Abstract
More than 15 years have elapsed since the identification of PLCzeta 1 (PLCζ) from a genomic search for mouse testis/sperm specific PLCs. This molecule was proposed to represent the sperm factor responsible for the initiation of calcium (Ca2+) oscillations required for egg activation and embryo development in mammals. Supporting evidence for this role emerged from studies documenting its expression in all mammals and other vertebrate species, the physiological Ca2+ rises induced by injection of its mRNA into mammalian and non-mammalian oocytes, and the lack of expression in infertile males that fail ICSI. In the last year, genetic animal models have added support to its role as the long sought-after sperm factor. In this review, we highlight the findings that demonstrated the role of Ca2+ as the universal signal of egg activation and the experimental buildup that culminated with the identification of PLCζ as the soluble sperm factor. We also discuss the structural-functional properties that make PLCζ especially suited to evoke oscillations in eggs. Lastly, we examine unresolved aspects of the function and regulation of PLCζ and whether or not it is the only sperm factor in mammalian sperm.
Keywords: Mammals, Egg Activation, Oocytes, Fertilization, Sperm, Sperm Factor, Calcium, IP3, Oscillations, Infertility
Introduction:
The question of how “animals come into being” has puzzled humans for thousands of years. While many alternative ideas were put forth, it was not until the identification of the gametes, during a two-hundred year stretch between the 17th and 19th centuries, that experimental evidence led to the discovery that the spermatozoon, the male gamete, enters the egg, the female gamete, to generate an embryo. Fertilization was first observed in species with external fertilization in which the process can be replicated under natural conditions, such as frogs, by Newport (Newport, 1853), and later in sea urchin and starfish, by Hertwig (Hertwig, 1876) and Fol (Fol, 1877). In contrast, in mammals the first direct observation of fertilization had to wait for nearly 80 years until M.C. Chang (Chang, 1959) developed the conditions that allowed successful in vitro fertilization (IVF), which was first accomplished in the rabbit model. These and subsequent studies documented the process of fertilization in a wide range of species and highlighted the complementary roles of gametes in the formation of a new organism. Nevertheless, these studies did not address the mechanism by which fertilization induces egg activation and initiation of development.
Jacques Loeb performed extensive studies in invertebrate species to address the question of how the sperm activates development during fertilization (1913) (Loeb, 1913). He recognized early on the dual function of the male gamete: “The spermatozoon has two kinds of effects upon the egg: in the first place, it induces development by promoting cell divisions, and secondly it transmits the paternal characters to the developing embryo.” As initially defined by Hertwig, fertilization represented the union of sperm and egg nuclei (Hertwig, 1876). This definition did not take into account the first premise of Loeb’s dictum, and it was quickly proven wrong by Boveri who found that embryo development was still possible if eggs deprived of their nuclear contents were fertilized (Boveri, 1889). Boveri’s proposed hypothesis, a crucial role for centrosomes as the initiators of development was also inaccurate, although it highlighted the two separate functions of the sperm (Loeb, 1913). After a long series of studies it was Loeb himself who conceived the notion that “ions” are responsible for the activation of development. While this observation highlighted the pivotal role of ions as the “signal” that drives the activation program, it ignored the mechanism(s) engaged by the sperm to promote their intracellular increase in eggs, which can be as simple as promoting plasma membrane voltage changes to allow activation of voltage-dependent channels (Jaffe, 1979) to co-opting the egg’s signaling machinery to induce repeated and sustained responses (Saunders et al., 2002). Continuing in this vein, Loeb noted, “nothing would more clearly demonstrate the sovereign role that electrolytes play in the phenomena of life than by causing, if possible, with this help unfertilized eggs to develop into larvae”. He experimented with a variety of ions and stimuli, and concluded that “The spermatozoon causes membrane formation [vitelline membrane elevation, for his work was on sea urchin eggs] by a substance which is comparatively soluble in the egg; in addition to this membrane-forming substance the spermatozoon carries a second substance into the egg which prevents the disintegration, which follows after mere membrane formation”. These hypotheses offered an ample framework for future research, and proved to be correct regarding the role of ions in egg activation as well as initiating the long search for the sperm substance responsible for embryo development in mammals, the so-called “sperm factor”.
Ca2+ is the common signal of egg activation
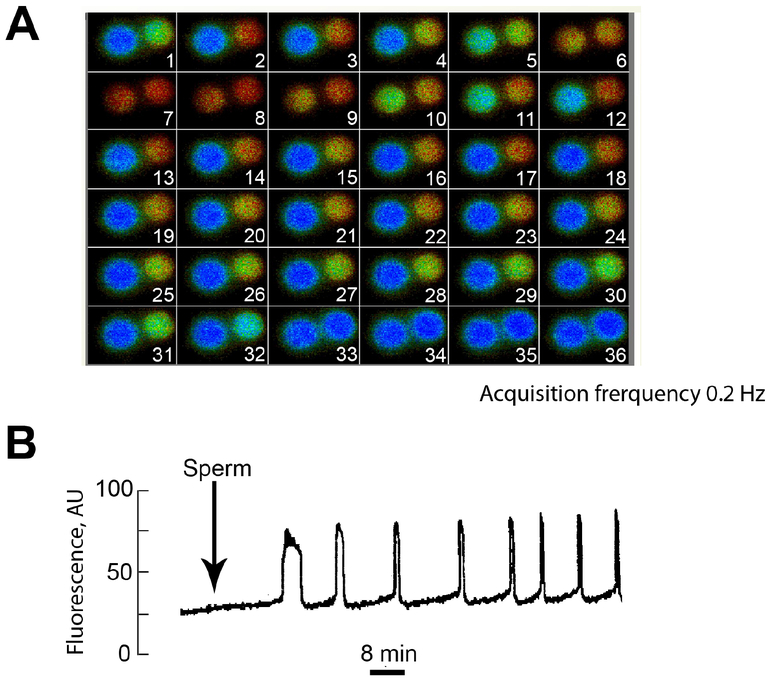
Despite extensive studies with a variety of salts and conditions, it was unclear what intracellular ionic change was responsible for the initiation of embryo development. Loeb was aware of Ringer’s studies on muscle cells and the effect of Ca2+ salts on inducing rhythmic contractions, as he had performed several studies on the topic. However, it was left to Lewis V. Heilbrunn to surmise that the activation of eggs of the marine worm Nereis sp that occurred following exposure to different physical and chemical conditions was due to increases in the levels of Ca2+ in the egg (Heilbrunn, 1937). The role of Ca2+ was further supported by Dan Mazia’s observation that an increase in the total intracellular concentration of Ca2+ was evident soon after fertilization in ultrafiltrates of egg homogenates of the sea urchin Arbacia sp (Mazia, 1937). Subsequently, several researchers showed that eggs could be activated in the absence of sperm simply by exposing them to Ca2+-containing solutions, or by inducing membrane disturbances but only in the presence of media containing Ca2+ (Yamamoto, 1954). These results were followed by the demonstration that divalent cation-transporting antibiotic ionophores induced all events of egg activation in echinoderm eggs (Steinhardt and Epel, 1974). It was shown that Ca2+ ionophores were capable of inducing egg activation independently of the divalent cation concentration of the extracellular media, which confirmed that increases in the intracellular concentration of Ca2+, but not of Mg2+, were responsible for inducing activation (Steinhardt and Epel, 1974). Exposure to these Ca2+ ionophores also activated eggs of other species including mammals (Steinhardt et al., 1974). Concomitant with the realization that changes in Ca2+ were an important cell signal, fluorescent probes were being developed to precisely measure changes in the intracellular concentration of ions. One of the first applications of this technology using the Ca2+-specific bioluminescent protein aequorin was the documentation of Ca2+ increases during fertilization in eggs of the medaka Oryzias latipes (Ridgway et al., 1977). A similar approach was used to demonstrate Ca2+ responses in sea urchin eggs (Steinhardt et al., 1977) and a few years later in mammals (Cuthbertson et al., 1981). Contemporary studies in mammals using electrophysiology confirmed the increases in intracellular Ca2+, and provided additional evidence regarding the oscillatory nature of the responses in this animal group (Igusa et al., 1983; Miyazaki and Igusa, 1981). The development of fluorescent Ca2+-sensitive dyes to measure intracellular Ca2+ changes facilitated and expanded the study of Ca2+ homeostasis in all cell types and in response to a variety of stimuli (Miyazaki and Ito, 2006). In mammalian eggs, those studies confirmed that sperm entry causes initiation of persistent Ca2+ oscillations, which last for at least 4 hours in rodents, the mammalian sub-group with the shortest cell-cycle duration, and more than 8 hours in larger mammals (Miyazaki and Ito, 2006) (Figure 1). Human eggs are not an exception, and the first demonstration of Ca2+ oscillations following fertilization in this species was reported using discarded IVF eggs (Taylor et al., 1993). Subsequent studies also showed that fertilization by intracytoplasmic sperm injection (ICSI), a technique used in the clinic to overcome certain types of male infertility, induced persistent Ca2+ oscillations with an overall pattern similar to that of natural fertilization, although with some differences such as delayed time to initiation of the first rise, which also unfolded with an altered spatiotemporal pattern (Nakano et al., 1997; Tesarik and Sousa, 1994). In summary, as a corollary to these studies a consensus emerged that Ca2+ oscillations induced by fertilization represent the egg-activating signal in mammals. This review will analyze the studies that conceptualized the mechanisms whereby the sperm induce Ca2+ oscillations, the molecules that emerged from these studies as candidate “sperm factors”, their active domains and their possible mechanism of action. We will also discuss recent findings that emerged from analysis of infertile human males that fail ICSI as well as from more recent genetic studies in the mouse. Finally, we will discuss some of the unanswered questions and the clinical applications that have surfaced from the discoveries in this field.
Figure 1. Sperm fusion with the egg induces repetitive Ca2+ oscillations in the mammalian zygote.
A. Eggs loaded with a Ca2+ fluorescent dye (Fluo-3) were monitored for changes in fluorescence after addition of sperm. Unfertilized eggs had low fluorescence (blue signal) whereas fertilized zygotes exhibited transient peaks of fluorescence (red signal). B. Quantification of the fluorescence changes recorded in a fertilized zygote.
