pasakinskiene I. Markers and mapping revisited: Finding your gene. New Phytol. 2009;183:935–966. doi: 10.1111/j.1469-8137.2009.02933.x. [DOI] [PubMed] [Google Scholar]
- 24.Yang C.H., Cheng Y.H., Chuang L.Y. A natural PCR-RFLP primer design for SNP genotyping using a genetic algorithm; Proceedings of the International MultiConference of Engineers and Computer Scientists, IMECS; Hong Kong, China. 17–19 March 2010; [(accessed on 1 March 2017)]. Available online: https://www.researchgate.net/publication/44260566_A_natural_PCR-RFLP_primer_design_for_SNP_genotyping_using_a_genetic_algorithm. [Google Scholar]
- 25.Ota M., Fukushima H., Kulski J.K., Inoko H. Single nucleotide polymorphism detection by polymerase chain reaction-restriction fragment length polymorphism. Nat. Protoc. 2007;2:2857–2864. doi: 10.1038/nprot.2007.407. [DOI] [PubMed] [Google Scholar]
- 26.Xu J., Sha T.A.O., Li Y.C., Zhao Z.W., Yang Z.L. Recombination and genetic differentiation among natural populations of the ectomycorrhizal mushroom Tricholoma matsutake from southwestern China. Mol. Ecol. 2008;17:1238–1247. doi: 10.1111/j.1365-294X.2007.03665.x. [DOI] [PubMed] [Google Scholar]
- 27.Antoni R. Applications of single nucleotide polymorphisms in crop genetics. Curr. Opin. Plant Biol. 2002;5:94–100. doi: 10.1016/S1369-5266(02)00240-6. [DOI] [PubMed] [Google Scholar]
- 28.Vignal A., Milan D., SanCristobal M., Eggen A. A review on SNP and other types of molecular markers and their use in animal genetics. Genet. Sel. Evol. 2002;34:275–305. doi: 10.1186/1297-9686-34-3-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J., Guo H., Yang Z.L. Single nucleotide polymorphisms in the ectomycorrhizal mushroom Tricholoma matsutake. Microbiology. 2007;153:2002–2012. doi: 10.1099/mic.0.2006/005686-0. [DOI] [PubMed] [Google Scholar]
- 30.Ferri L., Perrin E., Campana S., Tabacchioni S., Taccetti G., Cocchi P., Ravenni N., Dalmastri C., Chiarini L., Bevivino A., et al. Application of multiplex single nucleotide primer extension (mSNuPE) to the identification of bacteria: The Burkholderia cepacia complex case. J. Microbiol. Methods. 2010;80:251–256. doi: 10.1016/j.mimet.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Fu J.J., Mei Z.Q., Tania M., Yang L.Q., Cheng J.L., Khan M.A. Development of RAPD-SCAR markers for different Ganoderma species authentication by improved RAPD amplification and molecular cloning. Genet. Mol. Res. 2015;14:5667–5676. doi: 10.4238/2015.May.25.19. [DOI] [PubMed] [Google Scholar]
- 32.Wachtel-Galor S., Benzie I.F. Ganoderma lucidum (Lingzhi or Reishi): A medicinal mushroom. In: Wachtel-Galor S., Yuen J., Buswell J.A., Benzie I.F.F., editors. Herbal medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press/Taylor & Francis; Boca Raton, FL, USA: 2011. [(accessed on 1 March 2017)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92757/ [Google Scholar]
- 33.Cao H., But P.P., Shaw P.C. Methodological studies on genomic DNA extraction and purification from plant drug materials. J. Chin. Pharm. Sci. 1998;7:130–137. [Google Scholar]
- 34.Hong S.G., Jeong W., Jung H.S. Amplification of mitochondrial small subunit ribosomal DNA of polypores and its potential for phylogenetic analysis. Mycologia. 2002;94:823–833. doi: 10.1080/15572536.2003.11833176. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning: A Laboratory Manual. 4th ed. Volume 1. Cold Spring Harbor Laboratory Press; New York, NY, USA: 1989. pp. 157–174. [Google Scholar]
