Abstract
Objective
Low adenoma detection rates (ADR) are linked to increased postcolonoscopy colorectal cancer rates and reduced cancer survival. Devices to enhance mucosal visualisation such as Endocuff Vision (EV) may improve ADR. This multicentre randomised controlled trial compared ADR between EV-assisted colonoscopy (EAC) and standard colonoscopy (SC).
Design
Patients referred because of symptoms, surveillance or following a positive faecal occult blood test (FOBt) as part of the Bowel Cancer Screening Programme were recruited from seven hospitals. ADR, mean adenomas per procedure, size and location of adenomas, sessile serrated polyps, EV removal rate, caecal intubation rate, procedural time, patient experience, effect of EV on workload and adverse events were measured.
Results
1772 patients (57% male, mean age 62 years) were recruited over 16 months with 45% recruited through screening. EAC increased ADR globally from 36.2% to 40.9% (P=0.02). The increase was driven by a 10.8% increase in FOBt-positive screening patients (50.9% SC vs 61.7% EAC, P<0.001). EV patients had higher detection of mean adenomas per procedure, sessile serrated polyps, left-sided, diminutive, small adenomas and cancers (cancer 4.1% vs 2.3%, P=0.02). EV removal rate was 4.1%. Median intubation was a minute quicker with EAC (P=0.001), with no difference in caecal intubation rate or withdrawal time. EAC was well tolerated but caused a minor increase in discomfort on anal intubation in patients undergoing colonoscopy with no or minimal sedation. There were no significant EV adverse events.
Conclusion
EV significantly improved ADR in bowel cancer screening patients and should be used to improve colonoscopic detection.
Trial registration number
NCT02552017, Results; ISRCTN11821044, Results.
Keywords: colonoscopy, colonic adenomas, colorectal adenomas, colorectal cancer, colorectal cancer screening
Significance of this study.
What is already known about this subject?
We searched PubMed and MEDLINE for English language publications in humans up to October 2016 for randomised controlled trials (RCT), open and observational studies of Endocuff and Endocuff Vision. We identified four case series studies and four multicentre RCT using the original Endocuff. Findings from case series reported that Endocuff provided more stability during mucosectomy, improved Mean number of Adenomas detected per Procedure (MAP) and resulted in adenoma detection rates (ADR) of up to 44.7%. However, small, superficial, ‘scratch-like’ mucosal lesions were observed, especially in the ileocaecal region in 30% of patients. Two multicentre RCTs from Germany and one from the USA reported an ADR increase of 14%, 85% and 16.6% with Endocuff-assisted colonoscopy. However, the largest multicentre RCT was a Dutch study of 1063 procedures, which reported no significant difference in ADR but a higher MAP with Endocuff-assisted colonoscopy. A single- centre trial of Endocuff Vision has recently reported no improvement in ADR, but this was a small study. No multicentre RCTs of the second-generation Endocuff Vision, as used in this trial, have been published.
What are the new findings?
We present findings from the first multicentre RCT comparing Endocuff Vision-assisted colonoscopy with standard colonoscopy in patients attending for symptomatic, surveillance and Bowel Cancer Screening Programme colonoscopy. Thus, this is the first study to demonstrate improved ADR with Endocuff Vision.
Significance of this study.
How might it impact on clinical practice in the foreseeable future?
The results of the Accuracy of Detection using Endocuff Optimisation of Mucosal Abnormalities (ADENOMA) study demonstrate that Endocuff Vision is a safe device, which improves ADR in the faecal occult blood test positive screening population. It speeds up procedures and is generally well tolerated by patients.
Introduction
Adenoma detection rate (ADR) is the most important marker of colonoscopy quality.1 2 Low ADR correlates with higher postcolonoscopy colorectal cancer (PCCRC) rates and poorer outcomes.3–7 Measures to improve ADR such as optimising bowel preparation, slower withdrawal time, use of antispasmodics, improved training, position change and new technologies to improve mucosal visualisation have been developed.8–13
Lesions located on the proximal side of colonic folds present a particular problem and established manoeuvres such as retroflexion may not be possible in much of the colon.12 13 Devices that attach to the tip of the scope have been created to flatten folds but have not been demonstrated to consistently improve ADR.14
Endocuff Vision (EV) (figure 1) is a polypropylene device mounted onto the distal tip of a colonoscope. It consists of a fixed portion and a row of eight soft projections, which fold backwards during insertion but are pulled forwards during withdrawal to hold back colonic folds. EV is a second-generation device replacing the original Endocuff which had two rows of shorter, firmer projections. The original Endocuff (figure 2) demonstrated an improvement in ADR in some studies but this was not replicated in a large randomised controlled trial (RCT).15 16 The original Endocuff was reported to cause mucosal abrasions, therefore to minimise this and to further improve detection characteristics the EV was created.17
Figure 1.

Endocuff Vision (personal photograph by author).
Figure 2.
Endocuff (personal photograph by author).
Methods
Study design
Patients were recruited at seven hospitals (one academic and six community) in England between November 2014 and February 2016. Colonoscopists who perform colonoscopy on positive faecal occult blood (FOBt) patients as part of the English Bowel Cancer Screening Programme (BCSP) undergo additional accreditation and may not reflect typical colonoscopy practice.1 Therefore, each site was limited to four BCSP colonoscopists. A maximum of 10 colonoscopists per site were allowed to participate in the trial. A learning curve for EV has been reported18; therefore, all colonoscopists were required to perform a minimum of 20 cases with EV prior to study commencement and were trained by means of a presentation and video. Usual colonoscopy equipment as available in each site was used with no restrictions placed on type of equipment used. Left colon was defined as transverse colon, splenic flexure, descending colon, sigmoid and rectum. Right colon was defined as caecum, ascending colon and hepatic flexure. The study protocol has been published.19 The ADENOMA trial has been registered with clinicaltrials.gov NCT02552017, International Standard Randomised Controlled Trials Number ISRCTN11821044 and UK Clinical Research Network 17 718.
