Abstract
Background
Antimicrobial stewardship program (ASP) is a distinguished method to improve the prescription and efficacy of antibiotics.
Aim
The efficacy of ASP and conventional methods was compared to measure the effectiveness of meropenem (MPM) and vancomycin (VMN) antibiotics in pediatric patients.
Design
In an interventional quasi-experimental study, 135 children admitted in Children’s Hospital affiliated to University of Medical Sciences in time periods of 2014–2015 and 2015–2016 were assessed.
Methods
The conventional and ASP methods in 2014–2015 and 2015–2016 were respectively utilized to provide the best antimicrobial therapy of MPM and VMN antibiotics in patient children. The data of mortality rate (MR), antibiotic prescription (AP), antibiotic dose (ADe), antibiotic duration (ADn), length of hospital stay (LOHS), and blood cultures (BCs) were compared across the years using the Chi square, independent t test, and Fisher’s exact test.
Results
The levels of MR, AP, ADe, ADn, LOHS, and positive BCs using the ASP method in 2015–2016 were significantly lower those of in 2014–2015 using the conventional one (p < 0.05).
Conclusions
The ASP method versus conventional one with a better efficacy can be employed as an antibiotic administration guide for MPM and VMN in the therapy of patients in community-based hospitals.
Keywords: Antibiotic therapy, ASP intervention, Antimicrobial management, Antimicrobial resistance, Meropenem, Vancomycin
Background
One of the most common therapeutic agents or active pharmaceutical ingredients prescribed in healthcare and medical services is antimicrobial drugs [1]. It was clearly reported that 30–67% of children in their length of hospital stay (LOHS) consumed at least one antimicrobial medicine [2]. The consecutive antibiotic therapy may thus result in adverse drug reactions leading to the increased mortality and morbidity rate, longer hospital stay and higher health costs [3]. Also, it may lead to the severe antibiotic resistance and nosocomial infections [4]. Antibiotic resistance is a growing problem worldwide [5], so that it has been estimated that about half of antibiotic prescriptions in medical centers are incorrect or unessential [6].
There are several well-known ways to decrease the antibiotic resistance such as hand washing, disinfection of hands and hospital tools, prompted laboratory tests, continuous medical education, vaccination, preparation of antimicrobial resistance files, and use of antimicrobial stewardship program (ASP) method [7, 8]. The last one is an optimal use of antibiotics including true antimicrobial drug with an appropriate antibiotic dose (ADe), and duration (ADn) [9–11]. This program was initially introduced in 1996 [12–14] aiming to the improvement in health care quality with better use of antibiotics, and subsequently the reduction of antibiotic resistance rate and health care costs [6, 15–17]. Hence, implication of this method and assessment of its practical outcomes are essential to establish a comprehensive guideline for patients’ healthcare with a minimized toxicity and bacterial resistance [18].
The risk of antibiotic resistance versus the beneficial utilization of antibiotics should be balanced [19]. The routinely antibiotic prescription is preferred among the patients and considered as a medical skill in physicians [20]. A lack in antibiotic prescription may result in legal issues for the physician’s [21]. Some strategies to implement the ASP protocol include educational programs, antibiotic order forms, computerized programs, automatic stop orders, antibiotic cycling, and step-down therapy [22, 23]. An ASP team includes seven parts such as infectious disease specialist, professional center of infection control, hospital manager, clinical pharmacist for infectious diseases, clinical microbiologist, epidemiologist, and information technology expert subject [24, 25].
Published literatures in pediatrics earlier demonstrated the optimistic effect of ASP implementation on the use of antimicrobial agents [26–31]. However, this technique has not become pervasive in Iran due to the lack of proper and organized implementation in public and private pediatric hospitals. Besides, the consumption of drugs especially antibiotics in Iran based on the available data is three times more than the mean global rate [32]. On the other hand, there is a need to utilize a successful and multidisciplinary ASP model because of the restricted availability of data related to pediatric infectious diseases and institutional resources. Nowadays, meropenem (MPM) and vancomycin (VMN), as well as ciprofloxacin are the most common used antibiotics in children hospitalized for a wide range of diseases and health disorders. As ASP as an innovative and strategic method in Iran has been lower applied to improve the prescription and efficacy of antibiotics, the efficacy of this method versus conventional one in the current study was compared for MPM and VMN antibiotics in Iranian pediatric patients to optimize infection-related patient outcomes.