Hypotheses for how Ca2+ responses are initiated during fertilization
Once the role of Ca2+ as the universal ionic signal of egg activation was settled, researchers examined the possible mechanism(s) whereby the sperm might trigger Ca2+ release during fertilization. L.F. Jaffe first reviewed the available information and put forth a hypothesis (Jaffe, 1983). This study was largely based on the observation at the time in somatic cells that Ca2+ rises precede exocytosis (Rubin, 1982). The review analyzed the morphological changes caused by the wave of cortical granule exocytosis including the elevation of the vitelline membrane triggered by sperm entry as an overall proxy indicator for Ca2+ increases. It was proposed that in eggs of deuterostome species, which range from echinoderms to vertebrates, the fertilization Ca2+ signal occurred in a wave-like fashion and relied on Ca2+ release from internal stores, whereas in protostome eggs that did not display wave-like changes, the Ca2+ signal was underpinned by synchronized Ca2+ influx from the extracellular milieu. Although some of the conclusions of the review were inaccurate, for instance some protostome eggs display Ca2+ oscillations and rely both on Ca2+ influx and internal release of Ca2+, it highlighted two important aspects of the fertilization Ca2+ signal. First, it noted the regenerative nature of the Ca2+ rise, aptly called a “travelling explosion”, which we now know represents calcium induced-calcium release (CICR) (Bezprozvanny et al., 1991; Parker and Yao, 1996). During mammalian fertilization, it is also now known that CICR is largely dependent on the enhanced function of the inositol-1, 4, 5-trisphosphate receptor (IP3R1) (Miyazaki et al., 1992). Second, it pointed to the presence of specialized compartments of the endoplasmic reticulum (ER), deduced from previous studies in muscle cells (Fabiato and Fabiato, 1979), as the possible source of internal Ca2+. Subsequent studies confirmed the ER as the source of most of the Ca2+ release at fertilization (Gardiner and Grey, 1983). While this review turned out to be a classification of the source of Ca2+ required for the initial Ca2+ release at fertilization, it set the scene for the field to analyze the different theories about the mechanism(s) that triggered the release of intracellular Ca2+.
The Ca2+ bomb or conduit hypothesis
This model was based on the idea that the sperm introduces a bolus of Ca2+ during gamete fusion (Jaffe, 1983, 1991). This hypothesis was later modified to incorporate the suggestion that channels on the surface of the sperm membranes act as a conduit for Ca2+ entry from the extracellular media, with this Ca2+ then triggering CICR within the egg (Jaffe, 1991). However, a number of findings have undermined the validity of this model. For instance, injection of Ca2+ into sea urchin and ascidian eggs failed to induce further Ca2+ release (Swann and Whitaker, 1986). In mammals, a variety of methods were also used to increase intracellular Ca2+ levels and, without exception, this approach failed to induce oscillations (Ozil and Swann, 1995), although, in hamster eggs, it caused a single regenerative Ca2+ increase (Miyazaki et al., 1992). Further, timed imaging at the time of fertilization in the mouse failed to note an increase in the local cytoplasmic Ca2+ concentration at the site of gamete fusion, which would be expected if the sperm acted as a Ca2+ conduit (Jones et al., 1998). It cannot be discounted however that localized Ca2+ elevations failed to be picked up by the imaging methodologies employed, although given their modest magnitude it is doubtful they could have induced oscillations and activation. Remarkably, a recent study in C. elegans using high-speed in vivo imaging showed that sperm induces a rapid local Ca2+ rise that is followed by a Ca2+ wave. A TRP-3 channel, which is a sperm protein, is required for this local Ca2+ rise, which while detectable is not required for embryogenesis (Takayama and Onami, 2016). Collectively, research shows that in unfertilized mammalian eggs a bolus Ca2+ increase cannot act as a trigger of persistent oscillations, which is the hallmark of mammalian fertilization, although it may accelerate the initiation of the fertilization wave in a limited number of species.
The sperm-egg surface interaction hypothesis
This was the dominant hypothesis for many years. It proposed that egg activation is triggered by an interaction between a ligand associated with the sperm and a receptor on the egg (Evans and Kopf, 1998; Jaffe, 1990; Schultz and Kopf, 1995). One attractive feature of such a mechanism is that surface-mediated interactions are a normal feature of cell signaling in somatic cells. It also relied on demonstrations of functional expression of molecules associated with plasma membrane receptor signaling such as those linked to guanine nucleotide-binding protein (G-protein)-coupled receptors (GPCRs) or tyrosine kinase receptors. For example, injection of activators of heterotrimeric G-proteins as well as expression of exogenous GPCR receptors followed by addition of specific ligands induced Ca2+ responses in eggs of several species, suggesting a role for an egg phospholipase C beta during fertilization (PLCβ) (Miyazaki, 1988; Williams et al., 1998; Yim et al., 1994). Further, studies in echinoderms and other animals implicated a role for an egg PLCγ in the egg activation process (Shearer et al., 1999). For instance, injection of dominant negative PLCγ SH2 domains into ascidian and echinoderm eggs inhibited Ca2+ release at fertilization, suggesting that PLCγ activates the IP3 pathway in the egg of such invertebrates (Carroll et al., 1997; Giusti et al., 1999). There is also evidence for a role of PLCγ in frog eggs, as PLCγ associates with the Src family kinase (SFK) known as Xyk and becomes tyrosine phosphorylated shortly after fertilization (Sato et al., 2000). However what remains far less clear is whether the activation of PLCγ is the consequence of a surface-mediated interaction between a sperm ligand and an egg receptor. In addition, attempts to identify a role for either an egg PLCβ or PLCγ during egg activation in mammals have not been successful. For instance, injection of PLCγ SH2 domains into vertebrate eggs, including frogs and mammals, had no inhibitory effect on Ca2+ release and oscillations (Mehlmann et al., 1998; Runft et al., 1999), and neither did an antibody that blocked the Gq family of G-proteins (Williams et al., 1998). It is worth noting that activation of SFKs and their downstream signaling cascade have been recently shown to play a role in mammalian fertilization, but at later stages, including the incorporation of sperm (Wang et al., 2017). Therefore, whereas the signaling machinery to initiate Ca2+ responses associated with plasma membrane receptors is expressed in mammalian eggs and can be engaged by a variety of approaches, the resulting oscillations in mammals do not resemble those induced by the sperm (Miyazaki, 1988). Further, it is hard to imagine how the brief encounter between the sperm and the oolema during mammalian fertilization can be leveraged to support Ca2+ oscillations that last for hours in this species.
The soluble sperm factor hypothesis
Building on the original proposal of Jacques Loeb mentioned above, the ‘soluble sperm factor’ hypothesis proposed that the sperm contains a soluble factor that, upon gamete fusion, enters the egg, and triggers Ca2+ release from intracellular stores. In line with this notion, in 1985 Brian Dale showed that injection of a sea urchin sperm extract induced egg activation (Dale et al., 1985). A later study identified a similar putative egg activating factor in ascidian sperm (Dale, 1988). However, it was not clear from these studies whether the activating stimulus was a protein or a small molecular weight Ca2+-mobilizing messenger, or even Ca2+ itself, and the identity of the stimulus was not pursued further. Eventually these sperm activities were tested in mammals. The first evidence that a soluble sperm protein triggered the Ca2+ oscillations that induce embryo development in mammals was provided by Karl Swann, who injected sperm cytosolic extracts into mammalian eggs and showed that it triggered Ca2+ oscillations that were very similar to those seen at fertilization (Swann, 1990). Moreover, this physiological stimulus induced all the subsequent molecular events of egg activation (Stice and Robl, 1990; Swann, 1990). Analysis showed that the factor was apparently some sort of protein (Swann, 1990; Wu et al., 1997). Meanwhile, indirect evidence for the soluble sperm factor hypothesis in mammals already existed in the literature, as studies that preceded the advent of ICSI for decades, and involving injection of sperm into the ooplasm without obvious activation of plasma membrane receptors, resulted in egg activation and embryo cleavage in experimental animals (Uehara and Yanagimachi, 1976; Yanagida et al., 1991). This method was later perfected and extensively applied in fertility clinics where it has since been shown to overcome some cases of human male infertility (Palermo et al., 1992). Subsequent studies showed that ICSI induces Ca2+ oscillations in eggs with a similar pattern to those initiated by the sperm after IVF, albeit with differences in the timing of initiation of oscillations and the propagation of the first rise (Nakano et al., 1997; Tesarik and Souza, 1994). Therefore, these results were compatible with the idea that a soluble sperm factor was being released into the egg after fertilization. These findings were also hard to reconcile with the idea that an interaction between the oolema and the sperm could serve as the trigger of Ca2+ oscillations, since such an interaction is bypassed in ICSI. Despite these pieces of evidence, the soluble sperm factor hypothesis remained unpopular partly because it was far from clear how a protein in the sperm could induce Ca2+ release in an egg that is a thousand times bigger, in volume terms, than the sperm. In addition, the molecular identity of the sperm factor remained unclear for many years, with various candidates being proposed, but then failing to match the expected properties of the physiological agent of egg activation.
The first proposed candidate for the soluble sperm factor was a protein called glucosamine-6-phosphate deaminase that was identified as the primary component of a purified sperm extract that triggered Ca2+ oscillations in eggs (Parrington et al., 1996). This protein was also appropriately nicknamed “oscillin.” However, a recombinant version of this protein failed to trigger Ca2+ release when injected into the egg (Wolosker et al., 1998). Moreover, immunodepletion of glucosamine-6-phosphate deaminase from sperm extracts failed to abolish their ability to induce Ca2+ oscillations when injected into the egg (Wolny et al., 1999). It was therefore soon realized that oscillin did not meet the basic criteria to be the sperm factor, and presumably, the purified sperm extract that contained this protein, must also have contained another protein that was not identified – the bona fide sperm factor.
Another sperm factor candidate was tr-kit, which is a truncated version of the c-kit tyrosine kinase receptor. tr-kit has been shown to accumulate during the late stage of mouse spermatogenesis and localize to the residual sperm cytoplasm with maximal accumulation in the midpiece of the flagellum (Sette et al., 1997). Expression of Tr-kit mRNA in mouse eggs was shown to induce egg activation, which required association with the egg’s SFK Fyn kinase and activation of PLCγ (Sette et al., 2002). In human sperm, TR-KIT localizes to the equatorial region of the sperm head where it persists even after the acrosome reaction; its expression was associated with sperm of higher quality (Muciaccia et al., 2010). Nevertheless, Ca2+ responses, which are a hallmark of mammalian fertilization, were not detected in mouse eggs after injection of Tr-kit mRNA. Therefore, the role of tr-kit in mammalian fertilization is presently unknown, although it cannot be discounted that it plays a role in other functions of the sperm and/or affects its quality.
