Abstract
Background:
The effects of thyroid dysfunction in patients with pre-existing heart failure have not been adequately studied. We examined the prevalence of thyroid dysfunction and associations with cardiovascular outcomes in a large, prospective cohort of outpatients with pre-existing heart failure.
Methods and Results:
We examined associations between thyroid dysfunction and New York Heart Association (NYHA) class, atrial fibrillation, and a composite endpoint of ventricular assist device placement, heart transplantation, or death in 1365 participants with heart failure enrolled in the Penn Heart Failure Study. Mean age was 57 years, 35% were women, and the majority had NYHA class II (45%) or III (32%) symptoms. More severe heart failure was associated with higher thyroid stimulating hormone, higher free thyroxine (FT4), and lower total triiodothyronine (TT3) concentrations (p<0.001 all models). Atrial fibrillation was positively associated with higher levels of FT4 alone (p≤0.01 all models). There were 462 composite endpoints over a median 4.2 years of follow-up. In adjusted models, compared to euthyroidism, subclinical hypothyroidism (TSH 4.51–19.99 mIU/L with normal FT4) was associated with an increased risk of the composite endpoint overall (hazard ratio [HR], 1.82; 95% confidence interval [CI], 1.27–2.61; p=0.001) and in the subgroup with TSH ≥ 7.00 mIU/L (HR, 3.25; 95% CI, 1.96–5.39; p<0.001), but not in the subgroup with TSH 4.51–6.99 mIU/L (HR, 1.26; 95% CI, 0.78–2.06; p= 0.34). Isolated low T3 was also associated with the composite endpoint (HR, 2.12; 95% CI, 1.65–2.72; p<0.001).
Conclusions:
In patients with pre-existing heart failure, subclinical hypothyroidism with TSH ≥ 7 mIU/L and isolated low T3 levels are associated with poor prognosis. Clinical trials are needed to explore therapeutic effects of T4 and T3 administration in heart failure.
Keywords: Thyroid, heart failure, mortality, atrial fibrillation, subclinical hypothyroidism, T3
Thyroid hormone regulates multiple cardiovascular functions, directly affecting the myocardium, the conduction system, and the peripheral vasculature.1 Insufficient thyroid hormone causes hyperlipidemia and ventricular arrhythmias, excess thyroid hormone causes atrial arrhythmias, and both cause hypertension and heart failure. These cardiac abnormalities are generally reversible with treatment of the underlying thyroid condition.
Patients with subclinical thyroid dysfunction have levels of the thyroid hormone free thyroxine (FT4) within the reference range, but abnormal thyroid stimulating hormone (TSH) levels, suggesting that the amount of thyroid hormone present is not optimal for that patient. Multiple cohort studies have examined the relationship between thyroid function, within and outside the reference range, and incident atrial fibrillation, heart failure, and coronary heart disease.2–8 Subclinical hypothyroidism with TSH levels greater than 10 mIU/L has been associated with increased risk of ischemic heart disease, and subclinical hypothyroidism with TSH levels greater than 7 mIU/L has been associated with increased cardiovascular mortality.4 Subclinical thyroid dysfunction with TSH values greater than 10 mIU/L or below 0.1 mIU/L has also been associated with a higher risk of incident heart failure.5 In addition, low levels of the thyroid hormone triiodothyronine (T3) with normal levels of TSH and FT4, the low T3 syndrome, have been associated with increased mortality risk.1
However, the effects of thyroid dysfunction may differ depending on the underlying cardiac status of the patient. That is, the impact of subtle changes in thyroid function may be more pronounced in patients with pre-existing heart failure. In addition, acute illness directly alters thyroid function testing, which limits the causal inferences that can be derived from studying inpatients with heart failure. Despite recommendations by the American Heart Association to evaluate thyroid function in all patients presenting with heart failure,9 there have not been any studies to comprehensively examine the role of thyroid hormone abnormalities in exacerbating heart failure in the outpatient setting.
Therefore, we examined the prevalence of thyroid dysfunction and associations with cardiovascular outcomes in a large, prospective cohort of outpatients with pre-existing heart failure.
METHODS
Study Population
The Penn Heart Failure Study is a prospective cohort study of ambulatory patients with chronic heart failure recruited between October 2003 and November 2011 from referral centers at the University of Pennsylvania (Philadelphia, Pennsylvania), Case Western Reserve University (Cleveland, Ohio), and the University of Wisconsin (Madison, Wisconsin).10 Patients with a clinical diagnosis of heart failure as determined by a heart failure specialist were enrolled. At the time of study entry, standardized questionnaires were administered to participants and their physicians to obtain detailed clinical data as previously described.10 New York Heart Association (NYHA) class and cardiomyopathy etiology (ischemic versus non-ischemic) were determined by the physician based on all available clinical data. Participants with expected mortality of 6 months or less from a non-cardiac condition, as judged by their treating physician; a history of mechanical support with a ventricular assist device (VAD); or inability to provide informed consent were excluded. Each participant provided written informed consent. Institutional Review Boards from each participating institution approved the study protocol. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Due to the large number of participants treated with amiodarone, levothyroxine, and beta-adrenergic blocking agents at study entry, participants taking these medications were included in the present analyses. Small numbers of participants were taking other medications that could interfere with thyroid function tests—liothyronine (n=6), thyroid hormone extract (n=1), methimazole or propylthiouracil (n=14), lithium (n=4), or chronic intravenous dobutamine (n=7)—and were excluded.
Laboratory Assays
Venous blood samples were obtained at the time of study entry, processed, and stored at −80°C until the time of assay in 2014. Chemiluminescent immunoassays for TSH, FT4, total T3 (TT3) and anti-thyroid peroxidase (anti-TPO) antibody were performed using the Architect i2000SR instrument (Abbott Laboratories, Wiesbaden, Germany). The reference ranges for TSH, FT4 and TT3 were 0.45 to 4.5 mIU/L, 0.7 to 1.7 ng/dL and 80.0 to 159.0 ng/dL, respectively. Anti-TPO antibodies ≥ 5.6 IU/mL were classified as positive.