Materials and methods
Study design and participants
In this interventional quasi-experimental study, 135 children (mean age 12.2–20.6 months; mean weight, 7.8–8.7 kg) under treatment with MPM and VMN antibiotics in Children’s Hospital affiliated to University of Medical Sciences were eventually evaluated. The initial number of participants was 166. However, infants with a low birth weight or a LOHS lower than 1 week, birth at medical facilities other than NICUs, and having major congenital anomalies were excluded because most out-born infants after the first week of life were transferred and received variable nutritional feedings. In addition, several charts were missing key information, such as birth weight or discharge weight. Other exclusion criteria in this research were children referred to the surgery department and/or received antibiotics for prophylaxis. In general, 31 out of 166 people were excluded from the current study and (Fig. 1). Participation was completely voluntary and anonymous. Under parents’ consent, all obtained from questionnaire-based and clinical information were confidential and employed only for the blind study. We using a convenient and non-random sampling recognized all distinctive patients who received MPM and VMN antibiotics during two time periods from September 2014 to October 2016. The first period was considered as the 12 months before the ASP implementation from September 23, 2014 to September 22, 2015, while the second period was the first 12 months that the ASP was in place. This period was defined from October 23, 2015 to October 21, 2016. September 22, 2015–October 22, 2015 was also left out the research design as this time range was when the ASP was just getting begun.
Fig. 1.
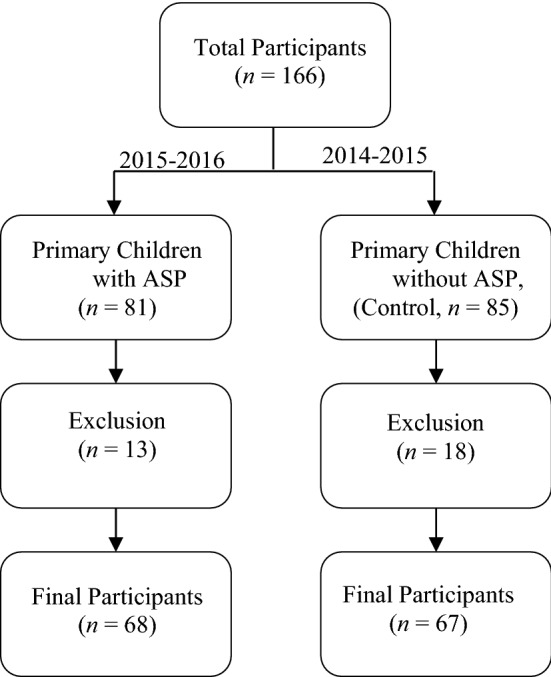
A schematic scheme of the whole and the adjusted comparisons of children groups studied in the current research
Antimicrobial stewardship program (ASP) setting
The core members of ASP team in this study was including specialist physicians and pharmacists in infectious diseases, skilled people in tracking outbreaks (epidemiologists), infection preventionists (IPs), clinical microbiologists, and two data scientists. From Saturday to Thursday, the ASP team officially inspects the primary instructions and applies daily and weekly usage arrangements of MPM and VMN. These antibiotics were purposefully prescribed according to the antimicrobial susceptibility patterns of clinical isolates or type of antibiotic-resistant infections, misapplication risk, and medical expenses. Besides, the ASP protocol incorporated pharmacist notification by personnel of clinical microbiology laboratory. After generating the real-time data collection, pharmacists present in the ASP team assessed each the documented order by evaluating the patient’s electronic medical record, subsequently issued some of key decisions/consultations after open communication with specialist physicians and sent a feedback to providers in terms of answers like stop, approve, and use of a sole antibiotic with a less/more dose.
