Abstract
Background
Natural killer cell responses to virally-infected or transformed cells depend on the integration of signals received through inhibitory and activating natural killer cell receptors. Human Leukocyte Antigen null cells are used in vitro to stimulate natural killer cell activation through missing-self mechanisms. On the other hand, CEM.NKr.CCR5 cells are used to stimulate natural killer cells in an antibody dependent manner since they are resistant to direct killing by natural killer cells. Both K562 and 721.221 cell lines lack surface major histocompatibility compatibility complex class Ia ligands for inhibitory natural killer cell receptors. Previous work comparing natural killer cell stimulation by K562 and 721.221 found that they stimulated different frequencies of natural killer cell functional subsets. We hypothesized that natural killer cell function following K562, 721.221 or CEM.NKr.CCR5 stimulation reflected differences in the expression of ligands for activating natural killer cell receptors.
Results
K562 expressed a higher intensity of ligands for Natural Killer G2D and the Natural Cytotoxicity Receptors, which are implicated in triggering natural killer cell cytotoxicity. 721.221 cells expressed a greater number of ligands for activating natural killer cell receptors. 721.221 expressed cluster of differentiation 48, 80 and 86 with a higher mean fluorescence intensity than did K562. The only ligands for activating receptor that were detected on CEM.NKr.CCR5 cells at a high intensity were cluster of differentiation 48, and intercellular adhesion molecule-2.
Conclusions
The ligands expressed by K562 engage natural killer cell receptors that induce cytolysis. This is consistent with the elevated contribution that the cluster of differentiation 107a function makes to total K562 induced natural killer cell functionality compared to 721.221 cells. The ligands expressed on 721.221 cells can engage a larger number of activating natural killer cell receptors, which may explain their ability to activate a larger frequency of these cells to become functional and secrete cytokines. The few ligands for activating natural killer cell receptors expressed by CEM.NKr.CCR5 may reduce their ability to activate natural killer cells in an antibody independent manner explaining their relative resistance to direct natural killer cell cytotoxicity.
Electronic supplementary material
The online version of this article (10.1186/s12865-018-0272-x) contains supplementary material, which is available to authorized users.
Keywords: NK cells, Activating NK receptor ligands, HLA null cells, K562, 721.221, CEM.NKr.CCR5
Background
Natural Killer (NK) cells are a subset of lymphocytes that direct innate immune responses to kill stressed, virally-infected, and transformed cells [1]. NK cells belong to the group 1 innate lymphoid cells (ILCs) as do ILC1s [2–4]. NK cells can interact with target cells and lyse them directly via the release of cytotoxic granules containing perforin and granzyme [5, 6]. Activated NK cells can also secrete a broad range of cytokines and chemokines that can activate adaptive immune cells to lyse target cells, bridging the innate and adaptive immune systems [7–9]. Regardless of the method used by NK cells to lyse target cells, they must first be activated to elicit a response. The activation state of an NK cell results from the integration of different signals transmitted through its activating and inhibitory natural killer cell receptors (aNKR and iNKR) [9, 10]. Activation can result from the loss of inhibitory signaling, when there are no ligands for iNKR to engage, in conjunction with sustained aNKR signaling, or from the engagement of aNKR by their ligands that overwhelms inhibitory signaling through iNKR [8, 10, 11]. However, in vivo, NK cells interacting with target cells will receive a variety of signals through both receptor types and whether this results in activation or not depends on the number and strength of each type of signal transmitted.
A common method to activate NK cells is to co-culture them with human leukocyte antigen (HLA)-null cells. The most frequently used HLA-null cells include the myelogenous leukemia K562 and B-lymphoblastoid 721.221 (.221) cells lines, which do not express the major histocompatibility complex class I (MHC-I) HLA-A, -B, or -C antigens on their surface [12–14]. As these cells are incapable of engaging the inhibitory killer immunoglobulin-like receptors (KIR) on NK cells, which recognize subsets of HLA-A, -B, and -C, inhibitory signaling through these receptors is abrogated. As signals from these inhibitory receptors oppose those from aNKR, their removal allows the engagement of aNKR by their ligands to activate NK cells [15].
