Abstract
Background
Intracerebral hemorrhage and ischemic stroke are increasingly recognized complications of central nervous system (CNS) infection by herpes simplex virus (HSV).
Aim of the study
To analyze clinical, imaging, and laboratory findings and outcomes of cerebrovascular manifestations of HSV infection.
Methods
Systematic literature review from January 2000 to July 2018.
Results
We identified 38 patients (median age 45 years, range 1–73) comprising 27 cases of intracerebral hemorrhage, 10 of ischemic stroke, and 1 with cerebral venous sinus thrombosis. Intracerebral hemorrhage was predominantly (89%) a complication of HSV encephalitis located in the temporal lobe. Hematoma was present on the first brain imaging in 32%, and hematoma evacuation was performed in 30% of these cases. Infarction was frequently multifocal, and at times preceded by hemorrhage (20%). Both a stroke-like presentation and presence of HSV encephalitis in a typical location were rare (25% and 10%, respectively). There was evidence of cerebral vasculitis in 63%, which was exclusively located in large-sized vessels. Overall mortality was 21% for hemorrhage and 0% for infarction. HSV-1 was a major cause of hemorrhagic complications, whereas HSV-2 was the most prevalent agent in the ischemic manifestations.
Conclusion
We found a distinct pathogenesis, cause, and outcome for HSV-related cerebral hemorrhage and infarction. Vessel disruption within a temporal lobe lesion caused by HSV-1 is the presumed mechanism for hemorrhage, which may potentially have a fatal outcome. Brain ischemia is mostly related to multifocal cerebral large vessel vasculitis associated with HSV-2, where the outcome is more favorable.
Keywords: Cerebrovascular complications, Herpes simplex virus encephalitis, Ischemic stroke intracerebral hemorrhage, Vasculopathy, Vasculitis, Mortality
Introduction
Herpes simplex virus (HSV) is the cause of acute viral encephalitis in 50–70% of cases where a virus can be identified [1]. Both HSV-1 and HSV-2 can cause encephalitis (HSE), which is predominantly located in the temporal lobes and the limbic system. From a histopathological viewpoint, HSE is a necrotizing process with perivascular inflammation and edema. Clinical care guidelines since the 1980s have emphasized increased awareness and swift initiation of treatment with acyclovir in encephalitic syndromes, lowering the mortality to below 20% [2, 3]. HSE, however, continues to result in substantial overall morbidity and mortality [4, 5]. Half of the survivors experience moderate to severe disability after 1 year [6]. Their quality of life is significantly impacted by epilepsy, psychiatric disorders, cognitive problems, dementia, headache, and alcohol abuse [5, 7, 8].
Cerebrovascular disease occurs as a complication of a variety of central nervous system (CNS) infections [9]. Many patients with infectious cerebral vasculopathies require intensive care with a generally poor prognosis [10, 11]. A recent analysis of 4871 cases of HSE in the USA reported intracranial hemorrhage in 2.7% and ischemic stroke in 5.6% [12]. However, there has been no systematic study conducted to evaluate clinical presentations, causative agents, pathogenesis, and outcome of cerebrovascular complications in HSE. Most knowledge about HSV-related cerebrovascular disease is provided by case reports. Therefore, an appraisal of the current state of understanding in this field is much needed. We systematically studied HSV-related cerebral vasculopathies reported in the literature.
Methods
We conducted a systematic review of medical literature to identify all published cases of cerebrovascular manifestations of HSV using MEDLINE/PubMed, Web of Science, and Google Scholar. The study period was January 2000 to July 2018. There were no language restrictions; non-English articles were included and translated using online resources such as Google Translate. Search terms used were “HSV,” “herpes,” “herpetic,” “meningoencephalitis,” or “encephalitis” and one of the following terms: “ischemia,” “infarction,” “stroke,” “hemorrhage,” “hematoma,” “vasculopathy,” or “vascular complication.” We reviewed titles, abstracts, and full articles. References in each identified article were reviewed to identify additional cases.
The inclusion criteria were (1) radiological evidence of cerebrovascular manifestations of HSV (infarction, hemorrhage, or vasculopathy characterized by features of vasculitis, thrombosis, or aneurysm) by computed tomography (CT) or magnetic resonance imaging (MRI), (2) mandatory confirmation of HSV infection by analysis of cerebrospinal fluid according to the diagnostic criteria proposed in a recent consensus paper [13], and (3) exclusion of other causes for stroke. We limited our search to publications addressing HSE in children and adults; thus, neonatal cases were excluded. Details of the evaluation and selection process are shown in Fig. 1.
Fig. 1.
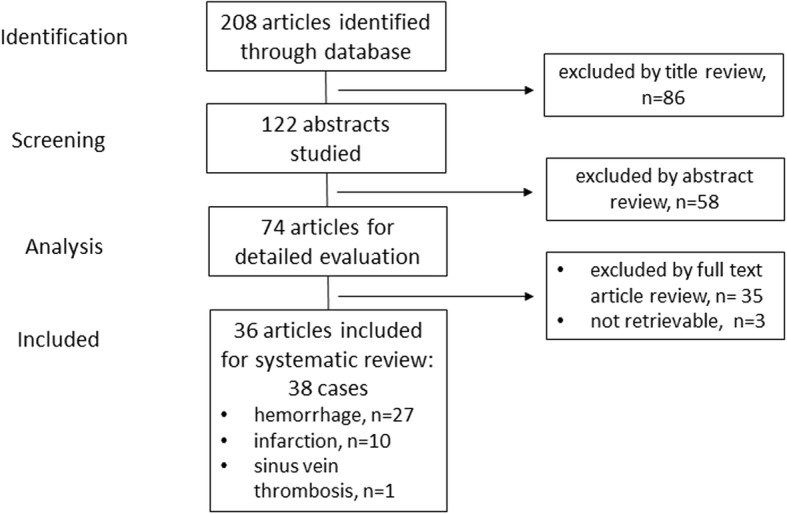
Flow chart of patient selection
We extracted the following data: demographics, time from onset of symptoms to admission, neurological symptoms at presentation (classification: encephalitis, meningitis, stroke, other), and presence of imaging surrogates of cerebrovascular disease on admission and during the acute course. Stroke was defined according to the World Health Organization as “rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 h or longer or leading to death, with no apparent cause other than of vascular origin.” Data on imaging included modality (CT or MRI), presence of encephalitis in a typical location (frontal or temporal lobe), characteristics of hemorrhage or ischemic lesion (unifocal or multifocal), distribution within vascular territories (anterior or posterior circulation or both), and features suggestive of vasculitis (small or large vessels or both). CSF data included cell count and the technique used to confirm CNS HSV infection (PCR, antibody, histology). Large vessel disease refers to involvement of the internal carotid artery (ICA), the vertebral artery (VA), the anterior cerebral artery (ACA) and its main branches, the middle cerebral artery (MCA) and its main branches, the posterior cerebral artery (PCA), and the basilar artery (BA) and its main branches [14]. Small vessels included the small penetrating arteries (e.g., the lenticulostriate arteries) that supply the deep structures of the brain. We also studied the use of steroids, as well as significant comorbidities. Outcome was classified according to the modified Rankin scale (mRS). Good outcome was defined as an mRS score of 0–2, and poor outcome as 3–5. Fatality (mRS 6) was categorized as an additional subgroup.
