Abstract
Mice are the pre-eminent research organism in which to model human diseases and study the involvement of the immune response. Rapidly accumulating evidence indicates a significant involvement of stress hormones in cancer progression, resistance to therapies, and suppression of immune responses. As a result, there has been a concerted effort to model human stress in mice. Here, we discuss recent literature showing how mice in research facilities are chronically stressed at baseline due to environmental factors. Focusing on housing temperature, we suggest that the stress of cool housing temperatures contributes to the impact of other imposed experimental stressors and therefore has a confounding effect on mouse stress models. Furthermore, we propose that manipulation of housing temperature is a useful approach for studying the impact of chronic stress on disease and the immune response and for testing therapeutic methods of reducing the negative effects of chronic stress.
Keywords: adrenergic stress, thermoneutral housing, destressing mouse models
Introduction:
For decades, it has been recognized, largely through epidemiological observations, that certain forms of chronic stress resulting from psychological conditions such as depression, lack of social support, and anxiety, suppress immunity and may serve as a risk factor for cancer progression (1–3). Research into the interrelationships between stress, the nervous system, and the immune system have given rise to the field of “psychoneuroimmunology”. Recent laboratory research in this area has begun to provide a mechanistic understanding of the pathways that mediate the negative impact of stress on cancer (4). Moreover, studies are now identifying behavioral (1) or pharmacological (5) interventions that reduce stress and improve cancer outcomes. As is the case for the study of other important human diseases, mouse models have been developed to carry out preclinical investigations into these relationships. However, recent reports have raised concerns that the physiology of control mice housed under standard vivarium conditions reflects the adverse effects of choices that have been made regarding several housing parameters (6–9); density, cage tops, cage color, bedding, temp, cage environment, husbandry, noise (10, 11); and light intensity (12). This concern is forcing researchers to re-examine presumptions that we have held about the physiology of mouse used for preclinical experiments and to consider how these factors affect experimental outcomes. In this brief review, we focus on evidence that mice are chronically stressed at baseline due to housing temperatures and discuss how this inherent stress may affect disease models and our efforts to understand how stress impacts these models. This is particularly important for any disease or therapy with an immune component considering that this baseline stress is known to be immunosuppressive (13–16). We also highlight the utility of manipulating housing temperature to model the impact of stress in murine models of cancer and other diseases.
Using mice to model human stress
To study stress, researchers have devised several different protocols for exposing mice to stressful stimuli. These diverse approaches are all based on the idea that an event, experience, or situation that is perceived to threaten the homeostatic balance of the animal elicits a complex, integrated stress response. This response is coordinated by two biological pathways: the hypothalamic-pituitary-adrenal gland (HPA) which signals for release of corticosteroids from the adrenal cortex and the sympathetic nervous system which causes release of the catecholamines epinephrine and norepinephrine (NE) from the adrenal medulla and NE from postganglionic sympathetic neurons which innervate cells and organs of the body (17–19). Receptors for glucocorticoids and catecholamines are expressed by almost all the cells in the body and so the stress response coordinates actions of cardiovascular, musculoskeletal, and immune systems (20).
Whether the stress response is beneficial or harmful to the animal depends on many factors. One important factor is the duration of the stress. An “acute” stress is considered to be a single event lasting minutes to hours, such as exposure to a predator, which may activate the sympathetic “fight or flight” response, and is then resolved, allowing the animal to return to its resting state. Acute stress has been shown to activate and support an immune response which may be needed in case of exposure to pathogens and/or wounding (20, 21). This is in contrast to the effects of a chronic stressor which lasts for extended periods of time with no resolution and is known to have suppressive effects on immunity (22–24).
Techniques for imposing stress in murine models are usually differentiated as being physical or psychological, although certain models may actually cause both. Physical stress is often imposed using “restraint stress” in which mice are held in ventilated conical tubes or bags to limit their movements (25, 26). To induce psychological stress, mice may be subjected to fear inducing stimuli such as scream (27), predator odor (28) or social isolation (25). Stress may also be imposed by social disruption in which submissive mice are exposed to aggressive “intruders” (29). Others have modeled stress using a chemical/pharmacological approach in which mice are injected daily with the stress hormone adrenaline (30) or adrenergic receptor antagonists (28, 31). These approaches have been used to induce either acute or chronic stress in mice depending on the duration of exposure. Avitsur et al, using the social disruption model, exposed a cage of mice to an intruder for 2 hrs; they used a single exposure for an acute stress and 6 exposures over 7 days for a longer duration, “repeated”, stress (32). Repeated exposure to brief stresses such as daily 1–2 hr restraint stress for several days allows for some level of recovery between exposures (21) in contrast to the continual, chronic delivery of norepinephrine by osmotic pump (33). None the less, longer duration stresses are all generally referred to as chronic stress and replicate at least some of the effects of the stress that humans face in chronic situations such as depression or isolation.