Logically, since PLCs are directly responsible for the production of IP3, which underlies the Ca2+ oscillations of fertilization (Jellerette et al., 2000; Miyazaki et al., 1992), they were potential sperm factor candidates. In fact it was a study of the Ca2+-releasing properties of mammalian sperm extracts using the cell-free sea urchin egg homogenate assay, that first suggested that the sperm factor might be a PLC (Jones et al., 1998). A further study indicated that mammalian sperm contained a PLC activity that correlated with ability to induce Ca2+ oscillations in mouse eggs following chromatographic fractionation (Parrington et al., 1999). Moreover, this PLC activity was only detected in testis and sperm and not in other tissues (Jones et al., 2000). Multiple PLC isoforms are expressed in sperm (Fukami et al., 2001; Parrington et al., 2002), and in vitro PLC assays demonstrated that the PLC activity present in sperm extracts is sensitive to the environmental Ca2+ concentrations prevailing in the cell, unlike other PLCs that require higher Ca2+ concentrations (Jones et al., 2000; Rice et al., 2000). Importantly, given the mentioned disparity in size between eggs and sperm, and the demonstration that expression in eggs of PLCs found in sperm failed to induce oscillations or caused it at non-physiological concentrations (Jones et al., 2000; Mehlmann et al., 1998), it became apparent that if the sperm factor was a PLC, it would have to be sperm-specific PLC(s) whose activity is differentially regulated under the prevailing condition of the ooplasm. In line with this, a survey of known PLCβ, γ, and δ isoforms failed to find evidence that any of these possessed the ability to induce the characteristic Ca2+ oscillations seen at fertilization in eggs (Parrington et al., 2002).
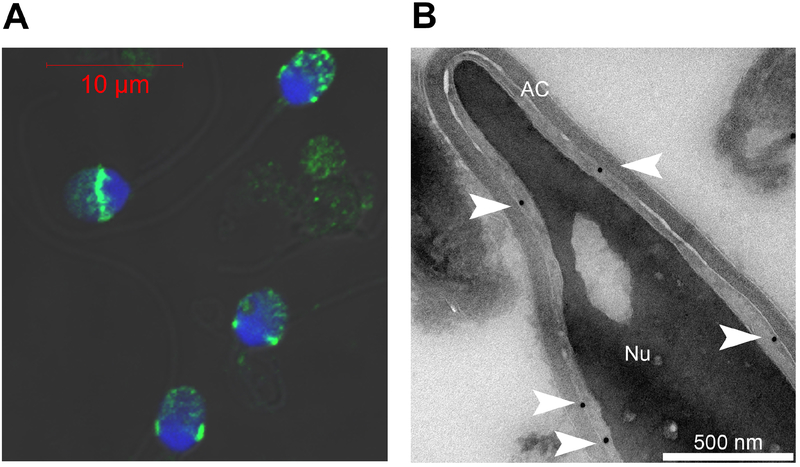
Subsequently, a genomic search for testis/sperm specific PLCs led to the discovery of a novel sperm-specific PLC, PLCζ, first reported in the mouse and then in other mammals (Cox et al., 2002; Saunders et al., 2002). As expected for a member of the PLC family, PLCζ shared many of its structural organization with other PLC isozymes except that it lacked the N-terminal pleckstrin homology (PH) domain (Cox et al., 2002; Saunders et al., 2002). Besides its specific expression in the sperm, PLCζ showed a series of unique features that made it an attractive candidate to be the sperm factor. First, injection of PLCζ mRNA into mouse eggs triggered fertilization-like Ca2+ oscillations, whereas injection of PLCδ1 mRNA at the same pipette concentrations failed to do so (Cox et al., 2002; Saunders et al., 2002). Second, depletion of PLCζ from hamster and porcine sperm extracts with specific antibodies largely removed their PLC activity and the ability of these extracts to initiate Ca2+ oscillations in eggs (Kurokawa et al., 2005; Saunders et al., 2002). Third, the ~70kDa molecular weight of PLCζ is within the 30–70 kDa range originally associated with the active fractions following chromatographic fractionation of the sperm extracts (Wu et al., 1997). Finally, immunofluorescence studies revealed that the protein was mostly located in the equatorial/post-acrosomal region of the human sperm head (Grasa et al., 2008; Yoon et al., 2012; Yoon et al., 2008; Young et al., 2009), which is the area that first undergoes fusion with the egg, consistent with a role in triggering Ca2+ release following sperm fusion with the egg (Figure 2). In support of this, EM experiments showed that PLCζ is located on the perinuclear theca facing the acrosome and likely associated with the inner acrosomal membrane (Escoffier et al., 2015). Remarkably, the distal part of the acrosome remains relatively unaltered during the acrosome reaction in humans (Zanetti and Mayorga, 2009), a location that preserves PLCζ from the acrosome reaction (Figure 2). Therefore, PLCζ has emerged as the strongest candidate to date for the sperm factor and extensive structural-functional studies ensued.
Figure 2. PLCζ is mostly located in the post-acrosomal area apposed to the inner acrosomal membrane.
A. Immunofluorescence studies of human sperm analyzed by confocal microscopy showing the localization of PLCζ in these cells. Control human sperm were stained with hPLCζ-antibodies (green) and Hoechst to identify nuclear DNA (blue). PLCζ has a ring-like shape at the base of the acrosome and surrounding the nucleus. B. Subcellular localization of PLCζ by EM. Gold particles targeting PLCζ antigenic sites were observed in the perinuclear matrix along the inner acrosomal membrane (white arrow heads). Gold particles targeting PLCζ antigenic sites were often observed at the base of the acrosome. Adapted from Escoffier et al, 2015.
Structural and functional studies of PLCζ
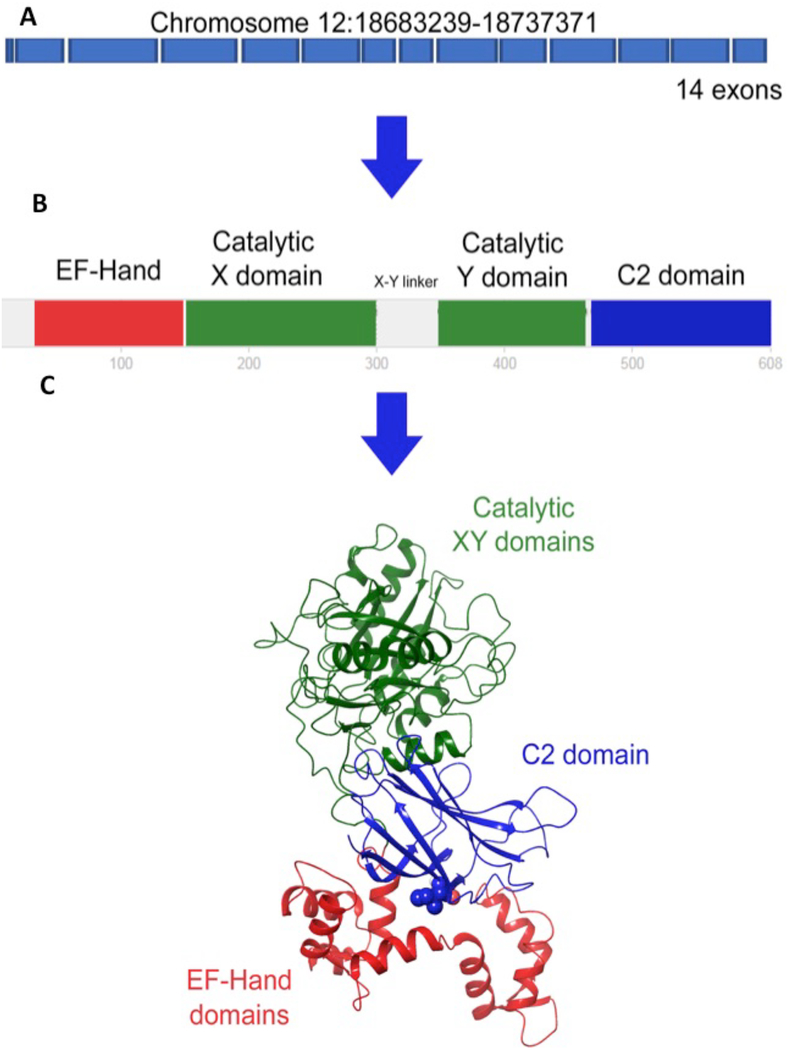
An important way of studying the mechanism of action of a protein is to investigate how its structure relates to its function. Extensive structure-function analysis of PLCζ has been carried out on this protein since its discovery in 2002. Like other PLCs (β, γ, δ, ε, η), PLCζ has a basic domain structure consisting of four EF hands, an X and Y catalytic domain, and a C2 domain (Figure 3). A key feature of PLCζ that allows it to be enzymatically highly active in mammalian eggs relative to other PLCs is its uniquely enhanced Ca2+ sensitivity (Rice et al., 2000); PLCζ is ~100-fold more sensitive to Ca2+ compared to PLδ1, possessing a half-maximal PLC activity (EC50) of ~80 nM, very close to the reported resting Ca2+ concentration in mammalian eggs of ~120 nM (Kouchi et al., 2004). This Ca2+ sensitivity of PLCζ appears to be largely due to its EF hand region. Thus, deletion of the four EF-hand domains dramatically increases the EC50 from 80 nM to 30 mM (Kouchi et al., 2005; Nomikos et al., 2005). In addition, replacement of PLCζ’s EF-hands with those from PLCδ1 results in a 10-fold decrease in the Ca2+ sensitivity of PLCζ without affecting its enzymatic activity. Deletion of EF1 and EF2 has limited impact on the EC50 of PLCζ (EC50~100 nM) but it negatively affects the ability of the modified enzyme to induce Ca2+ oscillations in eggs (Kouchi et al., 2005), suggesting that EF hand domains also modulate PLCζ’s interaction with its lipidic substrates. The 3D structure of PLCζ indicates (see Figure 3) that EF-hands are in close contact with the C2 domain, suggesting that their association is crucial for the ability of PLCζ to interact/process PIP2 (Nomikos et al., 2015).
Figure 3. Organization of the different domains of PLCζ.
A. The PLCZ1 gene contains 15 exons. The coding sequence starts at exon 2 and finishes at exon 15. B. PLCζ has three different domains, which are the EF-hands, the catalytic domain, and the C2 domain from the N terminus to the C terminus, respectively. The catalytic domain is subdivided into X and Y catalytic regions joined by the XY linker. The X and Y domains act together on the target as two mandibles of a jaw. C. The 3D structure of hPLCζ was modeled from the crystallographic structure of rPLCδ1. The model shows the close spatial interaction of the EF-hand and the C2 domains. The human I489F mutation is located at the EF-hands-C2 domain interface. Adapted from Escoffier et al, 2016.
The X and Y domains are the catalytic domains of PLCζ and, as expected, mutagenesis of conserved active site residues within them leads to loss of enzymatic activity and inability of the protein to induce Ca2+ release in mammalian eggs (Nomikos et al., 2011b). In all PLC isoforms, a discrete region known as the XY linker separates the X and Y domains, and the XY linker of PLCζ contains a distinctive cluster of basic amino acid residues that are not found in the XY-linker regions of other PLCs. Interestingly, the XY linker sequence is the least conserved region of PLCζ between different species (Nomikos et al., 2013). The diversity of this sequence may account for some of the species-specific differences in the pattern of Ca2+ oscillations triggered by PLCζ proteins of different mammalian species as well as for the varying relative potencies of these proteins in triggering cytosolic Ca2+ oscillations in eggs.