Thyroid function tests were used to define standard categories of thyroid function as follows (Figure 1): overt hyperthyroidism (TSH <0.45 mIU/L with elevated FT4 or elevated TT3 level), subclinical hyperthyroidism (TSH <0.45 mIU/L with FT4 level within the reference range), subclinical hypothyroidism (TSH 4.51–19.99 mIU/L with free T4 level within the reference range), overt hypothyroidism (TSH ≥ 20.00 mIU/L or TSH >4.50 mIU/L with free T4 level below the reference range), low T3 syndrome (TSH level and free T4 within the reference range with TT3 level below the reference range) and euthyroidism (TSH 0.45–4.50 mIU/L with FT4 and TT3 level within the reference range). Subclinical hypothyroidism was further classified into TSH 4.51–6.99 mIU/L and TSH 7.00–19.99 mIU/L, based on established subgroup classification. Meta-analyses have shown an increased risk of cardiovascular mortality in subclinical hypothyroidism with a TSH of 7 mIU/L or higher,4 and a non-statistically significant increase in risk of heart failure5 and stroke11 for TSH 7.0–9.9 mIU/L. We also performed analyses stratified by a TSH threshold of 10 mIU/L. Thyroid autoimmunity was defined by elevated anti-TPO antibody levels >5.61 IU/ml.
Figure 1.
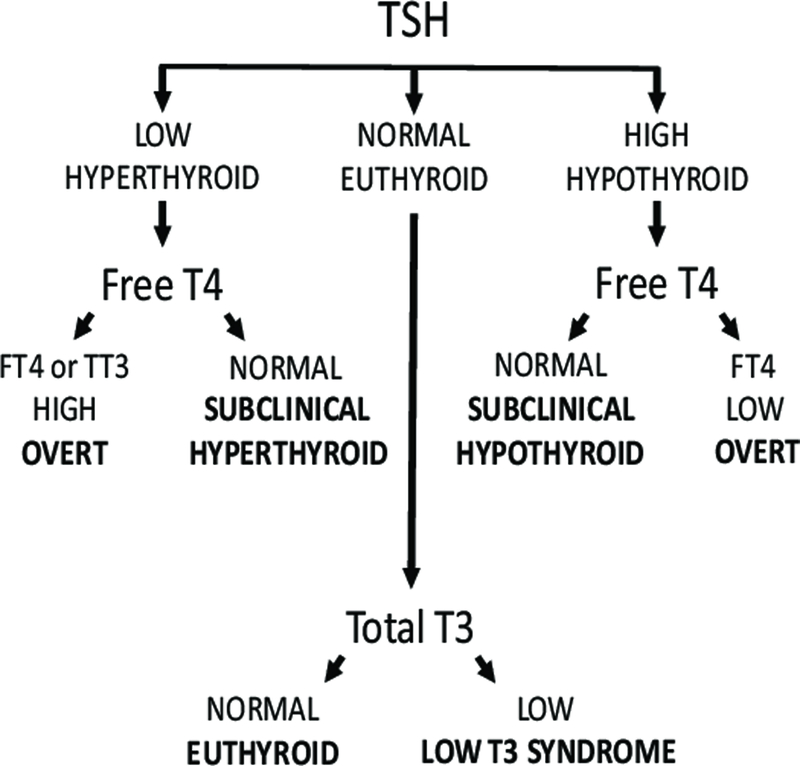
Categories of thyroid status.
Ascertainment of Outcomes
Prevalence of atrial fibrillation was assessed from electrocardiograms at the baseline study visit. NYHA class was determined by physician assessment. VAD placement, cardiac transplantation, and all-cause mortality were prospectively ascertained every 6 months via direct patient contact and verified through death certificates, medical records, and contact with patients’ family members. Outcomes were assessed through September 2013.
Covariate and Comorbidity Assessment
Race, medical conditions (hypertension, diabetes mellitus, hyperlipidemia, and chronic kidney disease), and medication and device use were documented through clinical history obtained from participants and their physicians via questionnaires. Race categories included Caucasian, African American, mixed Caucasian/African American, Native American, Asian, Arabic and Pacific Islander. Body weight and height were measured to compute body mass index (BMI). Cardiomyopathy etiology (ischemic or non-ischemic) was determined by the treating physician based on clinical assessment. All covariates were assessed at the baseline study visit.
Statistical Analyses
Patient demographics were summarized using standard descriptive statistics. Prevalence of thyroid dysfunction was stratified by amiodarone and levothyroxine use. Unadjusted means of each thyroid function test result were computed for each NYHA class and category of atrial fibrillation. One-way ANOVA was performed to examine cross-sectional associations between each thyroid function test and NYHA class and atrial fibrillation using log-transformed levels of TSH, FT4, and TT3. Additional models were examined 1) excluding amiodarone or levothyroxine users and 2) further limiting to euthyroid individuals. Multivariable logistic regression analyses were performed to confirm cross sectional associations between thyroid function and NYHA class and between thyroid function and atrial fibrillation, in separate models. Covariates included age, gender, race, BMI, and heart failure etiology. Cox proportional hazards models were used to examine the association between thyroid function at study entry and a composite outcome of time to first of VAD implantation, cardiac transplantation, and all-cause death. Models were examined using thyroid function tests both as continuous variables and by thyroid function categories and were adjusted for age, gender, race, BMI, amiodarone and levothyroxine use and heart failure etiology. Overt hyperthyroidism and overt hypothyroidism were not examined due to small numbers. An analysis additionally adjusting for anti-TPO status was performed to adjust for effects of thyroid autoimmunity, and an analysis additionally adjusting for estimated glomerular filtration rate (eGFR) calculated from the Modification of Diet in Renal Disease (MDRD) Study equation was performed. Participants who were alive and free of VAD placement or cardiac transplantation at the end of the follow up period were censored. Kaplan-Meier survival analyses were performed to study survival free of the composite primary outcome. All statistical analyses were performed using IBM SPSS for Windows version 24.0 and R for Mac OS version 3.4.0.
