Abstract
Introduction
Heme is a central molecule in mitochondrial respiration and ATP generation in neuronal cells. Thus, we assessed the importance of altered heme metabolism in Alzheimer's disease (AD) pathogenesis.
Methods
To investigate the role of altered heme metabolism in AD, we identified heme-related proteins whose expression is altered in AD patients and mouse models exhibiting amyloid pathology. We detected the levels of proteins involved in heme synthesis, uptake, degradation, and function during neuronal differentiation and characterized the effects of Aβ.
Results
We found that the expression levels of the rate-limiting heme synthetic enzyme ALAS1 and heme degradation enzyme HO-2 are selectively decreased in AD patients and mice. Aβ selectively reduces the levels of HO-2 and heme degradation, which are elevated to support neuronal functions in fully differentiated neuronal cells.
Discussion
Our data show that lowered heme metabolism, particularly the decreased levels of heme degradation and HO-2, is likely a very early event in AD pathogenesis.
Keywords: Alzheimer's disease, Amyloid β, Heme, Heme oxygenase, Heme degradation
1. Introduction
Despite decades of intense research on the basic biology and clinical pathophysiology of Alzheimer's disease (AD), there is still a lack of effective strategies to prevent and treat AD [1], [2]. From 1998 to 2011, about 100 compounds tested for the treatment of AD failed while in the clinical development phase. In 2012, 2 monoclonal antibodies against amyloid β (Aβ), bapineuzumab and solanezumab, failed to show desired clinical benefits in patients with mild-to-moderate AD in phase III clinical trials [3], [4]. Clearly, a better understanding of the molecular events leading to deficits in multiple brain functions is necessary [5]. Aβ has long been associated with AD. The formation of insoluble deposits, also known as senile plaques, by Aβ is a major hallmark of AD brains. Despite continuing debate about the Aβ hypothesis, overwhelming experimental evidence supports the idea that overproduction of Aβ is a very early, often initiating factor in AD [6]. It is conceivable that early events initiated by Aβ are crucial for AD pathogenesis, whereas late events, such as the formation of senile plaques, are merely symptomatic. Thus, antibodies that reduce the formation of senile plaques may not be effective in preventing AD initiation and progress.
Mitochondrial dysfunction and bioenergetic deficits have been identified as early and potentially causative events in AD pathogenesis [7], [8]. Importantly, heme is a key factor in mitochondrial function and bioenergetics. Heme is a central metabolic and signaling molecule regulating diverse molecular and cellular processes relating to oxygen utilization and metabolism [9]. Heme serves as a prosthetic group in proteins and enzymes involved in oxygen transport, utilization, and storage, such as globins and cytochromes. Multiple subunits in mitochondrial respiration or oxidative phosphorylation (OXPHOS) complexes II-IV contain heme. Furthermore, heme acts as a signaling molecule to coordinate the expression of genes encoding globins and cytochromes, as well as the translocation and assembly of these protein/enzyme complexes [10]. Heme binds to and directly regulates the activities of many proteins controlling processes ranging from tyrosine kinase signaling to microRNA processing [11], [12]. Thus, it is not surprising that multiple lines of evidence have indicated the association of heme and AD pathogenesis. First, heme has been shown to colocalize with Aβ deposits in AD tissues [13]. Second, lowered expression of heme synthetic enzymes is found in AD brains [14]. Third, Aβ binds to heme and causes functional heme deficiency. In addition, the Aβ-heme complex acts as a peroxidase and generates reactive oxygen species (ROS), which can generate an array of neurotoxic products [15], [16]. Clearly, compelling experimental and pathological evidence supports the involvement of heme in AD pathogenesis, although it is not clear which heme-related events play a causative role in AD pathogenesis.
To glean heme actions in neurogenesis and in AD pathogenesis, we first analyzed the data from two previously published genome-wide expression data sets from AD patient and control brains [17], [18], [19]. We also examined brain tissues from control and APPPS1 mice that coexpress KM670/671NL-mutated amyloid precursor protein and L166P-mutated presenilin-1 [20], [21]. These mice exhibit key features of amyloid pathology that allowed them to be applied in AD [22]. They are very useful for studying the effects of molecular and behavioral effects of Aβ, but the overexpression paradigm may cause additional phenotypes unrelated to AD. We found that the rate-limiting heme synthetic enzyme ALAS1 and heme degradation enzyme HO-2 are selectively reduced in AD brain hippocampi. Furthermore, to identify the potentially early events in Aβ action, we used an in vitro model previously shown to be useful for studying AD pathogenesis, the popular SH-SY5Y neuroblastoma cell line [23], [24]. SH-SY5Y cells can be differentiated to a more mature neuron-like phenotype that is characterized by neuronal markers, but not glial markers such as glial fibrillary acidic protein (GFAP) [25]. Using the SH-SY5Y cell line, we monitored heme synthesis, uptake, and degradation, as well as heme-related proteins and enzymes as neuronal differentiation progresses. We found that Aβ selectively reduces the levels of HO-2 and heme degradation in differentiated SH-SY5Y cells. These results provide novel insights into the early events underlying AD pathogenesis.
2. Methods
2.1. Reagents, SH-SY5Y cell culture, and differentiation
SH-SY5Y cells were purchased from ATCC and maintained according to ATCC protocols. Neuronal differentiation was induced by treatments with 10 μM of retinoic acid (RA; Sigma) and 20 ng/mL of nerve growth factor (NGF; Genentech) for 5 days followed by 50 ng/mL of brain-derived neurotrophic factor (Research Diagnosis) in serum-free medium for 3 days. Partially differentiated cells underwent the 5-day treatment of RA and NGF. For Aβ treatment, fully differentiated SH-SY5Y cells were treated with 5 μM Aβ for 3 days, as described [26]. Antibodies and MitoTracker Red CMXRos used were purchased from commercial vendors, including Abcam and Novus.
2.2. Measurement of heme synthesis, degradation, and uptake
Measurement of heme synthesis in SH-SY5Y cells was carried out exactly as described [27], [28]. The levels of heme degradation were calculated by subtracting the amounts of radiolabeled heme in cells grown for another 24 hours from the amounts of radiolabeled heme in cells after incubation with 4-14C-5-aminolevulinic acid (ALA) for 15 hours. For measuring heme uptake, a fluorescent analog of heme, ZnPP, was used, as described previously [29], [30]. Fluorescence intensity was measured with a Biotek Cytation 5 plate reader.
2.3. Confocal microscopy imaging and quantification
Cells were stained with primary and secondary antibodies according to the manufacturer's protocols. Cells were visualized using the laser confocal-Olympus FV3000RS. The Olympus FV21S Fluoview Software was used to draw regions of interest (ROIs) over multiple cells. The fluorescence intensity from all the ROIs was averaged, and the corresponding background average was subtracted to yield the signal intensity for each antigen.
2.4. Preparation of protein extracts and Western blotting
Protein extracts were prepared from SH-SY5Y cells as described previously [28]. 40 μg of proteins were electrophoresed, and Western blotting was carried out with a chemiluminescence Western blotting kit as described [28]. The signals were detected by using a Carestream image station 4000MM Pro, and quantitation was performed as described [28].