- 36.Boh B., Berovic M., Zhang J., Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- 37.Sanodiya B.S., Thakur G.S., Baghel R.K., Prasad G.B., Bisen P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 38.Sudheer S., Taha Z., Manickam S., Ali A., Cheng P.G. Development of antler-type fruiting bodies of Ganoderma lucidum and determination of its biochemical properties. Fungal Biol. 2018;122:293–301. doi: 10.1016/j.funbio.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Brown W.M., George M., Jr., Wilson A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peever T.L., Canihos Y., Olsen L., Ibanez A., Liu Y.C., Timmer L.W. Population genetic structure and host specificity of Alternaria spp. causing brown spot of Minneola tangelo and rough lemon in Florida. Phytopathology. 1999;89:851–860. doi: 10.1094/PHYTO.1999.89.10.851. [DOI] [PubMed] [Google Scholar]
- 41.Vakalounakis D.J., Fragkiadakis G.A. Genetic diversity of Fusarium oxysporum isolates from cucumber: Differentiation by pathogenicity, vegetative compatibility, and RAPD fingerprinting. Phytopathology. 1999;89:161–168. doi: 10.1094/PHYTO.1999.89.2.161. [DOI] [PubMed] [Google Scholar]
- 42.Yoder W.T., Christianson L.M. Species-specific primers resolve members of Fusarium section Fusarium: Taxonomic status of the edible “Quorn” fungus reevaluated. Fungal Genet. Biol. 1998;23:68–80. doi: 10.1006/fgbi.1997.1027. [DOI] [PubMed] [Google Scholar]
- 43.Manulis S., Kogan N., Reuven M., Yephet Y.B. Use of the RAPD technique for identification of Fusarium oxysporum f. sp. dianthi from carnation. Phytopathology. 1994;84:98–101. doi: 10.1094/Phyto-84-98. [DOI] [Google Scholar]
- 44.Goodwin P.H., Annis S.L. Rapid identification of genetic variation and pathotype of Leptosphaeria maculans by random amplified polymorphic DNA assay. Appl. Environ. Microbiol. 1991;57:2482–2486. doi: 10.1128/aem.57.9.2482-2486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang H.W., Park D.S., Go S.J., Eun M.Y. Fingerprinting of diverse genomes using PCR with universal rice primers generated from repetitive sequence of Korean weedy rice. Mol. Cells. 2002;13:281–287. [PubMed] [Google Scholar]
- 46.Aggarwal R., Singh V.B., Shukla R., Gurjar M.S., Gupta S., Sharma T.R. URP-based DNA fingerprinting of Bipolaris sorokiniana isolates causing spot blotch of wheat. J. Phytopathol. 2010;158:210–216. doi: 10.1111/j.1439-0434.2009.01603.x. [DOI] [Google Scholar]
- 47.González N., Godoy-Lutz G., Steadman J.R., Higgins R., Eskridge K.M. Assessing genetic diversity in the web blight pathogen Thanatephorus cucumeris (anamorph= Rhizoctonia solani) subgroups AG-1-IE and AG-1-IF with molecular markers. J. Gen. Plant Pathol. 2012;78:85–98. doi: 10.1007/s10327-012-0361-2. [DOI] [Google Scholar]
- 48.Aggarwal R., Gupta S., Banerjee S., Singh V.B. Development of a SCAR marker for detection of Bipolaris sorokiniana causing spot blotch of wheat. Can. J. Microbiol. 2011;57:934–942. doi: 10.1139/w11-089. [DOI] [PubMed] [Google Scholar]
- 49.Jana T.K., Singh N.K., Koundal K.R., Sharma T.R. Genetic differentiation of charcoal rot pathogen, Macrophomina phaseolina, into specific groups using URP-PCR. Can. J. Microbiol. 2005;51:159–164. doi: 10.1139/w04-122. [DOI] [PubMed] [Google Scholar]
- 50.Kang H.W., Park D.S., Park Y.J., You C.H., Lee B.M., Eun M.Y., Go S.J. Genomic differentiation among oyster mushroom cultivars released in Korea by URP-PCR fingerprinting. Mycobiology. 2001;29:85–89. doi: 10.1080/12298093.2001.12015766. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.