Data collection
An electronic and validated checklist including demographic (e.g., age, gender, and weight) and disease-related data were filled out for each patient and recorded in the pharmacy database. Clinical data included intensive care unit admission, disease type and intensity, antibiotic consumption history (such as receipt of past antibiotic therapy), ADe, ADn, LOHS, mortality, adverse effects, and results of bacterial cultures in MPM and VMN treatments, recommendations introduced by the ASP team about antibiotic prescription (AP) with a certain ADe, and patient’s agreement rate with consultations. The time difference (in day) between patient’s admission and discharge dates was considered as LOHS. The efficiency of used antibiotics against urinary tract infection (UTI), respiratory tract infection (RTI), and cerebrospinal fluid infection (CSFI) was respectively examined by the urine, tracheal, and cerebrospinal fluid (CSF) standard cultures. Gram staining from positive blood cultures was conducted to diagnose whether bacterial infections induced by Gram-positive cocci or by Gram-negative bacilli. The UTI was recognized from the clinical charts of all pediatric patients with substantial amounts of pathogens isolated in blood cultures. The UTI confirmation was also performed by checking their records to find the same pathogen in the urine and blood cultures, with pyuria by dipstick or sedimentary urine analysis and/or clinical symptoms (e.g., flank pain and fever) [33]. The diagnosis of lower/upper RTI was according to the following symptoms such as fever (T > 38.0 °C) or hypothermia (T < 35.5 °C), leukocytosis or leukopenia, and positive tracheal culture [34]. The CSFI was considered as a positive CSF culture with fever ≥ 38.0 °C [35]. In the ASP method, pathogenic results of early-positive blood cultures were quickly analyzed using a random-polymerase chain reaction (rPCR) [36, 37]. Thus, a couple of rPCR assays and BCs were determinant in the antibiotic continuation. The ADn was estimated from the beginning of appropriate antibiotic closest to collection date of BC. The stop date of scheduled antimicrobial treatment was also considered as the last day of appropriate antibiotics for patient’s children who were discharged alive.
Statistical analysis
The data were statistically assessed using the analysis of variance (ANOVA) with SPSS software package, version 13.0 (SPSS Inc., Chicago, IL, USA). The independent t-student test and Chi square (χ2) or Fisher’s exact tests were performed to assess between-group differences for numerical and categorical variables, respectively. The significant level was set at p < 0.05.
Results
Figure 2 depicts the number and percent of admission of patients in the various hospital wards such as internal medicine, neonatal (NICU), and pediatric (PICU) intensive care units and others. It can be seen in Fig. 2, the majority of patients were admitted in NICU and PICT wards. In details, the maximum admission was in the NICU (53.7%) in 2014–2015 and in the PICU (44.1%) in 2015–2016. The number of patient boys admitted in the time periods of 2014–2015 and 2015–2016 were 59.7 and 50.0%, respectively, whereas the corresponding number of patient girls was 40.3 and 50.0%, respectively (p > 0.05). Table 1 summarizes the frequency of 17 common diseases in in-patient children’s admitted in Bahrami Hospital in 2014–2016. This table reveals that the type of diseases was alike across the years (Table 1). Although pneumonia and sepsis in the investigated years were more prevalent diseases among the patient’s children, meningitis infection in 2015–2016 was highly detected (Table 1).
Fig. 2.
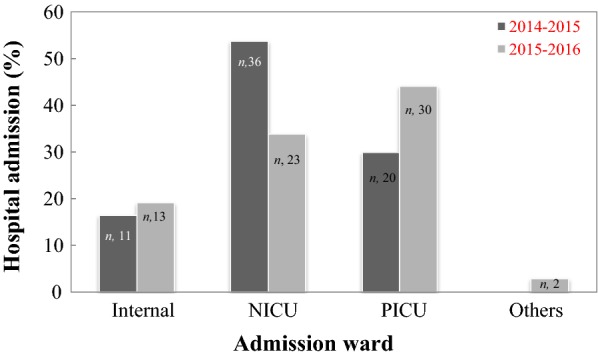
The admission percentage of pediatric patients (n, the patient number) in different wards of Bahrami Hospital during time periods of 2014–2015 and 2015–2016
Table 1.