2.5. Animals
The APPPS1 mice (Thy1-APPKM670/671NL, Thy1-PS1L166P) [20] were kindly provided by Mathias Jucker (University of Tubingen) and used in our previous studies [21], [31]. These mice exhibit key features of amyloid pathology that allowed them to be applied in AD. The APPPS1 mice colony (on C57BL/6 background) was established at the UTSW SPF barrier facility for small laboratory animals. Wild-type (WT) C57BL/6 were used in experiments as control mice, as in various previous studies [20], [21], [31]. All procedures involving mice were approved by the IACUC of UTSW, in accordance with NIH Guidelines for the Care and Use of Experimental Animals.
2.6. Immunohistochemistry
Four 6-month-old APPPS1, four 6-month-old WT, four 6-week-old APPPS1, and four 6-week-old WT mouse brains were sectioned in the cryostat and fixed, washed, and blocked. Tissue sections were incubated with anti-ALAS1 or anti-HO-2 antibodies overnight. Slides were washed and incubated with HRP-conjugated goat anti-rabbit IgG. Slides were washed and incubated with Cy3 Working Solution and then counterstained with DAPI. Slides were scanned with an Olympus VS120 slide scanner and quantified using cellSens software from Olympus. Multiple ROIs of equal area were drawn over the dentate gyrus and CA region of APPPS1 and WT mouse brains. Minimum and maximum thresholds were set to avoid any background signal. The mean signal intensity from all the ROIs was averaged, and the corresponding negative control average was subtracted to yield the signal intensity for each antigen.
2.7. Statistical analysis and analysis of microarray expression data from human patients
Data from different treatment groups of cells and mice were compared, and statistical analysis was performed with a Welch 2-sample t-test.
For expression data from human patient brains, raw CEL files were downloaded from Gene Expression Omnibus (accession numbers GSE36980 and GSE48350) [17], [18], [19]. Processing of the raw CEL files was performed using R and the Oligo, Affy, and Limma packages [32], [33], [34]. The Robust Multi-array Average function in Affy was used to background correct, perform quantile normalization, and calculate final expression value using median polish [35], [36], [37]. The Limma package was used to generate a linear fit model, contrast matrix, and overall differential expression analysis. Adjusted P-values were calculated using the “fdr” parameter.
3. Results
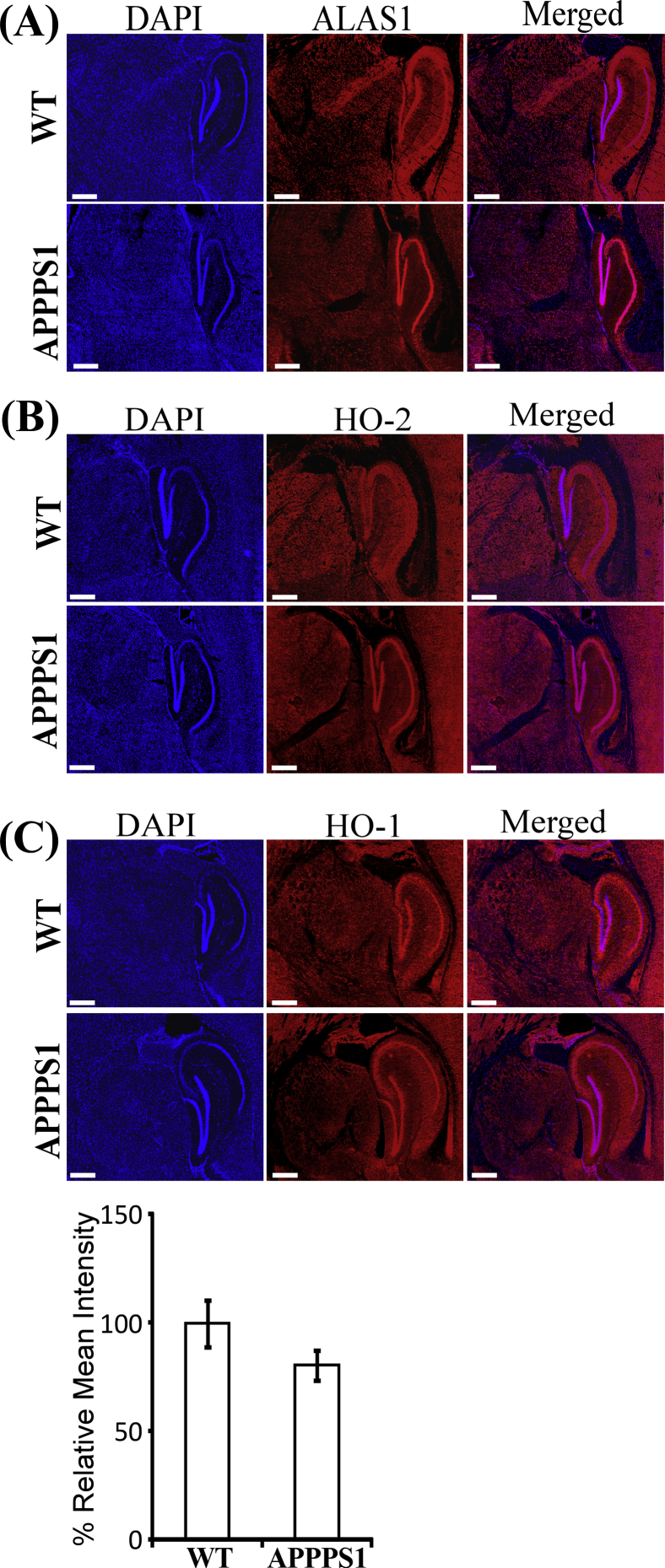
3.1. The expression levels of heme degradation enzyme HO2, as well as the heme synthetic enzyme ALAS1, were lowered in human AD hippocampi and in hippocampi of APPPS1 mouse brains
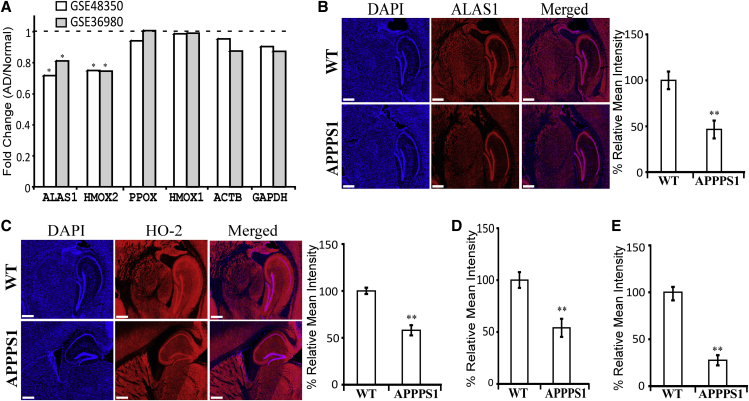
Previous studies on several heme-related genes showed that the transcript levels of heme synthesis enzymes such as ALAS1 are reduced in frontal cortical tissues of AD patient brains [14]. To further ascertain the link between AD and heme-related genes, we used two previously published genome-wide data sets from hippocampi of a number of AD patient and control brains [17], [18], [19]. Study GSE36980 [19] published 10 control and 7 AD gray matter hippocampus samples. Study GSE48350 [17], [18] published 26 control and 19 AD hippocampus samples. Each study used a different Affymetrix microarray, thus making our positive results more robust because they were validated using two different platforms, both of which are effective at gene quantification [38].
