Abstract
Basophils are evolutionarily conserved in vertebrates, despite their small numbers and short lifespan, suggesting that basophils have beneficial roles in maintaining health. However, these roles are not fully defined. Here, we demonstrate that basophil-deficient mice exhibited reduced bacterial clearance, and increased morbidity and mortality, in the cecal ligation and puncture (CLP) model of sepsis. Among the several pro-inflammatory mediators we measured, tumor necrosis factor (TNF) was the only cytokine that was significantly reduced in basophil-deficient mice after CLP. In accordance with that observation, we found that mice with genetic ablation of Tnf in basophils exhibited reduced systemic TNF concentrations during endotoxemia. Moreover, during CLP, mice whose basophils could not produce TNF exhibited reduced neutrophil and macrophage TNF production and effector functions, reduced bacterial clearance, and increased mortality. Taken together, our studies show that basophils can enhance the innate immune response against bacterial infection and help prevent sepsis.
INTRODUCTION
Basophils, the rarest granulocyte (<1% of peripheral blood leukocytes), were named by Paul Ehrlich more than 130 years ago based on the prominent basophilic granules in their cytoplasm1. We now know that basophils are evolutionarily conserved in many animal species2 and that basophils can help to enhance defenses against certain parasitic infections that induce adaptive immune responses associated with the production of immunoglobulin E (IgE) isotype antibodies3–7. Basophils activated by IgE-dependent mechanisms also contribute to pathology during allergic disorders8–11.
However, in part because of their rarity and their short life span (~2.5 days in mice)12, it has been challenging to identify important roles for basophils in settings other than those associated with IgE. A few studies have raised the possibility that basophils might participate in host responses to bacteria. For example, basophils can recognize and be activated by staphylococcal enterotoxins via antibody-mediated mechanisms13, and basophils can form extracellular traps that can immobilize and kill bacteria14. Yet, no studies have demonstrated that basophils can represent an important host defense component against bacteria in vivo, and so the extent and importance of basophils in contributing to bacterial infection resistance remains to be defined.
Here, we analyzed the potential roles of basophils in the cecal ligation and puncture (CLP) model of polymicrobial bacterial infection and sepsis in mice15–17. We show that basophils were one of the first leukocytes to appear at the infection site. Moreover, by using mice genetically deficient in basophils, we found that basophils reduced the morbidity and mortality associated with CLP, at least in part by enhancing bacterial clearance. Our studies with mice genetically deficient in basophil-derived TNF also indicate that basophils represent one important source of TNF during lipopolysaccharide (LPS)-induced endotoxemia and that basophil-derived TNF can enhance the ability of other myeloid cells to control bacterial infection and contribute to survival after CLP. These results provide novel insights into basophil-dependent mechanisms that can enhance innate immune responses to bacterial infection.
RESULTS
Basophils express active toll-like receptors (TLRs)
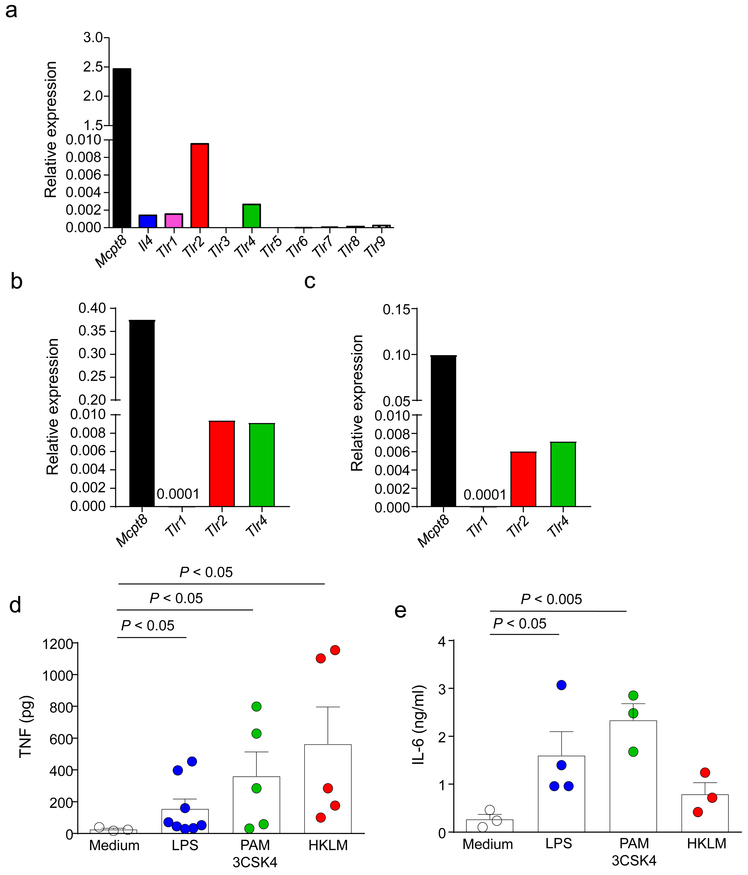
We first investigated whether mouse basophils express Toll-like receptors (TLRs) and can produce pro-inflammatory cytokines in response to bacterial products. In confirmation of prior work18, we found that mouse bone marrow-derived cultured basophils expressed abundant Tlr2 mRNA and moderate amounts of Tlr1 and Tlr4 mRNA (Fig. 1a). Similarly, we found comparable mRNA abundance of Tlr2 and Tlr4 and very low Tlr1 mRNA levels in bone marrow basophils harvested from naïve or CLP-treated mice (Fig. 1b,c). Moreover, basophils produced TNF and interleukin 6 (IL-6) in response to stimulation with TLR1/2, TLR2 and TLR4 ligands (Fig. 1d,e). These observations show that basophils express TLRs and can respond to TLR ligands by secreting pro-inflammatory cytokines.
Figure 1. Mouse basophils express TLRs and produce TNF and IL-6 after activation with TLR ligands.
(a-c) Tlr mRNA expression relative to GAPDH in FcεRIα+CD49b+c-Kit– basophils that were sorted from bone marrow-derived cells cultured in the presence of IL-3 (a) or from bone marrow cells harvested from naïve mice (b) or from mice at 24 h after CLP (c). The qPCR data are from one representative experiment of the n = 3 independent experiments performed, each of which gave similar results. (d, e) TNF (d) and IL-6 (e) concentrations in the supernatants of CD49b+ cells (1 × 106 cells) following 6 h of TLR ligand stimulation (TLR1/2 [PAM 3CSK4, 250 ng/ml], TLR2 [HKLM, 1 × 108/ml] or TLR4 [LPS, 1 μg/ml]). Data in d and e are shown as mean + SEM of the average of duplicate specimens with circles showing values obtained from individual experiments (n = 3 for medium, n = 8 for LPS and n = 5 for PAM 3CSK4 or HKLM in d, and n = 3 for medium, PAM 3CSK4 or HKLM, and n = 4 for LPS, in e. P values by Mann-Whitney test.
Basophils migrate early and enhance survival after CLP
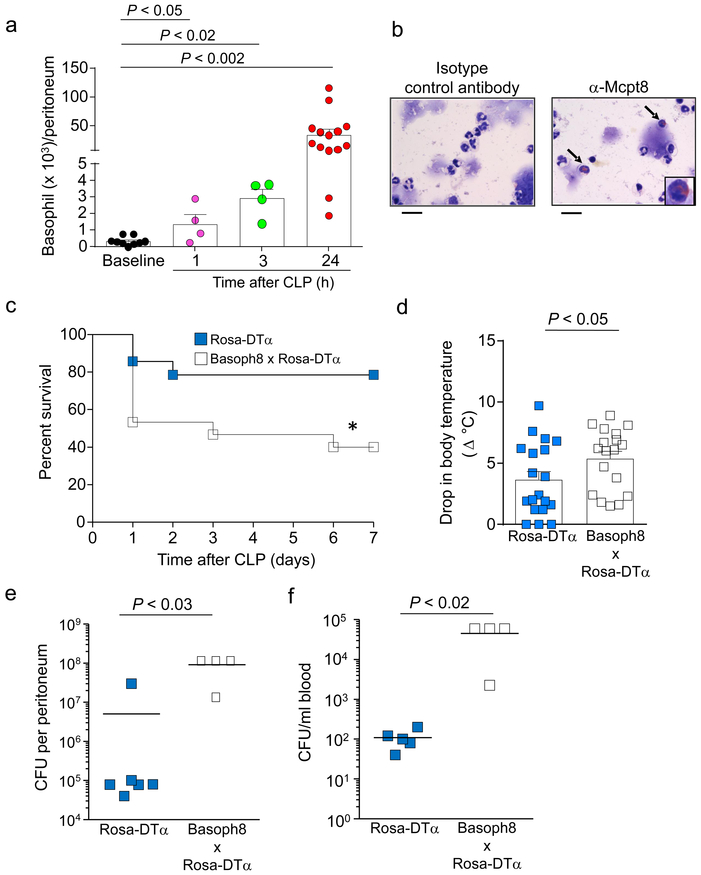
Basophils can migrate into tissues during chronic inflammatory processes10, but it is unknown to what extent basophils can infiltrate into sites of innate immune responses against bacterial infection. After the removal of doublets and dead cells, basophils were gated and defined as FcεRIα+ c-Kit− and CD49b+ cells to assess their numbers in the peritoneal cavity of naïve or CLP-treated mice (Supplementary Fig. 1). The numbers of intra-peritoneal basophils significantly increased as early as 1 h after induction of CLP of moderate severity (50% cecum ligation and one puncture with a 22G needle, Fig. 2a). Basophils remained elevated throughout the peak of the inflammatory response, which occurs at ~24 h after CLP in our model (Fig. 2a, and Supplementary Fig. 1). We confirmed that these cells were basophils by detecting increased mast cell protease 8 (Mcpt-8)-positive cells in the peritoneal fluid after CLP (Fig. 2b). Despite its name, Mcpt8 is expressed highly by basophils, but is expressed in lower amounts by certain mast cells and bone marrow eosinophils and neutrophils19. Importantly, peritoneal neutrophils and macrophages of naïve mice expressed very low amounts of Mcpt8 mRNA (Supplementary Fig. 2), indicating that basophils, but not other myeloid cells, may be the only substantial cell source of this protease in the peritoneum during CLP. Overall, these observations show that basophils migrate to the site of CLP as part of the early inflammatory response induced by this model, and progressively accumulate at that site as the inflammation reaches its peak.