Participants
Patients older than 18 years and referred for colonoscopy for clinical symptoms, as part of a postpolypectomy surveillance programme or with positive FOBt as part of BCSP, were invited.1 Patients were excluded if there was a pre-endoscopy suspicion of large bowel obstruction; known colon cancer or polyposis syndromes; known colonic stricture; known severe diverticular segment; known active colitis; on anticoagulants which had not been stopped preprocedure (meaning polypectomy might not be undertaken); if pregnant or attending for a therapeutic procedure or assessment of a known lesion. Some invited patients were not able to be recruited for logistical reasons such as unavailability of a research nurse or last-minute procedure cancellation.
Removal of EV during colonoscopy was indicated where: acute angulation in a fixed sigmoid colon rendered scope insertion more difficult; a new diagnosis of polyposis syndrome or active colitis was made; or a new stricture that might impede insertion was identified.
Randomisation and masking
Stratified randomisation based on age, gender, hospital site and BCSP status was performed using a dynamic adaptive algorithm created by the North Wales Organisation for Randomised Trials in Health Clinical Trials Unit.20 Randomisation was via a computerised internet-based platform. Patients, colonoscopists and research nurses were not blinded to randomisation arm, but all study analyses were conducted in a blinded fashion.
Outcomes
The primary aim was to ascertain if there was a difference in ADR between EV-assisted colonoscopy (EAC) and standard colonoscopy (SC).
The secondary aims were:
To ascertain if there was a difference in Mean number of Adenomas per Procedure (MAP) between EAC and SC.
To ascertain the distribution of polyps in the colon comparing EAC and SC (including assessment of cancer detection).
To ascertain if there was a difference in the detection of sessile serrated polyps (SSP) between EAC and SC.
To establish the rate of cuff exchange (ie, how often the cuff had to be removed).
To demonstrate non-inferiority of caecal intubation rate and insertion time to caecum comparing EAC and SC.
To demonstrate non-inferiority in complete withdrawal time in procedures where no polyps were detected comparing EAC and SC.
To demonstrate non-inferiority of patient experience when comparing EAC and SC.
To measure any difference in future colonoscopic workload due to increased ADR by generating follow-up surveillance procedures based on national (British Society of Gastroenterology (BSG)) guidelines comparing EAC and SC groups.
To measure any difference in ADR between BCSP and non-BCSP colonoscopists comparing EAC and SC.
To compare the ADR of the first 20% of patients scoped by each colonoscopist with the last 20% of patients in each arm to identify any changes in ADR throughout the trial.
To compare the baseline ADR of each colonoscopist before trial recruitment with that colonoscopist’s ADR during the trial in SC cases. Baseline was calculated over a period of 6 months pretrial.
Patients were followed up for 21 days and any adverse events (AE) and serious adverse events (SAE) were reported to the Data Monitoring Committee. The chair of the Data Monitoring Committee and two independent clinicians reviewed each case to determine if events were related to the trial. Patient comfort was assessed by a validated nurse assessment questionnaire and two patient questionnaires.21
Statistical analysis
The study was powered to demonstrate a difference in ADR between EAC and SC. In calculating the sample size, different ADRs were used for BCSP (FOBt positive) and non-BCSP patients. In BCSP, ADR is 45% and in non-BCSP it is 16%.1 22 A 10% increase in BCSP and 5% increase in non-BCSP were considered clinically significant. The ratio of BCSP to non-BCSP participants was projected to be 20:80. ADR for all patients combined was predicted to be 21.8% and a 6% increase was deemed clinically significant. To demonstrate a 6% increase with a 5% significance level and 90% power using a one-sided test, 886 patients per group were required. While patients were randomised to EAC or SC based on BCSP or non-BCSP status, restrictions to ensure that recruitment was in the 20:80 ratio were not mandated.
A one-sided Χ2 test was used to compare the primary outcome between groups. Additionally, as a sensitivity analysis, logistic regression was used to re-examine group differences adjusting for stratification factors included in the randomisation process. MAP was a secondary outcome and was analysed using the Mann-Whitney U test due to the positively skewed distribution. Χ 2 test and Mann-Whitney U test were used to analyse secondary outcomes where the objective was to examine the superiority of EAC. Other secondary outcomes were examined on a non-inferiority basis. For continuous outcomes, one-sided 97.5% CI for the mean difference between groups was calculated. For binary outcomes, a one-sided 97.5% CI for the difference in proportions was calculated. Non-inferiority was assumed when the bound of the CI did not cross the prespecified point of non-inferiority. All superiority analyses were performed on an intention-to-treat (ITT) basis. Per-protocol analyses were used for outcomes analysed on a non-inferiority basis and as a sensitivity analyses for the primary outcome.
Results
A total of 3928 patients were invited to participate in the trial; 2156 patients were excluded as they were ineligible (42%), declined participation (35%) or could not be recruited for logistical reasons (23%) (table 1). Patient characteristics of recruited and excluded patients were comparable.
Table 1.