The C2 domain comprises ~120 amino acid residues that form an eight-stranded antiparallel β-sandwich common to all C2 domains already described (Essen et al., 1996; Sutton et al., 1995). The C2 domain plays an important role in Ca2+-dependent subcellular membrane targeting in a variety of lipid-metabolizing enzymes (Clark et al., 1991). Consistent with this, in vitro studies have shown that the C2 domain of PLCζ binds to membrane phospholipids such as phosphatidylinositol 3-phosphate (PI3P) and phosphatidylinositol 5-phosphate (PI5P) (Kouchi et al., 2005). Further, deletion of the C2 domain or its substitution with the corresponding domain of PLCδ1 abolishes the ability of PLCζ to induce Ca2+ oscillations in intact eggs without affecting its ability to hydrolyze PIP2 (Nomikos et al., 2005). A cationic patch in the concave face of the β-sandwich that corresponds to strands β3- and β4 and the highly variable loops between β-strands have already been characterized to be important for lipid binding in other C2-domain bearing proteins (Corbalan-Garcia and Gomez-Fernandez, 2014). However, the sequence conservation among C2 domains is low and a significant number of C2 domains with little to no Ca2+ affinity have been identified, which is the case for the C2 domain of PLCζ (Corbalan-Garcia and Gomez-Fernandez, 2014; Guillen et al., 2013). Therefore, further studies are required to determine the binding sites and the physiological significance of the interactions of PLCζ with PI3P and PI5P, and to identify other potential molecular partners of the C2 domain that might regulate the function of PLCζ within the egg (Nomikos et al., 2017).
A big initial surprise when PLCζ was first identified was that it lacks a PH domain, a characteristic feature of other PLCs and of many other proteins. The main function of the PH domain is to facilitate the binding of PLCs to biological membranes to allow access to the membrane-bound substrate, PIP2. This raised the question of how PLCζ could bind PIP2 without a PH domain. Subsequent studies have shown that PLCζ is able to target the membrane(s) where the PIP2 substrate resides by an electrostatic interaction between the positively charged N-terminal lobe of the EF-hand domain and the basic amino acids on the C-terminal end of the XY-linker region with the negatively charged PIP2 (Nomikos et al., 2011b; Nomikos et al., 2015). Moreover, the EF hand domain of PLCζ has strong intramolecular interactions with the C2 domain, and disturbing them may affect the function of PLCζ, which may explain the infertility caused in humans by the naturally occurring Ile489Phe mutation. This mutation is located within the β1-strand of the C2 domain and is sufficient to disrupt the C2-EF hand domain interactions, possibly hindering the interactions of PLCζ with its lipidic targets/substrates (Escoffier et al., 2016). The absence of the PH domain may also explain why PLCζ appears to bind PIP2 located in intracellular membranes, as surmised by expression of a fluorescently–tagged PLCζ that attains a diffuse distribution in eggs, unlike the distribution observed following expression of other members of the PLC family, which show a clear association to the plasma membrane where most of the cellular PIP2 is found (Sanders et al., 2018; Yu et al., 2012).
Finally, the mouse PLCζ XY-linker region contains a predicted nuclear localization signal (NLS) sequence. Indicating a functional role for this signal, substitution of basic for acidic residues in the mouse PLCζ NLS led to loss of nuclear translocation ability without affecting Ca2+ oscillation-inducing activity, although allowing Ca2+ oscillations to continue beyond formation of the pronuclei (Ito et al., 2008; Kuroda et al., 2006). This finding suggested that nuclear sequestration might be a mechanism for terminating the Ca2+ oscillations at fertilization. However, interestingly, this mechanism is specific only to mouse zygotes, as bovine, rat and human PLCζs, which also possess a putative NLS, do not appear to undergo nuclear localization (Ito et al., 2008). As such, it currently remains unclear how Ca2+ oscillations are terminated in eggs of these and other mammalian species.
Human male infertility and PLCζ
One important piece of evidence in favor of a significant role for PLCζ in the egg activation process is the discovery of association between defects in PLCζ expression and/or function, and certain types of infertility in men. The use of ICSI has made it possible to treat many cases of male factor infertility, especially those caused by severe oligospermia, asthenospermia, teratospermia, or a combination of such disorders (Neri et al., 2014). However, fertilization failure after ICSI occurs in up to 3% of couples, and certain couples never achieve fertilization rates greater than 50%. Notably, nearly 80% of the eggs unsuccessfully fertilized by ICSI do not exit the MII stage, suggesting that such failure is caused by a defect in egg activation (Flaherty et al., 1998; Flaherty et al., 1995). While egg activation failure may be due to a variety of underlying causes, and in some cases defects are present in both egg and sperm, studies have shown that the success of ICSI is consistently reduced when sperm with certain morphological defects, for instance the round-headed sperm characteristic of the globozoospermia condition, are used.
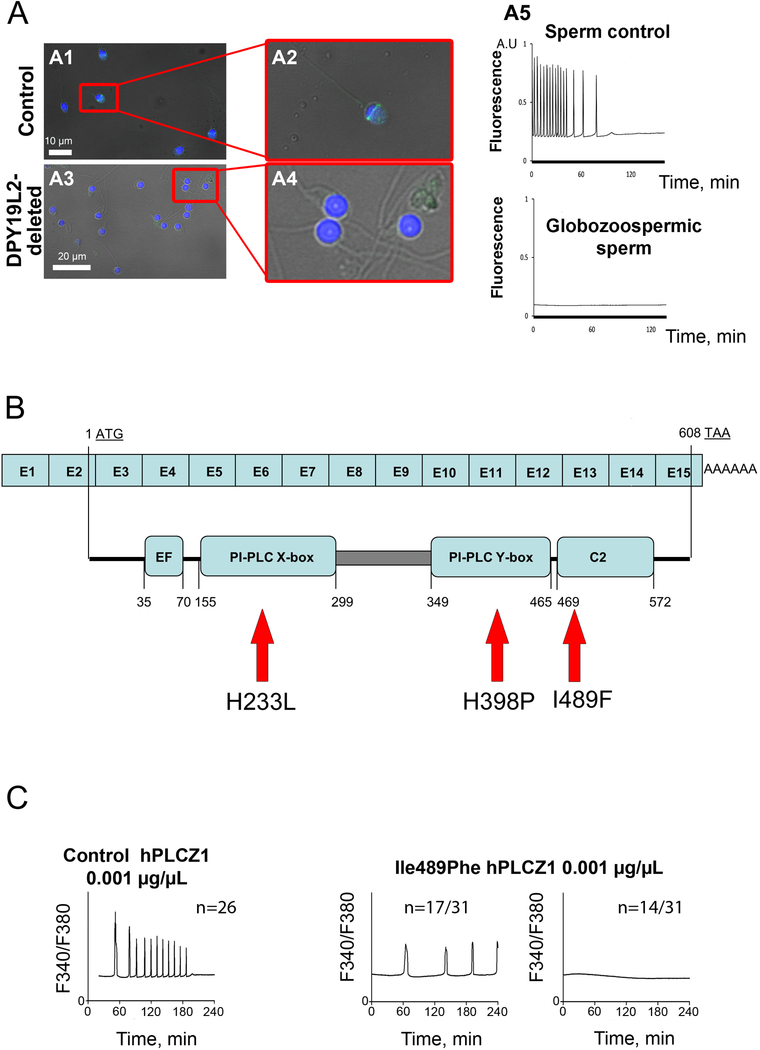
Yoon et al. (2008) first showed that sperm from certain patients that had failed ICSI, with some having globozoospermia, and others having sperm of normal morphology, also were unable to trigger Ca2+ oscillations and egg activation when injected into mouse eggs (Yoon et al., 2008). Analysis of PLCζ expression using immunofluorescence and western blotting indicated an absence of PLCζ protein in the sperm of patients who repeatedly failed ICSI and who were unable to induce Ca2+ oscillations (Figure 4). Injection of Plcz1 mRNA into eggs at the same time as the patients’ sperm triggered Ca2+ oscillations and egg activation, suggesting that the egg activation failure was due to a lack of expression of PLCζ, and not some defect in the egg’s ability to respond to a PLCζ stimulus (Yoon et al., 2008). Although the immunofluorescence and immunoblot data suggested some defect in PLCζ expression, analysis of the PLCZ1 gene in these patients failed to identify the molecular basis for such a lack of expression. Nevertheless, these results spurred research to understand the loss of PLCζ in globozoospermic patients. A significant finding was the discovery of the association of the loss of the DPY19L2 gene with human globozoospermia (Harbuz et al., 2011). These studies were later extended with a corresponding mouse knockout model that mimicked the human phenotype (Pierre et al., 2012). These studies showed using EM that the lack of Dpy19l2 causes severe morphological head anomalies with disjunction of the nuclear envelope and loss of the nuclear theca and consequently of PLCζ during spermiogenesis (Escoffier et al., 2015); thus, the late loss of PLCζ results in failure of egg activation in these patients. Genetic analysis of infertile men by Heytens et al. (2009) provided the first link between human male infertility and a mutation with a clearly debilitating functional effect on the PLCζ protein (Heytens et al., 2009). This study also looked at the sperm from a heterogeneous cohort of patients who had failed ICSI some of whom displayed globozoospermia and some others with normal sperm morphology. Immunoblot and immunofluorescence analysis identified defects both in the pattern of localization of PLCζ protein and its expression. In one of these non-globozoospermic patients, analysis of the PLCζ gene protein-coding sequence revealed a mutation His398Pro (H398P) that was predicted to have detrimental effects on PLCζ protein structure. Moreover, injection of a recombinant RNA version of the H398P mutant sequence failed to trigger Ca2+ oscillations in mouse eggs (Figure 4). One puzzling aspect of this study was that the H398P mutation was heterozygous, so while it should decrease the concentration of wild type PLCζ by approximately half, there should still be oscillations and only subfertility. However, subsequent analysis of this patient by Kashir et al. (2012) identified a second mutation, His233Leu (H233L), and showed that a recombinant PLCζ RNA carrying this mutation also failed to trigger Ca2+ oscillations in mouse oocytes (Kashir et al., 2012). Analysis of relatives of this patient showed that the H398P mutation had been inherited from the patient’s father and the H233L mutation from his mother and thus the patient showed compound heterozygosity. The H233L and H398P mutations occur in the X and Y catalytic domains of PLCζ. In line with these mutations affecting PLCζ’s catalytic activity, Nomikos et al. (2011a) showed that the H398 amino acid is conserved in the mouse sequence and that the injection of the mouse equivalent of the human H398P mutant (in mouse H435P) fails to trigger Ca2+ oscillations in mouse eggs. Moreover, Nomikos et al. also analyzed the enzymatic activity of the mouse PLCζ H435P protein using an in vitro assay of PLC activity, and showed the mutant protein to be enzymatically inert, in line with its location (and that of the H398P human equivalent) in the catalytic region of the PLCζ protein. Interestingly, this histidine is also conserved in PLCδ, further indicating an important functional role (Nomikos et al., 2011a).