Statistical analysis was performed using the GraphPad Prism 7 software (La Jolla, CA).
Results
Systematic review
Details of the selection process are outlined in Fig. 1. We were unable to include three potentially relevant manuscripts as they were not accessible. These papers exclusively reported hemorrhagic manifestations of HSE [15–17]. We eventually analyzed a total of 36 manuscripts comprising 38 patients (Fig. 1). There were 27 cases of intracerebral hemorrhage [18–42], 10 with cerebral infarction [43–52], and 1 patient with venous sinus thrombosis [53].
General and comparative analysis
The median age of the reported patients was 43 years (interquartile range (IQR) 27–65), and 19 (50%) were male. Six patients (15%) were younger than 18 years. There were no statistical differences of age and gender between patients with hemorrhagic and ischemic complications. Among cases where PCR distinguished between HSV-1 and HSV-2, HSV-1 was the predominant virus identified in cases with intracerebral hemorrhage (16/18, 89%). In contrast, cases with infarction were dominated by HSV-2 (5/7, 71%). There were patients with PCR-confirmed HSV CNS infection in whom the methodology did not distinguish between the two types (26% in the hemorrhage and 20% in the infarction group). Infectious comorbidities in patients with hemorrhage included HIV (n = 1) and hepatitis C (n = 1). There was a single case of systemic lupus erythematosus and immunosuppression in the infarction group. Demographics, clinical presentation, imaging, and outcome are presented separately for patients with hemorrhage and ischemia in Tables 1 and 2, respectively.
Table 1.
Overview of demographics, clinical, and radiological findings in patients with hemorrhagic manifestations of HSV CNS infection
| Demographics | |
| n | 27 |
| Median age (IQR), years | 40 (26–54) |
| < 18 years | 23% (4/27) |
| Male sex | 55% (15/27) |
| Days from symptom onset to admission (median, IQR), n = 24 | 3.5 (2–7) |
| Clinical presentation | |
| Encephalitis | 93% (25/27) |
| Stroke-like | 4% (1/27) |
| Unspecific | 4% (1/27) |
| Diagnostic testing | |
| HSV-1 (PCR of CSF) | 59% (16/27) |
| HSV-2 (PCR of CSF) | 7% (2/27) |
| HSV not distinguished (PCR of CSF) | 26% (7/27) |
| HSV not distinguished (antibody of CSF) | 4% (1/27) |
| PCR negative for HSV (CSF) | 4% (1/27) |
| CSF findings | |
| Pleocytosis (> 4 cells/μl) | 88% (21/24) |
| Median cell count#(cells/μl, IQR, n = 23) | 88 (25–387) |
| Neuroimaging | |
| Hemorrhage on first imaging | 32% (8/25) |
| Hemorrhage after admission | 68% (17/25) |
| Days from admission to detection of hemorrhage (median, IQR) | 10 (9–14) |
| Hemorrhage within HSE predilection sites | 89% (24/27) |
| Bilateral temporal lobe HSE | 33% (8/24) |
| Atypical localization of hemorrhage | 7% (2/27) |
| No encephalitic lesion | 4% (1/27) |
| Evidence for vasculitis | 0% (0/9) |
| Intervention | |
| Hematoma evacuation | 30% (8/27) |
| Outcome | |
| Good outcome (mRS 0–2) | 38% (8/24) |
| Unfavorable outcome (mRS 3–5) | 41% (11/24) |
| Fatality | 21% (5/24) |
#In patients with pleocytosis. IQR interquarile range
Table 2.
Overview of demographics, clinical, and radiological findings in patients with ischemic manifestations of HSV CNS infection
| Demographics | |
| n | 10 |
| Median age (IQR), years | 47 (26–69) |
| < 18 years | 20% (2/10) |
| Male gender | 30% (3/10) |
| Days from symptom onset to admission | n = 3 on day 1, n = 1 on day 2 |
| Clinical presentation | |
| Encephalitis | 50% (5/10) |
| Stroke-like | 30% (3/10) |
| Meningitis | 20% (2/10) |
| Diagnostic testing | |
| HSV-1 (PCR of CSF) | 20% (2/10) |
| HSV-2 (PCR of CSF) | 50% (5/10) |
| HSV not distinguished (PCR of CSF) | 20% (2/10) |
| PCR negative for HSV-1 (CSF) but antibody rise | 10% (1/10) |
| CSF findings | |
| Pleocytosis (> 4 cells/μl) | 100% (10/10) |
| Median cell count# (cells/μl, IQR, n = 10) | 42 (15–199) |
| Neuroimaging | |
| Ischemia on first brain imaging | 50% (5/10) |
| Hemorrhage on first imaging, ischemia later | 20% (2/10) |
| Presence of loco-typico HSV lesion | 10% (1/10) |
| Evidence for vasculitis | 63% (5/8) |
| Affected vessels | |
| Small-sized | 0% |
| Large-sized | 100% (5/5) |
| Affected areas of circulation | |
| Anterior | 30% (3/10) |
| Posterior | 20% (2/10) |
| Anterior and posterior | 50% (5/10) |
| Distribution of lesions | |
| Single | 10% (1/10) |
| Multiple | 90% (9/10) |
| Intervention | |
| Steroid treatment | 40% (4/10) |
| Outcome | |
| Good outcome (mRS 0–2) | 56% (5/9) |
| Unfavorable outcome (mRS 3–5) | 44% (4/9) |
| Fatality | 0% |
#In patients with pleocytosis. IQR interquartile range, PCR polymerase chain reaction, CSF cerebrospinal fluid, HSV herpes simplex virus
Intracerebral hemorrhage
The clinical syndrome preceding admission was almost exclusively of encephalitis (93%). We found a median time lag of 3.5 days from symptom onset to hospital admission. The hematoma developed as a complication of HSV encephalitis in a typical location in most of the patients (89%). The parietal and occipital lobe, as well as deep brain structures, were the remaining locations of hematoma. The majority of bleedings were classified as parenchymal hemorrhage (n = 26), and only one case was petechial. Ventricular and/or subarachnoid blood was present in four patients. Many patients had cerebral edema, occasionally complicated by subsequent midline shift (n = 2), herniation (n = 4), or brainstem compression (n = 2). There was no evidence of a vasculitic pathology in the nine patients with vascular imaging and the three with histological examination of brain biopsy. No aneurysms were seen. Brain hemorrhage was detected on the first neuroimaging in eight patients (32%). More frequent was the development of hemorrhage after admission and the initiation of antiviral therapy (68%), with a time lag of a median of 10 days. Hematoma evacuation was performed in 30%. Outcome was unfavorable in 62%. The course of individual patients is presented in Table 3.
Table 3.