Previous work from our lab demonstrated that the K562 and .221 HLA-null cell lines stimulated NK cells differentially to secrete the cytokine interferon-γ (IFN-γ), the chemokine CCL4, and to express the degranulation marker CD107a [16]. Specifically, we observed that .221 activated a greater fraction of NK cells than did K562 and that stimulation with .221 preferentially induced IFN-γ and CCL4 secretion, while K562 more potently stimulated degranulation [16]. In the absence of the expression of the major ligands for iNKR on the surface of K562 and .221, it is likely that ligands to aNKR regulate NK cell activation and differences in their expression profiles might explain their different capacities to activate NK cells. Indeed, the expression of some ligands to aNKR was shown to differ on both cell lines, although these differences remain incompletely characterized [17–22].
CEM.NKr.CCR5 cells are commonly used to stimulate NK cells by antibody dependent cellular cytotoxicity (ADCC) [23, 24]. They were derived from CEM.NKr cells that were selected from the parental CEM T cell line for their resistance to direct NK cell lysis [25]. CEM cells express HLA-A, B and C antigens and the aNKR CD16, whose engagement is important for ADCC activity [25, 26]. The aNKR profile of this cell line is poorly defined.
There are two major classes of aNKR. The first is the C-type lectin NKG2D receptor that binds to a family of MHC-I like molecules expressed on healthy cells only after periods of cellular stress [17, 27]. These include the human cytomegalovirus UL-16 binding proteins (ULBP) and MHC-I related chain (MIC) proteins [19, 28, 29]. The second class of receptors includes the natural cytotoxicity receptors (NCR) NKp30, NKp44, and NKp46, which can bind to membrane-associated heparan sulfate glycosaminoglycans, viral hemagglutinin and β-1,3-glucan [30–40]. Despite these findings, the cellular ligands to NCRs remain incompletely defined.
In addition to the two major aNKR families, signaling through several other receptors can contribute to NK cell activation. These include cluster of differentiation (CD)244/2B4 and the NK-T-B cell antigen (NTB-A), which are CD2 family receptors that engage CD48 to trigger NK cell cytotoxicity [41]. Another receptor, expressed on virtually all NK cells, is the leukocyte adhesion molecule DNAX accessory molecule-1 (DNAM-1) that binds to CD112 (Nectin-2) and CD155 (poliovirus receptor) ligands [18, 42, 43]. Signaling through DNAM-1 can stimulate NK cells when it is co-expressed with the lymphocyte function-associated antigen 1 (LFA-1). LFA-1 can bind the integrins intercellular adhesion molecule (ICAM)-1 and ICAM-2 on target cells, bridging NK and target cells and forming the immunological synapse [44–48]. Additionally, target cells expressing the T cell co-stimulatory B7 molecules CD80 and CD86 can stimulate NK cells [49–51]. The nature of this signaling is still poorly understood, but it has been suggested that NK cell activation by CD80 and CD86 depends on a CD28 variant expressed on NK cells [50, 52].
In contrast to HLA-A, -B, and -C, which signal through iNKRs, the non-classical HLA-E and -F can contribute to NK cell activation through the engagement of aNKRs. Much like its classical MHC-I counterparts, HLA-E interacts with the inhibitory C-type lectin-like receptor NKG2A, which heterodimerizes with CD94, and with the aNKRs NKG2E and -C [53–55]. HLA-F is a more recent addition to the family of ligands to aNKR and has been shown to stimulate degranulation and cytokine production through its binding to KIR3DS1 on NK cells [56–58].
To determine whether the differences observed in the frequency and function of NK cell subsets stimulated by K562, .221 and CEM.NKr.CCR5 cells are related to differences in their expression profiles of ligands to aNKRs, we analyzed the expression of a comprehensive panel of aNKR ligands on these cell lines by multi-parametric flow cytometry. As ligands to inhibitory KIR are not expressed on HLA-null cell lines, it is likely that expression of ligands to aNKRs plays a role in modulating differential NK cell responses to K562 and .221. Although others have proposed that the resistance of CEM.NKr.CCR5 cells to direct NK cell cytolysis is related to changes in cell surface marker expression that occurred when NK cell resistance to cytolysis was selected for, the ligands for aNKR have not been profiled on this cell line [25]. We report here a characterization of the aNKR ligand phenotype of three NK cell stimuli to contribute to a better understand the mechanisms governing their behavior as inducers of NK cell function and targets for NK cells cytolysis.