RESULTS
Of 2436 participants enrolled in the Penn Heart Failure Study, 1481 met eligibility criteria for analysis, and 1365 had complete covariates for the analyses. The mean age was 57 years, 35% were women and 71% were Caucasian (Table 1). The majority had NYHA class II (45%) or III (32%) symptoms of heart failure, with a predominance of systolic heart failure (85%) and heart failure of non-ischemic origin (71%). Physician-reported comorbid conditions included hypertension (62%), diabetes mellitus (29%), hyperlipidemia (49%) and chronic kidney disease (16%). Eleven percent (n=151) were taking amiodarone and 13% (n=173) were taking levothyroxine. Mean TSH level was 1.99 (SD 3.54) mIU/L, FT4 level was 1.13 (SD 0.20) ng/dL and TT3 level was 99 (SD 22) ng/dL. Overall, 74% were euthyroid, 5% had subclinical hypothyroidism, 5% had subclinical hyperthyroidism, 1% had overt hyperthyroidism and less than 1% had overt hypothyroidism (Table 2). Fourteen percent had low T3 syndrome.
Table 1.
Baseline characteristics of study population
| No. of individuals in the study | 1365 |
|---|---|
| Age, yrs, mean (SD) | 56.6 (14.5) |
| Female | 483 (35) |
| Race | |
| Caucasian | 962 (71) |
| African American | 332 (24) |
| Other | 35 (2) |
| BMI, kg/m2, mean (SD) | 30.4 (7.7) |
| Comorbid conditions | |
| Hypertension | 850 (62) |
| Diabetes | 397 (29) |
| Hyperlipidemia | 667 (49) |
| Chronic kidney disease | 220 (16) |
| Medications | |
| ACE Inhibitor/ARB | 1223 (90) |
| Beta blocker | 1199 (88) |
| Digoxin | 479 (35) |
| Loop diuretic | 957 (70) |
| Amiodarone | 151 (11) |
| Levothyroxine | 173 (13) |
| Corticosteroids | 45 (3) |
| Implantable cardioverter defibrillator | 256 (19) |
| Cardiac resynchronization therapy | 325 (24) |
| Ischemic etiology of heart failure | 393 (29) |
| Systolic heart failure | 1165 (85) |
| NYHA class | |
| I | 227 (17) |
| II | 614 (45) |
| III | 433 (32) |
| IV | 82 (6) |
| Atrial fibrillation | 92 (7) |
| Ejection fraction, %, mean (SD) | 34 (17) |
| Serum creatinine, mg/dL, mean (SD) | 1.3 (0.9) |
Data presented as n (%), except as indicated.
SD, Standard Deviation; BMI, body mass index; NYHA, New York Heart Association
Table 2.
Prevalence of thyroid dysfunction, stratified by amiodarone and levothyroxine use
| Thyroid category | Entire population n=1365 |
Amiodarone users n= 151 |
Levothyroxine users n=173 |
Excluding amiodarone & levothyroxine users n=1070 |
|---|---|---|---|---|
| Euthyroid | 1006 (73.7) | 64 (42) | 79 (45.7) | 870 (81.3) |
| Overt hypothyroid | 4 (0.3) | 2 (1.3) | 3 (1.7) | 1 (0.1) |
| Subclinical hypothyroid | ||||
| Overall | 74 (5.4) | 21 (13.9) | 28 (16.2) | 35 (3.3) |
| TSH 4.51 to 6.99 mIU/L | 48 (3.5) | 12 (7.9) | 13 (7.5) | 28 (2.6) |
| TSH 7.00 to 19.99 mIU/L | 26 (1.9) | 9 (6.0) | 15 (8.7) | 7 (0.7) |
| Subclinical hyperthyroid | 69 (5.1) | 4 (2.6) | 19 (11.0) | 47 (4.4) |
| Overt hyperthyroid | 15 (1.1) | 5 (3.3) | 8 (4.6) | 4 (0.4) |
| Low T3 syndrome | 197 (14.4) | 55 (36.4) | 36(20.8) | 113 (10.6) |
| Anti-TPO antibody positive | 123 (9.0) | 11 (7.3) | 41 (23.7) | 75 (7.0) |
Data presented as n (%).
TSH, thyroid stimulating hormone; T3, triiodothyronine; TPO, thyroid peroxidase
More severe heart failure (higher NYHA class) was associated with higher TSH, higher FT4, and lower TT3 overall (p< 0.001 for each thyroid function test), excluding amiodarone and levothyroxine users (p<0.001), and restricting to euthyroid individuals (p<0.001) (Table 3). Adjusted multinomial logistic regression analyses yielded similar results (Supplement Table 1). Atrial fibrillation was associated with higher FT4 overall (p = 0.002), excluding amiodarone and levothyroxine users (p<0.001), and restricting to euthyroid individuals (p=0.006)(Table 4). Atrial fibrillation was associated with lower TT3 overall (p=0.03) and after excluding amiodarone and levothyroxine users (p=0.04), but the association lost significance when restricting to euthyroid individuals. No significant associations were seen between atrial fibrillation and TSH in any of the models. In binary logistic regression analyses, after adjustment for covariates, only FT4, but not TSH or TT3, was associated with atrial fibrillation (p=0.004 overall, p=0.001 excluding amiodarone and levothyroxine users, and p=0.01 restricting to euthyroid individuals) (Supplement Table 2).
Table 3.