Figure 2. Basophil numbers increase in the peritoneal cavity and promote survival and bacterial clearance following CLP.
(a) Numbers of basophils (FcεRIα+ CD49b+ c-Kit– cells) in the peritoneal cavity at baseline (in naïve mice) or at 1, 3 or 24 h after induction of moderate CLP. Data were pooled from n = 3 independent experiments performed (n = 4 mice for 1 or 3 h after CLP and n = 12 mice for baseline or 24 h after CLP). Data in a are shown as mean + SEM with circles representing values from individual mice. P values by Mann-Whitney test. (b) Immunocytochemical analysis of basophils (some indicated by arrows) in cytospin preparations of CD49b+ peritoneal cells obtained 24 h after CLP and stained with anti-mouse Mcpt8 antibody (or with the same amount of an isotype control antibody of irrelevant antigen specificity) and counterstained with May-Grunwald/Giemsa stain. Scale bars=20 μm. Data are from one representative experiment of the n = 3 independent experiments performed, each of which gave similar results. (c) Survival after moderate CLP was higher in littermate control mice (Rosa-DTα; n = 14) than in basophil-deficient mice (Basoph8 x Rosa-DTα; n = 15). *P < 0.05 vs. Rosa-DTα by Logrank (Mantel-Cox test). (d-f) At 24 h after moderate CLP, drop in body temperature (d), and numbers of bacterial colony forming units (CFUs) in the blood (e) and peritoneal cavity (f), were greater in the basophil-deficient mice compared with their littermate controls. Data in d are shown as mean + SEM with squares representing values from individual mice (n = 19 for wild type mice and n = 18 for basophil-deficient mice). Data in e and f are shown as means with squares representing values from individual mice (n = 6 or 5 for wild type mice in e and f, respectively, and n = 4 for basophil-deficient mice in e and f). Data were pooled from the n = 3 independent experiments performed, each of which gave similar results. P values by Mann-Whitney test.
Next, we studied whether basophils can influence CLP outcomes, using Basoph8 x Rosa-DTα basophil-deficient mice20. In Basoph8 mice, the Mcpt8 gene was replaced at the start site with a reporter cassette expressing yellow fluorescent protein (YFP) followed by an IRES and humanized Cre. Then, basophils could be tracked in Basoph8 mice as YFP+ cells or deleted by crossing Basoph8 mice with Rosa-DTα mice (Basoph8 x Rosa-DTα), which contain the diptheria toxin-α gene inserted into the ubiquitous Rosa26 locus downstream of a loxp-flanked transcriptional site21. Our data indicate that basophil-deficient mice exhibit increased mortality and morbidity (drop in body temperature) (Figs. 2c,d), as well as reduced bacterial clearance at the infection site and in the blood (Figs. 2e,f), at 24 h after CLP. These findings support the conclusion that a beneficial role of basophils during CLP is to enhance bacterial clearance and that this response is associated with enhanced survival after CLP.
Basophils contribute to intraperitoneal TNF after CLP
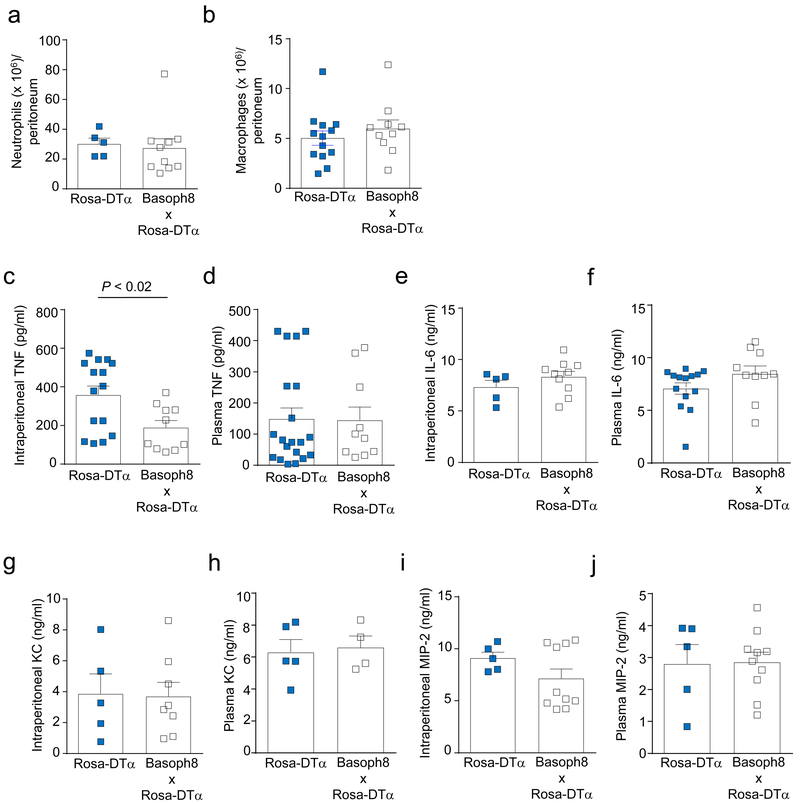
To investigate potential mechanisms by which basophils might contribute to bacterial clearance, we assessed whether the innate immune response following CLP is affected by a deficiency in basophils. Neutrophils and macrophages are essential for bacterial clearance following CLP22–24. Therefore, we investigated whether basophils might influence neutrophil or macrophage numbers at the infection site. However, we found that the numbers of neutrophils and macrophages in the peritoneal cavity were not significantly different between Basoph8 x Rosa-DTα basophil-deficient mice and the littermate controls at 24 h following CLP (Figs. 3a,b).
Figure 3. Basophils contribute to the increased TNF in the peritoneal cavity following CLP.
(a, b) Numbers of neutrophils (a) and macrophages (b) in the peritoneal live cell population analyzed by flow cytometry (Gr-1+ CD11b+ cells for neutrophils and F4/80+ CD11b+ for macrophages). Data in a and b are shown as mean + SEM with squares representing values from individual mice (n = 5 or 13 for wild type mice in a and b, respectively, and n = 10 for basophil-deficient mice in a and b). (c-j) Concentrations of TNF, IL-6, KC, and MIP-2 in the peritoneal cavity (c, e, g, i) and plasma (d, f, h, j) of ROSA-DTα and Basoph8 x ROSA-DTα mice at 24 h after CLP. Data are shown as means + SEM with squares representing values from individual mice (n = 15 in c, n = 20 in d, n = 5 in e, g–j and n = 14 in f for ROSA-DTα; and n = 10 in c-f, i, j, n = 8 in g, and n = 4 in h for Basoph8 x ROSA-DTα mice). Data were pooled from n = 3 independent experiments performed, each of which gave similar results. P values by Mann-Whitney test.
Among the several pro-inflammatory mediators we tested (IL-6, KC, MIP-2 and TNF), TNF was the only cytokine whose concentration was significantly reduced in the peritoneum of basophil-deficient mice at 24 h after CLP, whereas blood concentrations of TNF and the other mediators were similar in both the basophil-deficient mice and the littermate controls (Fig. 3c-j). These findings suggest that basophils might be an important local source of TNF, and/or may influence other cells to produce TNF, in the peritoneal cavity during the host’s response to bacteria. This observation is relevant because TNF can importantly contribute to the initiation and/or enhancement of the inflammatory response against certain bacterial infections25–27. Notably, we have shown that TNF-deficient mice subjected to CLP with the same severity we used in this study (i.e., moderately severe CLP) exhibited similar survival rates to those observed with basophil-deficient mice28, as well as significantly increased bacterial colony forming units in the peritoneum and blood after CLP (Supplementary Fig. 3). From these results, we can conclude that, in this model of CLP, endogenous TNF is required to limit bacterial growth and dissemination, and to survive peritonitis.