Methods
K562, .221 and CEM.NKr.CCR5 cell lines
K562 cells (American Type Culture Collection, Manassas, VA) .221 cells (a kind gift from Dr. Galit Alter, Harvard University) and CEM.NKr.CCR5 cells (National Institutes of Health [NIH] AIDS Reagent Program, Division of AIDS, NIAID, NIH, from Dr. Alexandra Trkola) were cryopreserved in 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) with 90% fetal bovine serum (FBS, Wisent Bio Porducts, St-Jean-Baptist, QC, Canada). K562 and .221 cells were verified to be HLA-null by staining with the pan HLA monoclonal antibody (mAb) W6/32 and a β2-microglobulin specific mAb [58]. CEM.NKr.CCR5 cells were typed for HLA allotypes using sequence based HLA typing methods and found to express HLA allotypes that were consistent with those previously reported for their parental CEM and CEM.NKr cell lines [25, 59]. Cell lines were thawed and cultured in RPMI 1640 medium supplemented with 10% FBS; 2 mM L-glutamine; 50 IU/mL penicillin; 50 mg/mL streptomycin (R10, all from Wisent) for at least one passage before staining. Cells were passaged three times a week and maintained in culture for a maximum of 1 month. Only healthy cells with a high viability were used in staining experiments.
Antibody staining and acquisition
Cell lines were stained in triplicate on 1 or 2 occasions or in replicates of six on 1 occasion with UV Live/Dead ® Fixable Blue cell stain kit, as per manufacturer’s directions (Thermofisher, Waltham, MA) and were stained with mAbs clones or chimeric proteins specific for cell surface aNKR ligands organized into 4 panels for the purpose of analysis. Table 1 shows for each mAb and chimeric protein used, its aNKR ligand specificity, the aNKR each ligand recognized, the designation of the antibody clone used for staining, its commercial source and to which fluorochrome antibodies or secondary antibodies recognizing chimeric proteins or primary antibodies were conjugated. Panel 1 included antibodies specific for ULBP-1-PerCP (170818), ULBP-2/5/6-APC (165903), ULBP-3-PE (166510), MIC-A-APC (159227), MIC-B-AlexaFluor 700 (236511; all from R&D Systems, Minneapolis, MN), Panel 2 was made up of antibodies to CD48-PE (BJ40), CD80-BV421 (2D10), CD86-PE-Dazzle594 (IT2.2; all from BioLegend, San Diego, CA), CD112-AlexaFluor 700 (610603; R&D), CD155-PE-Cy7 (SKII.4). Panel 3 comprised antibodies to ICAM-1-Pacific Blue (HCD54), and ICAM-2-PE (CBR-1C2/2; both from BioLegend). Cells were also stained with recombinant human IgG1 Fc chimeric proteins NKp30-Fc, NKp44-Fc, and NKp46-Fc (all from R&D) as a part of Panel 3. A final HLA Panel was composed of the fluorochrome conjugated antibody to HLA-E-PE-Cy7 (3D12; BioLegend), as well as an unconjugated recombinant human IgG1 Fc chimera KIR3DS1-Fc (R&D) and mAb 3D11, an unconjugated primary antibody against HLA-F (a kind gift from Dr. Daniel Geraghty, Fred Hutchison Research Institute, Seattle, WA). Briefly, cells were resuspended in a 96-well V-bottomed plate (Sarstedt, Nümbrecht, Germany) at a concentration of 1 × 106 cells per 100 μL of Dulbecco’s phosphate buffered saline (Wisent) and stained with the Live/Dead reagent. TruStain FcX reagent (BioLegend) was used to minimize non-specific Fc receptor interactions and cells were stained with conjugated mAbs from Panel 1, 2, 3, and HLA for 30 min in the