Characteristics of patients with hemorrhagic manifestations of HSV CNS infection
| No. (year), reference | Age, sex | HSV type, diagnostic test, CSF cells count | Initial clinical presentation, other findings | Localization of encephalitis | Localization of bleeding, other characteristics | Time from symptom onset to admission, time from admission to detection of hemorrhage | Presence of vasculitis (affected vessel), treatment | Outcome (mRS) |
|---|---|---|---|---|---|---|---|---|
| 1 (2001), Plantinga E [18] | 36, m | HSV-1, PCR, 33 cells/μl | Encephalitis (fever, consciousness, disorientation) | Left temporal, frontal, and insular areas | Left temporoparietal, hematoma, edema, midline shift | Day 6, day 10 | n.e., dexamethasone | Mild aphasia and short-term memory disturbances (3) |
| 2 (2001), Lee JW [19] | 15, m | HSV*, PCR, 15 cells/μl | Encephalitis (fever, aphasia, confusion) | Both temporal and frontal lobes | Left temporal and frontal lobe, petechial hemorrhage | Day 4, on first imaging | n.e. | Weakness upper extremity (2) |
| 3 (2001), Lee JW [19] | 6, m | HSV*, PCR, 5 cells/μl | Encephalitis (fever, vomiting, altered consciousness) |
Left medial temporal lobe | Left temporal and frontal lobe, hematoma | Day 17, on first imaging | n.e. | Severe bulbar dysfunction (4–5) |
| 4 (2002), Erdem G [20] | 1, f | HSV*, PCR, 33 cells/μl | Encephalitis (seizures, lethargy, fever) | Both temporal, frontal, and parietal lobes | Right temporal lobe, hematoma, edema | Day 6, on first imaging | No | Quadriparesis (4–5) |
| 5 (2004), Biswas A [21] | 38, m | HSV-1, PCR, 0 cells/μl | Encephalitis (headache, disturbed sleep) | Right inferior frontal and medial temporal region | Right frontal and temporal lobe, hematoma, edema | Not specified, not specified | No | Complete recovery (0) |
| 6 (2005), Kabakus N [22] | 3, m | HSV*, antibody, 450 cells/μl | Encephalitis (fever, headache hemiparesis) | Right temporal lobe | Left parietal lobe, hematoma, edema | Day 10, on first imaging | n.e. | Hemiparesis (3) |
| 7 (2005), Jabbour PM [23] | 27, m | HSV-1, PCR, 189 cells/μl | Encephalitis (fever, seizure) | Right mesial temporal lobe | Right temporal lobe, hematoma, uncal herniation, and ventricular blood | Day 2, day 9 | n.e., craniotomy and evacuation of hematoma | No focal neurological signs (0) |
| 8 (2006), Argyriou AA [24] | 22, m | HSV-1, PCR, 425 cells/μl | Encephalitis (seizure, fever, altered consciousness) | Left termporal lobe | Left parietal lobe, hematoma | Day 3, day 11 | n.e. | Complete recovery (0) |
| 9 (2007), Shelley BP [25] | 26, m | HSV*, PCR, 130 cells/μl | Encephalitis (fever, confusion, seizure) | Bilateral medial temporal lobe (left > right) | Left temporal lobe, hematoma | Day 1, day 18 | no | Complete recovery (0) |
| 10 (2008), Gkrania-Klotsas E [26] | 46, m | HSV-1, PCR, 0 cells/μl | Encephalitis (headache, fever, depersonalization) | No lesion | Left parietal lobe, hematoma | Day 7, on first imaging | n.e. | Returned to premorbid mental condition (0–1) |
| 11 (2009), Li JZ [27] | 56, m | HSV-1, PCR, 30 cells/μl | encephalitis (fever, seizure), HIV positive | Left medial temporal lobe | Left temporal lobe and basal ganglia, hematoma, edema, herniation | Day 1, day 6 (no hemorrhage on CT on day 1) | n.e., craniotomy and evacuation of hematoma | Mild neuropsychological deficits (2) |
| 12 (2010), Tonomura Y [28] | 30, f | HSV-1, PCR, 321 cells/μl | Encephalitis (headache, fever, neuropsychological deficits, altered mental state) | Bilateral (left > right) medial temporal and frontal lobes | Left amygdaloid body, hematoma, subarachnoid, and ventricular blood | Day 2, day 5 | n.e. | GCS 14, responds to simple orders (5) |
| 13 (2011), Takeuchi S [29] | 54, m | HSV-1, PCR, 86 cells/μl | Encephalitis (fever, walking difficulty, confusion, seizure) | Right temporal lobe | Temporal lobe, hematoma | Day 2, day 10 | n.e. | Hemiparesis, memory disturbances (3) |
| 14 (2013), Battaglia F [30] | 38, f | HSV-1, PCR, pleocytosis | Encephalitis (headache, fever, hallucinations, speech disturbances) | Diffuse cerebral edema on CT | Left temporal lobe, hematoma, edema with brainstem compression | Day 3, day 9 | n.e., craniotomy and evacuation of hematoma | Died 20 days from symptom onset (6) |
| 15 (2013), Rodriguez-Sainz A [31] | 45, f | HSV-1, PCR, 383 cells/μl | Encephalitis (headache, fever, incoherent speech), hepatitis C infection | Left medial temporal lobe | Left temporal lobe, hematoma, edema, brainstem compression | Day 2, day 9 (no lesion and no hemorrhage on CT on day 1) | n.e., craniotomy and evacuation of hematoma | Residual aphasia and right-sided hemiparesis (3–4) |
| 16 (2013), Rodriguez-Sainz A [31] | 53, f | HSV-1, PCR, 516 cells/μl | Encephalitis (fever, memory problems, headache) | Bilateral temporal lobes and insula | Left temporal lobe, hematoma, blood in subarachnoid space and midline shift | Day 6, day 8 | n.e. | Mild neuropsychological deficits (3) |
| 17 (2014), Yu W [32] | 64, f | HSV*, PCR and brain biopsy, not reported | Encephalitis (headache, leg pain, seizure 5 days later) | Bilateral temporal and frontal lobe | Right temporal lobe, hematoma, raised intracranial pressure | No details, on first imaging | No, craniotomy and evacuation of hematoma | Died on hospital day 25 (6) |
| 18 (2015), Zabroug S [33] | 28, f | HSV-1, PCR, 2/μl | Encephalitis (anterograde amnesia, insomnia), 4 month postpartum | Right temporal lobe | Supra-tentorial blood | No details, no details | No | Not reported |
| 19 (2015), Bhagchandania D [34] | 23, m | HSV*, PCR, 20/μl | Encephalitis (fever, seizures, altered sensorium) | Bilateral temporal and parietal lobe | Left temporal lobe, hematoma | Day 4, day 15 | n.e. | Behavioral abnormality (3–4) |
| 20 (2016), Gaye NM [35] | 53, f | HSV-2, PCR, 88 cells/μl | Encephalitis (recurrent seizures, fever on day 1) | Left mesial temporal lobe | Left temporal lobe, hematoma, ventricular blood | Day 2, day 18 | n.e. | Persistent severe neuropsychological deficits (5) |
| 21 (2017), Harada Y [36] | 71, f | HSV-1, PCR, 170 cells/μl | Encephalitis (fever, headache, altered mental status) | Right anterior medial temporal lobe and insular cortex | Right temporal lobe and right basal frontal lobe, hematoma, intraventricular blood | Day 8, day 14 | No | Near complete recovery (1) |
| 22 (2016), Mahale RR [37] | 71, m | HSV*, PCR, 5 cells/μl |
Encephalitis (fever, headache, altered mental status) | No loco-typico lesions | Left parieto-occipital and right occipital region, hematoma | Day 5, on first imaging | n.e. | Mild improvement of cortical blindness (4) |