Figure 4. Human infertility due to PLCζ deficiencies.
A. In contrast to what is observed in sperm of fertile men (A1-A2), in patients presenting globozoospermia (round sperm head devoid of acrosome), PLCζ is not detectable (A3-A4). Control and globozoospermic human sperm were stained with hPLCζ-antibodies (green) and Hoechst to evidence nuclear DNA (blue). A5. Whereas injection of the sperm head from a fertile patient leads to Ca2+ oscillations, injection of a globozoospermic sperm head does not trigger Ca2+ signaling. B. Localization of the different mutations found on the PLCZ1 gene. Two mutations (H233L and H398P) were found in the catalytic domains (from one compound heterozygote patient) and one mutation in the C2 domain (I489F) in two sterile brothers. C. Whereas injection of recombinant mRNA coding for WT hPLCζ triggers Ca2+ oscillations, injection of the mRNA coding for the C2 I489F mutant triggers abnormal or no Ca2+ signaling. Eggs injected with recombinant mRNAs coding for H233L and H398P mutants display similar reduced Ca2+ signaling. Adapted from Yoon et al 2008 and Escoffier et al 2016.
Most recently, Escoffier et al. (2016) studied two brothers, born from first cousin parents, who showed complete fertilization failure after ICSI despite normal sperm morphology (Escoffier et al., 2016). Whole exomic sequencing of these two patients’ DNA identified a homozygous mutation Ile489Phe (I489F), which occurs within the C2 domain of PLCζ. Analysis of a recombinant PLCZ1 containing the I489F mutation showed that it generated fewer Ca2+ transients than was the case with a wild type PLCZ1 mRNA when injected into mouse eggs. A tagged recombinant PLCζ containing the I489F mutation also showed an abnormal pattern of localization in GV and MII eggs, compared to wild type PLCζ. In addition, immunofluorescence and immunoblot analysis of sperm from the two patients showed an absence of expression of the mutant I489F PLCζ protein, suggesting that the mRNA or the protein might be unstable, although this proved not to be the case following injection of a tagged version of PLCζ mRNA containing the I489F mutation. Further analysis of the mutant I489F PLCζ by Nomikos et al. (2017) showed that the mutant PLCZ1 mRNA or protein failed to trigger Ca2+ oscillations in mouse eggs at physiological concentrations, but could trigger Ca2+oscillations if much higher, non-physiological concentrations of the mutant PLCζ protein were injected. In vitro biochemical analysis of the mutant I489F PLCζ protein by Nomikos et al. showed that it had similar enzymatic properties to wild-type PLCζ, but dramatically reduced binding to PI3P and PI5P-containing liposomes, confirming previous structure-function studies indicating the important role for the C2 domain binding to such lipids, which is required to trigger Ca2+ oscillations in the egg (Nomikos et al., 2017). This is consistent with modeling predictions that suggested that the I489F mutation creates an unusually large hydrophobic area in the C2 domain that might compromise the targeting and/or binding of PLCζ to specific substrate(s) (Escoffier et al., 2016). These studies of the effect of naturally occurring mutations in human PLCζ demonstrate their importance, both for identifying the molecular basis of certain types of male infertility, and also because they revealed important clues about the structure-function relationships of the PLCζ protein.
Escoffier et al (2016) also examined the expression of the protein PAWP in the sperm of these two patients. PAWP, which is coded by the WBP2NL gene, has been proposed as an alternative candidate for the physiological agent of egg activation (Wu et al., 2007). The initial claims were based on the findings that injection of the recombinant protein into eggs of several species caused egg activation (Wu et al., 2007). An additional study demonstrated that recombinant PAWP induced Ca2+ responses when injected into mouse and human eggs, although the shape of the individual rises was greatly abnormal (Aarabi et al., 2014). Further, injection of PAWP cRNA into mouse eggs by another group failed to induce Ca2+ oscillations (Nomikos et al., 2014; see also below for additional studies on the effects on fertility of genetic deletion of Wbp2nl). Consistent with this, analysis by Escoffier et al. of the WBP2NL gene failed to identify any mutations of this gene in the two patients. Moreover, immunoblot and immunofluorescence analysis of PAWP showed that it had normal expression and localization in the sperm of these two patients. Therefore, thus far PLCζ remains as the only protein whose presence is required for the initiation of Ca2+ oscillations during human fertilization.
Mutant mouse models of PLCζ function
The most definitive way to test the importance of a particular protein in a specific physiological process is to use genetic engineering to interfere with the expression of the gene coding for that protein in an animal model. Knott et al. (2005) carried out the first attempt to block expression of PLCζ using RNA interference (Knott et al., 2005). This study generated transgenic mice that expressed a short hairpin RNA (shRNA) targeting PLCζ. This led to a partial reduction of expression of PLCζ protein levels (~60% compared to controls) in sperm of transgenic males. Sperm from transgenic males were similar in number, morphology, and motility to those from control males. When transgenic sperm were used in IVF experiments, during which intracellular Ca2+ was monitored in the fertilized eggs, subtle differences emerged in the pattern of Ca2+ oscillations compared to controls. In addition, eggs fertilized by sperm from transgenic males showed reduced egg activation and development to blastocyst. Although transgenic males could produce offspring, genotyping of offspring showed no germline transmission of the transgene, suggesting that expression of the transgene had inhibited sperm egg activation capacity. Although this study indicated a role for PLCζ in egg activation, the partial nature of the gene knockdown left unresolved the issue of whether PLCζ is the physiological trigger of the Ca2+ oscillations that initiate embryogenesis in mammals.
A different transgenic approach was taken by Yoshida et al. (2007), who rather than knocking down PLCζ expression, created a transgenic mouse in which a PLCζ transgene construct was expressed in all cell types in both male and female mice (Yoshida et al., 2007). Oocytes from transgenic females underwent unperturbed meiotic maturation to the metaphase II stage, but unlike control eggs, these subsequently exhibited autonomous intracellular free Ca2+ oscillations, second polar body extrusion, pronucleus formation and parthenogenetic development. This finding was in line with a role for PLCζ as the trigger of the Ca2+ oscillations at fertilization, but did not address the issue of whether sperm PLCζ is the physiological trigger of such Ca2+ oscillations.
To definitively address the issue of PLCζ’s physiological importance during fertilization, a full gene knockout (KO) was required. The creation of the first Plcz1 gene KO mice was reported by Ito et al. (2010). The authors claimed that in this gene KO expression of PLCζ was abolished at the level of both mRNA and protein, as assessed by Northern blot and immunoblot, respectively. However, the authors also claimed that spermatogenesis was arrested at the round spermatid stage, and as this meant no spermatozoa were formed, this made it impossible to test the effect of PLCζ knockout on the capacity of sperm to trigger Ca2+ oscillations. It was however difficult to be sure of the validity of these claims since this report was only presented as a conference abstract and the findings were not published in a peer-reviewed journal. It is worth noting nevertheless that in mammals the genes Plcz1 and Capza3, an actin-capping protein controlling actin polymerization during spermiogenesis, are located back-to-back in the genome and they appear to contain a common bidirectional promoter (Coward et al., 2005). Further, a mutation in the Capza3 gene in mice caused severe defects in spermiogenesis and infertility (Geyer et al., 2009), and it is possible that at this first attempt of knocking out PLCζ might have also disrupted Capza3 gene expression, causing the unexpected spermatogenesis phenotype.
More recently, Hachem et al. (2017) used CRISPR/Cas9 genome editing to generate two Plcz1 gene KO lines (Hachem et al., 2017). One of these lines was generated using standard Cas9, which makes a double-stranded break in the DNA, and a single guide RNA (sgRNA), targeted to exon 5 in the PLCζ gene. The other line was generated using Cas9 nickase, which makes a cut in only a single strand of the DNA double helix, and two sgRNAs targeted at two sequences close together in exon 3 of the PLCζ gene. This approach was employed since Cas9 nickase has been shown to reduce the possibility of off-target effects. Both lines were predicted to lead to expression of truncated PLCζ proteins corresponding to small N-terminal portions lacking regions necessary for enzymatic activity. Complete absence of wild type Plcz1 mRNA expression in the testis, and lack of full length PLCζ protein in sperm, was confirmed by RT-PCR and immunoblot analysis, respectively, for both lines. Importantly, and in contrast to the report by Ito et al. (2011), spermatogenesis in both the Plcz1 KO lines was normal.
Given the presence of spermatogenesis, Hachem et al. (2017) examined the sperm of Plcz1 KO males; the sperm showed normal viability, motility, and ability to undergo capacitation and the acrosome reaction. Consequently, sperm from these males were cryopreserved and used for fertilization trials by ICSI or IVF. Remarkably, cryopreserved Plcz1 KO sperm failed to trigger Ca2+ oscillations in either case. This finding provides the first definitive evidence that PLCζ is the physiological trigger of the Ca2+ oscillations that normally initiate embryogenesis. Evaluation of eggs for signs of activation following IVF using KO sperm or natural mating with KO males showed greatly increased levels of polyspermy, likely due to the lack of Ca2+ oscillations failing to engage the Ca2+-sensitive mechanisms responsible for blocking polyspermy such as the cortical reaction, which is underpinned by the exocytosis of the cortical granules, and/or the plasma membrane block whose molecular underpinning(s) are not fully known (Avella et al., 2013). Surprisingly, however, a few zygotes obtained after IVF with KO sperm or natural mating with KO males, and then cultured in vitro developed to blastocysts, albeit with greatly reduced efficiency and after a significant delay. Moreover, male PLCζ KO mice were only subfertile, generating fewer pups per litter and less offspring per female over a 10-week mating period, rather than being totally infertile as might be expected based on the critical role of Ca2+ signals as the trigger of embryogenesis and the data from human patients discussed above. This constituted the first demonstration that in vivo fertilization without the normal physiological trigger of egg activation can result in offspring, at least in mice.