dark at room temperature (RT). For staining with any of the recombinant human IgG1 Fc chimeric proteins or unconjugated mAbs 3D11 and 3D12, cells were prepared as previously described, with the exception that they were stained with chimeric proteins or primary mAb on ice for 40 min. After washing, binding of Fc chimeric proteins was detected using a polyclonal anti-human IgG (Fcγ-specific)-PE conjugated secondary antibody (BioSciences), while 3D11 and 3D12 binding was detected using a polyclonal F (ab’)2 anti-mouse IgG-APC conjugated secondary antibody (eBioscience) for 20 min on ice. Following staining, all cells were washed and fixed with 2% paraformaldehyde (Santa Cruz, Dallas TX). Between 400,000 and 600,000 events were acquired on an LSRFortessa × 20 within 24 h (BD). Unstained, single stained controls (CompBead; BD), fluorescence minus one (FMO), and secondary antibody alone controls were used for multi-color compensation and gating purposes. An additional isotype control for the secondary antibodies used to detect KIR3DS1-Fc chimeric protein and unconjugated 3D11 mAb binding to HLA-F were also used. As staining with the antibody specific for ULBP-1 generated signals with a low mean fluorescence intensity (MFI), an isotope control for this mAb was also used as a control in addition to the above-mentioned controls. Flow cytometric analysis was performed using FlowJo software version 10 (TreeStar, Ashland OR).
Table 1.
Antibody/Chimeric Protein Panel Composition
| NK cell Receptor ligand specificity | aNKR/iNKR | Clone | Source | Fluorochrome |
|---|---|---|---|---|
| Panel 1 | ||||
| ULBP-1 | NKG2D | 170818 | R & D Systems | PerCP |
| ULBP-2/5/6 | NKG2D | 165903 | R & D Systems | APC |
| ULBP-3 | NKG2D | 166510 | R & D Systems | PE |
| MIC-A | NKG2D | 159227 | R & D Systems | APC |
| MIC-B | NKG2D | 236511 | R & D Systems | AlexaFluor 700 |
| Panel 2 | ||||
| CD48 | 2B4 | BJ40 | BioLegend | PE |
| CD80 | Unknown for NK cells | 2D10 | BioLegend | BV421 |
| CD86 | Unknown for NK cells | IT2.2 | BioLegend | PE-Dazzle 594 |
| CD112 | DNAM-1 | 610603 | R & D Systems | AlexaFluor 700 |
| CD155 | DNAM-1 | SKII.4 | BioLegend | PE-Cy7 |
| Panel 3 | ||||
| ICAM-1 | LFA-1 | HCD54 | BioLegend | Pacific Blue |
| ICAM-2 | LFA-1 | CBR-1C2/2 | BioLegend | PE |
| Unknown | NKp30 | NKp30-Fc | R & D Systems | Unconjugateda |
| Unknown | NKp44 | NKp44-Fc | R & D Systems | Unconjugateda |
| Unknown | NKp46 | NKp46-Fc | R & D Systems | Unconjugateda |
| HLA Panel | ||||
| HLA-F | KIR3DS1 | 3D11 | BioLegend | Unconjugatedb |
| HLA-F (KIR3DS1-Fc) | KIR3DS1 | – | R & D Systems | Unconjugateda |
| HLA-E | NKG2A | 3D12 | BioLegend | Unconjugatedb |
aChimeric protein binding was detected using a polyclonal anti-human IgG (Fcγ-specific)-PE conjugated secondary antibody (BioSciences)
b3D11 and 3D12 binding was detected using a polyclonal F(ab’)2 anti-mouse IgG-APC conjugated secondary antibody (eBioscience)
Results are presented as the MFI of cells stained with mAbs/Fc chimeric protein to each aNKR ligand versus FMO/isotype control/secondary antibody alone staining. The MFI of staining for individual ligands was reported as background subtracted FMO, isotype control or secondary antibody alone MFI. The control used to background subtract staining for each condition is indicated in Table 2.
Table 2.