| 23 (2016), Fisahn C [38] | 69, f | HSV*, brain biopsy, not reported | Stroke-like (acute onset of headache and right hemiparesis) | No loco-typico lesions | Left parietal lobe, hematoma, subarachnoid hemorrhage | Day 1, on first imaging | n.e. | Died (6) |
| 24 (2017), Mueller K [39] | 40, f | HSV-2, PCR, 558 cells/μl | Unspecific (headache, fever, nausea, vomiting) | CT on first day normal | Right temporal lobe, hemorrhage, midline shift | Day 7, day 14 | No, hemicraniectomy and temporal lobectomy | Survived, no further details reported |
| 25 (2017), El Shimy G [40] | 49, m | PCR negative, 45 cells/μl | Encephalitis (fever, headache, altered mental status) | Right temporal lobe and insula | Right medial temporal lobe, hematoma | Day 2, day 14 | No | Returned to baseline neurological status (0) |
| 26 (2013), Lo WB [41] | 46, m | HSV-1, PCR, 390 cells/μl | Encephalitis (fever, headache, confusion) | Left temporal lobe | Left temporal lobe, hematoma, edema, uncal herniation | Day 7, day 10 | Craniotomy on day 6, removal of anterior temporal lobe and evacuation of hematoma | Gradual improvement, no further details reported |
| 27 (2018), Sivasankar C [42] | 71, f | HSV-1, IHC, n.e. | Encephalitis (decreased responsiveness, hemiparesis, seizure) | Both temporal and parietal lobes | Right temporal lobe, hematoma, edema, uncal herniation | Day 1, day 8 (postoperative) | No, craniotomy for evacuation of hematoma on day 8 | Died on day 17 after hospital admission (6) |
*PCR methodology did not distinguish between HSV-1 and HSV-2. m male, f female, + yes, − no, n.a. not available, CSF cerebrospinal fluid, MCA middle cerebral artery, MP methylprednisone, n.e. not evaluated, MRI magnetic resonance imaging, CT computed tomography, HIV human immunodeficiency virus, IHC immunohistochemistry
Ischemic stroke
The initial clinical presentations of patients in the infarction group included encephalitis (50%), meningitis (20%), and stroke (30%). No relevant comorbidities were reported; one patient was 2 months postpartum. Forty percent of the patients were admitted within 2 days from symptom onset, but this information was missing for three patients. Initial brain imaging was performed with CT in five, with MRI in four, and using both in one case. Brain infarction was detected on the first brain imaging in 50%, and an encephalitic lesion in a typical location was present in one patient (10%). Hemorrhage preceded infarction in two patients (20%), who were assigned to the group with ischemic manifestation due to the overlap of radiological features and pathogenesis. Multiple ischemic lesions were found in 90%, located most commonly in both anterior and posterior circulations. CSF pleocytosis was abundant in all patients. Vascular imaging was performed in eight patients, with evidence for cerebral vasculitis found in 63%. This exclusively involved the large arteries. Forty percent of patients with infarction were treated with steroids. Outcome was unfavorable in 40 %, but no one died. The course of individual patients is presented in Table 4.
Table 4.
Characteristics of patients with ischemic manifestations of HSV CNS infection
| No. (year), reference | Age, sex | HSV type, diagnostic test, CSF cell count | Clinical presentation, special features | Presence of loco-typico HSV-encephalitis | Time from symptom onset to admission, ischemia on first imaging | Presence of vasculitis (affected vessel), affected brain region | Steroids, dosage (duration) | Outcome (mRS) |
|---|---|---|---|---|---|---|---|---|
| 1 (2004), Alexandri NM [43] | 31, m | HSV-1, PCR, 46 cells/μl | Encephalitis (fever, hallucinations, headache) | Yes | Not reported, no (CT) | No, multifocal (anterior and posterior) | − | Hemianopsia (2) |
| 2 (2009), Sas AM [44] | 3, f | HSV-1, PCR, 38 cells/μl | Encephalitis (fever, impaired vision, nausea, vomiting) | No | Day 5, yes (CT and MRI) | No, multifocal (posterior) | − | Blindness (3) |
| 3 (2012), Zepper P [45] | 72, m | HSV-2, PCR, 588 cells/μl | Stroke-like (aphasia, hemiparesis) | No | Unclear, no (hemorrhage on CT) | Yes (various vessels), multifocal (anterior and posterior) | − | Moderate cognitive deficits and hemiparesis (3–4) |
| 4 (2013), Guerrero WR [46] | 36, f | HSV*, PCR, 13 cells/μl | Encephalitis (headache, diplopia with skew deviation), 2 months postpartum | No | Day 2, yes (MRI) | Yes (basilar artery), single lesion (posterior) | MP 3 days, P 7 days, dosages not reported | Internuclear ophthalmoplegia (3) |
| 5 (2014), Terlizzi V [47] | 10, f | HSV-1, IgG antibodies appeared in CSF, 20 cells/μl | Stroke-like (headache and hemiparesis) | No | Day 1, no (CT) | Yes (distal ICA and MCA), multifocal (anterior) | − | Complete recovery (0) |
| 6 (2014), Snider SB [52] | 57, f | HSV-2, PCR, 1260 cells/μl | Meningitis (headache, nausea, vomiting, photophobia) | No | Unclear, no (hemorrhage on CT) | Yes (various vessels), multifocal (anterior and posterior) | Yes, dosage not reported, 21 days | Minimal deficits (1–2), resolution of stenoses |
| 7 (2016), Zis P [48] | 45, m | HSV-2, PCR, 64 cells/μl | Encephalitis (fever, confusion) | No | Day 1, no (CT) | Not studied, multifocal (anterior) | − | Mild cognitive deficits (2) |
| 8 (2016), Joshi P [49] | 48, f | HSV-2, PCR, 16 cells/μl | Meningitis (fever, headache, neck stiffness), later encephalitis | No | Day 120, yes (MRI) | Yes (various vessels), multifocal (anterior and posterior) | MP and P dosage/duration not reported | Relapsing course, outcome not reported |
| 9 (2017), Tsuboguchi S [50] | 73, f | HSV*, PCR (6000 copies/ml), 8 cells/μl | Stroke-like (hemiparesis) | No | Day 1, yes (MRI) | Not studied, multifocal (anterior and posterior) | Yes, MP 500 mg, 3 days | Modified Rankin scale 5 |
| 10 (2017), Zhang F [51] | 68, f | HSV-2, PCR, 649 cells/μl | Encephalitis (headache, numbness, hallucinations), systemic lupus, and immunosuppression | No | Day 21, yes (MRI) | No, multifocal (anterior) | − | Complete recovery (0) |
*PCR methodology did not distinguish between HSV-1 and HSV-2. m male, f female, + yes, − no, n.a. not available, CSF cerebrospinal fluid, MCA middle cerebral artery, MP methylprednisone
Venous sinus thrombosis
There was a single case of a 31-year-old man who had been suffering from fever, photophobia, and headache for 3 days. He was treated with acyclovir for suspected HSE (predominantly lymphocytic pleocytosis, HSV confirmed in CSF). Six days after admission, he developed bilateral upper limb weakness, ataxia, and bilateral headache. A brain MRI revealed features of encephalitis in the left temporal lobe and a superior sagittal sinus thrombosis. He was started on heparin and changed to warfarin for 1 year and was asymptomatic at follow-up.