The occurance rate of different diseases in in-patient children’s wards in 2014–2016
| Disease typea | Considered time period | |||
|---|---|---|---|---|
| 2014–2015 (non-ASP) | 2015–2016 (with ASP) | |||
| Frequency (n) | Percent (%) | Frequency (n) | Percent (%) | |
| Acute lymphocytic leukemia (ALL) | – | – | 3 | 4.41 |
| Acute gastroenteritis (AGE) | 1 | 1.49 | 3 | 4.41 |
| Left Congenital Diaphragmatic Hernia (CDH) | 1 | 1.49 | – | – |
| Bronchiolitis | 2 | 2.98 | – | – |
| Cerebral palsy (CP) | 2 | 2.98 | 3 | 4.41 |
| Leiomyosarcoma (LMS) tumor | 2 | 2.98 | – | – |
| Meningitis | 2 | 2.98 | 7 | 10.29 |
| Obstruction | 2 | 2.98 | 2 | 2.94 |
| Rheumatic fever (RF) | 2 | 2.98 | – | – |
| Tracheoesophageal (TE) fistula | 2 | 2.98 | – | – |
| Urosepsis | 2 | 2.98 | 1 | 1.47 |
| Posterior urethral valves (PUV) | 3 | 4.47 | – | – |
| Acute myeloid leukemia (AML) | 4 | 5.97 | 3 | 4.41 |
| Esophageal atresia (EA) | 4 | 5.97 | 1 | 1.47 |
| Necrotizing enterocolitis (NEC) | 5 | 7.46 | – | – |
| Pneumonia | 7 | 10.44 | 9 | 13.23 |
| Sepsis | 14 | 20.89 | 16 | 23.53 |
| Others | 12 | 17.91 | 20 | 29.41 |
aThe total number of children’s diseases in time periods of 2014–2015 and 2015–2016 was 67 and 68, respectively
The overall count of antibiotic prescriptions issued for the studied pediatric patients in time periods of 2014–2015 and 2015–2016 is given in Table 2. The antibiotic prescription was significantly reduced in 2015–2016 (p = 0.0001). Generally, a higher number (more than 3.4 times) of antibiotic prescriptions for VMN compared to MPM in 2014–2015 was issued. In addition, the percent of MPM and VMN prescriptions in 2015–2016 was significantly decreased from 10.44 to 1.47% and from 35.52 to 4.41%, respectively (Table 2). Interestingly, one patient who was treated in 2014–2015, did not experience any therapeutic adverse effect in 2015–2016 (p = 0.001). Also, the mortality rate was significantly reduced from 28.4% in 2014–2015 to 5.9% in 2015–2016 (p = 0.001). The results of different cell cultures for infectious diseases are also exhibited in Table 3. As shown in this table, a significant reduction in positive BCs from 2014–2015 to 2015–2016 was found (p = 0.001). Although a decrease in positive numbers of urine, tracheal, and CSF cultures was observed, this diminishing trend was not meaningful (Table 3). It is worth recalling that the implementation of ASP guideline could promote the management of UTI, RTI, and CSFI in pediatric patients. The ADe, ADn and LOHS amounts in 2015–2016 compared to 2014–2015 were significantly lowered using the ASP (Table 4).
Table 2.
The total number of antibiotic prescriptions for studied pediatric patients
| Time period | None | MPM | VMN | Both (MPM + VMN) |
|---|---|---|---|---|
| 2014–2015 (without ASP) | – | 7 (10.44%)a | 24 (35.82%)a | 36 (53.73%)a |
| 2015–2016 (with ASP) | 60 (88.23%) | 1 (1.47%)b | 3 (4.41%)b | 4 (5.88%)b |
Values in the same columns followed by different letters (a, b) are significantly different
Values inside and outside the parentheses respectively represent frequency (n, out of 67–68) and percent (%) of antibiotic prescriptions for the patient’s children
Table 3.
The frequency of infectious diseases diagnosed in microbial culture laboratories
| Culture type | Assessed periodb | p value | |
|---|---|---|---|
| 2014–2015 (non-ASP) | 2015–2016 (with ASP) | ||
| Blood culturea | 16 (23.88%)a | 3 (4.41%)b | 0.001 |
| Urine culture | 5 (7.46%) | 3 (4.41%) | > 0.05 |
| Tracheal culture | 2 (2.98%) | 1 (1.47%) | > 0.05 |
| Cerebrospinal fluid (CSF) culture | 1 (1.49%) | – | > 0.05 |
aValues in the same row followed by different letters (a, b) are significantly
bThe total number of tests in 2014–2015 and 2015–2016 was 67 and 68, respectively
Table 4.