Basoph8 x Tnffl/fl basophils produce less TNF
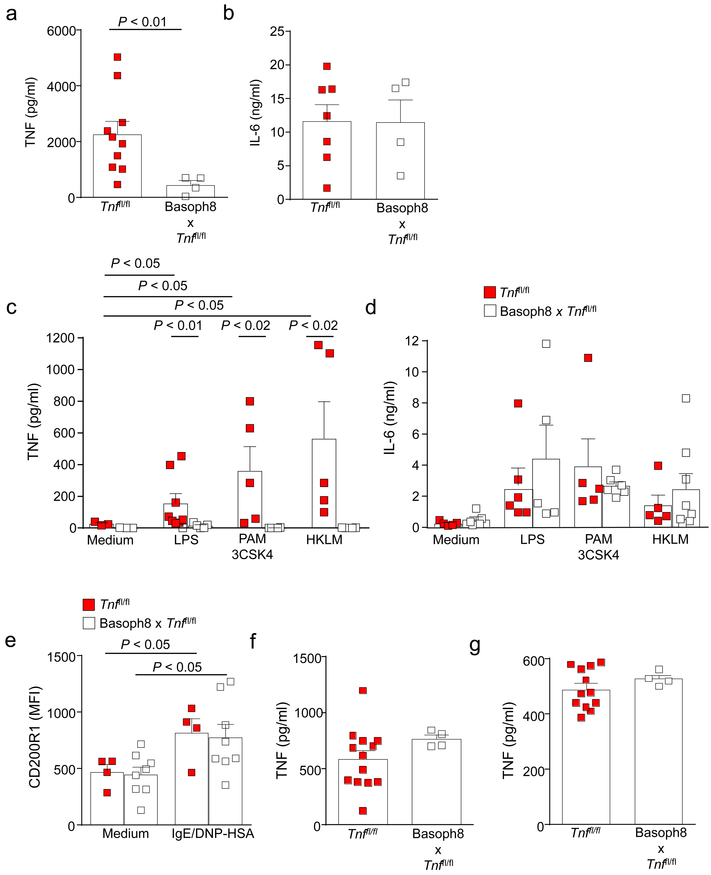
Next, we attempted to produce mice in which basophils were impaired in their ability to produce TNF. We crossed Basoph8 mice with mice containing loxP-flanked Tnf alleles29 (Basoph8 x Tnffl/fl mice). We found an approximately 80% reduction in TNF production but not in IL-6 production by Basoph8 x Tnffl/fl mouse bone marrow-derived CD49b+ cells (which consisted of 60–80% basophils) after activation with antigen [2,4 dinitrophenyl conjugated with human serum albumin (DNP-HSA)]-IgE complexes (Fig. 4a,b). Similarly, production of TNF, but not IL-6, in response to TLR ligands was significantly reduced in CD49b+ cells generated from Basoph8 x Tnffl/fl mice (Fig. 4c,d). Basophils from littermate controls and from Basoph8 x Tnffl/fl mice exhibited a similar increase in CD200R1 expression30 after activation mediated by IgE/antigen (Fig. 4e), suggesting that the reduced amounts of TNF that were produced by the Basoph8 x Tnffl/fl mouse basophils in vitro cannot be explained by an impairment in these basophils’ ability to undergo activation.
Figure. 4. TNF production is markedly diminished in CD49b+ cells generated from Basoph8 x Tnffl/fl mice.
TNF (a and c) and IL-6 (b and d) concentrations in the supernatants of CD49b+ cells (1 × 106 cells), following over-night sensitization with an IgE mAb to DNP (2 μg/ml) and 6 h of DNP-HSA (20 ng/ml) challenge (a, b) or TLR ligand stimulation (TLR1/2 [PAM 3CSK4, 250 ng/ml], TLR2 [HKLM, 1 × 108/ml] or TLR4 [LPS, 1 μg/ml)]) (c, d). CD49b+ cells enriched from bone marrow-derived cells of Basoph8 x Tnffl/fl mice were markedly diminished compared with those observed after challenge of the control Tnffl/fl mice, whereas IL-6 production was unchanged. Data in a and b are shown as mean + SEM of the average of duplicate specimens with squares showing values obtained from individual experiments (n = 10 and n = 8 for cells from wild type mice in a and b, respectively, and n = 4 in a and b for cells from mice with basophil-derived TNF deficiency). Data in c and d are shown as mean + SEM of the average of duplicate specimens with squares showing values obtained from individual experiments. In c, n = 3 for medium, n = 7 for LPS and n = 5 for PAM 3CSK4 or HKLM for cells from wild type mice, and n = 3 for medium, n = 6 for LPS and n = 4 for PAM 3CSK4 or HKLM for cells from mice with basophil-derived TNF deficiency. In d, n = 5 medium, n = 6 for LPS and n = 5 for PAM 3CSK4 or HKLM for cells from wild type mice; and n = 7 for medium, n = 5 for LPS and n = 7 for PAM 3CSK4 or HKLM for cells from mice with basophil-derived TNF deficiency. P values by Mann-Whitney test. (e) Mean fluorescence intensity (MFI) for CD200R1 in bone marrow-derived basophils (FcεRIα+ CD49b+ c-Kit−) sensitized overnight with IgE mAb to DNP (2 μg/ml) and then challenged with DNP-HSA (20 ng/ml) for 1 h. CD200R1 expression was assessed by flow cytometry. The data in e were pooled from n = 3 independent experiments that were performed, with each condition tested in duplicate. Data are shown as means + SEM with squares showing values for cells obtained from individual mice (n = 4 for wild type cells and n = 8 for cells from mice with basophil-derived TNF deficiency). P values by Mann-Whitney test. (f, g) TNF production by peritoneal cell-derived mast cells (PCMCs) (f) and thyoglycollate-elicited peritoneal macrophages (g). PCMCs were sensitized overnight with IgE mAb to DNP (2 μg/ml) and then challenged with DNP-HSA (20 ng/ml) for 6 h. Peritoneal macrophages were stimulated with LPS (100 ng/ml) for 6 h. Data in f and g are shown as means + SEM with squares showing values from individual experiments (n = 13 and n = 12 for Tnffl/fl mice in f and g, respectively, and n = 4 for Basoph8 x Tnffl/fl for f and g) with each condition tested in duplicate. P values by Mann-Whitney test.
TNF deletion in Basoph8 x Tnffl/fl mice appears to be basophil-specific, in that TNF production by peritoneal cell-derived mast cells (PCMCs) or thioglycollate-elicited peritoneal macrophages obtained from Basoph8 x Tnffl/fl mice was not significantly different from that of the corresponding cells obtained from the control mice (Fig. 4f,g). These findings are in agreement with a previous study that showed that basophils, but not eosinophils and neutrophils, in the bone marrow, or mast cells in the peritoneum, express mRNA for Mcpt819. Furthermore, our own observations showed that Mcpt8 mRNA abundance in primary neutrophils and macrophages sorted from peritoneal fluids obtained from wild-type and Basoph8 x Tnffl/fl mice were significantly lower than those found in basophils sorted from the bone marrow of wild-type mice (Supplementary Fig. 2). Overall, this evidence strongly suggests that Cre recombinase expression under the control of the Mcpt8 promoter and the consequent floxed Tnf gene deletion in mice occurs primarily in basophils and not in other myeloid cells.
We found no significant differences in the numbers of basophils or other leukocyte populations, including neutrophils in the spleen, bone marrow and peritoneal fluid, macrophages in the spleen and peritoneal fluid, monocytes and eosinophils in the blood and mast cells in the peritoneal fluid of Basoph8 x Tnffl/fl when compared with littermate controls (Supplementary Fig. 4a-e). In contrast, Basoph8 x Tnffl/fl mice exhibited ~60–66% increases in numbers of blood lymphocytes and neutrophils (Supplementary Fig. 4f), but they exhibited a similar reduction in the numbers of blood lymphocytes and neutrophils as their littermate controls at 24 h after CLP, as expected in mice subjected to this procedure31 (Supplementary Fig. 4g). These observations suggest that a modest inhibitory effect of basophil-derived TNF in the development and/or maintenance of normal numbers of blood granulocytes and lymphocytes may not have importantly influenced the results of our CLP studies.
Basophil-derived TNF during LPS-induced endotoxemia
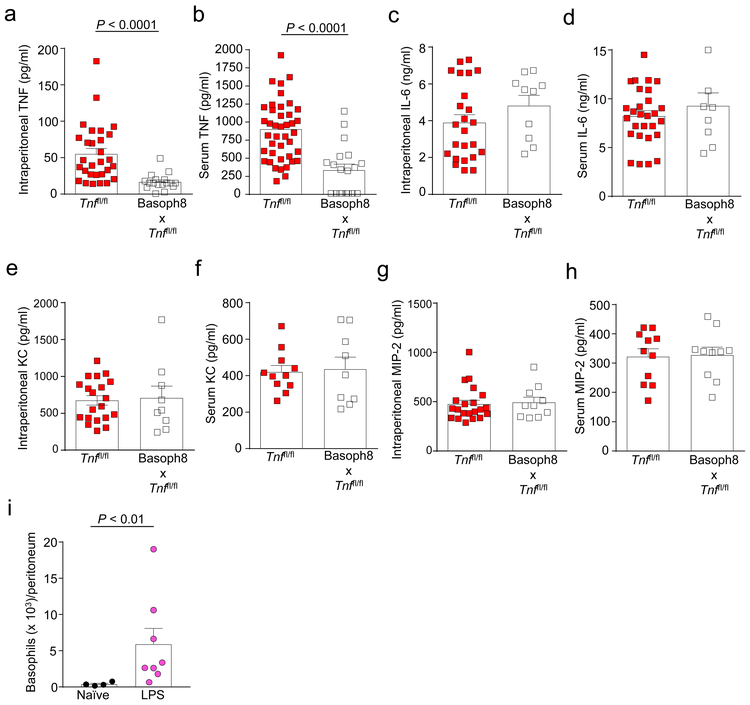
TNF production is an important downstream consequence of TLR signaling, in particular through TLR432. Moreover, TNF was reported to be a critical mediator of septic shock in humans and of LPS/D-Gal-induced acute systemic toxicity in mice33. Importantly, we found that Basoph8 x Tnffl/fl mice exhibited a significant reduction in the concentrations of both intraperitoneal (Fig. 5a) and serum (Fig. 5b) TNF 1 h after i.p. administration of LPS, indicating that basophil-derived TNF can contribute to the increased concentrations of TNF observed during LPS-induced endotoxemia. In contrast, Basoph8 x Tnffl/fl mice and their littermate controls exhibited similar intraperitoneal and serum concentrations of IL-6, KC, and MIP-2 at 1 h after LPS challenge (Fig. 5c-h), indicating that basophil-derived TNF does not detectably contribute to the increased concentrations of these mediators during LPS-induced endotoxemia.