Discussion
On the basis of this systematic review of published cases, we propose that intracerebral hemorrhage and brain infarction are two pathogenetically distinct manifestations of CNS HSV infection. Intracerebral hemorrhage almost exclusively occurred within the temporal lobe, was related to HSV-1, and caused life-threatening complications. Intracerebral hemorrhage is most likely a complication secondary to disintegration of vessels in the context of a necrotic encephalitic process. In contrast, brain infarction is associated with HSV-2, pathomechanistically related to large-vessel vasculitis leading to multifocal ischemia, but with a less detrimental prognosis. Our study also revealed that the occurrence of cerebrovascular complications of CNS HSV infection is independent of age and gender and is not associated with comorbidities or immunosuppression.
All three alphaviruses (HSV-1, HSV-2, and varicella-zoster virus (VZV)) are dormant in trigeminal and upper cervical ganglia that innervate the cerebral arteries, dural sinuses, and other brain structures [54, 55]. Upon reactivation, these viruses can then travel transaxonally via an immunoprivileged route to infect the brain and arteries. HSE is a necrotizing inflammatory process typically affecting the cortex and underlying white matter of the temporal lobe [3]. The insula, cingulate gyrus, and posterior orbital frontal lobe are involved less frequently. Extra-temporal involvement is well described in HSE, to occur in more than half of the cases, sometimes even without any temporal abnormalities [56]. CNS pathology in HSE can be attributed to a combination of cytolytic viral replication and immune-mediated mechanisms leading to axonal and glial damage [57]. Histologic examination in acute HSE often shows cytotoxic and vasogenic edema as well as necrosis with petechial hemorrhages [58, 59]. Accordingly, limited hemorrhage is an integral part of the disease process, but in a subgroup of patients, a proper intracerebral hematoma develops. This complication is likely to result from weakening of the neurovascular unit caused by the necrotizing process via increased permeability due to cytokine, chemokine, and protease action [60–62]. Modi et al. reported a 2.7% rate of intracerebral hemorrhage in a large cohort of HSE patients, also associated with an increased mortality [12]. Here, we expand the understanding of this complication by reporting a frequent association of hemorrhage with severe edema, midline shift, herniation, and brain stem compression. Indeed, Jouan et al. reported high rates of ICU admission (32%) and mechanical ventilation (17%) in patients with HSE and a two-fold increase in mortality in those requiring ICU care [6]. However, the aforementioned study could not find any predictive features for brain herniation on the first neuroimaging. In our analysis, hemorrhage occurred in most patients after admission (68%) at a median of 10 days. This indicates that the processes leading to vessel disruption requires some time. Additional factors potentially increasing susceptibility for the development of a hematoma could include the vicinity of the encephalitic process to penetrating vessels, an impaired coagulation state, and the extent of the inflammatory response. A direct link between antiviral therapy and intracerebral hemorrhage cannot be ruled out as there are cases of acyclovir-induced thrombocytopenia [63, 64]. The presence of thrombocytopenia carried a six-fold increased risk of mortality in a cohort of encephalitis of any type [65]. Thus, both monitoring of coagulation and cautious use of anticoagulants may be advisable in severe encephalitis.
From the immunological viewpoint, an innate immune response driven by monocytes and natural killer cells dominates the early phase [66]. This response is followed by extravasation and parenchymal entry of T cells, particularly cytotoxic CD8+ T cells. On a molecular level, matrix metalloproteinase-9 (MMP-9), a major regulator of collagen type IV and the main component of the neurovascular matrix, has been recognized as a key factor in this process. In a mouse model of HSE, MMP-9 activity increased during the early phase with peak levels in fully developed HSE, resulting in compromise of the neurovascular unit [67]. This phenomenon is also known from hemorrhagically transformed ischemic stroke in humans, where an upregulation of MMP-9 has also been linked to disruption of the neurovascular unit. [68] A few studies proposed a beneficial role of adjuvant steroids in HSE, which may restrict detrimental immune responses by mechanisms such as inducing apoptosis of immune cells in peripheral blood and within the CNS, or neutralization of MMP-9 activity by raising tissue inhibitor of MMP-1 (TIMP-1) in vascular endothelial cells [69, 70].
Our study also revealed that HSE is an important differential diagnosis of lobar hemorrhage as 32% had a hemorrhage detected on initial neuroimaging. This emphasizes the need for sufficient medical history, as the patients almost exclusively presented with an encephalitic syndrome. Moreover, it should be noted that hemorrhage in the temporal lobe needs to be investigated with appropriate vascular imaging in order to rule out vascular malformations or other causes of atypical hemorrhage.
It has been recognized since the early 1970s that HSE can occasionally present with a cerebral infarction [71]. An epidemiological study of HSE from the USA reported ischemic complications occurring twice as frequently (5.6%) as hemorrhage. Both cases with hemorrhage as the presenting imaging feature and brain infarction later during the course were related to HSV-2 with evidence of vasculitis. HSV-1 was the causative agent in the only patient with classical temporal lobe encephalitis who developed infarction. There was only one case of brain stem infarction caused by HSV-2, which is another encephalitic predilection site of HSV-2. Imaging findings of vasculitis include stenosis, vessel wall thickening, and enhancement [72]. If knowledge from VZV vasculopathy can be translated, the lack of CSF pleocytosis or of angiographic abnormalities should not preclude the diagnosis of vasculopathy. Indeed, in VZV vasculopathy, up to 33% of cases may have normal CSF and 30% may have negative vascular imaging [73]. Thus, confirmation of vasculitis on neuroimaging should not be the decisive factor for considering adjunctive steroid treatment. Little is known about the exact mechanisms of action leading to HSV vasculitis. In case of VZV disease, cerebral vessels demonstrate fibrinoid necrosis, intimal proliferation, loss of elastic lamina, and a lymphocytic or monocytic inflammatory infiltrate with surrounding multinucleated giant cells [74]. Additional mechanism of action may include an immune-mediated process leading to vasospasm and thrombosis and hypercoagulable state in combination with endothelial dysfunction resulting from activation of inflammatory and pro-coagulant cascades [9].