Mean fluorescence intensity (MFI) of activating NK receptor ligand staining on K562, .221 and CEM.NKr.CCR5 cells by specific antibodies and chimeric proteins
| Ligand ID | Cell Stained | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K562 | .221 | CEM.NKr.CCR5 | ||||||||||
| MFIa | Range MFI | Ctlb | Background subtracted MFI | MFI | Range MFI | Ctl | Background subtracted MFI | MFI | Range MFI | Ctl | Background subtracted MFIg | |
| Panel 1 | ||||||||||||
| ULBP-1 | 895 | 852, 935 | 586c | 309 | 389 | 382, 396 | 413c | − 24 | 276 | 271, 281 | 201 | 75c |
| ULBP-2/5/6 | 3779 | 3685, 4905 | 503d | 3276 | 93.5 | 85, 123 | 79d | 14.5 | 115 | 101, 127 | 24d | 90 |
| ULBP-3 | 1480 | 1219, 1895 | 749d | 731 | 396 | 374, 437 | 379d | 17 | 191 | 164, 223 | 159d | 32 |
| MIC-A | 1065 | 923, 1192 | 357d | 707 | 710 | 587, 1212 | 196d | 514 | 170 | 164, 183 | 156d | 16 |
| MIC-B | 786 | 784, 793 | 204d | 514 | 667 | 468, 877 | 113d | 554 | 107 | 94, 118 | 40d | 67 |
| Panel 2 | ||||||||||||
| CD48 | 1044 | 788, 2347 | 169.2d | 874.5 | 43,843 | 30,131, 54,430 | 299d | 43,544 | 10,684 | 5081, 16,047 | 90d | 10,594 |
| CD80 | 8980.5 | 7293, 10,778 | 527d | 8453 | 45,424 | 25,478, 65,004 | 526d | 44,898 | 469 | 425, 807 | 449d | 20 |
| CD86 | 355 | 317, 367 | 264d | 90 | 55,878 | 53,432, 56,184 | 22d | 55,856 | 85 | 52, 112 | 67d | 17 |
| CD112 | 3211 | 2485, 3923 | 296d | 2915 | 1.5 | 0.1, 12.1 | 1.6d | − 0.12 | 544 | 470, 675 | 270d | 234 |
| CD155 | 6187.5 | 5368, 6955 | 135d | 6052 | 70.8 | 67, 73 | 51d | 19.8 | 45 | 42, 54 | 28d | 18 |
| Panel 3 | ||||||||||||
| ICAM-1 | 5087 | 4656, 11,162 | 497d | 4590 | 16,875 | 11,852, 22,761 | 612d | 16,875 | 203 | 185, 220 | 117d | 86.5 |
| ICAM-2 | 54,429 | 50,356, 57,335 | 227d | 54,201 | 60,331 | 44,271, 78,093 | 379d | 59,952 | 61,416 | 47,186, 87,979 | 103d | 61,313 |
| NKp30 | 1173 | 1152, 1217 | 157e | 1016 | 182 | 165, 209 | 208e | − 25 | 160 | 116, 189 | 78e | 82 |
| NKp44 | 668 | 619, 836 | 157e | 511 | 406 | 373, 469 | 208e | 145 | 297 | 194, 350 | 78e | 218 |
| NPp46 | 302 | 165, 324 | 157e | 145 | 299 | 197, 450 | 208e | 91 | 268 | 209, 314 | 78e | 190 |
| HLA Panel | ||||||||||||
| HLA-F (3D11) | 10,690 | 7197, 13,867 | 1252c,e | 9438f | 5032 | 4905, 6218 | 236c,e | 4796f | 1710 | 1608, 1881 | 189e | 1521 |
| HLA-F (KIR3DS1-Fc) | 9630 | 6437, 12,587 | 416c,e | 9219f | 8093 | 5789, 9287 | 236c,e | 7856f | 2861 | 2578, 3600 | 187e | 3380 |
| HLA-E | 280 | 208, 359 | 12e | 267 | 171 | 99, 250 | 2.5e | 169 | 477 | 435, 690 | 48e | 428 |
aMedian of 6 replicates, MFI – mean fluorescence intensity
bCtl = Control staining
cControl staining where control was an isotype control
dControl staining where control was fluorescence minus one
eControl staining where control was a fluorochrome conjugated secondary antibody alone
fThe isotype control was used for background correction
gAverage of 6 replicates
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, Inc., La Jolla, CA). Mann-Whitney tests were used to assess the significance of differences in the MFI of each aNKR ligand’s cell surface expression level compared to their respective control staining conditions. Kruskal-Wallis tests with Dunn’s post tests were used to determine the significance of differences in the background subtracted MFI generated by staining K562, .221 and CEM.NKr.CCR5 for each of the cell surface aNKR ligands tested. The distribution of the MFI of ligand expression is reported as median (range) of the replicate values. P-values less than 0.05 were considered significant.