It is important to acknowledge that the effects of imposed stress are interpreted by comparing experimental outcomes in “stressed” mice to those of “control” mice who are already under substantial cold stress. As we discuss below, these widely used models of stress may fail to reveal the full range of stress-induced impairment because the control mice are already encountering substantial housing induced cold stress.
Housing temperatures for mice affect chronic adrenergic stress levels and experimental outcomes:
There are a myriad of factors that can affect the outcomes of pre-clinical studies. Many of these factors are specifics of the experimental design (including mouse strain, age, and sex as well as the source of mice and reagents) that are choices investigators make and report, enabling others to assess how these factors may affect outcomes. However, there are many other environmental factors that impact outcomes but are not reported because they are mandated by the Guide for Care and Use of Laboratory Animals (34) and implemented in animal facilities by the staff. Environmental factors such as the type of light, the type of cage, room temperature, humidity, diet, and noise levels are somewhat hidden variables that are seldom reported but are known to influence mouse physiology (10, 12, 35–38). Investigators naturally presume that these housing decisions are made based on optimizing the biology of the mice, but this is not always the case; many of these decisions are based, with good reason, on convenience and comfort of the people who work long hours in these facilities. However, it was pointed out almost a decade ago, that standard housing conditions provide a lifestyle for mice where they are “sedentary, have continuous access to food, and have virtually no environmental stimulation” and consequently are “metabolically morbid” being “overweight, insulin resistant, hypertensive” and at risk for premature death (6). These authors raised the alarm that presuming that these mice represent healthy baseline controls is problematic and could bias the outcomes of experiments. Soon after this report, Feldman et al(36) reported in a pivotal study that the outcomes of experiments studying obesity in UPC1 knockout mice differed depending on whether mice were housed at standard temperatures (~22˚C) or thermoneutrality (~30˚C) and that these mice demonstrated the expected obesity only when housed at 30˚C. Thus, the role for UCP1 in adaptive adrenergic thermogenesis, which had been questioned on the basis of negative results obtained in mice housed at 22˚C, was confirmed when chronic cold stress was alleviated by housing at 30˚C, clearly demonstrating the significance of considering ambient housing temperatures when planning and interpreting experiments. Our group first reported that mouse models of cancer and anti-tumor immunity (39–41), immune responses in graft vs host disease (42), dendritic cell biology (43), and radiosensitivity of hematopoietic stem cells (44) are each significantly influenced by room temperature. These are representative of a growing number of papers reporting how choice of housing temperature impacts experimental outcomes and reproducibility in several mouse models of disease and we have recently reviewed this topic (45–47). Since these reviews were published, similar effects on mouse models of Alzheimer’s (48), osteoporosis (49), fatty liver disease (50), and asthma (51) have also been reported.
With respect to the study of stress and cancer and anti-tumor immunity, we discovered that tumor growth is accelerated by chronic (mild) cold stress by standard room temperature of ~ 22˚C as compared to a thermoneutral 30˚; thus, the efficacy of the anti-tumor immune response differs significantly depending on the housing temperature (41). In this model, we observed a significant increase in anti-tumor effector CD8+ T-cells in the tumor microenvironment and in draining lymph nodes, and a decrease in both regulatory T cells and MDCS (immunosuppressive cells) at 30˚C, demonstrating that housing mice at 22˚C alone results in significant suppression of the anti-tumor immune response. We also observed that this effect is lost if tumors are grown in immunodeficient mice, implicating a role for the adaptive immune response (39, 41). We went on to show that this difference is also lost when mice (housed at 22˚C) were treated with β-adrenergic receptor antagonists (β-blockers) confirming that the degree of adrenergic stress is a function of room temperature (39, 41). These results suggest that trying to study the efficacy of immunotherapy when mice are housed at 22˚C is extremely problematic. In fact, we found that the effect of housing temperature on the efficacy of immunotherapy (the checkpoint inhibitor anti-PD-1) was dramatic. Both mammary and melanoma tumors showed little to no response at 22˚C but had a significant response at 30˚C (39). We also demonstrated that the increased adrenergic signaling at 22˚C has a direct effect on tumor cells, engaging survival mechanisms such as upregulation of anti-apoptotic molecules that increase tumor cell resistance to cytotoxic therapies(40) and could possibly increase resistance to cytotoxic immune cells. These issues have critical implications for interpreting the results of experiments studying the effects of adrenergic stress and the development of strategies for overcoming stress to improve response to immune or cytotoxic therapies in mice.