We acknowledge several limitations to our study. A publishing bias for more severe and atypical cases must be anticipated. This might also explain higher rates of hemorrhage than infarction. In addition, imaging techniques and time points of investigations differed within our cohort. Furthermore, vasculitic change in small vessels may not be picked up by non-invasive angiography. Future studies should ideally employ MRI with standardized vascular imaging and a PCR methodology which differentiates between HSV-1 and HSV-2. There was only a single case of venous sinus thrombosis, for which significant conclusions cannot be drawn.
Conclusion
We expand the understanding for HSV-related cerebrovascular disease and report distinct pathogenesis, cause, and outcome for cerebral hemorrhage and infarction. Further studies should focus on strategies to prevent intracerebral hemorrhage including avoidance of impaired coagulation and management of life-threatening consequences. In addition, HSV-related infarction is a rare but potentially treatable cause of stroke caused by large vessel vasculitis and usually does not clinically present as a stroke. Steroid treatment can be considered even in the absence of confirmation of vasculitis on neuroimaging.
Acknowledgements
The authors thank Prof. Eugen Trinka for continued support.
Funding
none
Availability of data and materials
all data generated or analysed during this study are included in this published article.
Abbrevations
- CNS
Central nervous system
- CT
Computer tomography
- HSE
Herpes simplex virus encephalitis
- HSV
Herpes simplex virus
- ICU
Intensive care unit
- MMP
Matrix metalloproteinase
- MRI
Magnetic resonance imaging
- mRS
Modified Rankin scale
- PCR
Polymerase chain reaction
- TIMP
Tissue-inhibitor of MMP
- VZV
Varicella-zoster virus
Authors’ contributions
LH, SP, and JS planned and conceived the study. LH, SP, and ECS collected the data. LH, SP, and JS interpreted the data. LH, SP, ECS, LKS, RN, and JS wrote and critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
not applicable
Consent for publication
all authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Larissa Hauer, Email: l.hauer@salk.at.
Slaven Pikija, Email: s.pikija@salk.at.
Eva C. Schulte, Email: eva.schulte@med.uni-muenchen.de
Laszlo K. Sztriha, Email: laszlo.sztriha@kcl.ac.uk
Raffaele Nardone, Email: raffale.nardone@gmail.com.
Johann Sellner, Phone: +43 (0)5 7255-34762, Email: j.sellner@salk.at.
References
- 1.Tyler KL. Acute viral encephalitis. N Engl J Med. 2018;379:557–566. doi: 10.1056/NEJMra1708714. [DOI] [PubMed] [Google Scholar]
- 2.Goulenok T, Buzele R, Duval X, Bruneel F, Stahl JP, Fantin B. Management of adult infectious encephalitis in metropolitan France. Med Mal Infect. 2017;47(3):206–220. doi: 10.1016/j.medmal.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Gnann JW, Jr, Whitley RJ. Herpes simplex encephalitis: an update. Curr Infect Dis Rep. 2017;19:13. doi: 10.1007/s11908-017-0568-7. [DOI] [PubMed] [Google Scholar]
- 4.Granerod J, Davies NW, Ramanuj PP, Easton A, Brown DW, Thomas SL. Increased rates of sequelae post-encephalitis in individuals attending primary care practices in the United Kingdom: a population-based retrospective cohort study. J Neurol. 2017;264:407–415. doi: 10.1007/s00415-016-8316-8. [DOI] [PubMed] [Google Scholar]
- 5.Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35:254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- 6.Jouan Y, Grammatico-Guillon L, Espitalier F, Cazals X, Francois P, Guillon A. Long-term outcome of severe herpes simplex encephalitis: a population-based observational study. Crit Care. 2015;19:345. doi: 10.1186/s13054-015-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellner J, Trinka E. Seizures and epilepsy in herpes simplex virus encephalitis: current concepts and future directions of pathogenesis and management. J Neurol. 2012;259:2019–2030. doi: 10.1007/s00415-012-6494-6. [DOI] [PubMed] [Google Scholar]
- 8.Sili U, Kaya A, Mert A, Group HSVES Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–118. doi: 10.1016/j.jcv.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Carod Artal FJ. Clinical management of infectious cerebral vasculitides. Expert Rev Neurother. 2016;16:205–221. doi: 10.1586/14737175.2015.1134321. [DOI] [PubMed] [Google Scholar]
- 10.Pillai SC, Hacohen Y, Tantsis E, Prelog K, Merheb V, Kesson A, Barnes E, Gill D, Webster R, Menezes M, et al. Infectious and autoantibody-associated encephalitis: clinical features and long-term outcome. Pediatrics. 2015;135:e974–e984. doi: 10.1542/peds.2014-2702. [DOI] [PubMed] [Google Scholar]
- 11.Chow FC, Marra CM, Cho TA. Cerebrovascular disease in central nervous system infections. Semin Neurol. 2011;31:286–306. doi: 10.1055/s-0031-1287658. [DOI] [PubMed] [Google Scholar]
- 12.Modi S, Mahajan A, Dharaiya D, Varelas P, Mitsias P. Burden of herpes simplex virus encephalitis in the United States. J Neurol. 2017;264:1204–1208. doi: 10.1007/s00415-017-8516-x. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, Stahl JP, Mailles A, Drebot M, Rupprecht CE, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garkowski A, Zajkowska J, Zajkowska A, Kulakowska A, Zajkowska O, Kubas B, Jurgilewicz D, Hladunski M, Lebkowska U. Cerebrovascular manifestations of Lyme neuroborreliosis-a systematic review of published cases. Front Neurol. 2017;8:146. doi: 10.3389/fneur.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukushima Y, Tsuchimochi H, Hashimoto M, Yubi T, Nakajima Y, Fukushima T, Inoue T. A case of herpetic meningoencephalitis associated with massive intracerebral hemorrhage during acyclovir treatment: a rare complication. No Shinkei Geka. 2010;38:171–176. [PubMed] [Google Scholar]
- 16.Politei JM, Demey I, Pagano MA. Cerebral haematoma in the course of herpes simplex encephalitis. Rev Neurol. 2003;36:636–639. [PubMed] [Google Scholar]
- 17.Sakaguchi J, Yonemura K, Hashimoto Y, Hirano T, Uchino M. Herpes simplex encephalitis originating from bilateral thalamic lesions with hemorrhagic component. Rinsho Shinkeigaku. 2005;45:368–371. [PubMed] [Google Scholar]
- 18.Plantinga EG, Vanneste JA. Mild herpes simplex encephalitis worsening despite acyclovir treatment. J Neurol. 2001;248:237–238. doi: 10.1007/s004150170235. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Kim IO, Kim WS, Yeon KM, Lee HJ, Hwang YS. Herpes simplex encephalitis: MRI findings in two cases confirmed by polymerase chain reaction assay. Pediatr Radiol. 2001;31:619–623. doi: 10.1007/s002470100508. [DOI] [PubMed] [Google Scholar]