Our differential gene expression analysis showed that among all heme-related genes, ALAS1 and HMOX2 (encoding HO-2) were significantly downregulated (Fig. 1A). Other heme-related genes, such as PPOX encoding protoporphyrinogen oxidase and HMOX1 encoding HO-1, were not changed significantly in AD brain tissues (Fig. 1A). Likewise, control genes, such as ACTB and GAPDH, were not changed significantly in AD brain tissues. These results strongly support that the changes in ALAS1 and HMOX2 are specific, not due to artifacts. In both data sets, the downregulation is significant but subtle. This may be attributable to the heterogeneity of the samples, as at least two studies have reported that hippocampus whole-tissue expression is suboptimal in terms of quantifying gene expression as well as predicting disease progression in AD [39], [40].
Fig. 1.
The expression levels of the rate-limiting heme synthetic enzyme ALAS1 and heme degradation enzyme HO-2 are decreased in hippocampi of AD human and mouse brains. (A) Analysis of genome-wide expression data shows that the transcript levels of ALAS1 and HMOX2 are decreased in hippocampi of AD human brains. (B) The levels of ALAS1 protein are decreased in the dentate gyrus of 6-week-old APPPS1 mouse brains. Scale bar: 500 μM. (C) The levels of HO-2 protein are decreased in the dentate gyrus of 6-week-old APPPS1 mouse brains. Scale bar: 500 μM. (D) The levels of ALAS1 protein are decreased in the dentate gyrus of 6-month-old APPPS1 mouse brains. (E) The levels of HO-2 protein are decreased in the dentate gyrus of 6-month-old APPPS1 mouse brains. In (B) and (C), representative DAPI-stained images and immunohistochemistry images of mouse brain tissues stained with the indicated antibodies are shown as examples. The data plotted are averages of data from 4 different APPPS1 and 4 wild-type mouse brain tissues and at least 10 regions of interest for each tissue. *P-value < .05; **P-value < .005.
To further ascertain the significance of lowered levels of ALAS1 and HO-2 in AD pathogenesis, we performed a series of in vivo experiments. We examined and compared the levels of heme synthesis and degradation enzymes in the APPPS1 mouse model for AD and WT controls. APPPS1 mice coexpress KM670/671NL-mutated amyloid precursor protein and L166P-mutated presenilin-1 and exhibit key features of amyloid pathology [20], [21]. Quantitation of immunohistochemistry data shows that levels of both ALAS1 (Fig. 1B and D) and HO-2 (Fig. 1C and E) were lowered in the dentate gyrus of 6-week-old (Fig. 1B and C) and 6-month-old (Fig. 1D and E; see immunohistochemistry images in Fig. S1A and B) APPPS1 mouse brain hippocampi compared with those in WT mouse brains. Similar reduction in ALAS1 and HO-2 levels was also detected in the CA region of APPPS1 mouse brain hippocampi. No significant changes in HO-1 levels were detected in APPPS1 mice (Fig. S1C). Together, these results from human and mice strongly support the idea that lowered levels of ALAS1 and HO-2 are associated with AD pathogenesis.
3.2. Heme synthesis and uptake intensified with the induction of neuronal differentiation and development
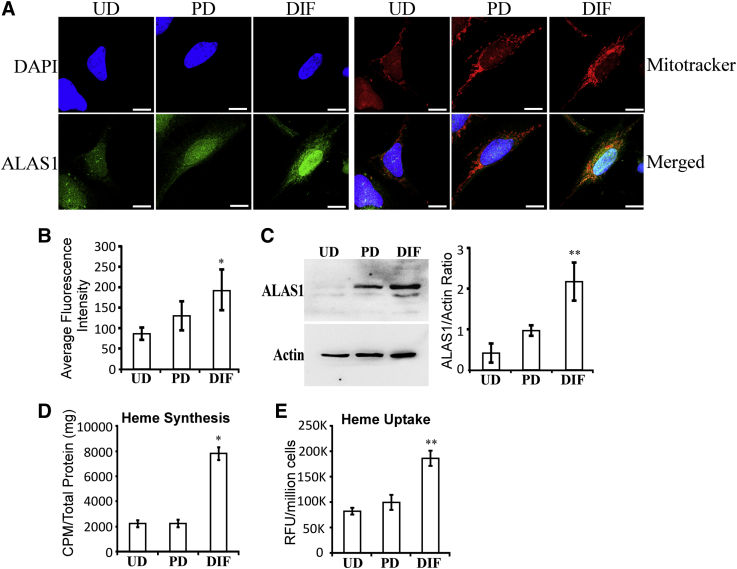
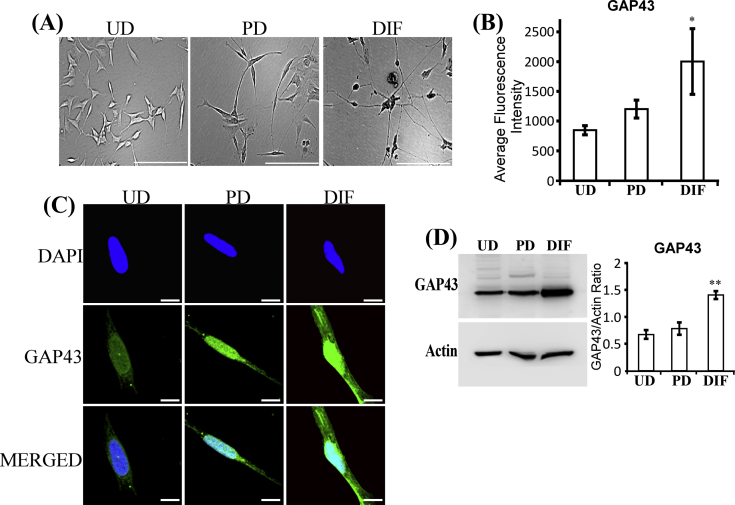
To further assess whether the changes in ALAS1 and HO-2 levels are early events during AD pathogenesis, we used a cell model for study neurobiology, the popular SH-SY5Y neuroblastoma cell line [23], [24]. Previous studies have characterized the positive effects of RA, nerve growth factor (NGF), and brain-derived neurotrophic factor on neuronal differentiation of SH-SY5Y cells [41], [42]. Based on these studies, we used RA and NGF to generate partially differentiated SH-SY5Y neuronal cells (Fig. S2A and B). Subsequently, brain-derived neurotrophic factor was added to partially differentiated cells, along with serum depletion, to generate fully differentiated cells. The levels of GAP43 were significantly higher in fully differentiated cells (Fig. S2C and D), as expected. Then, we examined the levels of cellular locations of the rate-limiting heme synthetic enzyme ALAS1 (Fig. 2A). As shown previously, ALAS1 was localized in the mitochondria, as well as in the nucleus (Fig. 2A). Importantly, quantification of both confocal imaging data (Fig. 2B) and Western blotting data (Fig. 2C) shows that the levels of ALAS1 proteins were not significantly increased in partially differentiated cells but were strongly increased in fully differentiated cells. Consistent with this dramatic increase in ALAS1 levels, the levels of heme synthesis were also strongly increased in fully differentiated cells (Fig. 2D). Likewise, we measured the levels of heme uptake and found that the levels of heme uptake were significantly increased in fully differentiated cells, but not partially differentiated cells (Fig. 2E). The results suggest that highly induced levels of heme synthesis and uptake are necessary for fully differentiated but not for partially differentiated neuronal cells.