Cross sectional association between thyroid function tests and NYHA class
| Entire Population n=1365 |
Excluding amiodarone and levothyroxine users n=1070 |
Restricting to euthyroid range excluding amiodarone and levothyroxine users n=973 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NYHA Class | NYHA Class | NYHA Class | |||||||||||||
| I n=227 |
II n=614 |
III n=433 |
IV n=82 |
p-value | I n=200 |
II n=495 |
III n=318 |
IV n=52 |
p-value | I n=182 |
II n=453 |
III n=288 |
IV n=46 |
p-value | |
| TSH mIU/L | 1.62 (1.5) |
1.82 (2.24) |
2.2 (5.0) |
3.11 (5.56) |
<0.001 | 1.54 (1.32) |
1.65 (2.04) |
1.71 (1.18) |
2.26 (1.40) |
<0.001 | 1.46 (0.77) |
1.51 (0.79) |
1.58 (0.78) |
2.15 (0.98) |
<0.001 |
| FT4 ng/dL | 1.08 (0.16) |
1.11 (0.18) |
1.15 (0.22) |
1.20 (0.25) |
<0.001 | 1.06 (0.13) |
1.07 (0.14) |
1.11 (0.19) |
1.15 (0.17) |
<0.001 | 1.06 (0.12) |
1.07 (0.14) |
1.1 (0.16) |
1.17 (0.15) |
<0.001 |
| TT3 ng/dL | 104.2 (18.8) |
100.9(20.8) | 95.7 (23.4) |
87.9 (26.0) |
<0.001 | 105.5(18.2) | 103.5 (19.5) |
99.6 (22.1) |
95.1 (26.6) |
<0.001 | 105.5 (18.2) |
104.2 (19.5) |
99.6 (20.2) |
96.4 (20.2) |
<0.001 |
Data are presented as mean (SD).
NYHA, New York Heart Association; TSH, thyroid stimulating hormone; FT4, free thyroxine; TT3, total triiodothyronine
One-way ANOVA was performed using log transformed values of each thyroid function test in separate models. See Supplement Table 1 for results of adjusted multinomial logistic regression analyses
Table 4.
Cross sectional association between thyroid function tests and atrial fibrillation
| Entire population | Excluding amiodarone and levothyroxine users | Restricting to euthyroid range excluding amiodarone and levothyroxine users | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Afib n=92 |
No Afib n=1273 |
p-value | Afib n=72 |
No Afib n=998 |
p-value | Afib n=62 |
No Afib n=911 |
p-value | |
| TSH mIU/L | 1.95 (1.36) |
2.00 (3.64) |
0.28 | 1.88 (1.36) |
1.67 (1.7) |
0.06 | 1.63 (0.82) |
1.55 (0.81) |
0.32 |
| FT4 ng/dL | 1.19 (0.24) |
1.12 (0.19) |
0.002 | 1.15 (0.23) |
1.08 (0.15) |
<0.001 | 1.13 (0.15) |
1.08 (0.14) |
0.006 |
| TT3 ng/dL | 94.6 (24.4) |
99.0 (21.8) |
0.03 | 98.2 (23.3) |
102.7 (20.5) |
0.04 | 99.2 (20.9) |
103.0 (19.6) |
0.10 |
Afib, atrial fibrillation; TSH, thyroid stimulating hormone; FT4, free thyroxine; TT3, total triiodothyronine
One-way ANOVA was performed using log transformed values of each thyroid function test in separate models. See Supplement Table 2 for results of adjusted binary logistic regression analyses
Over a median 4.2 years of follow-up, there were 462 composite endpoints with 31 VAD placements, 104 cardiac transplants and 327 deaths. After adjusting for age, gender, race, BMI, amiodarone use, levothyroxine use, and heart failure etiology, subclinical hypothyroidism (HR 1.82, 95% CI 1.27–2.61, p=0.001) and low T3 syndrome (HR, 2.12; 95% CI, 1.65–2.72; p<0.001) were each associated with increased risk of the composite endpoint compared to euthyroidism (Table 5). Kaplan-Meier survival analyses showed increasing risk of the composite outcome by category of increasing TSH (p<0.001 for log-rank test) and for low T3 syndrome compared to euthyroidism (p<0.001)(Figure 2). Subclinical hypothyroidism with a TSH level of 7.00 to 19.99 mIU/L was associated with increased risk of the composite endpoint (HR, 3.25; 95% CI 1.95–5.39; p<0.001), whereas subclinical hypothyroidism with TSH levels of 4.51 to 6.99 mIU/L was not (HR, 1.27; 95% CI 0.78– 2.06; p= 0.34) (Table 5). Additional models performed with different TSH cutoffs for subclinical hypothyroidism are presented in Supplement Table 3. Subclinical hyperthyroidism was not associated with the composite outcome (HR, 0.76; 95% CI 0.46–1.27; p=0.30). Similar hazard ratios were obtained in all models after adjusting for anti-TPO antibody status (Supplement Table 4) and for eGFR (Supplement Table 5).
Table 5.
Risk of time to first of VAD placement, cardiac transplantation or all-cause mortality by thyroid dysfunction category (n=1294).
| Category | Hazard ratio* | 95% CI | p-value |
|---|---|---|---|
| Subclinical hypothyroid | |||
| Overall | 1.82 | 1.27– 2.61 | 0.001 |
| TSH 4.51 to 6.99 mIU/L | 1.26 | 0.78– 2.06 | 0.34 |
| TSH 7.00 to 19.99 mIU/L | 3.25 | 1.96–5.39 | <0.001 |
| Subclinical hyperthyroid | 0.76 | 0.46–1.27 | 0.30 |
| Low T3 syndrome | 2.12 | 1.65–2.72 | <0.001 |
VAD, ventricular assist device; TSH, thyroid stimulating hormone; T3, triiodothyronine
Cox-proportional hazard models adjusted for age, sex, race, BMI, ischemic etiology, amiodarone use, and levothyroxine use.
Euthyroid group is the reference group.
Figure 2.