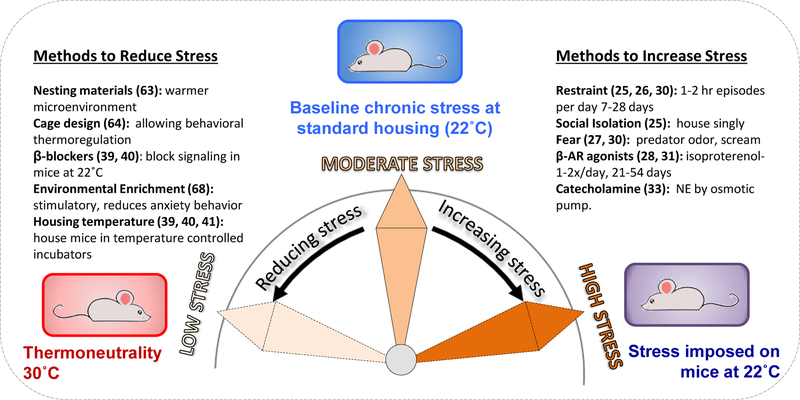
What is the appropriate baseline for identifying the immunological effects of stress in mouse models (Fig 1)?
Fig. 1: Manipulation of ambient housing temperature regulates the degree of baseline stress experienced by laboratory mice.
Mice housed at mandated sub-thermoneutral temperatures (~22˚C) experience chronic stress and have elevated levels of the sympathetic neurotransmitter norepinephrine (NE). Thus majority of studies designed to study the effects of stress in mouse models impose stress on mice which already are under a moderate degree of stress, sufficient to promote tumor growth and suppress immune responses. In contrast, by housing mice at thermoneutrality, baseline adrenergic stress is alleviated. Reducing baseline stress in mice housed at 22˚C can be achieved by alternative methods. Approaches for imposing and reducing stress in laboratory mice are indicated followed by representative references (25–28, 30, 31, 33, 39–41, 63, 64, 68).
The mild, but chronic cold stress that mice experience in 22˚C housing results in elevated levels of NE (the SNS neurotransmitter that drives nonshivering thermogenesis) (39, 40). These elevated NE levels are particularly concerning for preclinical tumor models because a growing literature in the last 15–20 years has developed demonstrating the tumor promoting effects of adrenergic stress signaling (52, 53). Additionally, it has become clear that adrenergic signaling suppresses immune responses and can skew the overall response away from a Th1 and CD8+ effector T-cell dependent immunity to a Th2 humoral response (19, 23, 27, 45, 54–60). Furthermore, effects of adrenergic stress on anti-tumor immunity have been reported mirroring the effects we have seen in response to 22˚C housing, that is, suppression of CD8+ T-cell proliferation, IFNγ expression, and cytotoxicity with a concurrent increase in pro-tumor immune suppressive cells, Tregs and MDSC (31, 61). Therefore, we predict that subthermoneutral housing which increases NE levels (and therefore, adrenergic signaling) has great potential for skewing results of experiments which are designed to help us understand the role of stress in immunity, particularly the anti-tumor immune response. In a recent review of the effects of housing mice below the thermoneutral zone, Ganeshan and Chawla (62) also expressed concern that in studies of mouse physiology and behavior, what is “considered the ‘basal state’ is probably representative of a ‘stressed state’. However, in most studies of stress, these mice are considered to be at “baseline” and stress protocols all move the “stress needle” from moderate stress to high stress (25, 26, 28, 30, 31). Therefore, the full impact that imposed stressors may have on endpoints such as tumor growth and anti-tumor immunity are likely attenuated. In other words, the “best” immune responses that the mouse can develop are expected to be seen at thermoneutrality and therefore thermoneutrality would be a more accurate baseline control. For that reason, we believe that housing temperature is a very biologically relevant way to model the impact of chronic sympathetic stress on immunity.