The ability of eggs fertilized by sperm lacking PLCζ to develop to blastocyst, albeit with greatly reduced efficiency and after a significant delay, and even develop into live offspring, was explained by Hachem et al. as being possibly due to spontaneous activation in vivo, something that has previously been reported in ovulated hamster and mouse eggs left to reside in the oviduct for extended periods of time in the absence of fertilization (Whittingham and Siracusa, 1978; Xu et al., 1997; Yanagimachi and Chang, 1961). Moreover, eggs from the C57BL/6 strain, the strain used by Hachem et al. (2017), have been shown to have a particularly high susceptibility to spontaneous activation during in vitro maturation (Cheng et al., 2012). While normally such parthenogenetic embryos would only develop to a very limited extent, penetration by a PLCζ KO sperm, even though this provided no apparent activating stimulus, might allow development to term following spontaneous egg activation. It is worth recalling that men presenting mutations in the PLCZ1 gene are completely infertile, despite years of unprotected sexual intercourse. This could reflect species differences between mice and men in terms of the ability of sperm to be able to activate the egg in the absence of PLCζ expression in the sperm, or the possibility that such infertile men have other defects that affect egg activation. To explore this point further, it would be useful to generate PLCζ deficient animals in different species to confirm whether or not the partial fertility displayed by PLCζ deficient mice is specific to rodents.
However, another explanation for the subfertility of PLCz1 KO males is that the KO sperm can trigger an atypical Ca2+ signal that was not detected by the study, or by acting on a signalling component downstream of the Ca2+ stimulus. Such a scenario has been strengthened by an even more recent study by Nozawa et al. (2018) that also used CRISPR/Cas9 genome editing to generate two Plcz1 gene KO lines as well as two Plcz1 knockin (KI) lines. In the Plcz1 KO mice, similar to the report of Hachem et al. (2017), spermatogenesis was normal (Nozawa et al., 2018). Nevertheless, using sperm from recently collected males and IVF, this study showed that KO sperm were able to initiate Ca2+ oscillations, although the oscillations started over an hour after fertilization and displayed an abnormal pattern that consisted of only a few Ca2+ transients. These highly abnormal Ca2+ oscillations could explain why Plcz1 KO sperm could trigger egg activation, albeit with lower efficiency, and with a considerable delay in pronuclear formation and embryo development, both in this study and in the work of Hachem et al. (2017). Remarkably, fresh Plcz1 KO sperm failed to induce Ca2+ transients if fertilization was performed using ICSI, just as reported by Hachem et al. Similarly, Nozawa et al. found that Plcz1 KO males were not infertile, only subfertile, producing smaller litters, and attributed this subfertility to the high rates of polyspermy due to failure of engaging the plasma membrane block mechanism for preventing polyspermy. Regarding the KI lines, homozygous male mice carrying Asp210Arg (D210R) or H435P mutations phenocopied Plcz1 KO male mice in spermatogenesis, fertility, IVF, and ICSI outcomes. The D210R mutation, which results in an enzymatically dead protein, could be detected as a full-length protein of ~74 kDa in similar amounts to PLCζ in WT mice following immunoblotting. However, the H435P protein, which is the mouse equivalent of the human H398P mutation, was not detected at ~74 kDa, suggesting that this mutant protein is unstable. This is line with a previous study that showed that the human PLCζ H398P protein also appears to be unstable when expressed in HEK cells (Kashir et al., 2011). Together, these studies show the importance of PLCζ in the initiation of Ca2+ oscillations and monospermic fertilization in mammals. Future studies should elucidate the mechanism(s) responsible for the PLCζ-independent Ca2+ oscillations in mouse fertilization, and the underlying reasons for why fertilization by ICSI cannot induce the Ca2+ these atypical responses.
A gene knockout approach has also been used to test the physiological importance of PAWP (gene name Wbp2nl) in mammalian egg activation. Satouh et al. (2015) generated a Wbp2nl KO mouse and showed that had no obvious defects in spermatogenesis (Satouh et al., 2015). Sperm produced by Wbp2nl KO males had normal morphology and could undergo the acrosome reaction. In addition, Wbp2nl KO sperm injected into oocytes by ICSI showed no defect in their ability to trigger Ca2+ oscillations. IVF experiments with Wbp2nl KO sperm showed no differences in either the time to complete pronuclear formation or time to complete fusion between the two pronuclei. Also, there appears to be no defects in the block to polyspermy following IVF with Wbp2nl KO sperm, as the number of normally fertilized embryos was comparable to that obtained after using WT sperm, which is in line with the ability of Wbp2nl KO sperm to trigger the normal pattern of Ca2+ oscillations. Mating studies with Wbp2nl KO males showed no defects in fertility, with normal size litters being produced by these males. These findings along with the previously discussed data in human sperm suggest that PAWP plays no role in egg activation in mammals, although it remains remotely possible that loss of PAWP expression is being compensated functionally by up regulation of another gene product, or by the recently reported ortholog Wbp2 (Hamilton et al., 2018). Hachem et al. (2017) also examined the issue of whether PAWP expression was altered following Plcz1 gene knockout. This study used RT-qPCR and immunoblot analysis to investigate levels of Wbp2nl mRNA and PAWP protein in Plcz1 KO testis and sperm, respectively. There were no observed differences in the levels of PAWP protein between wild type and Plcz1 KO sperm, although the level of Wbp2nl mRNA was slightly higher than in controls. Therefore, these findings indicate that the defect in the ability of PLCζ KO sperm to trigger Ca2+ oscillations in eggs is not due to some unintended off-target effect on PAWP.
Conclusions and Future Directions
Over the last 30 years, significant progress has been made in understanding the mechanisms of egg activation in all living animals and the universal role of Ca2+ in this process. Nevertheless, elucidation of how Ca2+ oscillations are initiated in mammals has remained elusive and controversial. The discovery of PLCζ nearly 15 years ago represented an important breakthrough and a starting point to objectively test hypotheses and examine the signaling mechanisms of fertilization. The emergence of affordable whole exome and genome sequencing procedures along with the advent of CRISPR technology has confirmed the pivotal role of PLCζ in mammalian fertilization. Nevertheless, significant questions remain. Despite its essential role for initiating oscillations, there appears to be, at least in the mouse, a secondary, PLCζ independent Ca2+ release mechanism(s). Whereas it purportedly plays a minor role, understanding its molecular nature, and determining whether its expression and function extends to other mammals would add fundamental insights in the understanding of fertilization. Further, we still do not know where PIP2 hydrolysis takes place in the ooplasm during fertilization (although see recent review by Sanders et. al. (Sanders et al., 2018)), or how PLCζ is targeted to its substrate. It also remains unresolved how the function of PLCζ is terminated, especially in species where PLCζ is not translocated to the pronuclei. Despite these lingering questions, the recent progress in the field and greater availability of tools ensures faster progress and discoveries. Importantly, the generation of Plcz1 KO mice provides an invaluable resource to address these known questions and pursue new ones that have gained urgency because of the phenotype of Plcz1 KO mice. For example, how are the gene expression profiles and epigenetic changes affected in embryos and pups conceived using Plcz1 KO males? What is the sequence of molecular change(s) underlying the plasma membrane block to polyspermy that are not induced following fusion of Plcz1 KO sperm? The Plcz1 KO experimental model should provide conclusive answers to these questions, which will greatly advance our understanding of the molecular mechanisms of fertilization.
In terms of clinical application, the absence of Ca2+ oscillations and infertility observed in some of the aforementioned patients is currently rescued in the clinic by treatment with Ca2+ ionophores (Mateizel et al., 2018). Exposure to ionophore causes a single and much larger Ca2+ response than those induced by fertilization, and whereas the treatment is effective at inducing egg activation and embryo development, additional studies are needed to evaluate the effects on the long term health of the offspring and their fertility. A less dramatic change in Ca2+ signaling is also observed following ICSI (Kurokawa and Fissore, 2003; Nakano et al., 1997; Tesarik and Sousa, 1994), which seems to be the fertilization method of choice in the clinic, even if sperm are suitable for IVF. Given the inherently higher risks of the ICSI procedure in terms of the health of the offspring (Davies et al., 2012) and the possible effects on the fertility of males offspring (Belva et al., 2016), it is worth conducting more thorough studies on how altered Ca2+ activating patterns might impact further embryo development, and in particular its effects on the epigenetic landscape of the embryo. Recombinant human PLCζ has been produced and studies have demonstrated that it is active and capable of inducing oscillations upon injection into human eggs (Sanusi et al., 2015; Yoon et al., 2012). Future studies should assess its clinical application and whether its use offers molecular and clinical advantages over the use of ionophores.
In closing, the discovery of PLCζ and the demonstration of its function have finally identified the sperm component that was suggested by Loeb more than 100 years ago to activate the egg metabolism during fertilization. Nevertheless, the identity, function and regulation of many other components of the Ca2+ toolkit of eggs and zygotes that crosstalk with PLCζ and are required to support the sperm-induced oscillations remain unresolved. Only when these unknown cellular components and mechanisms are identified, will we have a more complete understanding of the pathways of fertilization, which would optimize ART procedures and could make possible the development of effective contraceptive methods with minimal side effects.
Acknowledgements
The authors would like to thank current and former members of their laboratories and former and current collaborators on this subject for their contributions that our laboratories made to this field and that are summarized in this review. We apologize to all of those researchers whose work could not be not cited here because space limitation. The work in our laboratories was supported in part by grants from the NIH (HD051872; HD092499) and NIFA (Multistate grant #1727) to RAF, from a DPhil studentship from the Iraqi government for Alaa Hachem to JP and from ANR Genopat 2009, project ICG2I to CA.
Footnotes
Conflict of Interest
The authors declare that they do not have conflict of interest.