Patients excluded from the study
| Reasons | No. of patients | Gender | Age | |
| M (%) | F (%) | |||
| Not eligible | 909 | 499 (55) | 410 (45) | 62 (range 17–98) |
| Declined to participate | 749 | 347 (46) | 402 (54) | 63 (range 17–94) |
| Research team unavailable | 253 | 124 (49) | 129 (51) | 61 (range 22–88) |
| Procedure cancelled | 139 | 78 (56) | 61 (44) | 62 (range 18–88) |
| Did not attend | 100 | 60 (60) | 40 (40) | 56 (range 22–85) |
| Randomisation system maintenance | 6 | 4 (67) | 2 (33) | 67 (range 60–72) |
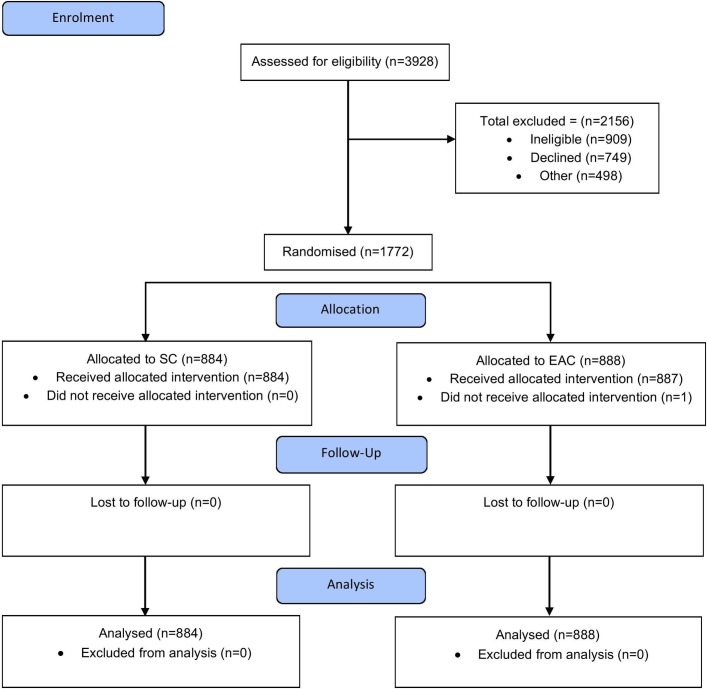
A total of 1772 patients were recruited, and the trial flow chart is illustrated in figure 3. Forty-eight colonoscopists participated in the trial, of which 17 were BCSP colonoscopists. No patients were lost to follow-up. Fifty-seven per cent patients were male with mean age 62 years. Patient characteristics were comparable in both groups (table 2). Bowel preparation was of an equivalent standard in both groups.
Figure 3.
Trial profile. EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
Table 2.
Demographics and colonoscopy indication for all patients
| SC (n=884)(%) | EAC (n=888)(%) | |
| Male | 502 (56.8) | 507 (57.1) |
| Female | 382 (43.2) | 381 (42.9) |
| Age—mean (SD) | 62.1 (11.1) | 61.7 (11.7) |
| Age <60 years | 273 (30.9) | 273 (30.7) |
| 60–73 years | 515 (58.3) | 514 (57.9) |
| 74+ years | 96 (10.9) | 101 (11.4) |
| Previous abdominal surgery | ||
| No | 542 (61.3) | 547 (61.6) |
| Yes | 342 (38.7) | 341 (38.4) |
| Recruitment | ||
| Non-BCSP patients | 481 (54.4) | 494 (55.6) |
| BCSP patients | 403 (45.6) | 394 (44.4) |
| Indications for colonoscopy | ||
| BCSP | 282 (32.0) | 274 (30.9) |
| BCSP surveillance | 88 (10.0) | 89 (10.0) |
| Colonoscopy conversion from bowel scope | 32 (3.6) | 31 (3.5) |
| Symptomatic diagnostic | 346 (39.1) | 357 (40.2) |
| Symptomatic surveillance | 135 (15.3) | 137 (15.4) |
BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
ADR was significantly higher with EAC (40.9% vs 36.2%, P=0.02) when analysed on ITT (table 3). The odds of adenoma detection were 22% higher with EAC. This was consistent when analysed per protocol. Model-based sensitivity analysis demonstrated a significant benefit of EAC in increasing ADR when adjusted by site, colonoscopist, indication for procedure and age. ADR improvement was driven by an increase in the BCSP subgroup with a 10.8% increase (61.7% with EAC vs 50.9% with SC, P<0.001) (table 4). There was a global rise in MAP for EAC (0.95 vs 0.75, P=0.02) that was driven by the BCSP subgroup (EAC 1.59 vs SC 1.20, P=0.004) (table 5).
Table 3.
Primary outcome for all patients
| Analysis | Adenoma detection | SC N (%) | EAC N (%) | % Difference (one-sided 95% CI) | One-sided P value |
| Intention to treat | No adenoma | 564 (63.8%) | 525 (59.1%) | ||
| 1+ adenomas | 320 (36.2%) | 363 (40.9%) | 4.7% (0.9% to ∞) | 0.02 | |
| PP 1* | No adenoma | 564 (63.8%) | 525 (59.2%) | ||
| 1+ adenomas | 320 (36.2%) | 362 (40.8%) | 4.6% (0.8% to ∞) | 0.02 | |
| PP 2† | No adenoma | 564 (63.8%) | 498 (58.5%) | ||
| 1+ adenomas | 320 (36.2%) | 353 (41.5%) | 5.3% (1.4% to ∞) | 0.01 |
*Omitting patients where Endocuff was not used.
†Omitting patients where Endocuff was not used and where Endocuff was removed.
EAC, EV-assisted colonoscopy; PP, per- protocol; SC, standard colonoscopy.
Table 4.
Primary outcome for subgroups
| Subgroup | SC | EAC | % Difference (one-sided 95% CI) | One-sided P value | ||
| N | % ADR | N | % ADR | |||
| BCSP patients | 403 | 50.9% | 394 | 61.7 | 10.8% (5.1% to ∞) | 0.001 |
| Non-BCSP patients | ||||||
| All | 481 | 23.9% | 494 | 24.3 | 0.4% (-4.1% to ∞) | 0.44 |
| Non-BCSP colonoscopists | 411 | 24.1% | 425 | 23.8 | −0.3% (-5.2% to ∞) | 0.54 |
| BCSP colonoscopists | 70 | 22.9% | 69 | 27.5 | 4.7% (-7.4% to ∞) | 0.26 |
ADR, adenoma detection rate; BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
Table 5.