Reducing stress in experimental mice:
The most obvious approach to reducing stress is housing mice at thermoneutrality. But, several other approaches have been suggested that don’t require having to increase room temperature or housing mice in incubators as we do. One option is to provide nesting materials for the mice to build nests and raise the temperature of their microenvironment to as high as 32˚C (63). Another idea is to house mice in specially designed cages with areas at different temperatures and that allows them to behaviorally thermoregulate as they might in nature (64). Several groups have reduced the tumor-promoting, immunosuppressive effects of cold-stress at 22˚C pharmacologically by treating mice with β-blockers (3, 25, 30, 39, 40, 65). Interestingly, although these results are interpreted as reducing stress back to baseline control levels, β-blockers may reduce stress signaling, not back to the levels experienced by untreated mice at 22˚C, but to the low levels experienced at thermoneutrality. This interpretation is suggested by our results in which β-blockers can improve responses seen at 22˚C to the level of those achieved at 30˚C, but β-blockers have no additional benefit in mice houses at 30˚C. Another approach to reducing baseline stress at 22˚C is by providing environmental enrichment (EE), giving mice a stimulating, socially interactive living environment which better resembles their freedom in the wild(66). EE is considered a model of “eustress”, a stress which is psychologically engaging and beneficial, as opposed to stresses that have a negative impact (“distress”). Several studies have reported that EE at 22˚C resulted in inhibition of tumor growth compared to mice in standard, non-enriched cages (67–70), although other studies have not been able to replicate this effect (71) indicating that other variables are likely involved in the EE effect. Interestingly, although a recent study of EE effects on a GEM model of colon cancer did not find a difference in the number or size of tumors, the EE mice had significantly extended lifespans and this was shown to be a result of vascular normalization and increased wound repair in mice housed in EE (72). Overall, the ability of EE to lower stress levels, reduce anxiety behavior, and reduce tumor growth in some models points to the likelihood that a significant amount of baseline stress derives from stressors other than housing temperature and EE helps to alleviate these other influences.
Number of mice/cage affects the degree of stress:
In what other ways might the effects chronic cold stress impact experimental outcomes? One way in which mice housed at 22˚C cope with the cold is to huddle together(73)and although current guidelines limit the maximum number of mice that can be housed together to 4–5 based on sex and size, there are many situations in which cages contain fewer mice. For example, as an experiment progresses, it is common for the number of mice/cage to change as mice are removed either due to morbidity/mortality or to collect specimens for serial analyses of a variety of parameters over time (e.g. to monitor changes in the tumor microenvironment such as immune cells, vessel or nerve growth, or hypoxia during tumor growth). This sequence of events is seldom, if ever, reported. One type of experimental design in which changes in number of mice/cage can be clearly seen are survival studies. In these experiments, whether reporting actual survival or a surrogate survival endpoint such as time to reach a particular tumor size, mice are removed from the group when the endpoint is reached (e.g. (41)). As the numbers of mice/cage are reduced, the remaining mice are subjected to r additional cold-stress as they are less able to huddle and keep each other warm. If experimental groups consist of more than one cage, this could result in different numbers of mice in each cage. Eventually, the remaining mice in a group might be consolidated into one cage-this disruption of social groups would further exacerbate the stress levels as exposure to stranger mice is one technique used to impose stress on mice (29, 74). However, an even more problematic approach is the social isolation protocol in which mice are housed singly to replicate psych-social stress. Here`, the experimental outcomes are attributed to the stress of social isolation itself and the potential role for increased cold-stress in singly housed mice is not taken into consideration. Another scenario in which mice are singly housed is to isolate male mice whose aggression is endangering cage-mates. As discussed in a recent review`, this is a complex problem with no easy solution`, but there is likely a balance to be achieved between increasing cold stress by removing the aggressive mouse and reducing the stress imposed on the submissive cage-mates (75).
Conclusions:
In comparison to other stress inducing protocols, altering housing temperature is a very convenient, reproducible, and biologically relevant method for modeling chronic stress in mice. We favor this model because the stress is actually “chronic” as opposed to shorter stresses repeated each day which require more handling of the mice. Additionally, because “reproducibility” of experiments between labs has become a critical issue in the re-assessment of how mouse models are used (76), it is likely that regulating the degree of housing-induced adrenergic stress and reporting environmental factors that affect this parameter (i.e. ambient temperature, type of bedding, and number of mice per cage throughout the experiment) could greatly facilitate reproducibility of experimental results between labs. We have also highlighted the value of stress models for testing pharmacological inhibitors of adrenergic stress receptor signaling (β-blockers) in combination with other therapies in light of recent retrospective epidemiological studies supporting the idea that cancer patients who are taking β-blockers for other indications have better outcomes (e.g. (5, 77–83)).