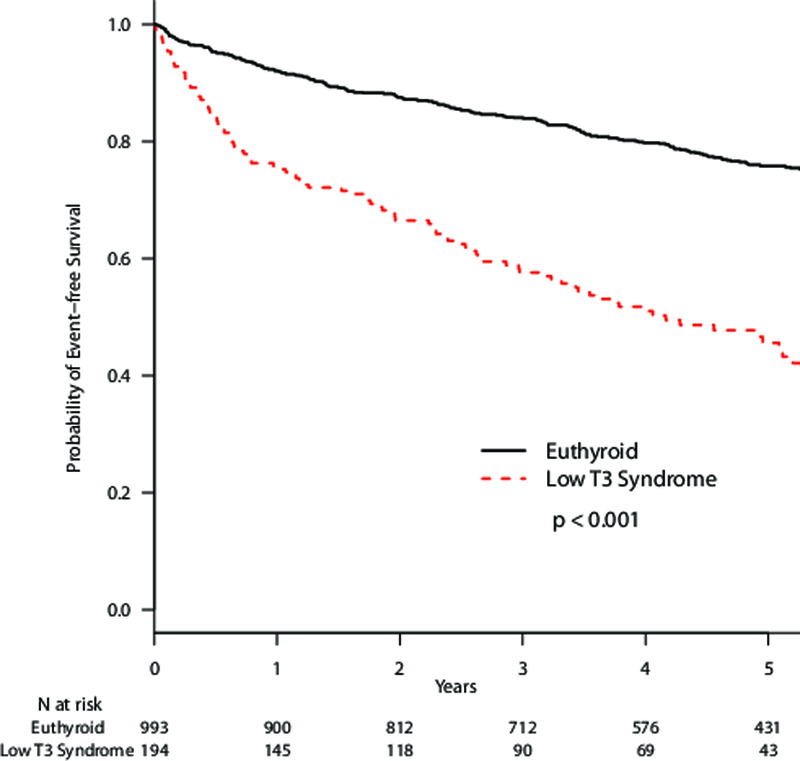
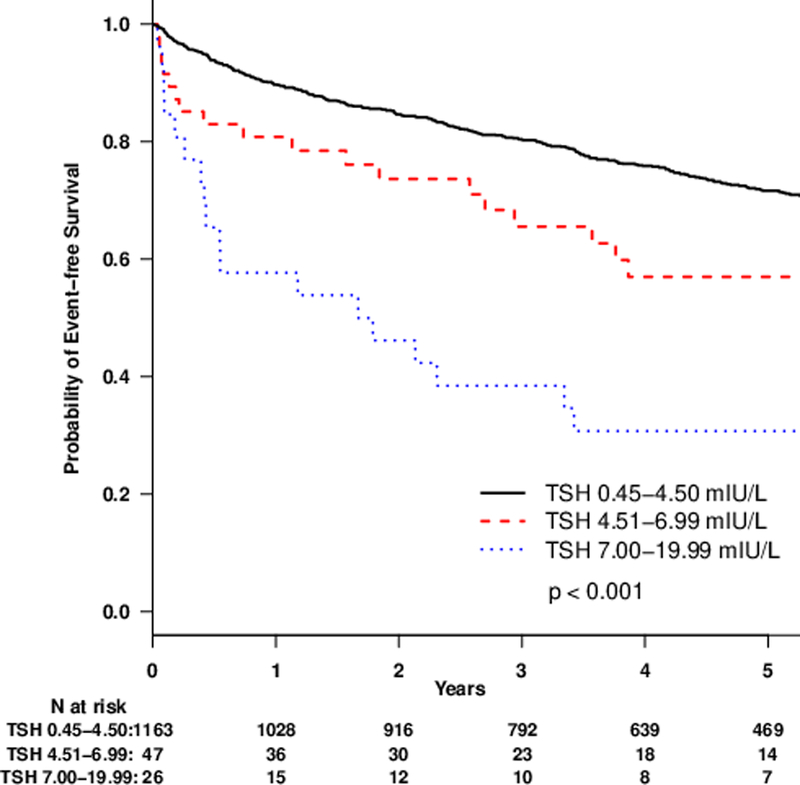
Kaplan-Meier plots of survival without VAD placement or cardiac transplantation by thyroid status comparing A. euthyroid and subclinical hypothyroid sub-categories and B. euthyroid and low T3 syndrome.
Additional Cox proportional hazards models using thyroid function tests as continuous measures showed that higher TSH, higher FT4 and lower TT3 were each associated with increased risk of the composite endpoint in the entire cohort (TSH: HR, 1.03 for a 1 mIU/L increase; 95% CI, 1.02–1.04; p <0.001; FT4: HR, 1.12 for a 0.1 ng/dL increase; 95% CI, 1.06–1.17; p <0.001; TT3: HR, 0.85 for a 10 ng/dL increase; 95% CI, 0.80 to 0.90; p <0.001) (Table 6).
Table 6.
Risk of time to first of VAD placement, cardiac transplantation or all-cause mortality by continuous measures thyroid function test individually
| Test | Hazard ratio | 95%CI | p-value |
|---|---|---|---|
| TSH per 1 mIU/L | 1.03 | 1.02–1.04 | <0.001 |
| FT4 per 0.1 ng/dL | 1.12 | 1.06–1.17 | <0.001 |
| TT3 per 10 ng/dL | 0.85 | 0.80–0.90 | <0.001 |
VAD, ventricular assist device; TSH, thyroid stimulating hormone; FT4, free thyroxine; TT3, total triiodothyronine
Cox-proportional hazard models adjusted for age, sex, race, BMI, ischemic etiology, amiodarone use, and levothyroxine use. N=1294 for TSH analyses, 1291 for FT4 analyses, and 1290 for TT3 analyses.
DISCUSSION
In a large prospective cohort of ambulatory patients with a broad spectrum of heart failure, thyroid function was significantly associated with clinically relevant outcomes, both at baseline and longitudinally. Higher TSH, higher FT4 and lower TT3 were each associated with more severe symptoms of heart failure at baseline, and also with increased risk of a composite outcome of VAD placement, cardiac transplant or all-cause mortality. Free thyroxine level showed a consistent association with atrial fibrillation across models. In adjusted models, more severe subclinical hypothyroidism was associated with a greater than 3-fold risk and low T3 syndrome with a greater than 2-fold risk of incident VAD placement, cardiac transplantation, or all-cause mortality.