Secondary outcomes
| Analysis | SC N (%) | EAC N (%) | Difference (one-sided 97.5% CI) | One-sided P value |
| Mean adenomas per procedure * | ||||
| Global | 0.75 (1.40) | 0.95 (1.89) | 0.20 (0.07 to ∞) | 0.02 |
| Non-BCSP | 0.37 (0.80) | 0.44 (1.24) | 0.07 (−0.04 to ∞) | 0.42 |
| BCSP | 1.20 (1.77) | 1.59 (2.32) | 0.39 (0.15 to ∞) | 0.004 |
| Polyp detection rate | ||||
| Global | 424 (48.0%) | 480 (54.1%) | 6.1% (2.2% to ∞) | 0.005 |
| Non-BCSP | 169 (35.1%) | 189 (38.3%) | 3.1% (−2.0% to ∞) | 0.16 |
| BCSP | 255 (63.3%) | 291 (73.9%) | 10.6% (5.2% to ∞) | <0.001 |
| Sessile serrated adenomas | ||||
| Global | 10 (1.1%) | 20 (2.3%) | 1.1% (0.1% to ∞) | 0.03 |
| Non-BCSP | 5 (1.0%) | 12 (2.4%) | 1.4% (0.0% to ∞) | 0.05 |
| BCSP | 5 (1.2%) | 8 (2.0%) | 0.8% (−0.7% to ∞) | 0.19 |
| Left colon adenomas | ||||
| Global | 196 (22.2%) | 232 (26.1%) | 4.0% (0.6% to ∞) | 0.03 |
| Non-BCSP | 64 (13.3%) | 71 (14.4%) | 1.1% (−2.6% to ∞) | 0.31 |
| BCSP | 132 (32.8%) | 161 (40.9%) | 8.1% (2.5% to ∞) | 0.009 |
| Right colon adenomas | ||||
| Global | 219 (24.8%) | 244 (27.5%) | 2.7% (−0.7% to ∞) | 0.10 |
| Non-BCSP | 66 (13.7%) | 74 (15.0%) | 1.3% (−2.4% to ∞) | 0.29 |
| BCSP | 153 (38.0%) | 170 (43.2%) | 5.2% (−0.5% to ∞) | 0.07 |
| Large adenomas (10+ mm) | ||||
| Global | 61 (6.9%) | 70 (7.9%) | 1.0% (−1.1% to ∞) | 0.21 |
| Non-BCSP | 11 (2.3%) | 16 (3.2%) | 1.0% (−0.8% to ∞) | 0.18 |
| BCSP | 50 (12.4%) | 54 (13.7%) | 1.3% (−2.6% to ∞) | 0.29 |
| Small adenomas (6–9 mm) | ||||
| Global | 68 (7.7%) | 94 (10.6%) | 2.9% (0.6% to ∞) | 0.02 |
| Non-BCSP | 25 (5.2%) | 19 (3.9%) | −1.4% (−3.5% to ∞) | 0.85 |
| BCSP | 43 (10.7%) | 75 (19.0%) | 5.4% (4.2% to ∞) | <0.001 |
| Diminutive adenomas (≤5 mm) | ||||
| Global | 272 (30.8%) | 307 (34.6%) | 3.8% (0.1% to ∞) | 0.04 |
| Non-BCSP | 92 (19.1%) | 102 (20.7%) | 1.5% (−2.7% to ∞) | 0.28 |
| BCSP | 180 (44.7%) | 205 (52.0%) | 7.4% (1.6% to ∞) | 0.02 |
*Mean and SD reported.
BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
Polyp detection was higher with EAC (54.1% vs 48%, P=0.005), again with the difference driven by BCSP. Left colon adenomas were significantly higher with EAC (26.1% vs 22.2%, P=0.03). Significant differences were demonstrated for patients with small (6–9 mm) and diminutive (≤5 mm) adenomas in favour of EAC. Again, the BCSP subgroup showed a significant difference between EAC and SC, while the non-BCSP subgroup did not. There were no differences for large adenomas (10+ mm), nor for the detection of right-sided adenomas. SSP detection rate was higher in the EAC arm (2.3% vs 1.1%, P=0.03). In contrast to the other outcomes, SSP detection was significantly increased with EAC only in the non-BCSP subgroup.
A total of 56 colorectal cancers (CRC) were detected with 36 patients in the EAC arm and 20 patients in the SC arm (4.1% vs 2.3%, P=0.02) (table 6). The increase in CRC detection with EAC was in the BCSP subgroup (6.6% vs 3.7%, P=0.03). There was no significant difference in the non-BCSP subgroup. When cancers were further subdivided into those diagnosed based on endoscopic appearances (recorded as a cancer endoscopically and confirmed histologically) and polyp cancers (recorded as a polyp but later found to contain cancer at histological assessment), EAC increased the detection of endoscopically found cancers globally, but polyp cancers only in the BCSP subgroup. Characteristics of cancers were comparable in the two groups with the the most common site being the sigmoid colon (17 patients).
Table 6.
Cancer detection rate
| SC (n=884) | EAC (n=888) | % Difference (one-sided 95% CI) | One-sided P value | |
| All cancers | ||||
| Global | 20 (2.3%) | 36 (4.1%) | 1.8 % (0.4% to ∞) | 0.02 |
| Non-BCSP | 5 (1.0%) | 10 (2.0%) | 1.0 % (−0.3% to ∞) | 0.11 |
| BCSP | 15 (3.7%) | 26 (6.6%) | 2.9 % (0.3% to ∞) | 0.03 |
| Endoscopically detected cancers | ||||
| Global | 19 (2.2%) | 32 (3.6%) | 1.5% (0.1% to ∞) | 0.03 |
| Non-BCSP | 4 (0.8%) | 9 (1.8%) | 1.0 % (−0.2% to ∞) | 0.09 |
| BCSP | 15 (3.7%) | 23 (5.8%) | 2.1 % (−0.3% to ∞) | 0.08 |
| Polyp cancers | ||||
| Global | 1 (0.1%) | 4 (0.5%) | 0.3 % (−0.1% to ∞) | 0.09 |
| Non-BCSP | 1 (0.2%) | 1 (0.2%) | 0.0 % (−0.5% to ∞) | 0.51 |
| BCSP | 0 (0.0%) | 3 (0.8%) | 0.8 % (0.0% to ∞) | 0.04 |
BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
EV cuff removal rate was 4.1%. The most common reason for removal was angulation in a fixed sigmoid colon (52.8% of removals). Other reasons included new cancer diagnosis (19.4%), identification of colonic strictures (16.7%) and a new diagnosis of active colitis (2.8%). The rate of EV removal was similar in both subgroups (BCSP 3.8% vs non-BCSP 4.3%).