Fig. 2.
Fully differentiated SH-SY5Y cells exhibit elevated levels of the rate-limiting heme synthetic enzyme ALAS1, heme synthesis, and heme uptake. (A) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI and antibodies against ALAS1, respectively. Scale bar: 10 μM. (B) The levels of ALAS1 in SH-SY5Y cells quantified from confocal images. (C) Western blotting data showing that ALAS1 levels are strongly increased in fully differentiated SH-SY5Y cells. (D) The levels of heme synthesis are strongly increased in fully differentiated SH-SY5Y cells. (E) The levels of heme uptake are strongly increased in fully differentiated SH-SY5Y cells. *P-value < .05; **P-value < .005.
3.3. The production of heme transporters and heme chaperones, as well as hemoproteins, increased with neuronal differentiation
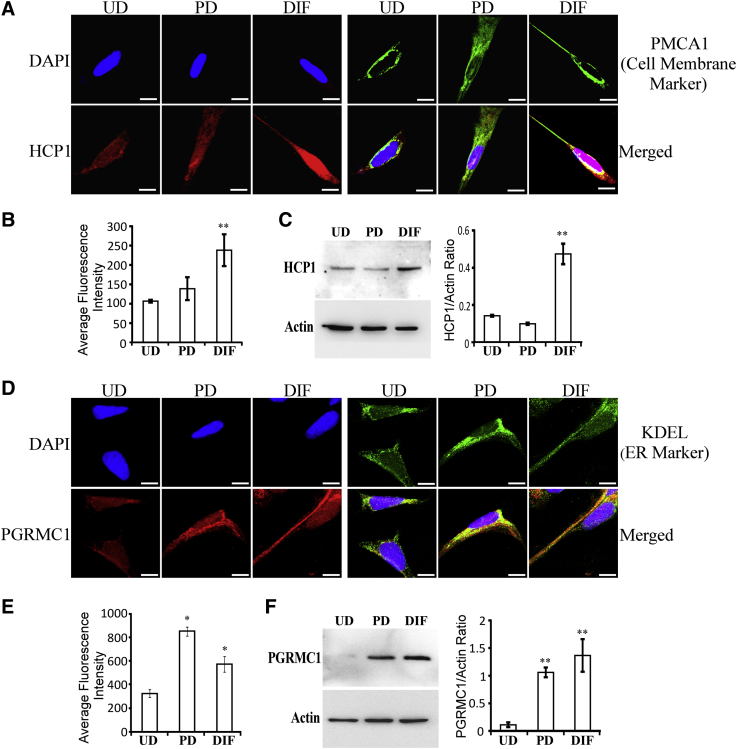
Furthermore, we detected the levels of the cell membrane heme transporter HCP1 and a putative heme sensor and heme chaperone necessary for maintenance of cellular heme and hemoprotein levels, PGRMC1 (progesterone receptor membrane component 1) [43]. PGRMC1 binds to heme and interacts with heme-related proteins such as ferrochelatase and cytochromes P450 to promote their functions. Fig. 3A shows that HCP1 was localized to the cell membrane and that its levels were significantly increased in fully differentiated cells (Fig. 3A and B). Likewise, Western blotting data show that HCP1 levels were significantly increased in fully differentiated SH-SY5Y cells (Fig. 3C). As expected, PGRMC1 was localized to the endoplasmic reticulum (Fig. 3D). Quantification of both confocal imaging data (Fig. 3E) and Western blotting data (Fig. 3F) shows that PGRMC1 levels were strongly increased in both partially and fully differentiated cells.
Fig. 3.
Fully differentiated SH-SY5Y cells exhibit elevated levels of the cell surface heme transporter HCP1 and the heme chaperone PGRMC1. (A) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI and antibodies against the cell surface marker PMCA1 and HCP1, respectively. Scale bar: 10 μM. (B) The levels of HCP1 in SH-SY5Y cells quantified from confocal images. (C) Western blotting data showing that HCP1 levels are strongly increased in fully differentiated SH-SY5Y cells. (D) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI and antibodies against the endoplasmic reticulum marker KDEL and PGRMC1, respectively. Scale bar: 10 μM. (E) The levels of PGRMC1 in SH-SY5Y cells quantified from confocal images. (F) Western blotting data showing that PGRMC1 levels are strongly increased in fully differentiated SH-SY5Y cells. *P-value < .05; **P-value < .005.
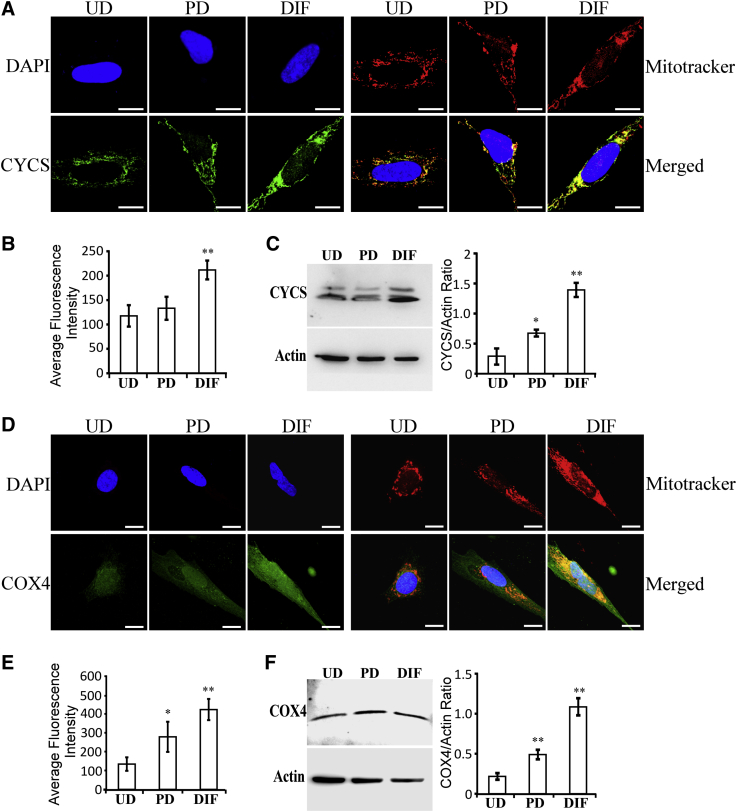
Elevated heme synthesis, uptake, and transport should lead to increased availability of cellular heme for generating hemoproteins, most of which are involved in oxygen utilization and metabolism, including certain subunits of OXPHOS complexes [44]. We therefore examined the levels of certain representative hemoproteins in SH-SY5Y cells. Two representative hemoproteins in OXPHOS complexes are cytochrome c and COX4. The levels of cytochrome c (Fig. 4A–C) and COX4 (Fig. 4D–F) were significantly and progressively increased in partially and fully differentiated SH-SY5Y cells, relative to undifferentiated cells. Clearly, our data show that as SH-SY5Y cells differentiate, they intensify the production of proteins and enzymes required for heme acquisition, enabling elevated production of hemoproteins, including those required for OXPHOS.