Figure 5. Basophils contribute to increased intraperitoneal and blood TNF concentrations during LPS-induced endotoxemia.
(a-h) Concentrations of TNF, IL-6, KC, and MIP-2 in the peritoneal cavity (a, c, e, f) and serum (b, d, f, h) of Tnffl/fl and Basoph8 x Tnffl/fl mice at 1 h after i.p. injection of LPS (10 μg). Data are shown as means + SEM with squares representing values from individual mice (n = 29 in a, n = 41 in b, n = 24 in c, n = 28 in d, n = 20 in e, n = 11 in f and h and n = 22 in g for Tnffl/fl mice; and n = 17in a, n = 19 in b, n = 10 in c, g and h, n = 8 in d and n = 9 in e and f for Basoph8 x Tnffl/fl mice). Data were pooled from n = 5 independent experiments performed, each of which gave similar results. P values by Mann-Whitney test. (i) Numbers of basophils (FcεRIα+ CD49b+ c-Kit− cells) in the peritoneal cavity at baseline (naïve mice) or at 1 h after i.p. injection of LPS (10 μg). Data are shown as means + SEM with squares representing values from individual mice. Data in i were pooled from n = 2 independent experiments performed (n = 4 and n = 8 mice for naïve and LPS-treated mice, respectively). P values by Mann-Whitney test.
As we showed for the CLP model, we found that basophils accumulate at the insult site (peritoneum) at 1 h after i.p. injection of a sub-lethal dose of LPS (Fig. 5i). Moreover, we found that peritoneal macrophages from Basoph8 x Tnffl/fl mice are impaired in their ability to produce TNF during LPS-induced endotoxemia (Supplementary Fig. 5). In contrast, macrophages obtained from Basoph8 x Tnffl/fl mice can produce TNF after stimulation with LPS ex vivo (Fig. 4g), indicating that the reduced TNF concentrations observed after LPS administration are not due to an intrinsic defect in the ability of myeloid cells to produce TNF. These data suggest that the overall reduced intraperitoneal concentrations of TNF detected in Basoph8 x Tnffl/fl mice may be explained by the impaired ability of resident myeloid cells to produce TNF during LPS-induced endotoxemia when not influenced by basophil-derived TNF. Taken together, our findings indicate that basophil-derived TNF can contribute, directly and indirectly, to the elevated concentrations of TNF seen during LPS-induced endotoxemia in mice.
Basophil-derived TNF enhances survival after CLP
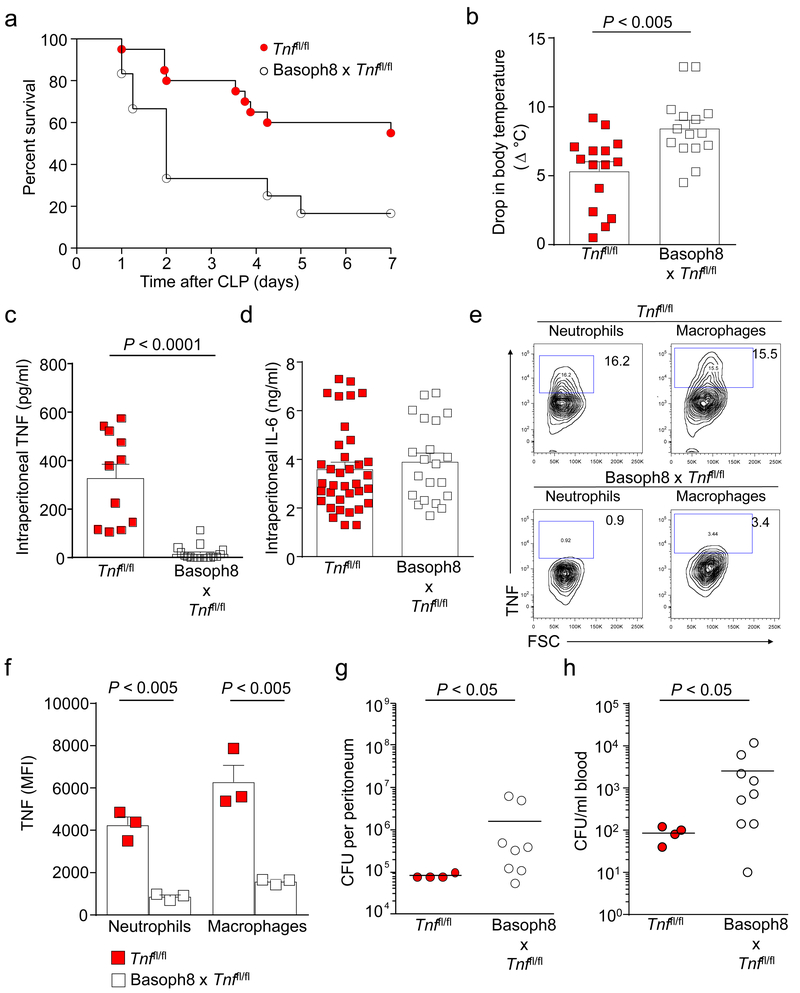
Next, we examined whether basophil-derived TNF can influence the outcome of CLP. Basoph8 x Tnffl/fl mice exhibited increased mortality and morbidity (as assessed by a drop in body temperature) at 24 h after CLP compared with the identically treated Basoph8 x Tnffl/fl mice (Figs. 6a,b). Basoph8 x Tnffl/fl mice also had significantly reduced concentrations of intraperitoneal TNF after CLP (Fig. 6c). Basoph8 x Tnffl/fl mice have reduced or absent Mcpt8 (i.e., Basoph8) as well as TNF (Supplementary Fig. 6a,b and Supplementary Fig. 7). However, morbidity, bacteria clearance, intraperitoneal TNF concentrations, and numbers of neutrophils and macrophages in the peritoneal cavity after CLP were not impaired in Basoph8 x Tnf+/+ mice, which lacked Mcpt8 but expressed TNF (Supplementary Fig. 6e-j), and no alterations in concentrations of intraperitoneal IL-6 were observed in Basoph8 x Tnffl/fl mice at 24 h after CLP (Fig. 6d).
Figure 6. Basophil-derived TNF contributes to reduced morbidity and mortality, increased TNF concentrations, and enhanced bacterial clearance following CLP.
(a) Survival after moderate CLP was higher in the Tnffl/fl mice (n = 20) than in the Basoph8 x Tnffl/fl mice (n = 12). *P < 0.02 vs. Tnffl/fl mice by Logrank (Mantel-Cox test). Data were pooled from n = 3 independent experiments performed, each of which gave similar results. (b-d) Drop in body temperature (b) and levels of intraperitoneal TNF (c) and IL-6 (d) in Tnffl/fl mice and Basoph8 x Tnffl/fl mice at 24 h after CLP. Data in b-d, shown as means + SEM with squares representing values for individual mice (n = 15, n = 11 and n = 36 for Tnffl/fl mice in b, c and d, respectively, and n = 15 in b and c, and n = 21 in d for Basoph8 x Tnffl/fl mice). Data were pooled from the n = 3 independent experiments performed, each of which gave similar results. P values by Mann-Whitney test. (e, f) Flow cytometry plots (e) and mean intensity fluorescence (MFI) (f) for TNF positive cells in the neutrophil and macrophage populations obtained from the peritoneal cavity of Tnffl/fl and Basoph8 x Tnffl/fl mice at 24 h after CLP and then incubated ex vivo for 4 h in the presence of GolgiPlug. Cells were immunostained for TNF in addition to surface Gr-1 and CD11b to identify neutrophils or surface F4/80 and CD11b to identify macrophages. Data in e are from one representative experiment of the n = 3 independent experiments performed, each of which gave similar results. The data in f were pooled from n = 3 independent experiments that were performed, with each condition tested in duplicate. Data are shown as means + SEM with squares showing values for cells obtained from individual mice (n = 3/group). P values by Mann-Whitney test. (g, h) Peritoneal cavity (g) and blood (h) colony forming units (CFU) in Tnffl/fl mice and Basoph8 x Tnffl/fl mice at 24 h after CLP. Data in g and h are shown as means with squares representing values from individual mice (n = 4 for Tnffl/fl mice, and n = 8 and n = 9 for Basoph8 x Tnffl/fl mice in g and h, respectively). Data were pooled from the n = 3 independent experiments performed, each of which gave similar results. P values by Mann-Whitney test.
There are multiple potential sources of TNF during CLP, including neutrophils and macrophages34. Moreover, in vitro studies have shown that exposure to exogenous TNF can enhance the ability of LPS to induce up-regulation of TNF mRNA expression in neutrophils35 and to increase cytotoxic activity in macrophages36. Notably, we found that, when assessed at 24 h after CLP, peritoneal neutrophils and macrophages from Basoph8 x Tnffl/fl mice exhibited a substantially reduced ability to produce TNF (Figs. 6e,f).