Mouse models are increasingly used to study the effects of stress on disease processes, responses to therapies, and the immune response, so it is timely to consider several ways in which environmental factors, and housing temperature in particular, can affect stress levels. At this point, however, several interesting questions remain to be addressed. For instance, researchers studying the effects of various imposed stressors (e.g., restraint stress) have not generally taken into account the fact that these mice are already under significant adrenergic stress due to standard ambient temperature prior to imposition of additional stress. Thus, it would be important to determine whether the stressors imposed on mice housed at 22˚C would have the same effects if they were imposed on mice in which cold stress has been alleviated by housing at thermoneutrality. Other questions include: Can we develop a reliable method or test for quantifying the degree of stress experienced by individual mice within a group and over the duration of an experiment that will enable direct comparisons between experiments and labs? It would also be especially interesting to be able to determine whether the variability in tumor growth rates seen within a group of mice is related to differences in the degree of stress experienced by different mice. Also, how do different protocols for inducing stress compare in terms of the degree of stress actually experienced by the mice? And how does thermoneutral housing compare to environmental enrichment in terms of stress reduction-what is a true baseline? Furthermore, it is important to determine whether there intrinsic immunological differences between mice that are raised from birth with chronic cold stress compared to those raised at thermoneutrality. As these questions are answered and strategies to reduce the stress levels in mice are incorporated into experimental designs, the full biological and physiological capabilities of our models will be more accurately represented in the results.
Acknowledgments
Grant Support:
The research summarized in this review was supported by The Peter T. Rowley Breast Cancer Research Grant C028252; The Harry J. Lloyd Charitable Trust; The Roswell Park Alliance Foundation; The National Institute of Health Grant T32CA085183, The National Institute of Health Grant R01 CA205246, and used Shared Resources supported by the Roswell Park Cancer Institute’s Comprehensive Cancer Center Support Grant CA016056.
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest
REFERENCES:
- 1.Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, Clevenger L, Lubaroff DM, Sood AK, and Cole SW 2011. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun 25: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreno-Smith M, Lutgendorf SK, and Sood AK 2010. Impact of stress on cancer metastasis. Future Oncol 6: 1863–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L, Sood AK, and Cole SW 2010. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70: 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green McDonald P., O’Connell M, and Lutgendorf SK 2013. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun 30 Suppl: S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powe D, Voss M, Zänker K, Habashy H, Green A, Ellis I, and Entschladen F 2010. Beta-Blocker Drug Therapy Reduces Secondary Cancer Formation in Breast Cancer and Improves Cancer Specific Survival. Oncotarget 1: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin B, Ji S, Maudsley S, and Mattson MP 2010. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A 107: 6127–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodhi IJ, and Semenkovich CF 2009. Why we should put clothes on mice. Cell Metab 9: 111–112. [DOI] [PubMed] [Google Scholar]
- 8.Maloney SK, Fuller A, Mitchell D, Gordon C, and Overton JM 2014. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 29: 413–420. [DOI] [PubMed] [Google Scholar]
- 9.Karp CL 2012. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med 209: 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth LA. The influence of the cage environment on rodent physiology and behavior: Implications for reproducibility of pre-clinical rodent research. Exp Neurol. 2015. [DOI] [PubMed]
- 11.Toth LA, Trammell RA, and Ilsley-Woods M 2015. Interactions Between Housing Density and Ambient Temperature in the Cage Environment: Effects on Mouse Physiology and Behavior. J Am Assoc Lab Anim Sci 54: 708–717. [PMC free article] [PubMed] [Google Scholar]