Fig. 4.
Fully differentiated SH-SY5Y cells exhibit elevated levels of heme-containing OXPHOS subunits cytochrome c and COX4. (A) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI, mitotracker red, and antibodies against cytochrome c, respectively. Scale bar: 10 μM. (B) The levels of cytochrome c in SH-SY5Y cells quantified from confocal images. (C) Western blotting data showing that cytochrome c levels are increased in fully differentiated SH-SY5Y cells. (D) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI, mitotracker red, and antibodies against COX-4, respectively. Scale bar: 10 μM. (E) The levels of COX4 in SH-SY5Y cells quantified from confocal images. (F) Western blotting data showing that COX4 levels are increased in fully differentiated SH-SY5Y cells. *P-value < .05; **P-value < .005.
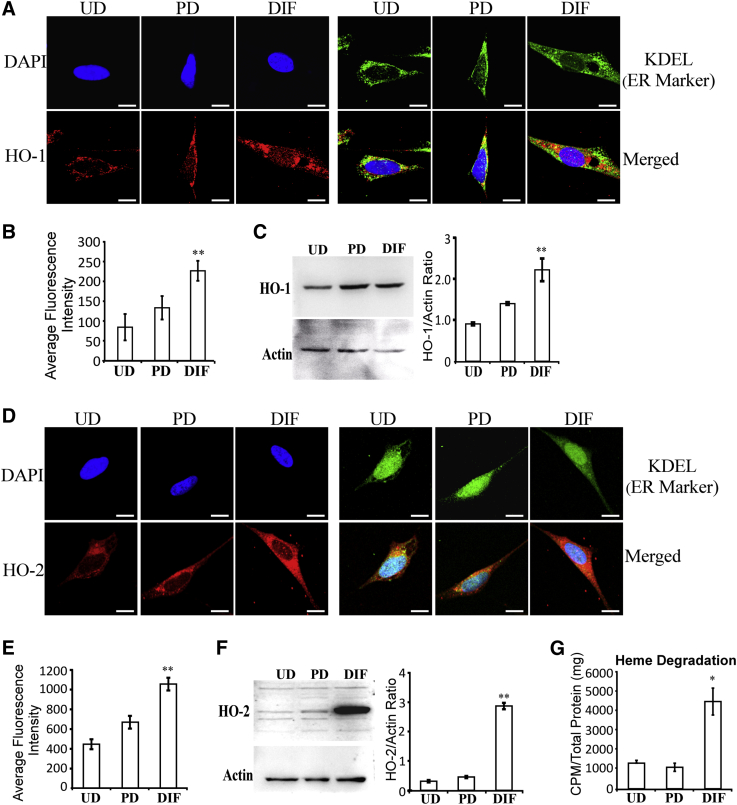
3.4. The levels of heme degradation enzymes, heme oxygenases, and rates of heme degradation increased with neuronal differentiation
Heme degradation is also an important factor in the maintenance of cellular homeostasis of heme. In mammals, there are two major isoforms of heme oxygenases, HO-1 and HO-2 [45]. Heme degradation generates potent antioxidants, biliverdin and bilirubin, neuronal modulator CO, and Fe2+ [46]. HO-2 is highly expressed in the brain. The developmental transcriptome data for human brains in Allen Brain Atlas show that HO-1 transcript levels are low in the brain (RPKM [Reads Per Kilobase Million] is about 2 in 4- to 12-month-old mice), whereas HO-2 transcript levels are high (RPKM is about 24 in 4- to 12-month-old mice) (see http://www.brainspan.org/rnaseq/search/index.html.)
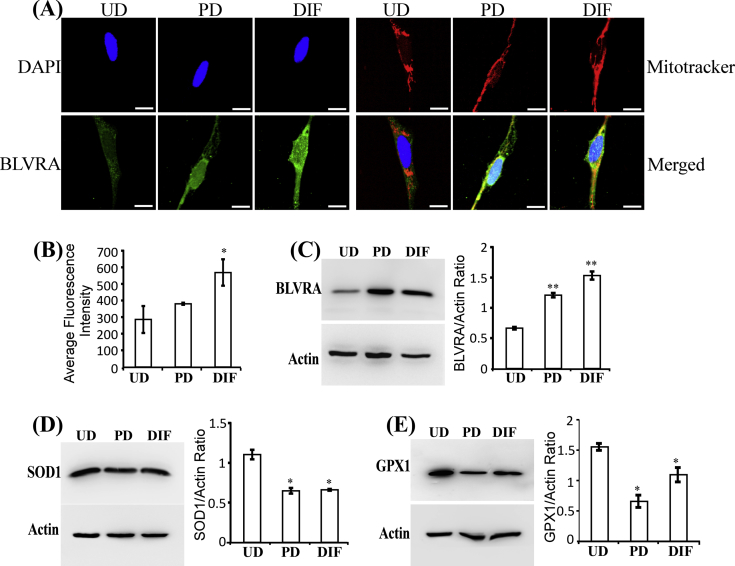
Here, we examined the levels of heme oxygenases, HO-1 and HO-2, in SH-SY5Y cells as differentiation progresses. Data from confocal microscopy imaging (Fig. 5A and B) and Western blotting (Fig. 5C) show that HO-1 protein levels were significantly increased in fully differentiated SH-SY5Y cells. Likewise, HO-2 protein levels were significantly increased in fully differentiated SH-SY5Y cells (Fig. 5D–F). Furthermore, measurements of heme degradation in SH-SY5Y cells show that the levels of heme degradation were strongly increased in fully differentiated cells (Fig. 5G). Confocal microscopy imaging (Fig. S3A and B) and Western blotting data (Fig. S3C) show that the next enzyme on the heme degradation pathway, biliverdin reductase BLVRA, was also increased in differentiated cells. The products of heme degradation, biliverdin and bilirubin, are potent antioxidants. These products are likely important for maintaining cellular reducing power as neuronal cells differentiate and become much more active in oxidative metabolism. Thus, we examined the levels of two widely expressed antioxidant enzymes, superoxidase dismutase (SOD1) and glutathione peroxidase (GPX1) [47]. The levels of these two enzymes were not increased but somewhat decreased in partially and fully differentiated SH-SY5Y cells (Fig. S3D and E). Together, these results show that the levels of heme degradation and enzymes required for heme degradation are preferentially increased in fully differentiated SH-SY5Y cells, likely leading to elevated levels of potent antioxidants biliverdin and bilirubin.
Fig. 5.