A summary of weight, age, and antibiotic dose and duration of therapy of patients stayed in the hospital in 2014–2016
| Numerical variable | Studied period | p value | |
|---|---|---|---|
| 2014–2015 (non-ASP) | 2015–2016 (with ASP) | ||
| Patient age (month) | 12.2 ± 3.0 | 20.6 ± 6.5 | > 0.05 |
| Patient weight (kg) | 7.8 ± 10.4 | 8.7 ± 6.7 | > 0.05 |
| LOHS (day)* | 22.7 ± 1.9a | 15.6 ± 2.8b | 0.015 |
| ADe (mg/kg)* | 23.36 ± 3.8a | 10.90 ± 3.6b | 0.043 |
| ADn (day)* | 11.9 ± 9.5a | 7.4 ± 4.6b | 0.001 |
LOHS length of hospital stay, ADe antibiotic dose, ADn antibiotic duration
*Values in the same row followed by different letters (a, b) are significantly
Discussion
The ASP method is a comprehensive approach to reduce the burden of the antibiotic resistance problem. This method as a practical strategy can notably improve the pattern and amount of antibiotic prescriptions in different hospital wards [38]. In this study, the outcomes of ASP and conventional method to control the antibiotic use of MPM and VMN in healthcare settings were compared in a community-based referral hospital in a developing country. Results showed that the ASP utilization not only can significantly reduce the ADe and ADn of MPM and VMN, but also can diminish the LOHS and number of positive cultures. Similar results were reported by Seah et al. [39], who studied the application effect of ASP method on the carbapenems use in a tertiary women’s and children’s hospital in Singapore. They found that the proper prescription of carbapenems using the ASP was increased from 55.9 to 70.3%. Also, the baseline ADe and ADn of carbapenems therapy were reduced up to 55.6 and 46.7%, respectively. Earlier, Di Pentima et al. [30] proved that the ASP protocol can be successfully applied to promote quality of care of hospitalized children by significantly decreasing targeted- and non-targeted-antimicrobial utilization up to 21%. Findings of Newland et al. [1] in the ASP efficiency assessment in pediatric hospitals in the United States were also in a good accordance with results of the present study. They noticed the higher antibiotic uphold rate with a monthly reduction rate of 7% during the ASP implementation compared to the control group. As well, the ADn and LOHS of antibiotic therapy were significantly reduced by applying the ASP. A significant decrease in ADn (33%) and LOHS (61–99%) for antibiotic treatments (e.g., ampicillin, cefotaxime, ceftazidime, eftriaxone, gentamicin, vancomycin, and MPM) was also reported by Lee et al. [40] in a tertiary children’s hospital after the guideline implementation of ASP. Song et al. [41] could successfully reduce the prescription of two antibiotics (~ 74%) against the infection of anaerobic bacteria by using the ASP method along with training course and inter-wards relationship enforcement. The considerable reduction in the antibiotic prescription was after the ASP utilization was comparable the antimicrobial efficiency of MPM and VMN in the present investigation. The prescription of a number of antibiotics including caspofungin, aztreonam, daptomaycin, ertapenem, voriconazole, linezolid, tigcycline, and MPM after the utilization of ASP method was assessed by Malani et al. [22]. Implementing the ASP led to a significant 50% decrease in the infection rate of Clostridium difficile with a reduced daily consumption dose (25.4%) and medical costs (15.2%). In the current study, the total dose of MPM and VMN was also reduced, but the calculation of costs was impossible.
Similar to results of our study, Morril et al. [42] reported the ASP was concomitant with a higher consultation count and a decreased time for consultation request leading to the improvement of patients’ health with a decreased rate of readmission and mortality. Thompson et al. [43] interestingly reported that use of ASP method would result in a better change from intravenous to oral antibiotic use. They achieved a significant success with an increased rate in oral antibiotics use. However, the pattern and route of antibiotic therapy were not considered in our study which may be a practical issue in further studies, especially in the pediatric population.