Results
The MFI of aNKR ligand expression on K562, .221 and CEM.NKr.CCR5 cells
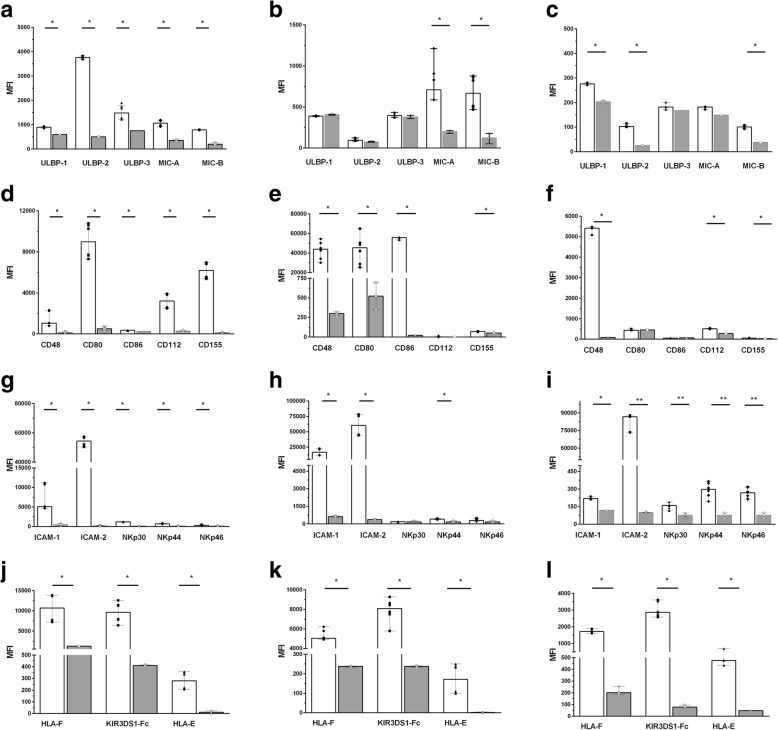
We hypothesized that differences in aNKR ligand levels expressed on K562, .221 and CEM.NKR.CCR5 cells could explain their differential abilities to stimulate NK cells. To address this, we determined the MFI of aNKR ligand expression by staining these cell lines with mAbs and chimeric proteins specific for these ligands and analyzing results by flow cytometry. The MFI of ligand staining was compared to that generated by the controls for each of the ligand specific reagents. Additional file 1: Figure S1 shows the gating strategy used to detect live singlet K562, .221 and CEM.NKr.CCR5 cells. Figure 1 shows examples of the histograms generated by staining K562, .221 and CEM.NKr.CCR5 cells with mAbs and chimeric proteins specific for aNKR ligands versus their FMO/isotype/secondary antibody controls. Table 2 and Fig. 2 show the median MFI obtained by staining these cell lines with the mAbs/chimeric proteins specific for aNKR ligands in Panels 1, 2, 3, and HLA versus their FMO/isotype/secondary antibody alone controls. Table 2 also shows the uncorrected, background corrected and control condition MFI staining levels of aNKR ligands on these three cell lines.
Fig. 1.