Caecal intubation rate was equivalent in both arms (table 7). Median insertion time to caecum was 8 min with EAC and 9 min with SC (P=0.001). There was no difference in withdrawal times for procedures without polyps. When asked specifically about discomfort on anal intubation, 8.6% patients found this more uncomfortable with EAC; however, for all other measures of comfort EAC was non-inferior to SC.
Table 7.
Caecal intubation rate, insertion time and withdrawal time
| SC | EAC | Difference (one-sided 97.5% CI) | Non-inferiority margin | |
| Caecal intubation rate: N (%) | ||||
| Global | 852 (96.4%) | 858 (96.7%) | 0.4% (−1.3% to ∞) | 5% |
| Non-BCSP | 458 (95.2%) | 474 (96.0%) | 0.7% (−1.8% to ∞) | 5% |
| BCSP | 394 (97.8%) | 384 (97.7%) | −0.1% (−2.1% to ∞) | 5% |
| Insertion time: median (IQR) | ||||
| Global | 9 (6, 15) | 8 (5, 12) | −1 (−∞ to 0) | 1 min |
| Non-BCSP | 12 (8, 17) | 10 (7, 14) | −2 (−∞ to −1) | 1 min |
| BCSP | 6 (4, 11) | 7 (4, 10) | 0 (−∞ to 1) | 1 min |
| Withdrawal time*: median (IQR) | ||||
| Global | 8 (6, 11) | 8 (6, 10) | 0 (−∞ to 0) | 1 min |
| Non-BCSP | 7 (5, 10) | 7 (5, 10) | 0 (−∞ to 1) | 1 min |
| BCSP | 9 (7, 12) | 8 (6, 10) | −1 (−∞ to 0) | 1 min |
*Figures for patients where no polyps were found only.
BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
The use of hyoscine-n-butylbromide to relax the colon was more common with EAC (627 vs 568, P=0.002). This difference was greater in the non-BCSP subgroup. There were no differences between groups in the use of carbon dioxide versus air insufflation, use of position change or rectal retroflexion (table 8). EAC met the criteria for all non-inferiority measures for the use of sedation and analgesia (table 9).
Table 8.
Use of hyoscine-n-butylbromide, carbon dioxide gas, position change and rectal retroflexion
| SC (n=884) | EAC (n=888) | % Difference (one-sided 97.5% CI) | One-sided P value | |
| Hyoscine-n-butylbromide use | ||||
| Global | 568 (64.3%) | 627 (70.6%) | 6.4% (2.7% to ∞) | 0.002 |
| Non-BCSP | 259 (53.9%) | 327 (66.2%) | 12.3% (7.2% to ∞) | <0.001 |
| BCSP | 309 (76.7%) | 300 (76.1%) | −0.5% (−5.5% to ∞) | 0.57 |
| Carbon dioxide gas use | ||||
| Global | 678 (76.7%) | 672 (75.7%) | −1.0% (−4.3% to ∞) | 0.69 |
| Non-BCSP | 311 (64.7%) | 315 (63.8%) | −0.9% (−5.9% to ∞) | 0.61 |
| BCSP | 367 (91.1%) | 357 (90.6%) | −0.5% (−3.8% to ∞) | 0.59 |
| Position change | ||||
| Global | 772 (87.5%) | 718 (81.3%) | −6.2% (−9.0% to ∞) | 1.00 |
| Non-BCSP | 413 (86.0%) | 392 (79.8%) | −6.2% (− 10.2% to ∞) | 0.99 |
| BCSP | 359 (89.3%) | 326 (83.2%) | −6.1% (−10.2% to ∞) | 0.99 |
| Rectal retroflexion | ||||
| Global | 785 (88.8%) | 723 (81.4%) | −7.4% (−10.1% to ∞) | 1.00 |
| Non-BCSP | 422 (87.7%) | 401 (81.2%) | −6.6% (−10.4% to ∞) | 1.00 |
| BCSP | 363 (90.1%) | 322 (81.7%) | −8.3% (−12.4% to ∞) | 1.00 |
BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
Table 9.
Use of nitrous oxide and oxygen gas, intravenous sedation and intravenous analgesia
| SC | EAC | Difference (one-sided 97.5% CI) | Non-inferiority margin or one-sided P value | |
| Nitrous oxide and oxygen gas | ||||
| Global | 291 (32.9%) | 283 (31.9%) | −1.0% (−∞ to 3.3%) | 10% |
| Non-BCSP | 209 (43.5%) | 180 (36.4%) | −7.0% (−∞ to −0.9%) | 10% |
| BCSP | 82 (20.4%) | 103 (26.2%) | 5.9% (−∞ to 11.7%) | 10% |
| Intravenous sedation | ||||
| Global | 591 (66.9%) | 586 (66.1%) | −0.8% (−∞ to 3.6%) | 10% |
| Non-BCSP | 349 (72.6%) | 357 (72.3%) | −0.3% (−∞ to 5.3%) | 10% |
| BCSP | 242 (60.1%) | 229 (58.3%) | −1.8% (−∞ to 5.0%) | 10% |
| Intravenous analgesia | ||||
| Global | 582 (65.8%) | 588 (66.3%) | 0.5% (−∞ to 4.9%) | 10% |
| Non-BCSP | 342 (71.1%) | 360 (72.9%) | 1.8% (−∞ to 7.4%) | 10% |
| BCSP | 240 (59.6%) | 228 (58.0%) | −1.5% (−∞ to −5.3%) | 10% |
BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
The BSG surveillance guidelines were used to determine whether patients with adenomas were low (requiring no surveillance or colonoscopy in 5 years), intermediate (colonoscopy in 3 years) or high (colonoscopy in 1 year) risk.23 In the EAC arm, 15.5% were intermediate or high risk requiring surveillance compared with 13% (P=0.03) in the SC arm (table 10).
Table 10.