In order to track basophils in Basoph8 mice, a yellow fluorescent protein (YFP) reporter was used as part of the cassette that contained humanized Cre recombinase, resulting in high YFP expression in basophils expressing the Mcpt8 gene (Basoph8+/− cells) (Supplementary Fig. 8a). In contrast, peritoneal neutrophils, macrophages, and mast cells obtained from Basoph8+/− x Tnffl/fl mice at 24 h after CLP induction exhibited low YFP expression, similar to that from cells obtained from the Tnffl/fl mice (Supplementary Fig. 8a). Moreover, we did not detect Mcpt8 gene expression by single-cell PCR in neutrophils, macrophages, or mast cells obtained from Basoph8+/− x Tnffl/fl mice 24 h after CLP (Supplementary Fig. 8b). These observations indicate that neutrophils, macrophages, and mast cells in the Basoph8+/− x Tnffl/fl mice did not up-regulate Cre recombinase for TNF deletion after the mice were subjected to CLP, suggesting that genetic deletion of TNF during CLP remained largely, if not entirely, basophil-specific. Overall, our findings support the conclusion that basophil-derived TNF can enhance the ability of neutrophils and macrophages to produce TNF during CLP, thus providing an explanation for the low TNF production detected in neutrophils and macrophages after CLP in Basoph8 x Tnffl/fl mice.
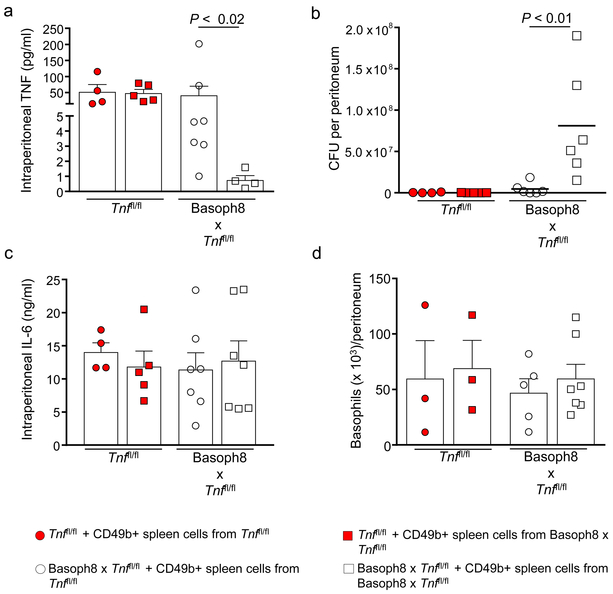
As observed in the basophil-deficient mice, we found that the reduced survival rates and increased morbidity in the Basoph8 x Tnffl/fl mice were associated with an impairment in the ability of these mice to clear bacteria, both at the infection site (peritoneal cavity) and systemically, as assessed at 24 h after CLP (Figs. 6g,h, respectively). Moreover, the adoptive transfer of CD49b+ basophil-enriched splenocytes from littermate control mice, but not from basophil TNF-deficient mice, increased intraperitoneal TNF (Fig. 7a) and enhanced the ability of Basoph8 x Tnffl/fl mice to clear bacteria at the infection site at 24 h after CLP (Fig. 7b). In contrast, we did not observe alterations in intraperitoneal IL-6 concentrations in mice deficient in basophil-derived TNF that underwent adoptive transfer of basophils from littermate controls at 24 h after CLP (Fig. 7c).
Figure 7. Adoptive transfer of CD49b+ spleen cells from control littermates enhances bacterial clearance in Basoph8 x Tnffl/fl mice.
Tnffl/fl and Basoph8 x Tnffl/fl mice received an adoptive transfer at 3 h after CLP with the CD49b+ basophil-enriched fraction of splenocytes (4 × 105 cells) obtained from Tnffl/fl mice or Basoph8 x Tnffl/fl mice. (a-d) Concentrations of TNF (a) and IL-6 (c), colony forming units (CFU) (b) and numbers of basophils (FcεRIα+ CD49b+ c-Kit− cells) (d) in the peritoneal cavity at 24 h after CLP. Data in a–d are shown as means + SEM with squares and circles representing values for individual mice (n = 4 in a–c and n = 3 in d for Tnffl/fl + CD49b+ spleen cells from Tnffl/fl; n = 5 in a–c and n = 3 in d for Tnffl/fl + CD49b+ spleen cells from Basoph8 x Tnffl/fl; n = 7 in a and c, n = 6 in b and n = 5 in d for Basoph8 x Tnffl/fl + CD49b+ spleen cells from Tnffl/fl; and n = 4 in a, n = 6 in b, n = 7 in c and d for Basoph8 x Tnffl/fl + CD49b+ spleen cells from Basoph8 x Tnffl/fl). Data were pooled from the n = 3 independent experiments performed, each of which gave similar results. P values by Mann-Whitney test.
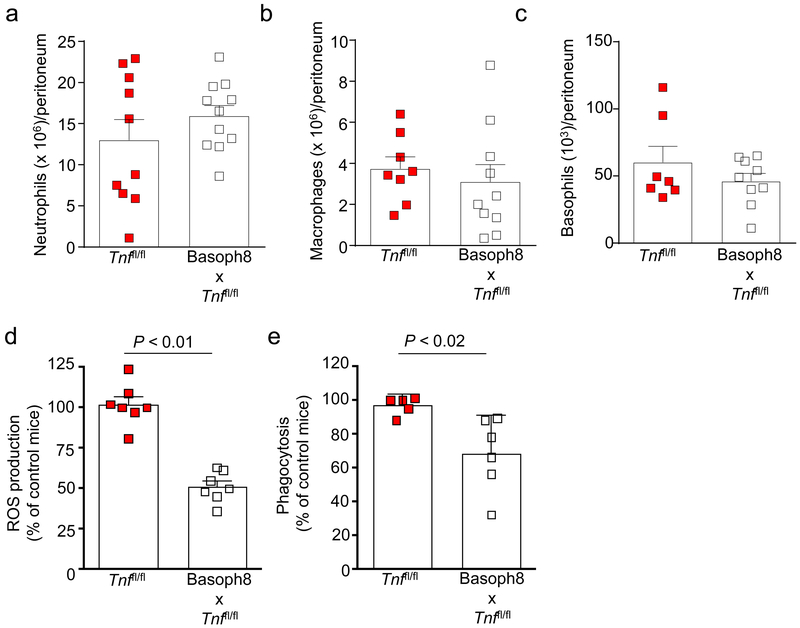
We also did not observe differences in TNF concentrations or bacterial clearance at the infection site at 24 h after CLP when we performed an adoptive transfer of basophils obtained from either wild-type littermate controls or basophil TNF-deficient mice into wild-type mice (Figs. 7a,b), suggesting that the endogenous TNF provided by host basophils (and perhaps other cells) is sufficient to enhance innate immunity during CLP. Importantly, basophil numbers in the peritoneal cavity were similar in all the mouse groups at 24 h after CLP (Fig. 7d). We think that these findings demonstrate a role for endogenous basophil-derived TNF in the enhancement of the innate immune response against bacteria during our model of CLP. Moreover, neutrophil, macrophage, and basophil numbers in the peritoneal cavity were similar in the control and Basoph8 x Tnffl/fl mice at 24 h after CLP (Fig. 8a-c), providing evidence that the impaired bacterial clearance in these mice cannot be explained by a generalized defect in myeloid cell mobilization to the infection site.
Figure 8. Basophil-derived TNF enhances neutrophil ROS production and macrophage phagocytosis after CLP.
Numbers of neutrophils (a), macrophages (b) and basophils (c), and ROS production (d) and macrophage phagocytosis (e) in peritoneal lavage fluids at 24 h after inducing moderate CLP in Tnffl/fl mice and Basoph8 x Tnffl/fl mice. Data are shown as means + SEM with squares representing values for individual mice (n = 10 in a, n = 8 in b, n = 7 in c and d, and n = 5 in e for Tnffl/fl mice; and n = 11 in a, n = 10 in b, n = 9 in c, n = 7 in d and n = 6 in e for Basoph8 x Tnffl/fl. Data were pooled from n = 3 independent experiments performed, each of which gave similar results. P values by Mann-Whitney test.
TNF can influence bacterial clearance by increasing neutrophil production of reactive oxygen species (ROS) and macrophage phagocytosis37. We observed that at 24 h after CLP Basoph8 x Tnffl/fl peritoneal neutrophils produced diminished amounts of ROS (Fig. 8d) and peritoneal macrophages were impaired in their ability to phagocytose bacteria (Fig. 8e). Importantly, neutrophils and macrophages of naïve Basoph8 x Tnffl/fl mice produced ROS or phagocytosed bacteria like those of littermate controls to stimuli such as PMA or opsonized FITC-labeled Escherichia coli (Supplementary Fig. 9a,b), suggesting that basophil-derived TNF can improve mouse survival during CLP in part by enhancing the ability of neutrophils and macrophages to clear bacteria. IL-6 also can improve survival in mouse models of sepsis, and the mortality of IL-6-deficient mice is higher than that of wild type mice following bacterial infections38–40. We found that peritoneal macrophages from Basoph8 x Tnffl/fl mice exhibited diminished production of IL-6 during CLP (Supplementary Fig. 10), suggesting that basophil-derived TNF also induces IL-6 production by myeloid cells that can enhance their activity against bacteria.