There are multiple mechanisms through which thyroid hormones affect cardiovascular function. Thyroid hormones bind to thyroid hormone receptor-α in cardiac myocytes to regulate gene expression.12 Specific effects include upregulation of myosin heavy chain α and downregulation of myosin heavy chain β to affect the contractile apparatus, regulation of cycling through induction of SERCA2a and downregulation of phospholamban, and enhancement of adrenergic responsiveness through upregulation of the β1-adrenergic receptor. Interestingly, the absence of thyroid hormone also affects transcription of thyroid hormone-responsive genes, due to repression of these genes by thyroid hormone receptor when ligand is absent.13 In addition, thyroid hormones exert multiple nongenomic effects on the cardiomyocyte by directly binding to specific targets, including ion channels on cell and mitochondrial membranes.12 Thyroid hormones stimulate vasodilation through increasing nitric oxide production in vascular smooth muscle and calcium reuptake in arterioles, leading to decreased coronary vascular tone and decreased systemic vascular resistance. In total, thyroid hormone deficiency causes decreased contractility, increased systemic vascular resistance, and bradycardia, whereas thyroid hormone excess causes increased contractility, increased blood volume from activation of the renin-angiotensin-aldosterone axis, pulmonary hypertension, and tachycardia.14
Both overt hyperthyroidism and overt hypothyroidism increase risk of incident heart failure. The more subtle alterations in thyroid status found with subclinical thyroid dysfunction have also been associated with incident heart failure. A meta-analysis of individual data from 25,390 participants in six observational studies demonstrated an association between subclinical thyroid dysfunction with TSH values above 10.0 mIU/L or below 0.1 mIU/L and an increased risk of incident heart failure.5 However, the impact of thyroid dysfunction may depend on the underlying cardiac status. Specifically, patients who already have heart failure may not have adequate cardiac capacity to tolerate minor changes in thyroid hormone. Two studies have been conducted in outpatients with pre-existing heart failure, but they have been unable to effectively examine the relationship between subclinical thyroid dysfunction and mortality due to lack of measurement of T4 or T3 levels.15,16 The prevalence of subclinical hypothyroidism in our cohort was lower and of subclinical hyperthyroidism was higher than has been reported in population-based cohorts without heart failure, even after exclusion of participants taking levothyroxine or amiodarone.5 We did not detect an association between subclinical hyperthyroidism and the composite outcome. A possible explanation is the high rate of beta-adrenergic blocking agent use in our study, which mitigates the effects of thyroid hormone on the adrenergic system and has been shown to reverse adverse cardiac sequelae of subclinical hyperthyroidism.17
An analysis of the Third National Health and Nutrition Survey (NHANES III) supports our finding of an association between subclinical hypothyroidism and mortality in patients with heart failure.18 Participants with self-reported heart failure with subclinical hypothyroidism had higher mortality than their euthyroid counterparts, whereas there was no association between subclinical hypothyroidism and mortality in patients without self-reported heart failure at baseline. There were insufficient participants with pre-existing heart failure to stratify by severity of subclinical hypothyroidism. Studies have also been performed in inpatients admitted with acute forms of cardiac disease19 and heart failure20 showing associations between subclinical thyroid dysfunction and mortality. However, acute illness affects the thyroid axis and leads to thyroid testing abnormalities, and, therefore, these findings may not extrapolate to chronic heart failure in the outpatient setting. Cohorts selectively recruiting patients with heart failure are required to examine questions related to pre-existing disease. Of 14,879 participants analyzed in the NHANES cohort, only 470 had pre-existing heart failure, and that was determined by self-report, without assessment of severity.18 Similarly, of 25,390 participants included in the meta-analysis of thyroid dysfunction and heart failure, only 440 had pre-existing heart failure.5 Our analysis of 1365 ambulatory patients with chronic heart failure is the largest and best-characterized cohort to examine thyroid function. Our study clearly shows an association between subclinical hypothyroidism in outpatients and progression of heart failure as indicated by the need for VAD, a heart transplant, or mortality. Furthermore, our study had sufficient power to perform stratified analyses by degree of subclinical hypothyroidism, showing that highest risk of adverse events was in participants with TSH levels of 7 mIU/L or higher. Although this is an observational study, these findings refine the group of patients with heart failure who should undergo additional study to assess potential benefits from treatment with thyroid hormone.
Production of thyroid hormone is controlled by the pituitary gland, which, in the setting of insufficient thyroid hormone levels, releases TSH to stimulate the thyroid to produce thyroid hormone. The thyroid axis operates as a classic negative feedback loop, in which thyroid hormone feeds back to decrease TSH production and maintain an individualized pituitary-thyroid set point. It follows that outcomes associated with higher TSH levels should also be associated with lower FT4 and TT3 levels. The associations between higher TSH levels and lower TT3 levels and adverse outcomes in our study are consistent with anticipated effects of thyroid hormone insufficiency on the failing heart. The associations with FT4 are not as easily explained. Concordant with our study, prior population-based cohort studies have demonstrated associations between higher FT4 levels in the euthyroid range and incident atrial fibrillation,6,8 incident heart failure,6 and sudden cardiac death.21 Higher FT4 levels may reflect lower peripheral deiodination of T4 to T3 due to increases in cytokines, free fatty acids, and cortisol, leading to inadequate T3 due to unavailability of T4 precursor.
In patients with heart failure, low T3 levels have been associated with myocardial fibrosis and abnormalities in myocardial perfusion and metabolism.22 The low T3 syndrome, defined as a low T3 level with levels of TSH and FT4 in the reference range, is present in 20–30% of patients with heart failure.12, 23 The prevalence of low T3 syndrome in our study was 14%. In studies of hospitalized patients with heart failure, low T3 syndrome was independently associated with higher all-cause mortality.24–27 Our study validates this association in outpatients with chronic heart failure.