- 12.Suckow MA, Wolter WR, and Duffield GE 2017. The Impact of Environmental Light Intensity on Experimental Tumor Growth. Anticancer Res 37: 4967–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messmer MN, Kokolus KM, Eng JW, Abrams SI, and Repasky EA 2014. Mild cold-stress depresses immune responses: Implications for cancer models involving laboratory mice. Bioessays 36: 884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin RL 2017. Mice Housed at Elevated Vivarium Temperatures Display Enhanced T-cell Response and Survival to Francisella tularensis. Comp Med 67: 491–497. [PMC free article] [PubMed] [Google Scholar]
- 15.Stemmer K, Kotzbeck P, Zani F, Bauer M, Neff C, Muller TD, Pfluger PT, Seeley RJ, and Divanovic S 2015. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. Int J Obes (Lond) 39: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian Xiao Y., Ganeshan K, Hong C, Nguyen Khoa D., Qiu Y, Kim J, Rajendra K. Tangirala, Tonotonoz P, and Chawla A 2015. Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metabolism [DOI] [PMC free article] [PubMed]
- 17.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, and Sood AK 2006. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chrousos GP 2009. Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374–381. [DOI] [PubMed] [Google Scholar]
- 19.Elenkov IJ, and Chrousos GP 2006. Stress system--organization, physiology and immunoregulation. Neuroimmunomodulation 13: 257–267. [DOI] [PubMed] [Google Scholar]
- 20.Dhabhar FS 2014. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res 58: 193–210. [DOI] [PubMed] [Google Scholar]
- 21.Dhabhar FS 2018. The short-term stress response-Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front Neuroendocrinol 49: 175–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padgett DA, and Glaser R 2003. How stress influences the immune response. Trends in Immunology 24: 444–448. [DOI] [PubMed] [Google Scholar]
- 23.Glaser R, and Kiecolt-Glaser JK 2005. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5: 243–251. [DOI] [PubMed] [Google Scholar]
- 24.Padro CJ, and Sanders VM 2014. Neuroendocrine regulation of inflammation. Semin Immunol 26: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, and Sood AK 2006. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12: 939–944. [DOI] [PubMed] [Google Scholar]
- 26.Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, Hollande F, and Sloan EK 2014. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 40: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou N, Zhang X, Zhao L, Zhao X, Li Z, Song T, and Huang C 2013. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun 439: 471–476. [DOI] [PubMed] [Google Scholar]
- 28.Hulsurkar M, Li Z, Zhang Y, Li X, Zheng D, and Li W 2017. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene 36: 1525–1536. [DOI] [PubMed] [Google Scholar]
- 29.Hanke ML, Powell ND, Stiner LM, Bailey MT, and Sheridan JF 2012. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun 26: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R Jr., Danial N, Datta SR, and Kulik G 2013. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 123: 874–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nissen MD, Sloan EK, and Mattarollo SR 2018. beta-Adrenergic Signaling Impairs Antitumor CD8(+) T-cell Responses to B-cell Lymphoma Immunotherapy. Cancer Immunol Res 6: 98–109. [DOI] [PubMed] [Google Scholar]
- 32.Avitsur R, Stark JL, Dhabhar FS, Padgett DA, and Sheridan JF 2002. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J Neuroimmunol 124: 54–61. [DOI] [PubMed] [Google Scholar]
- 33.Palm D, Lang K, Niggemann B, Drell T. L. t., Masur K, Zaenker KS, and Entschladen F 2006. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer 118: 2744–2749. [DOI] [PubMed] [Google Scholar]
- 34.Animals, N. R. C. U. C. f. t. U. o. t. G. f. t. C. a. U. o. L. 2011. Guide for the Care and Use of Laboratory Animals (National Academies, Washington, DC: ), 8th ed. [Google Scholar]
- 35.Animals., C. f. t. U. o. t. G. f. t. C. a. U. o. L. 2012. Guide for the Cared and Use of Laboratory Animals 8th ed. The National Academies Press, Washington, DC. [Google Scholar]
- 36.Feldmann HM, Golozoubova V, Cannon B, and Nedergaard J 2009. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209. [DOI] [PubMed] [Google Scholar]
- 37.Hogan MC, Norton JN, and Reynolds RP 2018. Environmental Factors: Macroenvironment versus Microenvironment. In Management of Animal Care and Use Programs in Research, Education, and Testing nd, Weichbrod RH, Thompson GAH, and Norton JN, eds. CRC Press/Taylor & Francis (c) 2018 by Taylor & Francis Group, LLC., Boca Raton (FL) 461–478. [Google Scholar]