To explore a potential contribution of basophils to the outcome of human sepsis, we investigated whether basophil-specific gene expression levels in whole blood associate with clinical outcomes using a dataset obtained from both 35 healthy subjects and 167 severely injured patients, ages 16–55 (dataset available at http://web.mgh.harvard.edu/TRT/) (Supplemental Table 1). In agreement with our observation that circulating basophil numbers increase during infection, we found that expression of FCERIA positively correlated with the presence of a nosocomial infection (FDR adjusted P value = 0.0362 and Spearman correlation coefficient = 0.27). Importantly, at d 7 of hospitalization, FCERIA expression inversely correlated with potential negative outcomes of nosocomial infections, such as multiple organ failure, time to recovery, and hospital length of stay (Supplemental Table 1).
DISCUSSION
We used genetically-manipulated mice to reveal a potentially important role for basophils in enhancing inflammation at early stages of the innate immune response to CLP, and in enhancing survival in mice subjected to this long-standing model of polymicrobial infection and sepsis.
Our in vivo studies indicate that the presence of basophils reduces mortality and morbidity after CLP by enhancing bacterial clearance. Interestingly, we found that TNF was the only cytokine examined that was significantly reduced in the peritoneum of basophil-deficient mice after CLP. There is evidence that TNF can be pathogenic during sepsis, such observations generally having been made in those with severe sepsis or septic shock33. In other settings, death due to sepsis occurs in subjects with impaired immunity, including in their ability to produce TNF41. Consistent with such observations, the use of anti-TNF antibodies and genetic approaches in preclinical models of sepsis, including CLP, has consistently shown that the contribution of TNF is critical for an efficient innate antimicrobial defense28, 42–44. Despite this evidence, the cellular sources of the TNF which can help to initiate inflammatory responses during the early stages of infections have yet to be fully defined.
Having shown that the presence of basophils significantly contributes to TNF concentrations in the peritoneum after CLP and that basophils can produce substantial amounts of TNF in vitro, we then assessed the extent to which basophil-derived TNF might be important in vivo. To do this, we generated mice with basophils lacking TNF (Basoph8 x Tnffl/fl mice).
Basoph8 x Tnffl/fl mice exhibited significantly increased morbidity, reduced concentrations of intraperitoneal TNF and increased CFUs in the peritoneal cavity and blood after moderately severe CLP. It was reported that TNF can prime neutrophils and macrophages to produce ROS and to phagocytose bacteria, respectively37. Therefore, we hypothesized that the reduced ability of Basoph8 x Tnffl/fl mice to clear bacteria may be associated with impaired host response to infection. We observed that, at 24 h after CLP, Basoph8 x Tnffl/fl mice displayed reduced peritoneal neutrophil ROS production and macrophage phagocytosis. Basoph8 x Tnffl/fl mice also exhibited reduced concentrations of TNF in the peritoneal cavity and blood (but not reduced concentrations of IL-6, KC or MIP-2) 1 h after i.p. injection of LPS, indicating that basophils represent a significant source of the TNF produced in this setting as well.
Considering that basophils constitute <1% of peripheral blood leukocytes, it was initially surprising to find that peritoneal levels of TNF were almost undetectable after CLP and blood levels of TNF were greatly diminished during LPS-induced endotoxemia in Basoph8 x Tnffl/fl mice. Because there is mounting evidence that basophils are essential initiators of certain inflammatory responses, e.g., IgE-dependent chronic allergic inflammation45, we considered the possibility that basophil-derived TNF plays a major role in the first stages of host defense against bacteria by priming macrophages and neutrophils to produce large amounts of TNF35,36 that, in an autocrine and/or paracrine fashion, can influence important functions of these myeloid cells, e.g., ROS production and phagocytosis. Indeed, we found that neutrophil and macrophage TNF production, and macrophage IL-6 production, were reduced in Basoph8 x Tnffl/fl mice after CLP.
It is unclear to us how basophil-derived TNF influences myeloid function during CLP, but it may do so indirectly by controlling the expression of third-party cytokines. In support of this hypothesis, we found that peritoneal macrophages from Basoph8 x Tnffl/fl mice exhibited a reduced ability to produce IL-6 during CLP. It has been shown that IL-6 administration can improve survival in mouse models of sepsis. Moreover, the mortality of IL-6-deficient mice is high following bacterial infections compared with that of wild type mice38–40. Accordingly, there is a possibility that basophil-derived TNF induces IL-6 production by macrophages that, in an autocrine manner, enhances macrophage activity against bacteria.
The contribution of basophils to innate immunity during bacterial infections and sepsis in humans is not yet clear. Humans with a total selective lack of basophils have not been observed. However, it has been suggested that alterations in basophil numbers and/or basophil-associated molecules are consistent with specific roles for basophils in the pathophysiology of human diseases, such as anaphylaxis46.
We found in a dataset generated by the Inflammation and Host Response to Injury Program (Glue Grant)47, 48 that thirty-nine patients with severe traumatic injuries who participated in this study were evaluated for basophil counts at admission (no infection present) and at a time when the same patients developed a nosocomial infection. We found that numbers of basophils were significantly increased in patients who acquired a nosocomial infection (median: 0.07 basophils x 109/L of blood [range: 0–0.4] versus 0.04 basophils x 109/L of blood [range: 0–0.11] at baseline, P < 0.001). This finding, as well as the results of our investigation of whole blood expression of FCERIA, suggests that nosocomial infection stimulates a blood basophil response in humans.
In summary, we have demonstrated in mice that basophils are among the first leukocytes to migrate to the site of infection after induction of CLP and have provided several lines of evidence that basophils, at least in part by releasing TNF, can enhance the inflammatory response against bacteria and promote bacterial clearance via effects on other myeloid cells. These effects of basophils and basophil-derived TNF are not trivial, in that the basophil-TNF axis can significantly diminish the morbidity and the mortality associated with this model of polymicrobial infection and sepsis. These findings show that basophils, despite their low numbers, can trigger a cascade of events that both helps to initiate an innate immune response against polymicrobial infection and enhances the effectiveness of this response. Together, these findings provide novel insights into how basophils, and basophil-derived TNF, might have key roles in the early stages following bacterial infections and in resisting the progression of such infections to bacteremia and sepsis.
METHODS
Mice
C57BL/6 mice were purchased from Jackson Laboratories. Mice with transgenic Basoph8-Cre expression20 (Basoph8 mice) on the C57BL/6 background were kindly provided by R. Locksley (University of California, San Francisco). In order to track basophils in Basoph8 mice, the reporter, YFP, was used as part of the cassette that contained humanized Cre recombinase, resulting in high YFP expression in basophils expressing the Mcpt8 gene20. Basoph8 mice were crossed with mice containing loxP-flanked Tnf alleles29. Basoph8 mice were also crossed with Rosa-DTα mice purchased from Jackson Laboratories to generate basophil-deficient mice as described20. A combination of Basoph8+/− and Basoph8+/+ mice were used, unless otherwise specified.
All of these mice were bred and maintained in the Seattle Children’s Research Institute animal facility. Unless specified otherwise, all of the experiments were performed using male mice that were 12-weeks old at the beginning of the experiment. All animal care and experimentation were conducted in accordance with the current National Institutes of Health guidelines and with the approval of the Seattle Children’s Research Institute Institutional Animal Care and Use Committee.
LPS-induced endotoxemia
For LPS-induced endotoxemia, mice were injected intraperitoneally (i.p.) with 10 μg of LPS (LPS from E. coli, Serotype O55:B5, catalog number ALX-581–013, Enzo Life Sciences, Inc.) in sterile saline (pyrogen-free 0.9% NaCl), and the serum and peritoneal fluids were collected 1 h after challenge.
Cecal ligation and puncture (CLP)
CLP was performed as described previously49. Briefly, mice were deeply anaesthetized by an intraperitoneal (i.p.) injection of 100 μg/kg Ketamine and 20 μg/kg Xylazine. The cecum was exposed by performing a 1–2 cm midline incision on the anterior abdomen, and the distal half of the cecum was subjected to ligation (moderate CLP) and punctured once with a 22G needle in the ligated segment. The cecum was then placed back into the abdomen, 1 ml of sterile saline (pyrogen-free 0.9% NaCl) was administered into the peritoneal cavity, and the incision was closed using 9-mm steel wound clips. To assess mouse morbidity, mouse rectal temperatures were measured at 24 h after CLP. For survival experiments, mice were observed for mortality at least 4 times daily for the first 3 days, and then twice a day for up to seven days. Mice that were clearly moribund were euthanized by CO2 inhalation.
Adoptive transfer of basophils
Adoptive transfer of basophils was performed using a protocol previously described50. Briefly, spleen cells were isolated from mice and the basophil-enriched CD49b+ fraction was prepared by using a CD49b (DX5) microbead magnetic cell sorting kit (catalog number 130–052-501, Miltenyi Biotec). Mice were injected i.p. with the CD49b+ fraction (4 × 105 cells) at 3 h after CLP.