Our study has many strengths, including the large number of participants with advanced heart failure, adjustment for or exclusion of amiodarone and levothyroxine use, exclusion of participants taking other medications that could potentially alter thyroid function test results, long duration of follow up, careful assessment of longitudinal outcomes, and the robustness of results as exemplified by the consistency and dose-response effects across all models. Thyroid function testing was performed on banked blood, and results were not provided to study participants or their physicians. In current practice, many end stage heart failure patients who would have otherwise died are rescued by VAD placements and cardiac transplants; hence, we considered VAD placement and cardiac transplantation as mortality equivalents when defining clinical outcomes of heart failure, as we have in previous analyses of this cohort.10 This is the first study to evaluate prognostic effects of thyroid function on this composite clinical end point.
Our study has some limitations. First, we did not have a second confirmatory blood test for thyroid function after baseline. Hence we were unable to exclude the possibility of transient changes in thyroid function. Second, participants in our study were recruited from academic centers. The prospective, standardized data collection maximizes the internal validity of our analyses, but it is possible that they may not be generalizable to patients with less severe heart failure or patients in a non-referral based heart failure practice. Third, our study is an observational study, and we cannot draw definitive conclusions about whether thyroid function testing abnormalities are a marker or mediator of adverse events. Interventional studies are required to determine whether normalizing thyroid function improves health in patients with heart failure.
There are several implications from our study. Our data indicate that thyroid function is a key prognostic indicator in patients with preexisting heart failure. Subclinical hypothyroidism with TSH ≥ 7 mIU/L was associated with poor outcomes whereas TSH < 7 mIU/L was not. Studies to date evaluating levothyroxine supplementation in heart failure have reported discrepant results; our findings suggest that levothyroxine trials in heart failure could be limited to patients with subclinical hypothyroidism with TSH ≥ 7.0 mIU/L. The association of FT4, even in the euthyroid range, with poor outcomes in our study raises the potential utility of monitoring FT4 levels during levothyroxine supplementation. Our study also supports the role of low T3 syndrome in heart failure prognostication. A potential implication of this observation is the therapeutic utility of T3 in heart failure, which has been underexplored despite reports of improved contractility with intravenous infusion of T3 in a rat model of heart failure28 and acceptable safety of short term infusions of T3 in hospitalized patients with heart failure and long-term safety in patients with mild reductions in ejection fraction (mean left ventricular ejection fraction 43%).29–31
CONCLUSIONS
In conclusion, we found that subclinical hypothyroidism with TSH ≥ 7 mIU/L and low T3 syndrome are poor prognostic indicators in ambulatory patients with heart failure. Our findings indicate the need for future studies to explore therapeutic effects of T4 and T3 administration in heart failure.
Supplementary Material
What is new?
We present analyses of thyroid function and outcomes in 1365 outpatients with heart failure enrolled in the Penn Heart Failure Study. This is the largest and best-characterized prospective cohort of pre-existing heart failure to examine thyroid status.
We found significant associations between thyroid function and heart failure severity, atrial fibrillation, and a composite endpoint of ventricular assist device placement, cardiac transplantation, or all-cause mortality.
In adjusted models, more severe subclinical hypothyroidism (TSH ≥ 7 mIU/L) was associated with a greater than 3-fold risk and isolated low T3 with a greater than 2-fold risk of the composite endpoint.
What are the clinical implications?
Our data are observational, but they provide the rationale for clinical trials to explore the therapeutic effects of thyroid hormone supplementation in patients with heart failure and subclinical hypothyroidism with TSH ≥ 7 mIU/L or with isolated low T3.
ACKNOWLEDGMENTS:
SOURCES OF FUNDING: Supported by R01HL088577 from the National Heart, Lung, and Blood Institute and K24AG042765 from the National Institute on Aging.
Footnotes
DISCLOSURES: None
Contributor Information
Lakshmi Kannan, Division of Endocrinology, Diabetes, and Metabolism.
Pamela A. Shaw, Department of Biostatistics, Epidemiology, and Informatics.
Michael P. Morley, Cardiovascular Institute, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
Jeffrey Brandimarto, Cardiovascular Institute, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
James C. Fang, Division of Cardiovascular Medicine, University of Utah School of Medicine, Salt Lake City, UT.
Nancy K. Sweitzer, Division of Cardiology, University of Arizona College of Medicine, Tucson, AZ.
Thomas P. Cappola, Division of Cardiovascular Medicine, Cardiovascular Institute, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA.
Anne R. Cappola, Division of Endocrinology, Diabetes, and Metabolism.