Fully differentiated SH-SY5Y cells exhibit elevated levels of heme oxygenases and heme degradation. (A) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI, antibodies against KDEL (endoplasmic reticulum marker), and antibodies against HO-1, respectively. Scale bar: 10 μM. (B) The levels of HO-1 in SH-SY5Y cells quantified from confocal images. (C) Western blotting data showing that HO-1 levels are increased in fully differentiated SH-SY5Y cells. (D) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI, antibodies against KDEL (endoplasmic reticulum marker), and antibodies against HO-2, respectively. Scale bar: 10 μM. (E) The levels of HO-2 in SH-SY5Y cells quantified from confocal images. (F) Western blotting data showing that HO-2 levels are increased in fully differentiated SH-SY5Y cells. (G) The levels of heme degradation were strongly increased in fully differentiated cells. *P-value < .05; **P-value < .005.
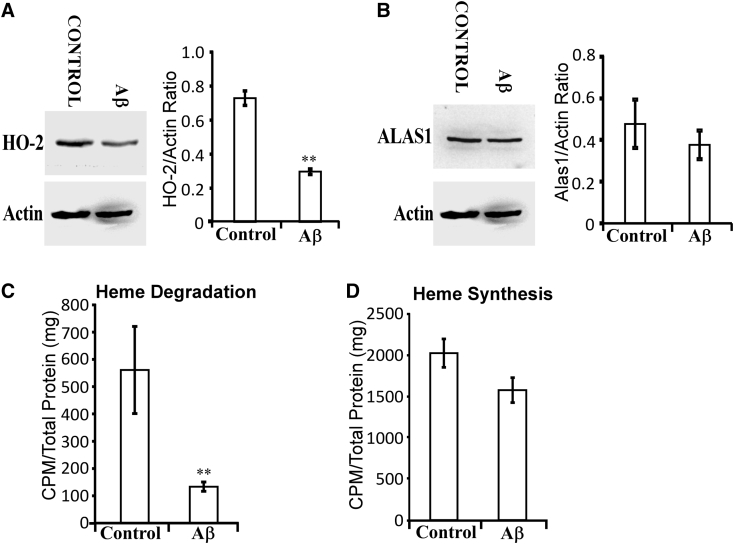
3.5. Aβ interferes with heme degradation in differentiated neuronal cells
Evidently, elevated heme synthesis and degradation are important for neuronal functions. Thus, it is plausible that Aβ may interfere with neuronal functions by disturbing heme synthesis and/or degradation. Extracellular Aβ is internalized in neuronal cells and distributed in mitochondrial, endoplasmic reticulum, and cytosol [48]. We therefore directly examined the effects of Aβ on heme synthesis and degradation in fully differentiated SH-SY5Y cells. Western blotting data show that the levels of HO-2 (Fig. 6A), but not ALAS1 (Fig. 6B), were decreased by Aβ. Consistent with this result, measurements of heme synthesis and degradation show that heme degradation (Fig. 6C), but not heme synthesis (Fig. 6D), was significantly decreased by Aβ. These results show that HO activity and heme degradation are more sensitive to the interference of Aβ than ALAS activity and heme synthesis.
Fig. 6.
Aβ selectively decreases the levels of HO-2 and heme degradation in fully differentiated SH-SY5Y cells. The cells were treated with 5 μM of Aβ for 3 days and were collected and analyzed. (A) Western blotting data showing that HO-2 levels are significantly reduced by Aβ treatment in fully differentiated SH-SY5Y cells. (B) Western blotting data showing that ALAS1 levels are not significantly reduced by Aβ treatment in fully differentiated SH-SY5Y cells. (C) Aβ treatment causes a statistically significant reduction in heme degradation levels. (D) Aβ treatment does not significantly affect heme synthesis levels. *P-value < .05; **P-value < .005.
4. Discussion
Aβ has been a major focus of AD research because mutations in the APP or the presenilin-1 gene cause rare cases of inherited early-onset AD. However, several anti-Aβ drug candidates failed in late-stage clinical trials [49], [50]. These failures do not necessarily suggest that the amyloid cascade hypothesis for Alzheimer's disease is incorrect, but they suggest that it is important to consider mechanisms of action of potential drug candidates and the timeline of neuropathological events induced by Aβ [2], [6]. Aβ causes many changes at the cellular and molecular levels, ranging from bioenergetic events and oxidative stress to Ca2+ homeostasis disruption and inflammation [7], [8], [51]. If a drug acts on late events induced by Aβ, it may not be effective at alleviating neurological deficits associated with AD. Thus, it is crucial not only to characterize various potentially neurotoxic events induced by Aβ but also to illuminate the hierarchical orders of these events.
Here, using a commonly used cell model for neuronal differentiation, we characterized the changes in the levels of heme synthesis, uptake, and degradation, as well as proteins/enzymes involved in heme acquisition and utilization, as cells undergo neuronal differentiation. We found that heme synthesis, uptake, and degradation, along with the related proteins and enzymes, are all intensified in fully differentiated neuronal cells, compared with undifferentiated and partially differentiated cells (Fig. 2, Fig. 3, Fig. 4, Fig. 5). The intensification of heme acquisition is presumably a prerequisite for enhanced production of mitochondrial respiratory or OXPHOS complexes, which are required for neuronal cells to generate ATP via oxidative metabolism. Neuronal cells require high levels of OXPHOS to supply ATP necessary for neuronal activities. The brain uses 20% of all oxygen used by the human body although it has only 2% of the body's mass. Thus, elevated heme availability is necessary for brain functions.
Importantly, our studies also show that the levels of heme degradation, along with heme oxygenases HO-1 and HO-2, are highly elevated in fully differentiated cells (Fig. 5). As neuronal cells must use a large amount of oxygen, the levels of side products of oxidative metabolism, ROS, should increase. Intriguingly, the levels of conventional antioxidant enzymes [47], including SOD1 and GPX1, which play important roles in many cells, did not significantly increase in fully differentiated SH-SY5Y cells (Fig. S3). Importantly, the products of heme degradation include two potent antioxidants, biliverdin and bilirubin [45], [46]. The elevated levels of heme degradation will lead to increased levels of biliverdin and bilirubin in fully differentiated neuronal cells, which likely provide antioxidants for neutralizing higher levels of ROS generated by elevated OXPHOS.
A plethora of previous studies have linked heme and altered heme synthesis and metabolism to AD pathogenesis [13], [14], [15], [16]. Here, taking advantage of two previously published large data sets for gene expression analysis of AD vs. normal brain tissues, we identified that ALAS1 and HMOX2 are two heme-related genes whose expression is significantly lowered in hippocampi of AD brains (Fig. 1A). Furthermore, we show that the levels of these two enzymes are selectively lowered in hippocampi of APPPS1 mouse brains (Fig. 1B–E). Together, these results strongly suggest that lowered expression of ALAS1 and HMOX2 is an important feature of AD hippocampi.