Patient risk group
| Patient group | Risk | SC N (%) | EAC N (%) | Two-sided P value |
| All patients | No adenoma | 564 (63.8%) | 525 (59.1%) | 0.03 |
| Low | 205 (23.2%) | 225 (25.3%) | ||
| Intermediate | 87 (9.8%) | 95 (10.7%) | ||
| High | 28 (3.2%) | 43 (4.8%) | ||
| Non-BCSP | No adenoma | 366 (76.1%) | 374 (75.7%) | 0.78 |
| Low | 93 (19.3%) | 89 (18.0%) | ||
| Intermediate | 18 (3.7%) | 25 (5.1%) | ||
| High | 4 (0.8%) | 6 (1.2%) | ||
| BCSP | No adenoma | 198 (49.1%) | 151 (38.3%) | 0.004 |
| Low | 112 (27.8%) | 136 (34.5%) | ||
| Intermediate | 69 (17.1%) | 70 (17.8%) | ||
| High | 24 (6.0%) | 37 (9.4%) |
BCSP, Bowel Cancer Screening Programme; EAC, EV-assisted colonoscopy; SC, standard colonoscopy.
There were no differences in individual colonoscopists’ ADR between the first 20% and last 20% of procedures. In addition, when comparing pretrial ADR of all colonoscopists with trial ADR in the SC arm to help assess for the Hawthorn effect, there was only an increase in ADR for one out of the 48 colonoscopists with their ADR increasing by 23.3% (P<0.01). There was no difference in experience of colonoscopists between the two arms of the study. Olympus colonoscopes were used in 1760/1772 cases and there was no difference in type of scope found between both study arms.
There were 23 AEs of which 11 were in the EAC arm. AEs were reported to the Data Monitoring Committee and analysed by two independent clinicians. No AEs were judged to be related to use of EV.
Discussion
This multicentre RCT demonstrated that EV significantly improved ADR, MAP and cancer detection, driven by improvement in bowel cancer screening patients. ADR is widely accepted as the most important contemporaneous marker of colonoscopy quality with low ADR strongly linked to higher PCCRC rates. These results are highly significant with major potential clinical impact.3 24 In a Polish study, colonoscopists with an ADR of <20% had an HR for PCCRC 10 times higher than colonoscopists with an ADR of >20% (ADR ≥20%, absolute risk 0.011% vs ADR <20% absolute risk 0.115%).3 An American study found an inverse relationship between ADR and risk of PCCRC, advanced-stage PCCRC and fatal PCCRC.4 A 1% increase in ADR was associated with a 3% reduction in PCCRC and a 5% reduction in fatal PCCRC.4 If results of the current trial mirrored this study, EV could reduce the risk of PCCRC by 14% and fatal PCCRC by 24%.
The increase in ADR, MAP and cancer in the EAC arm was driven by BCSP patients. These patients were FOBt positive and consequently had high rates of neoplastic pathology. These results suggest that EV improves visualisation and ADR in a population where neoplastic pathology is more common. ADR also relies on colonoscopists being observant, understanding pathology and rigorous in removing lesions. BCSP colonoscopists are accredited to a minimum standard in these areas and it may be that EV works most effectively when used by colonoscopists with these skills. ADR in both subgroups was higher than predicted but mirrors recent improvements in English data. These higher rates might also represent improved performance in a trial setting; however, most colonoscopists did not significantly improve their ADR above pretrial levels in the SC arm making this explanation unlikely. While this trial stratified for the BCSP subgroup in both arms, it did not mandate the proportion of overall patients recruited as BCSP and non-BCSP meaning that recruitment differed from the anticipated 20/80 ratio. Over-recruitment of BCSP patients most likely occurred because of the optimal research environment found in BCSP, with research motivated clinicians and nurses, leading to high levels of recruitment in this setting.
Increased detection of adenomas with EV was in the left colon where colonic folds are most prominent. EV assists detection by holding back and everting colonic folds and allowing them to slowly revert to their anatomical position, thereby improving mucosal visualisation. Improved detection was not mirrored in the right colon where the colon is generally straighter with less folds. Right-sided cancers and the failure of screening programmes to protect against them are becoming an increasing problem and it is unlikely EV would address this issue. As might be expected, EV improved detection of small and diminutive polyps but not larger polyps; however, somewhat surprisingly cancer detection was improved. While this was not expected, previous studies have demonstrated that while the greatest miss rates are for small lesions, miss rates for large lesions are still significant.25 In addition to the holding back of mucosal folds, the EV also stabilises colonoscope position and can help prevent slippage back of the scope avoiding rapid slide by of areas of mucosa. This stabilisation could provide an explanation for improved cancer detection; however, one would expect cancer detection to mirror detection of large polyps. Therefore, although the increase in cancer detection was significant and should not be ignored (with the authors unable to find another explanation for this finding), the disparity between cancer and large polyp detection warrants cautious interpretation of the increased cancer detection. EV did increased the detection of SSPs; however, this was a small change and the overall detection rate of SSPs was low. Therefore, the clinical significance of this finding should not be overinterpreted.
The benefits of improving ADR on reducing PCCRC rates have been demonstrated in populations directly screened by colonoscopy. However, it is not possible to fully quantify the effect of a 10.8% rise in ADR in FOBt-positive BCSP patients as no data exist on long-term outcomes in this population. In addition, the ceiling at which further improvement in ADR confers no additional patient benefit has not been established. Nevertheless, an increase of 10.8% in ADR in a screening population is likely to be highly significant.
ADR is the most widely used quality marker but the importance of MAP is growing as high-quality colonoscopy should find all adenomas in a patient, whereas where ADR is used a single adenoma positively affects this key performance indicator.16 Parallel improvement in both ADR and MAP in this study add to its weight and suggest genuine clinical benefit associated with EV use. Withdrawal times were equivalent in both groups. The improved detection without prolongation of the procedure may reflect improved efficiency with EV conferred by stabilisation of the colonoscope tip and less need for backward and forward manoeuvring of the tip to see around folds. Insertion times were quicker with EV and this may be due to the ability of EV to stabilise the scope tip during scope straightening manoeuvres.