REFERENCES
- 1.Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation 2010;122:385–393. [DOI] [PubMed] [Google Scholar]
- 2.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 2006;295:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Asvold BO, Sgarbi JA, Volzke H, Gencer B, Maciel RM, Molinaro S, Bremner A, Luben RN, Maisonneuve P, Cornuz J, Newman AB, Khaw KT, Westendorp RG, Franklyn JA, Vittinghoff E, Walsh JP, Rodondi N, Thyroid Studies Collaboration. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med 2012;172:799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J, Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010;304:1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N, Thyroid Studies Collaboration. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012;126:1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab 2015;100:1088–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asvold BO, Vatten LJ, Bjoro T, Bauer DC, Bremner A, Cappola AR, Ceresini G, den Elzen WP, Ferrucci L, Franco OH, Franklyn JA, Gussekloo J, Iervasi G, Imaizumi M, Kearney PM, Khaw KT, Maciel RM, Newman AB, Peeters RP, Psaty BM, Razvi S, Sgarbi JA, Stott DJ, Trompet S, Vanderpump MP, Volzke H, Walsh JP, Westendorp RG, Rodondi N, Thyroid Studies Collaboration. Thyroid function within the normal range and risk of coronary heart disease: an individual participant data analysis of 14 cohorts. JAMA Intern Med 2015;175:1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaker L, Heeringa J, Dehghan A, Medici M, Visser WE, Baumgartner C, Hofman A, Rodondi N, Peeters RP, Franco OH. Normal thyroid function and the risk of atrial fibrillation: the Rotterdam Study. J Clin Endocrinol Metab 2015;100:3718–3724. [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161.. [DOI] [PubMed] [Google Scholar]
- 10.Ky B, Kimmel SE, Safa RN, Putt ME, Sweitzer NK, Fang JC, Sawyer DB, Cappola TP. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation 2009;120:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaker L, Baumgartner C, den Elzen W, Ikram MA, Blum MR, Collet TH, Bakker SJ, Dehgha A, Drechsler C, Luben RN, Hofman A, Portegies ML, Medici M, Iervasi G, Stott DJ, Ford I, Bremner A, Wanner C, Ferrucci L, Newman A, Dullaart RP, Sgarbi JA, Ceresini G, Maciel RM, Westendorp R, Jukema JW, Imaizumi M, Franklyn JA, Bauer DC, Walsh JP, Rasvi S, Khaw KT, Cappola AR, Völzke H, Franco OH, Gussekloo J, Rodondi N, Peeters RP. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015;100: 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 2017;14:39–55. [DOI] [PubMed] [Google Scholar]
- 13.Brent GA. Mechanisms of thyroid hormone action. J Clin Invest 2012;122:3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, Francis GS, Lenihan D, Lewis EF, McNamara DM, Pahl E, Vasan RS, Ramasubbu K, Rasmusson K, Towbin JA, Yancy C, American Heart Association Committee on Heart Failure and Transplantation of the Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention & and Council on Quality of Care and Outcomes Research. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016;134:e579–e646. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JE, Hellkamp AS, Mark DB, Anderson J, Johnson GW, Poole JE, Lee KL, Bardy GH. Thyroid function in heart failure and impact on mortality. JACC Heart Fail 2013;1:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez AC, Jhund PS, Stott DJ, Gullestad L, Cleland JG, van Veldhuisen DJ, Wikstrand J, Kjekshus J, McMurray JJ. Thyroid-stimulating hormone and clinical outcomes: the CORONA trial (controlled rosuvastatin multinational study in heart failure). JACC Heart Fail 2014;2:35–40. [DOI] [PubMed] [Google Scholar]
- 17.Fazio S, Biondi B, Carella C, Sabatini D, Cittadini A, Panza N, Lombardi G, Sacca L.Diastolic dysfunction in patients on thyroid stimulating hormone suppressive therapy with levothyroxine: beneficial effect of β–blockade. J Clin Endocrinol Metab 1995;80:2222–2226. [DOI] [PubMed] [Google Scholar]
- 18.Rhee CM, Curhan GC, Alexander EK, Bhan I, Brunelli SM. Subclinical hypothyroidism and survival: the effects of heart failure and race. J Clin Endocrinol Metab 2013;98:2326–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iervasi G, Molinaro S, Landi P, Taddei MC, Galli E, Mariani F, L’Abbate A, Pingitore A. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med 2007;167:1526–1532. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Yang X, Wang Y, Ding L, Wang J, Hua W. The prevalence and prognostic effects of subclinical thyroid dysfunction in dilated cardiomyopathy patients: a single-center cohort study. J Card Fail 2014;20:506–512. [DOI] [PubMed] [Google Scholar]
- 21.Chaker L, van den Berg ME, Niemeijer MN, Franco OH, Dehghan A, Hofman A, Rijnbeek PR, Deckers JW, Eijgelsheim M, Stricker BH, Peeters RP. Thyroid function and sudden cardiac death: a prospective population-based cohort study. Circulation 2016;134:713–722. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Guan H, Fang W, Zhang K, Gerdes AM, Iervasi G, Tang YD. Free triiodothyronine level correlates with myocardial injury and prognosis in idiopathic dilated cardiomyopathy: Evidence from cardiac MRI and SPECT/PET Imaging. Sci Rep 2016;6:39811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ascheim DD, Hryniewicz K. Thyroid hormone metabolism in patients with congestive heart failure: the low triiodothyronine state. Thyroid 2002;12:511–515. [DOI] [PubMed] [Google Scholar]
- 24.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L’Abbate A, Donato L. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation 2003;107:708–713. [DOI] [PubMed] [Google Scholar]
- 25.Pingitore A, Landi P, Taddei MC, Ripoli A, L’Abbate A, Iervasi G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med 2005;118:132–136. [DOI] [PubMed] [Google Scholar]
- 26.Passino C, Pingitore A, Landi P, Fontana M, Zyw L, Clerico A, Emdin M, Iervasi G. Prognostic value of combined measurement of brain natriuretic peptide and triiodothyronine in heart failure. J Card Fail 2009;15:35–40. [DOI] [PubMed] [Google Scholar]
- 27.Frey A, Kroiss M, Berliner D, Seifert M, Allolio B, Güder G, Ertl G, Angermann CE, Störk S, Fassnacht M. Prognostic impact of subclinical thyroid dysfunction in heart failure. Int J Cardiol 2013;168:300–5. [DOI] [PubMed] [Google Scholar]
- 28.Henderson KK, Danzi S, Paul JT, Leya G, Klein I, Samarel AM. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction-induced congestive heart failure. Circ Heart Fail 2009;2:243–252. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton MA, Stevenson LW, Fonarow GC, Steimle A, Goldhaber JI, Child JS, Chopra IJ, Moriguchi JD, Hage A. Safety and hemodynamic effects of intravenous triiodothyronine in advanced congestive heart failure. Am J Cardiol 1998;81:443–447. [DOI] [PubMed] [Google Scholar]
- 30.Pingitore A, Galli E, Barison A, Iervasi A, Scarlattini M, Nucci D, L’abbate A, Mariotti R, Iervasi G. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab 2008;93:1351–1358. [DOI] [PubMed] [Google Scholar]
- 31.Holmager P, Schmidt U, Mark P, Andersen U, Dominguez H, Raymond I, Zerahn B, Nygaard B, Kistorp C, Faber J. Long-term L-Triiodothyronine (T3) treatment in stable systolic heart failure patients: a randomised, double-blind, cross-over, placebo-controlled intervention study. Clin Endocrinol 2015;83:931–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