Cytometric analysis
Peritoneal cells were collected from mice 24 h following CLP. Basophils were then enriched by CD49b+ magnetic bead (Miltenyi Biotec) separation. Approximately 2 × 105 CD49b+ peritoneal cells were then allowed to adhere to glass slides with cytospin funnels and dried for at least 1 h. The cells were then fixed with methanol followed by incubation with 0.3% H2O2 in methanol to inhibit endogenous peroxidase reactions. The cells were then blocked with 5% goat serum and 5% bovine serum albumin in phosphate buffered saline, treated with 5 μg/ml of rat IgG2a anti-mouse Mcpt8 antibody (clone TUG8, catalog number 647401, BioLegend) or with the same amount of an rat isotype control antibody of irrelevant antigen specificity (clone RA3–6B2, catalog number 103201, BioLegend) overnight at 4 °C, and then treated with HRP-conjugated goat anti-rat IgG (catalog number 7077 Cell Signaling Technology) that was diluted 1:500. The cells were subsequently incubated with DAB solution (catalog number D4293, Sigma-Aldrich) and counterstained with May-Grunwald/Giemsa stain. The images were captured with a Leica DM6000 B microscope using a Leica DFC300 FX camera that was run by the Leica LAS X software.
Complete blood counts
For complete blood count (CBC) measurements, blood was obtained via cardio-puncture an analyzed with an automatic analyzer (Siemens Advia 120).
Bacterial CFU quantification
Peritoneal fluid or blood dilutions were performed, and the samples were plated on Luria broth LB agar (peritoneal fluids) or tryptic soy agar (TSA) with 5% sheep blood plates (blood). The colonies were counted after overnight incubation at 37 °C.
Oxidative burst
Production of reactive oxygen species (ROS) was assessed by flow cytometry using a modified protocol, as previously described51. Briefly, peritoneal cells were collected at 24 h after CLP. After red blood cell lysis, the peritoneal cells were loaded in the dark for 30 min at 37 °C with 1 μM of CM-H2DCFDA, which is a general oxidative stress indicator (catalog number C6827, ThermoFisher). After washing, the cells were stained for neutrophil and macrophage specific surface markers and flow cytometry analysis was performed immediately thereafter.
Phagocytosis
Phagocytosis was assessed by flow cytometry using a modified protocol, as previously described52. Briefly, peritoneal cells were collected at 24 h after CLP, the red blood cells were lysed, and the cells were stained for neutrophil- and macrophage-specific surface markers. The stained cells were incubated with opsonized FITC-labeled E. coli (K-12 strain) bioparticles (catalog number E2861, ThermoFisher) for 10 min at 37 °C and flow cytometry analysis was performed immediately thereafter.
Peritoneal cell-derived mast cell (PCMC) generation
Peritoneal cells from mice were maintained in vitro for two weeks in medium containing IL-3 (10 ng/ml, catalog number 130–099-510, Miltenyi Biotec) and SCF (50 ng/ml, catalog number 250–03, Peprotech) until mast cells (May-Grünwald-Giemsa stained cells) represented >95% of the total non-adherent cells, as assessed by flow cytometry (c-Kit+ FcεRIα+), as previously described53.
Thioglycollate-elicited macrophages
Thioglycollate-elicited macrophages were obtained 3 days after the i.p. injection of 1 ml of BBL fluid thioglycollate medium (catalog number 221195, Becton Dickinson).
Bone marrow-derived basophil generation
For functional studies, CD49b+ cells were isolated from the bone marrow of naïve mice using a CD49b (DX5) microbead magnetic cell sorting kit (catalog number 130–052-501, Miltenyi Biotec), according to the manufacturer’s instructions, and then they were cultured in the presence of IL-3 (10 ng/ml) for 5–7 days. Using this protocol, basophils accounted for the 85–90% of the CD49b+ cells that produced TNF, as assessed by flow cytometry (FcεRIα+ CD49b+ c-Kit− TNF+).
For qPCR, FcεRIα+, CD49b+ and c-Kit− cells were sorted from bone marrow-derived cells that were cultured in the presence of IL-3 (10 ng/ml) for 5–7 days.
Flow cytometry
Single-cell suspensions were stained with a combination of the following antibodies: Alexa Fluor 700-conjugated mAb to Ly-6G/Ly-6C (clone RB6–8C5, catalog number 108421), allophycocyanin (APC)-conjugated mAb to F4/80 (clone BM8, catalog number 123115), APC-conjugated mAb to CD49b (clone DX5, catalog number 108909), phycoerythrin (PE)-conjugated mAb to FcεRIα (clone MAR-1, catalog number 134307), and APC/Cy7-conjugated mAb to c-Kit (clone 2B8, catalog number 105825), all from BioLegend; and eFluor 450-conjugated mAb to CD11b (clone M1/70, catalog number 48–0112-82), perCP-eFluor 710 (PE-Cy5.5)-conjugated mAb to CD200R (clone OX110, catalog number 46–5201-82), from eBioscience. 4’, 6-diamidino-2-phenylindole (DAPI) (catalog number D9542, Sigma-Aldrich) was used to detect dead cells. Only cells that were negative for DAPI were used for the cell population analysis. For intracellular cytokine expression measurements, cells from CLP-treated mice were maintained in media alone in the presence of GolgiPlug (catalog number BDB555029, Fisher Scientific) (1:1,000) at 37 °C for 4 h. The cells were surface-stained with a combination of the antibodies used above, fixed and permeabilized using a commercially available kit (catalog number 554722, BD Biosciences), and then stained with either a PE/Cy7-conjugated mAb to TNF (clone MP6-XT22, catalog number 506323) or a PE-conjugated mAb to IL-6 (clone MP5–20F3, catalog number 504503), from BioLegend. The cells were acquired on a BD LSR II with the FACSDiva software and analyzed with the FlowJo software (version 8.8.7, Tree Star).
Cytokine release assays
For IgE/antigen-mediated activation, basophils and mast cells (1 × 105/100 μl) were sensitized with IgE mAb to DNP (H1-ε−26)54 (2 μg/ml) overnight at 37 °C. The cells then were challenged with DNP-HSA (10 ng/ml) for 18 h at 37 °C. For TLR-mediated activation, basophils were incubated with either synthetic triacylated lipoprotein (PAM 3CSK4, TLR1/2 ligand, catalog number tlrl-pms, InvivoGen), heat-killed listeria monocytogenes (HKLM, TLR2 ligand, catalog number tlrl-hklm, InvivoGen), or LPS (TLR4 ligand, Sigma Aldrich) for 6 h. The cell supernatants were then collected for cytokine measurements.
Cytokine and chemokine measurements
Cytokine measurements in the various figures were performed at different time intervals, which are indicated in the legends for the respective figures.
Concentrations of mouse TNF and IL-6 produced by bone marrow-derived cultured CD49b+ cells were measured using the ProcartaPlex Multiplex Immunoassay (Essential Th1/Th2, 6plex, catalog number EPX060–20831-901, Fisher Scientific) with detection limits of 0.39 pg/ml and 0.21 pg/ml, respectively. Concentrations of TNF produced by basophils isolated from the bone marrow were measured with a high sensitivity ELISA kit from eBioscience (catalog number BMS607HS) with a detection limit of 0.75 pg/ml.
Concentrations of TNF, IL-6, KC, and MIP-2 (for serum and intraperitoneal lavage fluids) were measured with ELISAs. The TNF and IL-6 ELISAs were from BioLegend (catalog numbers 430904 and 431304, respectively); and the KC and MIP-2 ELISAs were from Peprotech (catalog numbers 900-K127 and 900-K152, respectively). The detection limits for these assays were 4 pg/ml for TNF, 2 pg/ml for IL-6, 4 pg/ml for KC and 16 pg/ml for MIP-2.
qPCR
RNA (1 μg) was isolated from cells with the RNeasy mini kit (catalog number 74104, Qiagen) and converted to first-strand cDNA with the iScript™ cDNA Synthesis Kit (catalog number 1708890, BioRad). cDNA was analyzed for Tlr1–9, Mcpt8, Il4, and Gapdh quantitative expression mRNA abundance with the Maxima™ SYBR Green/ROX qPCR Master Mix (catalog number K-0222, ThermoFisher) on a 7500-Fast-Real-Time PCR System or a Step One Plus Real-Time PCR System Thermal Cycling Block (Applied Biosystems). The primers used were as follows: Tlr1 forward, 5′-GGCGAGCAGAGGCAATTGTGGA-3′; Tlr1 reverse, 5′-CGAGAACCGCTCAACCCCAGG-3′; Trl2 forward, 5′-GGTGACCTCCGAGCGTGTGC-3′; Tlr2 reverse, 5′-TCGCCGAGCCCATTGAGGGT-3’; Tlr3 forward, 5′TGCTCAGGAGGGTGGCCCTT-3′; Tlr3 reverse, 5′-CCGGGGTTTGCGCGTTTCCA-3′; Tlr4 forward, 5′-AGGAGTGCCCCGCTTTCACC-3′; Tlr4 reverse, 5′-GCCAGAGCGGCTGCTCAGAAA-3′; Tlr5 forward, 5′-CCCCTTCAGAGGCGTGAGGCT-3′; Tlr5 reverse, 5′-CTGGAGCTGGGGGACTCGCA-3′; Tlr6 forward, 5′-ACCGTCAGTGCTGGAAATAGAGCTT-3′; Tlr6 reverse, 5′-CCACCGGTCGGGGCCAAAAG-3′; Tlr7 forward, 5’-AGCTGTCCCCACCACTTTGCC-3′; Tlr7 reverse, 5′-GACCTGGGGCCCATGACGGT-3’; Tlr8 forward, 5′-AGCAGGGGTAACGCACCGTC-3′; Tlr8 reverse, 5′-AGGTGGTGAACCAGAGCAGCCA-3′; Tlr9 forward, 5′ACGCAGCGCCCAAACTCTCC-3′, Tlr9 reverse, 5′-GCGGTCTTCCAACAGGCGCT-3′; Mcpt8 forward, 5′-GTGGGAAATCCCAGTGAGAA-3′; Mcpt8 reverse, 5′-TCCGAATCCAAGGCATAAAG-3′; Il4 forward, 5′-GGGACGCCATGCACGGAGATG-3′; Il4 reverse, 5′-TGCGAAGCACCTTGGAAGCCC-3′; Gapdh forward, 5′-TCACCACCATGGAGAAGGC-3′; Gapdh reverse, 5′- GCTAAGCAGTTGGTGGTGCA-3′. The results were analyzed using the dCt method normalized to Gapdh.