- 20.Erdem G, Vanderford PA, Bart RD. Intracranial hemorrhage in herpes simplex encephalitis: an unusual presentation. Pediatr Neurol. 2002;27:221–223. doi: 10.1016/S0887-8994(02)00428-9. [DOI] [PubMed] [Google Scholar]
- 21.Biswas A, Das SK, Roy T, Dhibar T, Ghorai SP. Acute intracerebral haematoma--an unusual presentation of herpes simplex encephalitis. J Assoc Physicians India. 2004;52:69–71. [PubMed] [Google Scholar]
- 22.Kabakus N, Gurgoze MK, Yildirim H, Godekmerdan A, Aydin M. Acute hemorrhagic leukoencephalitis manifesting as intracerebral hemorrhage associated with herpes simplex virus type I. J Trop Pediatr. 2005;51:245–249. doi: 10.1093/tropej/fmh109. [DOI] [PubMed] [Google Scholar]
- 23.Jabbour PM, Ojemann SG. Herpes simplex encephalitis. Is anticoagulation safe? Neurologist. 2005;11:187–189. doi: 10.1097/01.nrl.0000159761.57148.70. [DOI] [PubMed] [Google Scholar]
- 24.Argyriou AA, Tsota I, Solomou E, Marangos M, Kalogeropoulou C, Petsas T, Dimopoulos PA, Chroni E. Intracerebral haemorrhage as a rare complication of HSV-1 meningoencephalitis: case report and review of the literature. Scand J Infect Dis. 2006;38:63–66. doi: 10.1080/00365540500264019. [DOI] [PubMed] [Google Scholar]
- 25.Shelley BP, Raniga SB, Al-Khabouri J. An unusual late complication of intracerebral haematoma in herpes encephalitis after successful acyclovir treatment. J Neurol Sci. 2007;252:177–180. doi: 10.1016/j.jns.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Gkrania-Klotsas E, Lever AM. Herpes simplex I encephalitis presenting as a brain haemorrhage with normal cerebrospinal fluid analysis: a case report. J Med Case Rep. 2008;2:387. doi: 10.1186/1752-1947-2-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JZ, Sax PE. HSV-1 encephalitis complicated by cerebral hemorrhage in an HIV-positive person. AIDS Read. 2009;19:153–155. [PubMed] [Google Scholar]
- 28.Tonomura Y, Kataoka H, Yata N, Kawahara M, Okuchi K, Ueno S. A successfully treated case of herpes simplex encephalitis complicated by subarachnoid bleeding: a case report. J Med Case Rep. 2010;4:310. doi: 10.1186/1752-1947-4-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi S, Takasato Y. Herpes simplex virus encephalitis complicated by intracerebral hematoma. Neurol India. 2011;59:594–596. doi: 10.4103/0028-3886.84344. [DOI] [PubMed] [Google Scholar]
- 30.Battaglia F, Noudel R, Roche PH. Herpes simplex virus encephalitis requiring emergency surgery. Rev Neurol (Paris) 2013;169:182–183. doi: 10.1016/j.neurol.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Sainz A, Escalza-Cortina I, Guio-Carrion L, Matute-Nieves A, Gomez-Beldarrain M, Carbayo-Lozano G, Garcia-Monco JC. Intracerebral hematoma complicating herpes simplex encephalitis. Clin Neurol Neurosurg. 2013;115:2041–2045. doi: 10.1016/j.clineuro.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Yu W, Lee A, Welch B. Herpes simplex encephalitis presents as large temporal lobe hemorrhage. Neurol Cases. 2014;1:12–15. [Google Scholar]
- 33.Zabroug S, Idalene M, Azmoun S, Ihbibane F, Tassi N. Postpartum herpetic encephalitis complicated by cerebral hematoma. Rev Neurol (Paris) 2015;171:680–682. doi: 10.1016/j.neurol.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Bhagchandania D, Atama V, Thadanib S, Kumara S, Atama I. Acute viral encephalitis with intracerebral bleed: an atypical presentation. J Neurol Res. 2015;5:261–263. doi: 10.14740/jnr349w. [DOI] [Google Scholar]
- 35.Gaye NM, Grimaud J. HSV-2 encephalitis complicated by cerebral hemorrhage in an immunocompetent person. Rev Neurol (Paris) 2016;172:169–170. doi: 10.1016/j.neurol.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Harada Y, Hara Y. Herpes simplex encephalitis complicated by cerebral hemorrhage during acyclovir therapy. Intern Med. 2017;56:225–229. doi: 10.2169/internalmedicine.56.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahale RR, Mehta A, Shankar AK, Miryala A, Acharya P, Srinivasa R. Bilateral cerebral hemorrhage in herpes simplex encephalitis: rare occurrence. J Neurosci Rural Pract. 2016;7:S128–S130. doi: 10.4103/0976-3147.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisahn C, Tkachenko L, Moisi M, Rostad S, Umeh R, Zwillman ME, Tubbs RS, Page J, Newell DW, Delashaw JB. Herpes simplex encephalitis of the parietal lobe: a rare presentation. Cureus. 2016;8:e785. doi: 10.7759/cureus.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller K, Ryan JE, Tai A, Armonda RA. Delayed temporal lobe hemorrhage after initiation of acyclovir in an immunocompetent patient with herpes simplex Virus-2 encephalitis: a case report. Cureus. 2017;9:e980. doi: 10.7759/cureus.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ElShimy G, Mariyam Joy C, Berlin F, Lashin W. Intracranial hemorrhage complicating herpes simplex encephalitis on antiviral therapy: a case report and review of the literature. Case Rep Infect Dis. 2017;2017:6038146. doi: 10.1155/2017/6038146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo WB, Wilcock DJ, Carey M, Albanese E. Neurological picture. Herpes encephalitis complicated by cerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2013;84:1404–1406. doi: 10.1136/jnnp-2013-305552. [DOI] [PubMed] [Google Scholar]
- 42.Sivasankar C, Whyte K, Ayodele M. An unusual etiology of acute spontaneous intracerebral hemorrhage. Neurohospitalist. 2018; in press. [DOI] [PMC free article] [PubMed]
- 43.Alexandri NM, Tavernarakis A, Potagas C, Molari H, Koutra H. Ischemic stroke and herpes simplex virus type-1 associated meningoencephalitis. Rev Neurol (Paris) 2004;160:579–581. doi: 10.1016/S0035-3787(04)70991-1. [DOI] [PubMed] [Google Scholar]
- 44.Sas AM, Niks EH, Lequin MH, Catsman-Berrevoets CE, de Wit MC. Herpes simplex virus type-1 encephalitis and occipital ischemic stroke. Pediatr Neurol. 2009;41:294–296. doi: 10.1016/j.pediatrneurol.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Zepper P, Wunderlich S, Forschler A, Nadas K, Hemmer B, Sellner J. Pearls & oy-sters: cerebral HSV-2 vasculitis presenting as hemorrhagic stroke followed by multifocal ischemia. Neurology. 2012;78:e12–e15. doi: 10.1212/WNL.0b013e31823fcd4d. [DOI] [PubMed] [Google Scholar]