The use of the SH-SY5Y cell model enabled us to identify potentially early events in AD pathogenesis. Interestingly, Aβ causes a strong decrease in heme degradation, but not heme synthesis (Fig. 6). The lack of Aβ effect on heme synthesis in differentiated SH-SY5Y cells may be attributable to the short-term nature of the treatment (3 days) or the absence of astrocytes and glial cells. Both heme oxygenase and Aβ bind to heme readily. Thus, Aβ may be in contact with HO-2 quickly via heme. Heme-Aβ interaction will generate potent ROS, as previous studies showed [15], [16], which would inactivate HO-2. Aβ is imported and distributed in various cellular compartments, mitochondria, endoplasmic reticulum, and cytosol [48]. Thus, Aβ can potentially interact with both HO-2 and ALAS1. However, there is not a direct way for ALAS1 to be in contact with Aβ, as in the case of HO-2. This may explain why HO-2 and heme degradation are more sensitive to Aβ treatment than ALAS1 and heme synthesis. Lowered heme degradation should also lead to lowered levels of biliverdin and bilirubin in neuronal cells, which would likely lead to oxidative stress in early stages of AD pathogenesis. Our data suggest that one potentially effective way to prevent AD pathogenesis is to strengthen antioxidant or reducing power in brain cells.
Our results are consistent with previous studies indicating that oxidative stress is an early event in AD pathogenesis [52], [53]. Our data provide a molecular basis for this conclusion. Notably, Aβ also binds to heme and sequesters heme [15], [16]. The Aβ-heme complex acts as a peroxidase and generates ROS. Both lowered levels of HOs and heme can lead to reduced heme degradation and oxidative stress. Thus, even at the cellular level in early stages of exposure of neuronal cells to Aβ, multiple mechanisms can reduce heme degradation and increase oxidative stress. The interaction of heme and Aβ, lowered levels of HO and heme degradation, and the formation of ROS likely forms a vicious positive feed-forward cycle [15]. Thus, stopping this feed-forward cycle would be crucial for designing therapeutic strategies to treat AD. Drugs that act on and dispose Aβ beyond this initiation of this cycle may not work to alleviate the neurological deficits associated with AD.
Research in Context.
-
1.
Systematic review: We reviewed (using PubMed) all publications relating to the involvement of heme in Alzheimer's disease (AD) pathogenesis. Over 100 articles have described the interaction between Aβ and heme and the relevance to AD pathogenesis. It is unclear whether Aβ-heme interaction and the various changes relating to heme in AD brain tissues are early events in AD pathogenesis or if they are merely side effects of AD pathogenesis.
-
2.
Interpretation: We combined data from human patients and mouse and cell models for studying AD to show that the levels of heme oxygenase and heme degradation are selectively decreased in Aβ-treated neuronal cells and in AD human and mouse brain hippocampi.
-
3.
Future directions: These results set the stage for further studies to illuminate the role of altered heme metabolism in initiating AD pathogenesis and to test decreased heme degradation as a target of disease-modifying interventions in AD.
Acknowledgments
This work is in part supported by the Cecil H. and Ida Green Distinguished Chair fund. I.B. is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer's Disease Research, and his laboratory is supported by the National Institutes of Health grant R01AG055577.
Footnotes
The authors have no competing interests to declare.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2018.12.003.
Supplementary Data
Figure S1.
The expression levels of the rate-limiting heme synthetic enzyme ALAS1 and heme degradation enzyme HO-2 are decreased in hippocampi of AD human and mouse brains. In (A) and (B) representative DAPI-stained images and IHC images of 6-month-old mouse brain tissues stained with the indicated antibodies are shown as examples. (C) The levels of HO-1 protein are not significantly changed in the dentate gyrus of 6-week-old APPPS1 mouse brains. The data plotted are averages of data from 4 different APPPS1 and 4 wild-type mouse brain tissues and at least 10 regions of interest for each tissue. *, P-value < 0.05; **, P-value < 0.005.
Figure S2.
SH-SY5Y cells undergo progressive neuronal differentiation when treated with retinoic acid (RA), nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF). (A) Images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells. Scale bar: 200 μM. (B) The levels of GAP43 in SH-SY5Y cells quantified from confocal images. Scale bar: 10 μM. (C) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI and antibodies against neuronal marker GAP43, respectively. (D) Western blotting data showing that GAP43 levels are increased in fully differentiated SH-SY5Y cells. *, p-value < 0.05; **, p-value < 0.005.
Figure S3.
The levels of enzymes involved in generating antioxidants in SH-SY5Y cells as neuronal differentiation proceeds. (A) Fluorescent images of undifferentiated (UD), partially differentiated (PD), and fully differentiated (DIF) SH-SY5Y cells stained with DAPI, mitotracker, and antibodies against biliverdin reductase BLVRA, respectively. Scale bar: 10 μM. (B) The levels of BLVRA in SH-SY5Y cells quantified from confocal images. (C) Western blotting data showing that BLVRA levels are increased in partially and fully differentiated SH-SY5Y cells. (D) Western blotting data showing that SOD1 levels are not increased in partially and fully differentiated SH-SY5Y cells. (E) Western blotting data showing that GPX1 levels are not increased in partially and fully differentiated SH-SY5Y cells. *, P-value < 0.05; **, P-value < 0.005.
References
- 1.Graham W.V., Bonito-Oliva A., Sakmar T.P. Update on Alzheimer's Disease Therapy and Prevention Strategies. Annu Rev Med. 2017;68:413–430. doi: 10.1146/annurev-med-042915-103753. [DOI] [PubMed] [Google Scholar]
- 2.Cappa S.F. The Quest for an Alzheimer Therapy. Front Neurol. 2018;9:108. doi: 10.3389/fneur.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 5.Folch J., Ettcheto M., Petrov D., Abad S., Pedros I., Marin M. Review of the advances in treatment for Alzheimer disease: Strategies for combating beta-amyloid protein. Neurologia. 2018;33:47–58. doi: 10.1016/j.nrl.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onyango I.G. Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer's disease. Neural Regen Res. 2018;13:19–25. doi: 10.4103/1673-5374.224362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoshan-Barmatz V., Nahon-Crystal E., Shteinfer-Kuzmine A., Gupta R. VDAC1, mitochondrial dysfunction, and Alzheimer's disease. Pharmacol Res. 2018;131:87–101. doi: 10.1016/j.phrs.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L. World Scientific Publishing Company; Singapore: 2011. HEME BIOLOGY: The Secret Life of Heme in Regulating Diverse Biological Processes. [Google Scholar]
- 10.Komar A.A., Kommer A., Krasheninnikov I.A., Spirin A.S. Cotranslational folding of globin. J Biol Chem. 1997;272:10646–10651. doi: 10.1074/jbc.272.16.10646. [DOI] [PubMed] [Google Scholar]
- 11.Yao X., Balamurugan P., Arvey A., Leslie C., Zhang L. Heme controls the regulation of protein tyrosine kinases Jak2 and Src. Biochem Biophys Res Commun. 2010;403:30–35. doi: 10.1016/j.bbrc.2010.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F. Heme and microRNA biogenesis. In: Zhang L., editor. HEME BIOLOGY: The Secret Life of Heme in Regulating Diverse Biological Processes. World Scientific Publishing Company; Singapore: 2011. pp. 127–138. [Google Scholar]