Earlier trials of the original Endocuff reported improvements in ADR of up to 14.7%,26 27 but results were not replicated in a large Dutch trial.16 Studies of the original Endocuff were also limited by the reporting of high rates of mucosal abrasions. EV differs from the original Endocuff in having only one row of projections that are softer and 2 mm longer. The single ring of softer projections appears to be more manoeuvrable and do not cause the same abrasions. Abrasions were not observed in this study. The results of the current trial are similar to those of pilot studies, which showed a 16% improvement in ADR in a screening setting.28 In contrast, a single-centre trial of EV has recently reported no improvement in ADR.29 This was, however, a smaller trial with numbers that may have been insufficient to demonstrate the results shown in the current trial. Additionally, this trial reported an extremely high ADR in the control arm and increasing that ADR with EV would have been very difficult.
Devices attached to colonoscopes should not hinder the procedure, increase discomfort or cause AEs. In addition, it is important that devices should not need to be removed often. EV did not hinder colonic intubation and intubation time was quicker when EV was used—an EV cuff removal rate of 4.1% is an acceptably low level. Colonic spasm may hinder insertion during any colonoscopy and this may be more of an issue when EV is used. Use of hyoscine-n-butylbromide was higher in the EAC arm and it is likely that colonoscopists were more likely to use antispasmodics to aid insertion. Hyoscine-n-butylbromide is used widely in BCSP as standard practice and this is likely to explain the lack of increase in use in this group.
EAC was non-inferior in almost all measures of patient experience with patients reporting no difference in experience overall when EV was used. When asked specifically regarding anal insertion, EAC was reported to be slightly more uncomfortable. Where colonoscopy is undertaken under deep sedation, this is not likely to be an issue but where light or no sedation is used, adequate cuff lubrication and gentle insertion technique should be optimised to minimise any anal discomfort. EV was safe and did not cause any AEs in this trial.
Many studies purporting to demonstrate benefits of new technologies are conducted in expert (often single) centres. This study was undertaken in a mixture of academic and community settings meaning that results are generalisable to standard clinical practice. Other studies have reported adenoma miss rate or other markers of quality; however, ADR is the most widely used marker and has been shown to correlate strongly with PCCRC rates and therefore was chosen as the primary outcome measure. One limitation of this study is that despite being an RCT it could not be performed with operator blinding as the cuff is visible on insertion, the projections can be clearly seen holding back folds and indeed EV can only be used correctly if the endoscopists knows it is there. Tandem studies have previously been used to compare devices in colonoscopy to identify missed lesions. However, an RCT comparing ADR in two equivalent arms allowing for confounders is the optimal way to study this intervention. As this study compared ADR in both arms and both arms involved a single colonoscopy, missed adenomas are unlikely to have a significant impact on results. Other measures known to improve ADR were comparable in the two groups.
EV provides a method of improving ADR that is simple, safe, well tolerated and at relatively low cost. EV improves detection of left-sided adenomas and has potential to benefit flexible sigmoidoscopy screening programme as well as those that focus on colonoscopy.30
Conclusion
The ADENOMA study demonstrated that EV significantly improved ADR, MAP and cancer detection, most clearly noticeable in FOBt-positive screening patients. EV facilitated quicker colonic intubation and was non-inferior in all aspects of patient comfort other than causing minimal discomfort on anal intubation. EV should be recommended for use in patients with high risk of having adenomas such as those undergoing colonoscopy following a positive FOBt.
Acknowledgments
The authors would like to thank members of the Trial Steering Committee (Professor Mark Hull, Dr James East, Mr Colin Everett and Mrs Carol West), Data Monitoring Committee (Professor Michael Bramble, Professor John McLaughlin, Dr Ben Carter and Dr Anjan Dhar), research nurses, members of the research team and patients at all participating sites who took part in the study.
Footnotes
Contributors: WSN: trial manager. Contributed to study design, ran the study, recruited patients to the study, analysed results, reviewed and was the main author of the manuscript. RB: designed the study, contributed to running the study, reviewed and contributed to the writing of the manuscript. ZPT: contributed to study design, analysed results, reviewed and contributed to the writing of the manuscript. PB: trial statistician. Contributed to study design, analysed results, reviewed and contributed to the writing of the manuscript. ZH: clinical trials unit statistician. Contributed to study design, reviewed analysis, reviewed and contributed to the writing of the manuscript. MDR: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. GC: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. NT: clinical trials unit data manager. Contributed to study design, data custodian during trial conduct, reviewed analysis, reviewed and contributed to the writing of the manuscript. TJL: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. AR: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. JGS: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. JP: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. LJN: contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. BPS: designed the study, contributed to running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript. CJR: Chief Investigator. Designed the study, contributed to the running of the study, recruited patients to the study, analysed results, reviewed and contributed to the writing of the manuscript.
Funding: This was an investigator-led, industry funded trial adopted onto the UK National Institute for Health Research Portfolio. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Competing interests: CJR has received research grants from ARC Medical, Olympus Medical, Aquilant Endoscopy, Norgine and travel grants from Boston Scientific and Cook Medical. He is an advisory board member for Ai4Gi. BPS has received speaker grants from Olympus Medical and research support from Norgine, Aquilant and Diagmed. He is an advisory board member for Creo Medical. ZPT was a non-paid speaker for Norgine Pharmaceutical. He has received research and educational grants from Norgine Pharmaceutical and medical equipment support from Olympus. He holds a Consultant Agreement for Creo Medical.