- 46.Guerrero WR, Dababneh H, Hedna S, Johnson JA, Peters K, Waters MF. Vessel wall enhancement in herpes simplex virus central nervous system vasculitis. J Clin Neurosci. 2013;20:1318–1319. doi: 10.1016/j.jocn.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Terlizzi V, Improta F, Di Fraia T, Sanguigno E, D'Amico A, Buono S, Raia V, Boccia G. Primary herpes virus infection and ischemic stroke in childhood: a new association? J Clin Neurosci. 2014;21:1656–1658. doi: 10.1016/j.jocn.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Zis P, Stritsou P, Angelidakis P, Tavernarakis A. Herpes simplex virus type 2 encephalitis as a cause of ischemic stroke: case report and systematic review of the literature. J Stroke Cerebrovasc Dis. 2016;25:335–339. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Joshi P. Multiple strokes associated with herpes simplex virus type-2 infection: case report. J Neuro-Oncol. 2016;22:251–253. doi: 10.1007/s13365-015-0385-4. [DOI] [PubMed] [Google Scholar]
- 50.Tsuboguchi S, Wakasugi T, Umeda Y, Umeda M, Oyake M, Fujita N. Herpes simplex encephalitis presenting as stroke-like symptoms with atypical MRI findings and lacking cerebrospinal fluid pleocytosis. Rinsho Shinkeigaku. 2017;57:387–390. doi: 10.5692/clinicalneurol.cn-001033. [DOI] [PubMed] [Google Scholar]
- 51.Zhang F, Sumida A, Margolesky J, Tornes L, Ramos A, Koch S. HSV-2 encephalitis presenting as multifocal ischemic stroke. Neurol Sci. 2017;38:2229–2230. doi: 10.1007/s10072-017-3100-9. [DOI] [PubMed] [Google Scholar]
- 52.Snider SB, Jacobs CS, Scripko PS, Klein JP, Lyons JL. Hemorrhagic and ischemic stroke secondary to herpes simplex virus type 2 meningitis and vasculopathy. J Neuro-Oncol. 2014;20:419–422. doi: 10.1007/s13365-014-0253-7. [DOI] [PubMed] [Google Scholar]
- 53.Lal A, Dhamne MC, Hui AC, Ahmad A. Herpes simplex virus (HSV) encephalitis in a young man: an unusual course. BMJ Case Rep. 2018;2018:bcr-2017–bc222499. doi: 10.1136/bcr-2017-222499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- 55.Mayberg MR, Zervas NT, Moskowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984;223:46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- 56.Chow FC, Glaser CA, Sheriff H, Xia D, Messenger S, Whitley R, Venkatesan A. Use of clinical and neuroimaging characteristics to distinguish temporal lobe herpes simplex encephalitis from its mimics. Clin Infect Dis. 2015;60:1377–1383. doi: 10.1093/cid/civ051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piret J, Boivin G. Innate immune response during herpes simplex virus encephalitis and development of immunomodulatory strategies. Rev Med Virol. 2015;25:300–319. doi: 10.1002/rmv.1848. [DOI] [PubMed] [Google Scholar]
- 58.Deigendesch N, Stenzel W. Acute and chronic viral infections. Handb Clin Neurol. 2017;145:227–243. doi: 10.1016/B978-0-12-802395-2.00017-1. [DOI] [PubMed] [Google Scholar]
- 59.Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13:493–508. doi: 10.1007/s13311-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamei S, Taira N, Ishihara M, Sekizawa T, Morita A, Miki K, Shiota H, Kanno A, Suzuki Y, Mizutani T, et al. Prognostic value of cerebrospinal fluid cytokine changes in herpes simplex virus encephalitis. Cytokine. 2009;46:187–193. doi: 10.1016/j.cyto.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Ichiyama T, Shoji H, Takahashi Y, Matsushige T, Kajimoto M, Inuzuka T, Furukawa S. Cerebrospinal fluid levels of cytokines in non-herpetic acute limbic encephalitis: comparison with herpes simplex encephalitis. Cytokine. 2008;44:149–153. doi: 10.1016/j.cyto.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y, Lu ZN, Guo YJ, Mei YW. Favorable effects of MMP-9 knockdown in murine herpes simplex encephalitis using small interfering RNA. Neurol Res. 2010;32:801–809. doi: 10.1179/016164110X12644252260556. [DOI] [PubMed] [Google Scholar]
- 63.Kamboj J, Wu F, Kamboj R, Suzue K, Khosla P. A rare case of acyclovir-induced thrombocytopenia. Am J Ther. 2014;21:e159–e162. doi: 10.1097/MJT.0b013e31826fc4be. [DOI] [PubMed] [Google Scholar]
- 64.Tsappa I, Missouris C, Psarellis S. Acyclovir-induced thrombocytopaenia in a patient with SLE. BMJ Case Rep. 2018;2018:bcr-2018–bc225118. doi: 10.1136/bcr-2018-225118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thakur KT, Motta M, Asemota AO, Kirsch HL, Benavides DR, Schneider EB, McArthur JC, Geocadin RG, Venkatesan A. Predictors of outcome in acute encephalitis. Neurology. 2013;81:793–800. doi: 10.1212/WNL.0b013e3182a2cc6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spatola M, Du Pasquier RA. Immune system's role in viral encephalitis. Rev Neurol (Paris) 2014;170:577–583. doi: 10.1016/j.neurol.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Sellner J, Simon F, Meyding-Lamade U, Leib SL. Herpes-simplex virus encephalitis is characterized by an early MMP-9 increase and collagen type IV degradation. Brain Res. 2006;1125:155–162. doi: 10.1016/j.brainres.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 68.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, Sharp FR. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forster C, Kahles T, Kietz S, Drenckhahn D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J Physiol. 2007;580:937–949. doi: 10.1113/jphysiol.2007.129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gold R, Linker RA, Chan A. Termination of inflammation in the nervous system. Rev Neurol (Paris) 2007;163:672–676. doi: 10.1016/S0035-3787(07)90478-6. [DOI] [PubMed] [Google Scholar]
- 71.Ule G, Ametani T. Acute necrotizing herpes-encephalitis presenting ischemic brain infarct (author's transl) Zentralbl Allg Pathol. 1973;117:282–288. [PubMed] [Google Scholar]
- 72.Kuker W, Gaertner S, Nagele T, Dopfer C, Schoning M, Fiehler J, Rothwell PM, Herrlinger U. Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis. 2008;26:23–29. doi: 10.1159/000135649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, Safdieh JE, Kamenkovich E, Ostrow LW, Levy M, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidbauer M, Budka H, Pilz P, Kurata T, Hondo R. Presence, distribution and spread of productive varicella zoster virus infection in nervous tissues. Brain. 1992;115(Pt 2):383–398. doi: 10.1093/brain/115.2.383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
all data generated or analysed during this study are included in this published article.