- 13.Cullen K.M., Kocsi Z., Stone J. Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging. 2006;27:1786–1796. doi: 10.1016/j.neurobiolaging.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer B.E., Smith M.A., Richardson S.L., Perry G., Zhu X. Down-regulation of aminolevulinate synthase, the rate-limiting enzyme for heme biosynthesis in Alzheimer's disease. Neurosci Lett. 2009;460:180–184. doi: 10.1016/j.neulet.2009.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh C., Seal M., Mukherjee S., Ghosh Dey S. Alzheimer's Disease: A Heme–Aβ Perspective. Acc Chem Res. 2015;48:2556–2564. doi: 10.1021/acs.accounts.5b00102. [DOI] [PubMed] [Google Scholar]
- 16.FitzGerald K.E., Lal S., Kalainayakan S.P., Zhang L. Alzheimer’s Disease. SMGroup; Dover, DE, USA: 2016. Molecular Mechanisms Underlying Heme Action in Promoting the Pathogenesis of Alzheimer's Disease; pp. 1–13. [Google Scholar]
- 17.Berchtold N.C., Coleman P.D., Cribbs D.H., Rogers J., Gillen D.L., Cotman C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer's disease. Neurobiol Aging. 2013;34:1653–1661. doi: 10.1016/j.neurobiolaging.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berchtold N.C., Cribbs D.H., Coleman P.D., Rogers J., Head E., Kim R. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;105:15605–15610. doi: 10.1073/pnas.0806883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hokama M., Oka S., Leon J., Ninomiya T., Honda H., Sasaki K. Altered expression of diabetes-related genes in Alzheimer's disease brains: the Hisayama study. Cereb Cortex. 2014;24:2476–2488. doi: 10.1093/cercor/bht101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radde R., Bolmont T., Kaeser S.A., Coomaraswamy J., Lindau D., Stoltze L. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H., Sun S., Herreman A., De Strooper B., Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. J Neurosci. 2010;30:8566–8580. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaguri H., Nilsson P., Hashimoto S., Nagata K., Saito T., De Strooper B. APP mouse models for Alzheimer's disease preclinical studies. EMBO J. 2017;36:2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agholme L., Lindstrom T., Kagedal K., Marcusson J., Hallbeck M. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis. 2010;20:1069–1082. doi: 10.3233/JAD-2010-091363. [DOI] [PubMed] [Google Scholar]
- 24.Kovalevich J., Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. 2013;1078:9–21. doi: 10.1007/978-1-62703-640-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shipley M.M., Mangold C.A., Szpara M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J Vis Exp. 2016:53193. doi: 10.3791/53193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stine W.B., Jungbauer L., Yu C., LaDu M.J. Preparing synthetic Abeta in different aggregation states. Methods Mol Biol. 2011;670:13–32. doi: 10.1007/978-1-60761-744-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y., Hon T., Ye W.Z., Zhang L. Heme Deficiency Selectively Interferes with the Ras-MAPK Signaling Pathway and the Expression of a Subset of Neuronal Genes. Cell Growth Differ. 2002;13:431–439. [PubMed] [Google Scholar]
- 28.Hooda J., Cadinu D., Alam M.M., Shah A., Cao T.M., Sullivan L.A. Enhanced heme function and mitochondrial respiration promote the progression of lung cancer cells. PLoS One. 2013;8:e63402. doi: 10.1371/journal.pone.0063402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailao E.F., Parente J.A., Pigosso L.L., de Castro K.P., Fonseca F.L., Silva-Bailao M.G. Hemoglobin uptake by Paracoccidioides spp. is receptor-mediated. Plos Negl Trop Dis. 2014;8:e2856. doi: 10.1371/journal.pntd.0002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan X., Protchenko O., Philpott C.C., Hamza I. Topologically conserved residues direct heme transport in HRG-1-related proteins. J Biol Chem. 2012;287:4914–4924. doi: 10.1074/jbc.M111.326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J., Supnet C., Sun S., Zhang H., Good L., Popugaeva E. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels (Austin) 2014;8:230–242. doi: 10.4161/chan.27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gautier L., Cope L., Bolstad B.M., Irizarry R.A. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho B.S., Irizarry R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26:2363–2367. doi: 10.1093/bioinformatics/btq431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolstad B.M., Irizarry R.A., Astrand M., Speed T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 36.Irizarry R.A., Bolstad B.M., Collin F., Cope L.M., Hobbs B., Speed T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 38.Robinson M.D., Speed T.P. A comparison of Affymetrix gene expression arrays. BMC Bioinformatics. 2007;8:449. doi: 10.1186/1471-2105-8-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsberg S.D., Alldred M.J., Che S. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer's disease. Neurobiol Dis. 2012;45:99–107. doi: 10.1016/j.nbd.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stempler S., Waldman Y.Y., Wolf L., Ruppin E. Hippocampus neuronal metabolic gene expression outperforms whole tissue data in accurately predicting Alzheimer's disease progression. Neurobiol Aging. 2012;33:2230 e13–e21. doi: 10.1016/j.neurobiolaging.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Encinas M., Iglesias M., Liu Y., Wang H., Muhaisen A., Cena V. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 42.Simpson P.B., Bacha J.I., Palfreyman E.L., Woollacott A.J., McKernan R.M., Kerby J. Retinoic acid evoked-differentiation of neuroblastoma cells predominates over growth factor stimulation: an automated image capture and quantitation approach to neuritogenesis. Anal Biochem. 2001;298:163–169. doi: 10.1006/abio.2001.5346. [DOI] [PubMed] [Google Scholar]
- 43.Piel R.B., 3rd, Shiferaw M.T., Vashisht A.A., Marcero J.R., Praissman J.L., Phillips J.D. A Novel Role for Progesterone Receptor Membrane Component 1 (PGRMC1): A Partner and Regulator of Ferrochelatase. Biochemistry. 2016;55:5204–5217. doi: 10.1021/acs.biochem.6b00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alam M.M., Lal S., FitzGerald K.E., Zhang L. A holistic view of cancer bioenergetics: mitochondrial function and respiration play fundamental roles in the development and progression of diverse tumors. Clin Transl Med. 2016;5:3. doi: 10.1186/s40169-016-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maines M.D. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 46.Barone E., Di Domenico F., Mancuso C., Butterfield D.A. The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: It's time for reconciliation. Neurobiol Dis. 2014;62:144–159. doi: 10.1016/j.nbd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mates J.M., Perez-Gomez C., Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 48.Chen J.X., Yan S.S. Role of mitochondrial amyloid-beta in Alzheimer's disease. J Alzheimers Dis. 2010;20:S569–S578. doi: 10.3233/JAD-2010-100357. [DOI] [PubMed] [Google Scholar]
- 49.Feldman H.H., Haas M., Gandy S., Schoepp D.D., Cross A.J., Mayeux R. Alzheimer's disease research and development: a call for a new research roadmap. Ann N Y Acad Sci. 2014;1313:1–16. doi: 10.1111/nyas.12424. [DOI] [PubMed] [Google Scholar]
- 50.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 51.Mattson M.P., Arumugam T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018;27:1176–1199. doi: 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakrabarti S., Sinha M., Thakurta I.G., Banerjee P., Chattopadhyay M. Oxidative stress and amyloid beta toxicity in Alzheimer's disease: intervention in a complex relationship by antioxidants. Curr Med Chem. 2013;20:4648–4664. doi: 10.2174/09298673113209990152. [DOI] [PubMed] [Google Scholar]