Ethics approval: North East-York Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Lee TJ, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: experience from the NHS Bowel cancer screening programme. Gut 2012;61:1050–7. 10.1136/gutjnl-2011-300651 [DOI] [PubMed] [Google Scholar]
- 2. Rees CJ, Thomas Gibson S, Rutter MD, et al. UK key performance indicators and quality assurance standards for colonoscopy. Gut 2016;65:1923–9. 10.1136/gutjnl-2016-312044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362:1795–803. 10.1056/NEJMoa0907667 [DOI] [PubMed] [Google Scholar]
- 4. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–306. 10.1056/NEJMoa1309086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 2007;132:96–102. 10.1053/j.gastro.2006.10.027 [DOI] [PubMed] [Google Scholar]
- 6. Cooper GS, Xu F, Barnholtz Sloan JS, et al. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer 2012;118:3044–52. 10.1002/cncr.26602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut 2014;63:957–63. 10.1136/gutjnl-2013-304880 [DOI] [PubMed] [Google Scholar]
- 8. Clark BT, Rustagi T, Laine L. What level of bowel prep quality requires early repeat colonoscopy: systematic review and meta-analysis of the impact of preparation quality on adenoma detection rate. Am J Gastroenterol 2014;109:1714–23. 10.1038/ajg.2014.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee TJ, Blanks RG, Rees CJ, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the Bowel Cancer Screening Programme in England. Endoscopy 2013;45:20–6. 10.1055/s-0032-1325803 [DOI] [PubMed] [Google Scholar]
- 10. Corte C, Dahlenburg L, Selby W, et al. Hyoscine butylbromide administered at the cecum increases polyp detection: a randomized double-blind placebo-controlled trial. Endoscopy 2012;44:917–22. 10.1055/s-0032-1310009 [DOI] [PubMed] [Google Scholar]
- 11. Munroe CA, Lee P, Copland A, et al. A tandem colonoscopy study of adenoma miss rates during endoscopic training: a venture into uncharted territory. Gastrointest Endosc 2012;75:561–7. 10.1016/j.gie.2011.11.037 [DOI] [PubMed] [Google Scholar]
- 12. East JE, Bassett P, Arebi N, et al. Dynamic patient position changes during colonoscope withdrawal increase adenoma detection: a randomized, crossover trial. Gastrointest Endosc 2011;73:456–63. 10.1016/j.gie.2010.07.046 [DOI] [PubMed] [Google Scholar]
- 13. Dik VK, Moons LM, Siersema PD. Endoscopic innovations to increase the adenoma detection rate during colonoscopy. World J Gastroenterol 2014;20:2200–11. 10.3748/wjg.v20.i9.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morgan J, Thomas K, Lee-Robichaud H, et al. Transparent Cap Colonoscopy versus Standard Colonoscopy for Investigation of Gastrointestinal Tract Conditions. Cochrane Database Syst Rev 2011;2:CD008211 10.1002/14651858.CD008211.pub2 [DOI] [PubMed] [Google Scholar]
- 15. Biecker E, Floer M, Heinecke A, et al. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol 2015;49:413–8. 10.1097/MCG.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 16. van Doorn SC, van der Vlugt M, Depla A, et al. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut 2017;66:438–45. 10.1136/gutjnl-2015-310097 [DOI] [PubMed] [Google Scholar]
- 17. Lenze F, Beyna T, Lenz P, et al. Endocuff-assisted colonoscopy: a new accessory to improve adenoma detection rate? Technical aspects and first clinical experiences. Endoscopy 2014;46:610–4. 10.1055/s-0034-1365446 [DOI] [PubMed] [Google Scholar]
- 18. Marsano J, Tzimas D, Razavi F, et al. The Learning Curve for Endocuff Assisted Colonoscopy [abstract]. Gastrointest Endosc 2014. [Google Scholar]
- 19. Bevan R, Ngu WS, Saunders BP, et al. The ADENOMA Study. Accuracy of Detection using Endocuff Vision™ Optimization of Mucosal Abnormalities: study protocol for randomized controlled trial. Endosc Int Open 2016;4:E205–12. 10.1055/s-0041-107900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Russell D, Hoare ZS, Whitaker R, et al. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:n/a–34. 10.1002/sim.4175 [DOI] [PubMed] [Google Scholar]
- 21. Rostom A, Ross ED, Dubé C, et al. Development and validation of a nurse-assessed patient comfort score for colonoscopy. Gastrointest Endosc 2013;77:255–61. 10.1016/j.gie.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 22. Rajasekhar PT, Rutter MD, Bramble MG, et al. Achieving high quality colonoscopy: using graphical representation to measure performance and reset standards. Colorectal Dis 2012;14:1538–45. 10.1111/j.1463-1318.2012.03057.x [DOI] [PubMed] [Google Scholar]
- 23. Atkin WS, Saunders BP. British Society for Gastroenterology Association of Coloproctology for Great Britain and Ireland. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002;51(Suppl 5):v6–v9. 10.1136/gut.51.suppl_5.v6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rees CJ, Bevan R, Zimmermann-Fraedrich K, et al. Expert opinions and scientific evidence for colonoscopy key performance indicators. Gut 2016;65:2045–60. 10.1136/gutjnl-2016-312043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006;101:343–50. 10.1111/j.1572-0241.2006.00390.x [DOI] [PubMed] [Google Scholar]
- 26. Floer M, Biecker E, Fitzlaff R, et al. Higher adenoma detection rates with endocuff-assisted colonoscopy - a randomized controlled multicenter trial. PLoS One 2014;9:e114267 10.1371/journal.pone.0114267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Palma GD, Giglio MC, Bruzzese D, et al. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: a randomized back-to-back study. Gastrointest Endosc 2018;87:232–40. 10.1016/j.gie.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 28. Tsiamoulos ZP, Misra R, Rameshshanker R, et al. Impact of a new distal attachment on colonoscopy performance in an academic screening center. Gastrointest Endosc 2017;5107:31789–3. [DOI] [PubMed] [Google Scholar]
- 29. Bhattacharyya R, Chedgy F, Kandiah K, et al. Endocuff-assisted vs. standard colonoscopy in the fecal occult blood test-based UK Bowel Cancer Screening Programme (E-cap study): a randomized trial. Endoscopy 2017;49:1043–50. 10.1055/s-0043-111718 [DOI] [PubMed] [Google Scholar]
- 30. Bevan R, Rubin G, Sofianopoulou E, et al. Implementing a national flexible sigmoidoscopy screening program: results of the English early pilot. Endoscopy 2015;47:225–31. 10.1055/s-0034-1378119 [DOI] [PubMed] [Google Scholar]