References
- Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R, 2014. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J 28:4434–4440. [DOI] [PubMed] [Google Scholar]
- Avella MA, Xiong B, Dean J, 2013. The molecular basis of gamete recognition in mice and humans. Mol Hum Reprod 19, 279–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belva F, Bonduelle M, Roelants M, Michielsen D, Van Steirteghem A, Verheyen G, Tournaye H, 2016. Semen quality of young adult ICSI offspring: the first results. Hum Reprod 31, 2811–2820. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE, 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351, 751–754. [DOI] [PubMed] [Google Scholar]
- Boveri T, 1889. An organism produced sexually without characteristics of the mother. Am Naturalist 27, 222–232. [Google Scholar]
- Carroll DJ, Ramarao CS, Mehlmann LM, Roche S, Terasaki M, Jaffe LA, 1997. Calcium release at fertilization in starfish eggs is mediated by phospholipase Cgamma. J Cell Biol 138, 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, 1959. Fertilization of rabbit ova in vitro. Nature 184(Suppl 7), 466–467. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Zhong Z, Latham KE, 2012. Strain-specific spontaneous activation during mouse oocyte maturation. Fertil Steril 98, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL, 1991. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 65, 1043–1051. [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S, Gomez-Fernandez JC, 2014. Signaling through C2 domains: more than one lipid target. Biochim Biophys Acta 1838, 1536–1547. [DOI] [PubMed] [Google Scholar]
- Coward K, Ponting CP, Chang HY, Hibbitt O, Savolainen P, Jones KT, Parrington J, 2005. Phospholipase Czeta, the trigger of egg activation in mammals, is present in a non-mammalian species. Reproduction 130, 157–163. [DOI] [PubMed] [Google Scholar]
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA, 2002. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 124, 611–623. [DOI] [PubMed] [Google Scholar]
- Cuthbertson KS, Whittingham DG, Cobbold PH, 1981. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature 294, 754–757. [DOI] [PubMed] [Google Scholar]
- Dale B, 1988. Primary and secondary messengers in the activation of ascidian eggs. Exp Cell Res 177, 205–211. [DOI] [PubMed] [Google Scholar]
- Dale B, DeFelice LJ, Ehrenstein G, 1985. Injection of a soluble sperm fraction into sea-urchin eggs triggers the cortical reaction. Experientia 41, 1068–1070. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A, 2012. Reproductive technologies and the risk of birth defects. N Engl J Med 366, 1803–1813. [DOI] [PubMed] [Google Scholar]
- Escoffier J, Lee HC, Yassine S, Zouari R, Martinez G, Karaouzene T, Coutton C, Kherraf ZE, Halouani L, Triki C, Nef S, Thierry-Mieg N, Savinov SN, Fissore R, Ray PF, Arnoult C, 2016. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum Mol Genet 25, 878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffier J, Yassine S, Lee HC, Martinez G, Delaroche J, Coutton C, Karaouzene T, Zouari R, Metzler-Guillemain C, Pernet-Gallay K, Hennebicq S, Ray PF, Fissore R, Arnoult C, 2015. Subcellular localization of phospholipase Czeta in human sperm and its absence in DPY19L2-deficient sperm are consistent with its role in oocyte activation. Mol Hum Reprod 21, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen LO, Perisic O, Cheung R, Katan M, Williams RL, 1996. Crystal structure of a mammalian phosphoinositide-specific phospholipase C delta. Nature 380, 595–602. [DOI] [PubMed] [Google Scholar]
- Evans JP, Kopf GS, 1998. Molecular mechanisms of sperm-egg interactions and egg activation. Andrologia 30, 297–307. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F, 1979. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol 41, 473–484. [DOI] [PubMed] [Google Scholar]
- Flaherty SP, Payne D, Matthews CD, 1998. Fertilization failures and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod 13 Suppl 1, 155–164. [DOI] [PubMed] [Google Scholar]
- Flaherty SP, Payne D, Swann NJ, Mattews CD, 1995. Aetiology of failed and abnormal fertilization after intracytoplasmic sperm injection. Hum Reprod 10, 2623–2629. [DOI] [PubMed] [Google Scholar]
- Fol H, 1877. Sur le commencement de l’hénogénie chez divers animaux. Archives des sciences physiques et naturelles 58. [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, Takenawa T, 2001. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science 292, 920–923. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Grey RD, 1983. Membrane junctions in Xenopus eggs: their distribution suggests a role in calcium regulation. J Cell Biol 96, 1159–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CB, Inselman AL, Sunman JA, Bornstein S, Handel MA, Eddy EM, 2009. A missense mutation in the Capza3 gene and disruption of F-actin organization in spermatids of repro32 infertile male mice. Dev Biol 330, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA, 1999. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J Biol Chem 274, 29318–29322. [DOI] [PubMed] [Google Scholar]
- Grasa P, Coward K, Young C, Parrington J, 2008. The pattern of localization of the putative oocyte activation factor, phospholipase Czeta, in uncapacitated, capacitated, and ionophore-treated human spermatozoa. Hum Reprod 23, 2513–2522. [DOI] [PubMed] [Google Scholar]
- Guillen J, Ferrer-Orta C, Buxaderas M, Perez-Sanchez D, Guerrero-Valero M, Luengo-Gil G, Pous J, Guerra P, Gomez-Fernandez JC, Verdaguer N, Corbalan-Garcia S, 2013. Structural insights into the Ca2+ and PI(4,5)P2 binding modes of the C2 domains of rabphilin 3A and synaptotagmin 1. Proc Natl Acad Sci U S A 110, 20503–20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem A, Godwin J, Ruas M, Lee HC, Ferrer Buitrago M, Ardestani G, Bassett A, Fox S, Navarrete F, de Sutter P, Heindryckx B, Fissore R, Parrington J, 2017. PLCzeta is the physiological trigger of the Ca(2+) oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 144, 2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LE, Suzuki J, Acteau G, Shi M, Meinsohn MC, Sutivsky P, Oko R, 2018. WBP2 shares a common location in mouse spermatozoa with WBP2NL/PAWP and like its descendent is a candidate mouse oocyte activating factor Biol Reprod Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbuz R, Zouari R, Pierre V, Ben Khelifa M, Kharouf M, Coutton C, Merdassi G, Abada F, Escoffier J, Nikas Y, Vialard F, Koscinski I, Triki C, Sermondade N, Schweitzer T, Zhioua A, Zhioua F, Latrous H, Halouani L, Ouafi M, Makni M, Jouk PS, Sele B, Hennebicq S, Satre V, Viville S, Arnoult C, Lunardi J, Ray PF, 2011. A recurrent deletion of DPY19L2 causes infertility in man by blocking sperm head elongation and acrosome formation. Am J Hum Genet 88, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbrunn LV, and Wilbur Karl M., 1937. Stimulation and Nuclear Breakdown in the Nereis Egg. Biological Bulletin 73, 557–564. [Google Scholar]
- Hertwig O, 1876. Beiträge zur Kenntniss der Bildung, Befruchtung und Theilung des thierischen Eies.. Morphol. Jahrb 1, 347–434 [Google Scholar]
- Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, Deane CM, Ruas M, Grasa P, Soleimani R, Cuvelier CA, Gerris J, Dhont M, Deforce D, Leybaert L, De Sutter P, 2009. Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod 24, 2417–2428. [DOI] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S, Yamashita N, 1983. Periodic hyperpolarizing responses in hamster and mouse eggs fertilized with mouse sperm. J Physiol 340, 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Ikawa M, 2010. Program and Abstracts of the 11th International Symposium on Spermatology (p. p:). Presented at 11. International Symposium on Spermatology, Okinawa, JPN (2010-06-24–2010-06-29). [Google Scholar]
- Ito M, Shikano T, Oda S, Horiguchi T, Tanimoto S, Awaji T, Mitani H, Miyazaki S, 2008. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol Reprod 78, 1081–1090. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, 1990. First messengers at fertilization. J Reprod Fertil Suppl 42, 107–116. [PubMed] [Google Scholar]
- Jaffe LF, 1979. Control of development by ionic currents. Soc Gen Physiol Ser 33, 199–231. [PubMed] [Google Scholar]
- Jaffe LF, 1983. Sources of calcium in egg activation: a review and hypothesis. Dev Biol 99, 265–276. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, 1991. The path of calcium in cytosolic calcium oscillations: a unifying hypothesis. Proc Natl Acad Sci U S A 88, 9883–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellerette T, He CL, Wu H, Parys JB, Fissore RA, 2000. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol 223, 238–250. [DOI] [PubMed] [Google Scholar]
- Jones KT, Cruttwell C, Parrington J, Swann K, 1998. A mammalian sperm cytosolic phospholipase C activity generates inositol trisphosphate and causes Ca2+ release in sea urchin egg homogenates. FEBS Lett 437, 297–300. [DOI] [PubMed] [Google Scholar]
- Jones KT, Matsuda M, Parrington J, Katan M, Swann K, 2000. Different Ca2+-releasing abilities of sperm extracts compared with tissue extracts and phospholipase C isoforms in sea urchin egg homogenate and mouse eggs. Biochem J 346 Pt 3, 743–749. [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Soeller C, Cannell MB, 1998. The passage of Ca2+ and fluorescent markers between the sperm and egg after fusion in the mouse. Development 125, 4627–4635. [DOI] [PubMed] [Google Scholar]
- Kashir J, Jones C, Lee HC, Rietdorf K, Nikiforaki D, Durrans C, Ruas M, Tee ST, Heindryckx B, Galione A, De Sutter P, Fissore RA, Parrington J, Coward K, 2011. Loss of activity mutations in phospholipase C zeta (PLCzeta) abolishes calcium oscillatory ability of human recombinant protein in mouse oocytes. Hum Reprod 26, 3372–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee HC, Hamer R, Heindryckx B, Deane CM, De Sutter P, Fissore RA, Parrington J, Wells D, Coward K, 2012. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCzeta) leads to male infertility. Hum Reprod 27, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ, 2005. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol Reprod 72, 992–996. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S, 2004. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 279, 10408–10412. [DOI] [PubMed] [Google Scholar]
- Kouchi Z, Shikano T, Nakamura Y, Shirakawa H, Fukami K, Miyazaki S, 2005. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Czeta. J Biol Chem 280, 21015–21021. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Ito M, Shikano T, Awaji T, Yoda A, Takeuchi H, Kinoshita K, Miyazaki S, 2006. The role of X/Y linker region and N-terminal EF-hand domain in nuclear translocation and Ca2+ oscillation-inducing activities of phospholipase Czeta, a mammalian egg-activating factor. J Biol Chem 281, 27794–27805. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Fissore RA, 2003. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol Hum Reprod 9, 523–533. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Wu H, He C, Malcuit C, Black SJ, Fukami K, Fissore RA, 2005. Functional, biochemical, and chromatographic characterization of the complete [Ca2+]i oscillation-inducing activity of porcine sperm. Dev Biol 285, 376–392. [DOI] [PubMed] [Google Scholar]
- Loeb J, 1913. Artificial Parthenogenesis and Fertilization The University of Chicago Press. [Google Scholar]
- Mateizel I, Verheyen G, Van de Velde H, Tournaye H, Belva F, 2018. Obstetric and neonatal outcome following ICSI with assisted oocyte activation by calcium ionophore treatment. J Assist Reprod Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D, 1937. The release of calcium in Arbacia eggs upon fertilization. J. Cell. Comp. Physiol 10, 291–304. [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA, 1998. SH2 domain-mediated activation of phospholipase Cgamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol 203, 221–232. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, 1988. Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium rises in golden hamster eggs. J Cell Biol 106, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Igusa Y, 1981. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature 290, 702–704. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Ito M, 2006. Calcium signals for egg activation in mammals. J Pharmacol Sci 100, 545–552. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K, 1992. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 257, 251–255. [DOI] [PubMed] [Google Scholar]