Single-cell sorting and PCRs
Peritoneal neutrophils (Gr-1+ F4/80−), macrophages (Gr-1− F4/80+) and mast cells (FcεRIα+ c-Kit+ CD49b+), as well as basophils (FcεRIα+ c-Kit− CD49b+), were identified after elimination of doublets and dead cells (BD FACS Aria IIu). Single cells were sorted into 96-well PCR plates (with the presence of a single cell in each well confirmed by microscopy) containing 10 μl mix of SuperScript III Platinum One-Step qRT-PCR Kit (Invitrogen) and 0.2 × pool of TaqMan probes (Applied Biosystems) for the genes of interest. Mcpt8 (Mm00484933_m1) and Gapdh (Mm99999915_g1) gene probes were used. Cells underwent reverse transcription followed by 22 cycles of pre-amplification. The resulting amplified cDNA was analyzed for gene expression with the aforementioned TaqMan probes and the TaqMan Universal PCR Master Mix (Applied Biosystems).
Immunoblot analysis
Bone marrow-derived basophils were lysed with M-PER (Thermo Scientific) plus 1X halt protease inhibitor and EDTA (Thermo Scientific). Then, the samples were spun at 15,000xg for 15 min at 4 °C to remove cellular debris. Cell lysates were denatured by boiling for 10 min at 70 °C with 1X sample buffer (NuPage 4X LDS sample buffer, Life Technologies) and 1X reducing agent (NuPage 10X sample reducing agent, Life Technologies) and separated with SDS/PAGE (4–20% Bis-Tris-gels with MOPS running buffer, Genscript), electroblotted onto Invitrolon poly (vinylidene difluoride) membranes (Invitrogen), and then probed with a rat IgG2a anti-mouse Mcpt8 antibody (clone TUG8, catalog number 647401, BioLegend) along with a mouse IgG1 anti-GAPDH antibody (clone GA1R, Cell Sciences) as a loading control.
Statistical analyses
We assessed differences in the survival rates after CLP using the Logrank (Mantel-Cox test). All of the other data were analyzed for statistical significance using the Mann Whitney U-test. P < 0.05 was considered statistically significant. Unless otherwise specified, all of the data are presented as mean ± SEM or mean + SEM.
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Figures. Additional data are available from the corresponding authors upon request.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank R. M. Locksley for providing the Basoph8 mice and members of the Piliponsky and Galli labs for helpful discussions. Research in the Piliponsky lab was supported by the National Institute of Health (NIH) (R01 HL113351, R01 HL141094) and the American Heart Association (AHA) (12GRNT9680021). Research in the Galli lab was supported by the NIH (R01 CA72074, R37 AI23990, R01 AI070813 and R01 AR067145) and by the Department of Pathology, Stanford University. Research in the Nedospasov lab was supported by a grant from the Russian Science Foundation (14–50-00060).
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Ehrlich P Ueber die specifischen Granulationen des Blutes. Archiv fuer Anatomie und Physiologie: Physiologische Abteilung 571–579 (1879). [Google Scholar]
- 2.Canfield PJ Comparative cell morphology in the peripheral blood film from exotic and native animals. Aust Vet J 76, 793–800 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Eberle JU & Voehringer D Role of basophils in protective immunity to parasitic infections. Semin Immunopathol 38, 605–613 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Karasuyama H & Yamanishi Y Basophils have emerged as a key player in immunity. Curr Opin Immunol 31, 1–7 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Mukai K, Karasuyama H, Kabashima K, Kubo M & Galli SJ Differences in the Importance of Mast Cells, Basophils, IgE, and IgG versus That of CD4+ T Cells and ILC2 Cells in Primary and Secondary Immunity to Strongyloides venezuelensis. Infect Immun 85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reitz M et al. Mucosal mast cells are indispensable for the timely termination of Strongyloides ratti infection. Mucosal Immunol 10, 481–492 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Siracusa MC, Kim BS, Spergel JM & Artis D Basophils and allergic inflammation. J Allergy Clin Immunol 132, 789–801; quiz 788 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton RG, MacGlashan DW Jr. & Saini SS IgE antibody-specific activity in human allergic disease. Immunol Res 47, 273–284 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Marone G, Borriello F, Varricchi G, Genovese A & Granata F Basophils: historical reflections and perspectives. Chem Immunol Allergy 100, 172–192 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Mukai K et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23, 191–202 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Sato E et al. Chronic inflammation of the skin can be induced in IgE transgenic mice by means of a single challenge of multivalent antigen. J Allergy Clin Immunol 111, 143–148 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Ohnmacht C & Voehringer D Basophil effector function and homeostasis during helminth infection. Blood 113, 2816–2825 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Leung DY et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest 92, 1374–1380 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yousefi S et al. Basophils exhibit antibacterial activity through extracellular trap formation. Allergy 70, 1184–1188 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Dejager L, Pinheiro I, Dejonckheere E & Libert C Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19, 198–208 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Poli-de-Figueiredo LF, Garrido AG, Nakagawa N & Sannomiya P Experimental models of sepsis and their clinical relevance. Shock 30 Suppl 1, 53–59 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Rittirsch D, Hoesel LM & Ward PA The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 81, 137–143 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto T et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol 10, 706–712 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Ugajin T et al. Basophils preferentially express mouse Mast Cell Protease 11 among the mast cell tryptase family in contrast to mast cells. J Leukoc Biol 86, 1417–1425 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Sullivan BM et al. Genetic analysis of basophil function in vivo. Nat Immunol 12, 527–535 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voehringer D, Liang HE & Locksley RM Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J Immunol 180, 4742–4753 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobbetti T et al. Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proc Natl Acad Sci U S A 111, 18685–18690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoesel LM et al. Harmful and protective roles of neutrophils in sepsis. Shock 24, 40–47 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Huang X et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 106, 6303–6308 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn JL et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Rothe J et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364, 798–802 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Tite JP, Dougan G & Chatfield SN The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol 147, 3161–3164 (1991). [PubMed] [Google Scholar]
- 28.Maurer M et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med 188, 2343–2348 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grivennikov SI et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22, 93–104 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Torrero MN, Larson D, Hubner MP & Mitre E CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy 39, 361–369 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remick DG, Newcomb DE, Bolgos GL & Call DR Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13, 110–116 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Poltorak A et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Tracey KJ et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330, 662–664 (1987). [DOI] [PubMed] [Google Scholar]
- 34.Mannel DN & Echtenacher B TNF in the inflammatory response. Chem Immunol 74, 141–161 (2000). [PubMed] [Google Scholar]
- 35.Singh RK & Sodhi A Effect of TNF priming of murine peritoneal macrophages on their activation to a tumoricidal state. Immunol Lett 28, 127–133 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Wright HL, Thomas HB, Moots RJ & Edwards SW RNA-seq reveals activation of both common and cytokine-specific pathways following neutrophil priming. PLoS One 8, e58598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullen PG, Windsor AC, Walsh CJ, Fowler AA 3rd & Sugerman HJ Tumor necrosis factor-alpha and interleukin-6 selectively regulate neutrophil function in vitro. J Surg Res 58, 124–130 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Barton BE & Jackson JV Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun 61, 1496–1499 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barton BE, Shortall J & Jackson JV Interleukins 6 and 11 protect mice from mortality in a staphylococcal enterotoxin-induced toxic shock model. Infect Immun 64, 714–718 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Poll T et al. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 176, 439–444 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Boomer JS et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eskandari MK et al. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 148, 2724–2730 (1992). [PubMed] [Google Scholar]
- 43.Echtenacher B, Falk W, Mannel DN & Krammer PH Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol 145, 3762–3766 (1990). [PubMed] [Google Scholar]
- 44.Echtenacher B, Weigl K, Lehn N & Mannel DN Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect Immun 69, 3550–3555 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obata K et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110, 913–920 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Korosec P et al. Basophils, high-affinity IgE receptors, and CCL2 in human anaphylaxis. J Allergy Clin Immunol 140, 750–758 e715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao W et al. A genomic storm in critically injured humans. J Exp Med 208, 2581–2590 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweeney TE, Shidham A, Wong HR & Khatri P A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci Transl Med 7, 287ra271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-only references
- 49.Piliponsky AM et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol 176, 926–938 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wada T et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest 120, 2867–2875 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weighardt H et al. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J Immunol 177, 5623–5630 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Kasten KR et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun 78, 4714–4722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piliponsky AM et al. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am J Pathol 181, 875–886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu FT et al. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol 124, 2728–2737 (1980). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Figures. Additional data are available from the corresponding authors upon request.