Conclusions
In conclusion, because the ASP compared to the conventional method showed a superior effectiveness for MPM and VMN antibiotics, a culture-guided ASP is seriously proposed to community-based local hospitals owing to the assurance of implementation and performance of a proper antimicrobial therapy. A significant reduction in antibiotic consumption also can highly promote patients’ satisfaction and their expectations by decreasing medical costs and long-term disability for patients. Although the present research was limited by its quasi-experimental design, the obtained results in pediatric patients along with the evaluation of care quality can be verified by conducting further studies with larger sample size and multi-center sampling. Furthermore, the efficiency and satisfaction with the available ASP may potentially improve the therapy optimization by incorporating a prospective audit-and-feedback intervention.
Authors’ contributions
AR, SYM, PS, MGH, SK, LKH, SJMS, SKY, and AI participated in the design of the study, executed data collection, draft of the manuscript, performed the statistical analysis and contributed to the draft manuscript, conceived and designed the study, participated in its co-ordination and prepared the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All authors have consented for publication.
Ethics approval and consent to participate
The present investigation was approved by “The Research Ethics Committee of the Tehran University of Medical Sciences” and was performed in accordance with the ethical standards laid down in the operative version of the Declaration of Helsinki. All participants included in this study read and signed an informed consent form.
Funding
No funding reported.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ASP
antimicrobial stewardship program
- MPM
meropenem
- VMN
vancomycin
- MR
mortality rate
- APn
antibiotic prescription
- ADe
antibiotic dose
- AD
nantibiotic duration
- LOHS
length of hospital stay
- BCs
blood cultures
- IPs
infection preventionists
- UTI
urinary tract infection
- RTI
respiratory tract infection
- CSFI
cerebrospinal fluid infection
- CSF
cerebrospinal fluid
- rPCR
random-polymerase chain reaction
- ANOVA
analysis of variance
References
- 1.Newland JG, Stach LM, De Lurgio SA, Hedican E, Yu D, Herigon JC, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatr Infect Diseases Soc. 2012;1(3):179–186. doi: 10.1093/jpids/pis054. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JM, Shah ND, Vermeulen LC, Doloresco F, Martin PK, Blake S, et al. Projecting future drug expenditures-2009. Am J Health Syst Pharm. 2009;66(3):237–257. doi: 10.2146/ajhp080636. [DOI] [PubMed] [Google Scholar]
- 3.Masters BR. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Graefes Arch Clin Exp Ophthalmol. 2016;254:2285–2287. doi: 10.1007/s00417-015-2950-1. [DOI] [Google Scholar]
- 4.Hicks LA, Taylor TH, Jr, Hunkler RJ. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368(15):1461–1462. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- 5.MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infect Control Hosp Epidemiol. 2008;29(3):203–211. doi: 10.1086/528810. [DOI] [PubMed] [Google Scholar]
- 6.Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 7.Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- 8.Vogtlander NP, van Kasteren ME, Natsch S, Kullberg BJ, Hekster YA, van der Meer JW. Improving the process of antibiotic therapy in daily practice: interventions to optimize timing, dosage adjustment to renal function, and switch therapy. Arch Intern Med. 2004;164(11):1206–1212. doi: 10.1001/archinte.164.11.1206. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove S, Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis. 2003;36(11):1433–1473. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]
- 10.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42(5):657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006. Geneva: World Health Organization; 2009. [Google Scholar]