- Muciaccia B, Sette C, Paronetto MP, Barchi M, Pensini S, D’Agostino A, Gandini L, Geremia R, Stefanini M, Rossi P, 2010. Expression of a truncated form of KIT tyrosine kinase in human spermatozoa correlates with sperm DNA integrity. Hum Reprod 25, 2188–2202. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Shirakawa H, Mitsuhashi N, Kuwabara Y, Miyazaki S, 1997. Spatiotemporal dynamics of intracellular calcium in the mouse egg injected with a spermatozoon. Mol Hum Reprod 3, 1087–1093. [DOI] [PubMed] [Google Scholar]
- Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD, 2014. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium 55, 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport G, 1853. On the impregnation of the ovum in amphibia. Philos Trans R Soc Lond 143, 233–290. [Google Scholar]
- Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA, 2005. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem 280, 31011–31018. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Elgmati K, Theodoridou M, Calver BL, Cumbes B, Nounesis G, Swann K, Lai FA, 2011a. Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP(2) hydrolysis activity of sperm PLCzeta. Biochem J 434, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Elgmati K, Theodoridou M, Calver BL, Nounesis G, Swann K, Lai FA, 2011b. Phospholipase Czeta binding to PtdIns(4,5)P2 requires the XY-linker region. J Cell Sci 124, 2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Kashir J, Swann K, Lai FA, 2013. Sperm PLCzeta: from structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett 587, 3609–3616. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Sanders JR, Theodoridou M, Kashir J, Matthews E, Nounesis G, Lai FA, Swann K, 2014. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol Hum Reprod; 20, 938–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Sanders JR, Parthimos D, Buntwal L, Calver BL, Stamatiadis P, Smith A, Clue M, Sideratou Z, Swann K, Lai FA, 2015. Essential Role of the EF-hand Domain in Targeting Sperm Phospholipase Czeta to Membrane Phosphatidylinositol 4,5-Bisphosphate (PIP2). J Biol Chem 290, 29519–29530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos M, Stamatiadis P, Sanders JR, Beck K, Calver BL, Buntwal L, Lofty M, Sideratou Z, Swann K, Lai FA, 2017. Male infertility-linked point mutation reveals a vital binding role for the C2 domain of sperm PLCzeta. Biochem J 474, 1003–1016. [DOI] [PubMed] [Google Scholar]
- Nozawa K, Satouh Y, Fujimoto T, Oji A, Ikawa M, 2018. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci Rep 8, 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozil JP, Swann K, 1995. Stimulation of repetitive calcium transients in mouse eggs. J Physiol 483 (Pt 2), 331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC, 1992. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340, 17–18. [DOI] [PubMed] [Google Scholar]
- Parker I, Yao Y, 1996. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J Physiol 491 (Pt 3), 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Jones KT, Lai A, Swann K, 1999. The soluble sperm factor that causes Ca2+ release from sea-urchin (Lytechinus pictus) egg homogenates also triggers Ca2+ oscillations after injection into mouse eggs. Biochem J 341, 1–4. [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Jones ML, Tunwell R, Devader C, Katan M, Swann K, 2002. Phospholipase C isoforms in mammalian spermatozoa: potential components of the sperm factor that causes Ca2+ release in eggs. Reproduction 123, 31–39. [DOI] [PubMed] [Google Scholar]
- Parrington J, Swann K, Shevchenko VI, Sesay AK, Lai FA, 1996. Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature 379, 364–368. [DOI] [PubMed] [Google Scholar]
- Pierre V, Martinez G, Coutton C, Delaroche J, Yassine S, Novella C, Pernet-Gallay K, Hennebicq S, Ray PF, Arnoult C, 2012. Absence of Dpy19l2, a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus. Development 139, 2955–2965. [DOI] [PubMed] [Google Scholar]
- Rice A, Parrington J, Jones KT, Swann K, 2000. Mammalian sperm contain a Ca(2+)-sensitive phospholipase C activity that can generate InsP(3) from PIP(2) associated with intracellular organelles. Dev Biol 228, 125–135. [DOI] [PubMed] [Google Scholar]
- Ridgway EB, Gilkey JC, Jaffe LF, 1977. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci U S A 74, 623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RP, 1982. Calcium-phospholipid interactions in secretory cells: a new perspective on stimulus-secretion coupling. Fed Proc 41, 2181–2187. [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA, 1999. Calcium release at fertilization of Xenopus eggs requires type I IP(3) receptors, but not SH2 domain-mediated activation of PLCgamma or G(q)-mediated activation of PLCbeta. Dev Biol 214, 399–411. [DOI] [PubMed] [Google Scholar]
- Sanders JR, Ashley B, Moon A, Woolley TE, Swann K, 2018. PLCzeta Induced Ca(2+) Oscillations in Mouse Eggs Involve a Positive Feedback Cycle of Ca(2+) Induced InsP3 Formation From Cytoplasmic PIP2. Front Cell Dev Biol 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanusi R, Yu Y, Nomikos M, Lai FA, Swann K, 2015. Rescue of failed oocyte activation after ICSI in a mouse model of male factor infertility by recombinant phospholipase Czeta. Mol Hum Reprod 21, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Tokmakov AA, Iwasaki T, Fukami Y, 2000. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev Biol 224, 453–469. [DOI] [PubMed] [Google Scholar]
- Satouh Y, Nozawa K, Ikawa M, 2015. Sperm postacrosomal WW domain-binding protein is not required for mouse egg activation. Biol Reprod 93, 94. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA, 2002. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129, 3533–3544. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS, 1995. Molecular basis of mammalian egg activation. Curr Top Dev Biol 30, 21–62. [DOI] [PubMed] [Google Scholar]
- Sette C, Bevilacqua A, Bianchini A, Mangia F, Geremia R, Rossi P, 1997. Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development 124, 2267–2274. [DOI] [PubMed] [Google Scholar]
- Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P, 2002. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J 21, 5386–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, De Nadai C, Emily-Fenouil F, Gache C, Whitaker M, Ciapa B, 1999. Role of phospholipase Cgamma at fertilization and during mitosis in sea urchin eggs and embryos. Development 126, 2273–2284. [DOI] [PubMed] [Google Scholar]
- Steinhardt R, Zucker R, Schatten G, 1977. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol 58, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Epel D, 1974. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A 71, 1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Epel D, Carroll EJ Jr., Yanagimachi R, 1974. Is calcium ionophore a universal activator for unfertilised eggs? Nature 252, 41–43. [DOI] [PubMed] [Google Scholar]
- Stice SL, Robl JM, 1990. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev 25, 272–280. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR, 1995. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell 80, 929–938. [DOI] [PubMed] [Google Scholar]
- Swann K, 1990. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 110, 1295–1302. [DOI] [PubMed] [Google Scholar]
- Swann K, Whitaker M, 1986. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol 103, 2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama J, Onami S, 2016. The Sperm TRP-3 Channel Mediates the Onset of a Ca(2+) Wave in the Fertilized C. elegans Oocyte. Cell Rep 15, 625–637. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Lawrence YM, Kingsland CR, Biljan MM, Cuthbertson KS, 1993. Oscillations in intracellular free calcium induced by spermatozoa in human oocytes at fertilization. Hum Reprod 8, 2174–2179. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Sousa M, 1994. Comparison of Ca2+ responses in human oocytes fertilized by subzonal insemination and by intracytoplasmic sperm injection. Fertil Steril 62, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Testart J, 1994. Treatment of sperm-injected human oocytes with Ca2+ ionophore supports the development of Ca2+ oscillations. Biol Reprod 51, 385–391. [DOI] [PubMed] [Google Scholar]
- Uehara T, Yanagimachi R, 1976. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol Reprod 15, 467–470. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo J, Carlton C, McGinnis LK, Kinsey WH, 2017. Sperm-oocyte contact induces outside-in signaling via PYK2 activation. Dev Biol 428, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham DG, Siracusa G, 1978. The involvement of calcium in the activation of mammalian oocytes. Exp Cell Res 113, 311–317. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Mehlmann LM, Jaffe LA, Kopf GS, Schultz RM, 1998. Evidence that Gq family G proteins do not function in mouse egg activation at fertilization. Dev Biol 198, 116–127. [PubMed] [Google Scholar]
- Wolny YM, Fissore RA, Wu H, Reis MM, Colombero LT, Ergun B, Rosenwaks Z, Palermo GD, 1999. Human glucosamine-6-phosphate isomerase, a homologue of hamster oscillin, does not appear to be involved in Ca2+ release in mammalian oocytes. Mol Reprod Dev 52, 277–287. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Kline D, Bian Y, Blackshaw S, Cameron AM, Fralich TJ, Schnaar RL, Snyder SH, 1998. Molecularly cloned mammalian glucosamine-6-phosphate deaminase localizes to transporting epithelium and lacks oscillin activity. FASEB J 12, 91–99. [DOI] [PubMed] [Google Scholar]
- Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, Park KW, Yi YJ, Xi YW, Prather RS, Oko R, 2007. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem, 282, 12164–75. [DOI] [PubMed] [Google Scholar]
- Wu H, He CL, Fissore RA, 1997. Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol Reprod Dev 46, 176–189. [DOI] [PubMed] [Google Scholar]
- Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T, 1997. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod 57, 743–750. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, 1954. Physiological studies on fertilization and activation of fish eggs. V. The role of calcium ions in activation of Oryzias eggs. Exp Cell Res 6, 56–68. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Bedford JM, Yanagimachi R, 1991. Cleavage of rabbit eggs after microsurgical injection of testicular spermatozoa. Hum Reprod 6, 277–279. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Chang MC, 1961. Fertilizable life of golden hamster ova and their morphological changes at the time of losing fertilizability. J Exp Zool 148, 185–203. [DOI] [PubMed] [Google Scholar]
- Yim DL, Opresko LK, Wiley HS, Nuccitelli R, 1994. Highly polarized EGF receptor tyrosine kinase activity initiates egg activation in Xenopus. Dev Biol 162, 41–55. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Eum JH, Lee JE, Lee HC, Kim YS, Han JE, Won HJ, Park SH, Shim SH, Lee WS, Fissore RA, Lee DR, Yoon TK, 2012. Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum Reprod 27, 1768–1780. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, Mager J, Fissore RA, 2008. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest 118, 3671–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Amanai M, Fukui T, Kajikawa E, Brahmajosyula M, Iwahori A, Nakano Y, Shoji S, Diebold J, Hessel H, Huss R, Perry AC, 2007. Broad, ectopic expression of the sperm protein PLCZ1 induces parthenogenesis and ovarian tumours in mice. Development 134, 3941–3952. [DOI] [PubMed] [Google Scholar]
- Young C, Grasa P, Coward K, Davis LC, Parrington J, 2009. Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil Steril 91, 2230–2242. [DOI] [PubMed] [Google Scholar]
- Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K, 2012. PLCzeta causes Ca(2+) oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P(2). Mol Biol Cell 23, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti N, Mayorga LS, 2009. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod 81, 396–405. [DOI] [PubMed] [Google Scholar]