- 38.Paigen B, Svenson KL, Von Smith R, Marion MA, Stearns T, Peters LL, and Smith AL 2012. Physiological effects of housing density on C57BL/6J mice over a 9-month period. J Anim Sci 90: 5182–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, Attwood K, Abrams SI, Hylander BL, and Repasky EA 2017. beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8+ T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res 77: 5639–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, and Hylander BL 2015. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta-adrenergic receptor activation. Nat Commun 6: 6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, and Repasky EA 2013. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A 110: 20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leigh ND, Kokolus KM, O’Neill RE, Du W, Eng JW, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, and Repasky EA 2015. Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. J Immunol 195: 5045–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, Lee KP, and Repasky EA 2014. Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naive and tumor-bearing mice. Front Immunol 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Povinelli BJ, Kokolus KM, Eng JW, Dougher CW, Curtin L, Capitano ML, Sailsbury-Ruf CT, Repasky EA, and Nemeth MJ 2015. Standard sub-thermoneutral caging temperature influences radiosensitivity of hematopoietic stem and progenitor cells. PLoS One 10: e0120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bucsek MJ, Giridharan T, MacDonald CR, Hylander BL, and Repasky EA 2018. An overview of the role of sympathetic regulation of immune responses in infectious disease and autoimmunity. Int J Hyperthermia 34: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hylander BL, and Repasky EA 2016. Thermoneutrality, Mice and Cancer: A Heated Opinion. Trends in Cancer 2. [DOI] [PubMed] [Google Scholar]
- 47.Qiao G, Chen M, Bucsek MJ, Repasky EA, and Hylander BL 2018. Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response. Front Immunol 9: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandal M, White PJ, Tournissac M, Tremblay C, St-Amour I, Drouin-Ouellet J, Bousquet M, Traversy MT, Planel E, Marette A, and Calon F 2016. Impaired thermoregulation and beneficial effects of thermoneutrality in the 3×Tg-AD model of Alzheimer’s disease. Neurobiol Aging 43: 47–57. [DOI] [PubMed] [Google Scholar]
- 49.Iwaniec UT, Philbrick KA, Wong CP, Gordon JL, Kahler-Quesada AM, Olson DA, Branscum AJ, Sargent JL, DeMambro VE, Rosen CJ, and Turner RT 2016. Room temperature housing results in premature cancellous bone loss in growing female mice: implications for the mouse as a preclinical model for age-related bone loss. Osteoporos Int 27: 3091–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Graspeuntner S, Cappelletti M, Wu D, Mukherjee R, Chan CC, Lawson MJ, Klarquist J, Sunderhauf A, Softic S, Kahn CR, Stemmer K, Iwakura Y, Aronow BJ, Karns R, Steinbrecher KA, Karp CL, Sheridan R, Shanmukhappa SK, Reynaud D, Haslam DB, Sina C, Rupp J, Hogan SP, and Divanovic S 2017. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med 23: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao W, Zhou L, Zhao X, Song L, Lu Y, Zhong N, Yang P, Sun B, and Zhang X 2017. Thermoneutral housing temperature regulates T-regulatory cell function and inhibits ovabumin-induced asthma development in mice. Sci Rep 7: 7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole SW, and Sood AK 2012. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 18: 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, and Sood AK 2015. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 15: 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elenkov IJ, and Chrousos GP 1999. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab 10: 359–368. [DOI] [PubMed] [Google Scholar]
- 55.Nance DM, and Sanders VM 2007. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21: 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellinger DL, and Lorton D 2014. Autonomic regulation of cellular immune function. Auton Neurosci 182: 15–41. [DOI] [PubMed] [Google Scholar]
- 57.Lorton D, and Bellinger DL 2015. Molecular Mechanisms Underlying beta-Adrenergic Receptor-Mediated Cross-Talk between Sympathetic Neurons and Immune Cells. Int J Mol Sci 16: 5635–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estrada LD, Agac D, and Farrar JD 2016. Sympathetic neural signaling via the beta2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8(+) T-cell effector function. Eur J Immunol 46: 1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, and Sinigaglia F 1997. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest 100: 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanders VM 1998. The role of norepinephrine and beta-2-adrenergic receptor stimulation in the modulation of Th1, Th2, and B lymphocyte function. Adv Exp Med Biol 437: 269–278. [DOI] [PubMed] [Google Scholar]