- 12.Avorn J, Solomon DH. Cultural and economic factors that (mis) shape antibiotic use: the nonpharmacologic basis of therapeutics. Ann Intern Med. 2000;133(2):128–135. doi: 10.7326/0003-4819-133-2-200007180-00012. [DOI] [PubMed] [Google Scholar]
- 13.Leibovici L, Paul M, Ezra O. Ethical dilemmas in antibiotic treatment. J Antimicrob Chemother. 2011;67(1):12–16. doi: 10.1093/jac/dkr425. [DOI] [PubMed] [Google Scholar]
- 14.Metlay JP, Shea JA, Crossette LB, Asch DA. Tensions in antibiotic prescribing: pitting social concerns against the interests of individual patients. J Gen Intern Med. 2002;17(2):87–94. doi: 10.1046/j.1525-1497.2002.10711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Légaré F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. Can Med Assoc J. 2012;184:E726–E734. doi: 10.1503/cmaj.120568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGowan JJ, Gerding DN. Does antibiotic restriction prevent resistance? New Horiz. 1996;4(3):370–376. [PubMed] [Google Scholar]
- 17.Sunenshine RH, Liedtke LA, Jernigan DB, Strausbaugh LJ. Role of infectious diseases consultants in management of antimicrobial use in hospitals. Clin Infect Dis. 2004;38(7):934–938. doi: 10.1086/382358. [DOI] [PubMed] [Google Scholar]
- 18.Fishman N. Society for Healthcare Epidemiology of America, Infectious Diseases Society of America. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS) Infect Control Hosp Epidemiol. 2012;33(4):322–327. doi: 10.1086/665010. [DOI] [PubMed] [Google Scholar]
- 19.Struelens MJ. Multidisciplinary antimicrobial management teams: the way forward to control antimicrobial resistance in hospitals. Curr Opin Infect Dis. 2003;16(4):305–307. doi: 10.1097/00001432-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 20.McGowan JE., Jr Minimizing antimicrobial resistance: the key role of the infectious diseases physician. Clin Infect Dis. 2004;38(7):939–942. doi: 10.1086/382363. [DOI] [PubMed] [Google Scholar]
- 21.Harris DJ. Initiatives to improve appropriate antibiotic prescribing in primary care. J Antimicrob Chemother. 2013;68(11):2424–2427. doi: 10.1093/jac/dkt360. [DOI] [PubMed] [Google Scholar]
- 22.Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145–148. doi: 10.1016/j.ajic.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control. 2012;1(1):32. doi: 10.1186/2047-2994-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzales R, Sauaia A, Corbett KK, Maselli JH, Erbacher K, et al. Antibiotic treatment of acute respiratory tract infections in the elderly: effect of a multidimensional educational intervention. J Am Geriatr Soc. 2004;52(1):39–45. doi: 10.1111/j.1532-5415.2004.52008.x. [DOI] [PubMed] [Google Scholar]
- 25.Jump RL, Olds DM, Seifi N, Kypriotakis G, Jury LA, Peron EP, et al. Effective antimicrobial stewardship in a long-term care facility through an infectious disease consultation service: keeping a LID on antibiotic use. Infect Control Hosp Epidemiol. 2012;33(12):1185–1192. doi: 10.1086/668429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agwu AL, Lee CK, Jain SK, Murray KL, Topolski J, Miller RE, et al. A World Wide Web–based antimicrobial stewardship program improves efficiency, communication, and user satisfaction and reduces cost in a tertiary care pediatric medical center. Clin Infect Dis. 2008;47(6):747–753. doi: 10.1086/591133. [DOI] [PubMed] [Google Scholar]
- 27.Metjian TA, Prasad PA, Kogon A, Coffin SE, Zaoutis TE. Evaluation of an antimicrobial stewardship program at a pediatric teaching hospital. Pediatr Infect Dis J. 2008;27(2):106–111. doi: 10.1097/INF.0b013e318158603a. [DOI] [PubMed] [Google Scholar]
- 28.Di Pentima MC, Chan S, Eppes SC, Klein JD. Antimicrobial prescription errors in hospitalized children: role of antimicrobial stewardship program in detection and intervention. Clin Pediatr. 2009;48(5):505–512. doi: 10.1177/0009922808330774. [DOI] [PubMed] [Google Scholar]
- 29.Di Pentima MC, Chan S. Impact of antimicrobial stewardship program on vancomycin use in a pediatric teaching hospital. Pediatr Infect Dis J. 2010;29(8):707–711. doi: 10.1097/INF.0b013e3181d683f8. [DOI] [PubMed] [Google Scholar]
- 30.Di Pentima MC, Chan S, Hossain J. Benefits of a pediatric antimicrobial stewardship program at a children’s hospital. Pediatrics. 2011;128:1062–1070. doi: 10.1542/peds.2010-3589. [DOI] [PubMed] [Google Scholar]
- 31.Pollack LA, van Santen KL, Weiner LM, Dudeck MA, Edwards JR, Srinivasan A. Antibiotic stewardship programs in US acute care hospitals: findings from the 2014 National Healthcare Safety Network Annual Hospital Survey. Rev Infect Dis. 2016;63(4):443–449. doi: 10.1093/cid/ciw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseinzadeh K, Azimian J. Iranians’ self-report knowledge and practice about arbitrary use of antibiotics. J Clin Diagn Res. 2017;11(8):FC06–FC09. doi: 10.7860/JCDR/2017/25368.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanai M, Ogasawara M, Hayashi Y, Suzuki K, Takahashi H, Satomura A. Impact of interventions by an antimicrobial stewardship program team on appropriate antimicrobial therapy in patients with bacteremic urinary tract infection. J Infect Chemother. 2018;24(3):206–211. doi: 10.1016/j.jiac.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Khalili H, Shojaei L, Mohammadi M, Beigmohammadi MT, Abdollahi A, Doomanlou M. Meropenem/colistin versus meropenem/ampicillin–sulbactam in the treatment of carbapenem-resistant pneumonia. J Compar Effect Res. 2018;7(09):901–911. doi: 10.2217/cer-2018-0037. [DOI] [PubMed] [Google Scholar]
- 35.Park YK, Yi HJ, Choi KS, Lee YJ, Chun HJ, Kwon SM. Predictive factors of fever after aneurysmal subarachnoid hemorrhage and its impact on delayed cerebral ischemia and clinical outcomes. World Neurosurg. 2018;114:e524–e531. doi: 10.1016/j.wneu.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 36.Bauer KA, West JE, Balada-Llasat JM, Pancholi P, Stevenson KB, Goff DA. An antimicrobial stewardship program’s impact. Clin Infect Dis. 2010;51(9):1074–1080. doi: 10.1086/656623. [DOI] [PubMed] [Google Scholar]
- 37.Parta M, Goebel M, Thomas J, Matloobi M, Stager C, Musher DM. Impact of an assay that enables rapid determination of Staphylococcus species and their drug susceptibility on the treatment of patients with positive blood culture results. Infect Control Hosp Epidemiol. 2010;31(10):1043–1048. doi: 10.1086/656248. [DOI] [PubMed] [Google Scholar]
- 38.Liew YX, Lee W, Cai YY, Teo J, Tang SL, Ong RQ, et al. Utility and safety of procalcitonin in an antimicrobial stewardship program (ASP) in patients with malignancies. Eur J Clin Microbiol Infect Dis. 2012;31(11):3041–3046. doi: 10.1007/s10096-012-1662-2. [DOI] [PubMed] [Google Scholar]
- 39.Seah XF, Ong YL, Tan SW, Krishnaswamy G, Chong CY, Tan NW, et al. Impact of an antimicrobial stewardship program on the use of carbapenems in a tertiary women’s and children’s hospital, Singapore. Pharmacotherapy. 2014;34(11):1141–1150. doi: 10.1002/phar.1490. [DOI] [PubMed] [Google Scholar]
- 40.Lee KR, Bagga B, Arnold SR. Reduction of broad-spectrum antimicrobial use in a tertiary children’s hospital post antimicrobial stewardship program guideline implementation. Pediatr Crit Care Med. 2016;17(3):187–193. doi: 10.1097/PCC.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 41.Song YJ, Kim M, Huh S, Lee J, Lee E, Song KH, et al. Impact of an antimicrobial stewardship program on unnecessary double anaerobic coverage prescription. Infect Chemother. 2015;47(2):111–116. doi: 10.3947/ic.2015.47.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrill HJ, Gaitanis MM, LaPlante KL. Antimicrobial stewardship program prompts increased and earlier infectious diseases consultation. Antimicrob Resist Infect Control. 2014;3(1):12. doi: 10.1186/2047-2994-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson C, Zahradnik M, Brown A, Fleming DG, Law M. The use of an IV to PO clinical intervention form to improve antibiotic administration in a community based hospital. BMJ Qual Improv Rep. 2015;4(1):u200786. doi: 10.1136/bmjquality.u200786.w2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