- 61.Siska PJ, van der Windt GJ, Kishton RJ, Cohen S, Eisner W, MacIver NJ, Kater AP, Weinberg JB, and Rathmell JC 2016. Suppression of Glut1 and Glucose Metabolism by Decreased Akt/mTORC1 Signaling Drives T Cell Impairment in B Cell Leukemia. J Immunol 197: 2532–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganeshan K, and Chawla A 2017. Warming the mouse to model human diseases. Nat Rev Endocrinol 13: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, and Garner JP 2013. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110–111: 87–95. [DOI] [PubMed] [Google Scholar]
- 64.Gordon CJ, Puckett ET, Repasky ES, and Johnstone AF 2017. A Device that Allows Rodents to Behaviorally Thermoregulate when Housed in Vivariums. J Am Assoc Lab Anim Sci 56: 173–176. [PMC free article] [PubMed] [Google Scholar]
- 65.Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H, Pimentel MA, Chai MG, Karnezis T, Rotmensz N, Renne G, Gandini S, Pouton CW, Ferrari D, Moller A, Stacker SA, and Sloan EK 2016. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun 7: 10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slater AM, and Cao L 2015. A Protocol for Housing Mice in an Enriched Environment. J Vis Exp: e52874. [DOI] [PMC free article] [PubMed]
- 67.Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, Lin B, and During MJ 2010. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li G, Gan Y, Fan Y, Wu Y, Lin H, Song Y, Cai X, Yu X, Pan W, Yao M, Gu J, and Tu H 2015. Enriched Environment Inhibits Mouse Pancreatic Cancer Growth and Down-regulates the Expression of Mitochondria-related Genes in Cancer Cells. Sci Rep 5: 7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nachat-Kappes R, Pinel A, Combe K, Lamas B, Farges MC, Rossary A, Goncalves-Mendes N, Caldefie-Chezet F, Vasson MP, and Basu S 2012. Effects of enriched environment on COX-2, leptin and eicosanoids in a mouse model of breast cancer. PLoS One 7: e51525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Y, Gan Y, Yuan H, Wang Q, Fan Y, Li G, Zhang J, Yao M, Gu J, and Tu H 2016. Enriched environment housing enhances the sensitivity of mouse pancreatic cancer to chemotherapeutic agents. Biochem Biophys Res Commun 473: 593–599. [DOI] [PubMed] [Google Scholar]
- 71.Westwood JA, Darcy PK, and Kershaw MH 2013. Environmental enrichment does not impact on tumor growth in mice [v1; ref status: indexed, http://f1000r.es/18c] F1000Res 2: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bice BD, Stephens MR, Georges SJ, Venancio AR, Bermant PC, Warncke AV, Affolter KE, Hidalgo JR, and Angus-Hill ML 2017. Environmental Enrichment Induces Pericyte and IgA-Dependent Wound Repair and Lifespan Extension in a Colon Tumor Model. Cell Rep 19: 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon CJ 2012. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol 37: 654–685. [Google Scholar]
- 74.Sommershof A, Scheuermann L, Koerner J, and Groettrup M 2017. Chronic stress suppresses anti-tumor TCD8+ responses and tumor regression following cancer immunotherapy in a mouse model of melanoma. Brain Behav Immun 65: 140–149. [DOI] [PubMed] [Google Scholar]
- 75.Weber EM, Dallaire JA, Gaskill BN, Pritchett-Corning KR, and Garner JP 2017. Aggression in group-housed laboratory mice: why can’t we solve the problem? Lab Anim (NY) 46: 157–161. [DOI] [PubMed] [Google Scholar]
- 76.2009. Troublesome variability in mouse studies. Nat Neurosci 12: 1075. [DOI] [PubMed] [Google Scholar]
- 77.Barron TI, Connolly RM, Sharp L, Bennett K, and Visvanathan K 2011. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol 29: 2635–2644. [DOI] [PubMed] [Google Scholar]
- 78.De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, and Geppetti P 2011. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 171: 779–781. [DOI] [PubMed] [Google Scholar]
- 79.Diaz ES, Karlan BY, and Li AJ 2012. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol 127: 375–378. [DOI] [PubMed] [Google Scholar]
- 80.Giampieri R, Scartozzi M, Del Prete M, Faloppi L, Bianconi M, Ridolfi F, and Cascinu S 2015. Prognostic Value for Incidental Antihypertensive Therapy With beta-Blockers in Metastatic Colorectal Cancer. Medicine (Baltimore) 94: e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, and Gonzalez-Angulo AM 2011. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 29: 2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, Zhuang Y, Levy LB, Lu C, and Gomez DR 2013. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 24: 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, Drabick JJ, and Schell TD 2018. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7: e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]