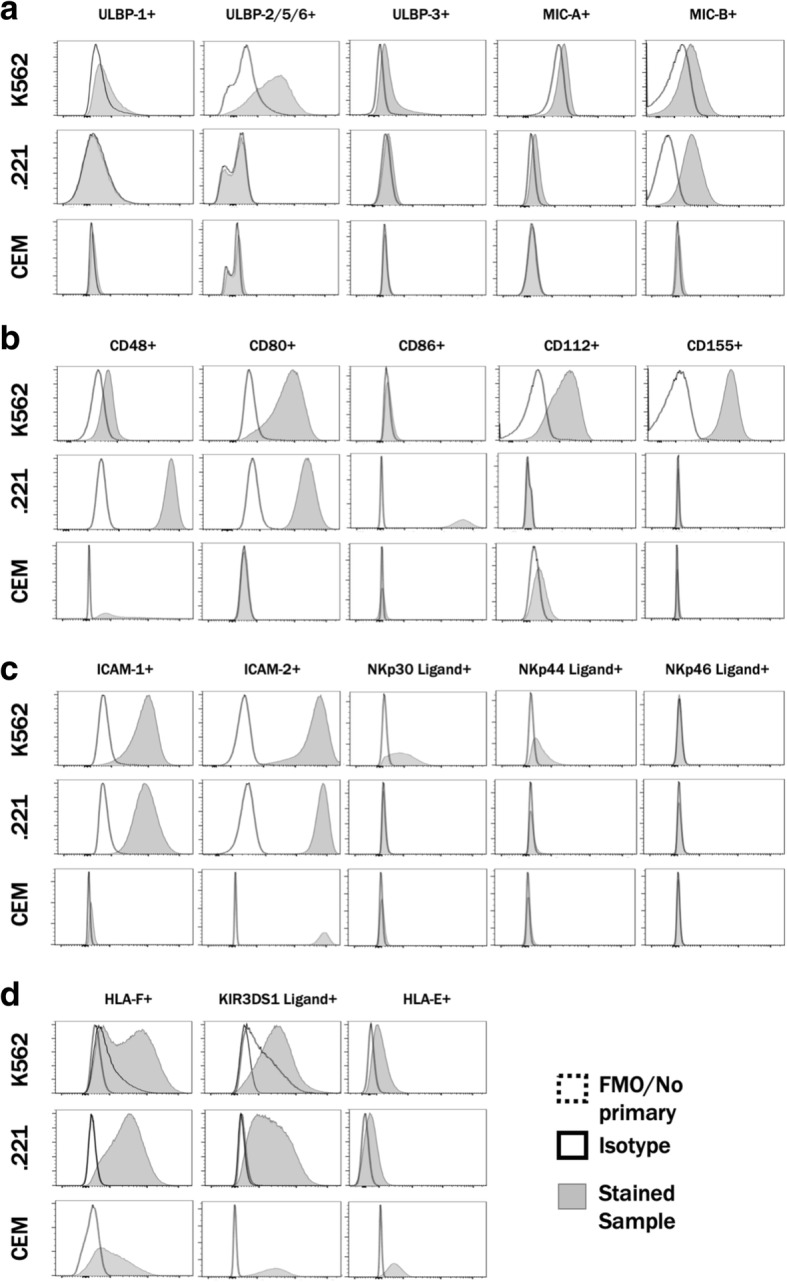
Staining K562, .221 and CEM.NKr.CCR5 cells with monoclonal antibodies (mAbs) and chimeric proteins specific for activating NK cell receptors (aNKR). Shown are examples of flow cytometry plots generated by binding fluorochrome conjugated mAbs, chimeric proteins or unconjugated mAbs with fluorochrome conjugated secondary antibodies specific for aNKRs to K562 (top rows in each panel), .221 (middle rows in each panel) and CEM.NKr.CCR5 (bottom rows in each panel) cells. Staining was done using Panel 1 (a) Panel 2 (b), Panel 3 (c) and Panel HLA (d) mAbs or chimeric protein reagents. Grey histograms represent staining with mAbs or chimeric proteins binding to aNKR. White histograms represent staining with fluorescence minus one, isotype control or secondary antibody alone controls. For information on which control was used for staining with each mAb or chimeric protein see Table 2
Fig. 2.
Mean fluorescence intensity (MFI) generated by staining K562, .221 and CEM.NKr.CCR5 cells with monoclonal antibodies (mAbs) and chimeric proteins to activating natural killer cell receptor (aNKR) versus their respective background controls. The y-axis shows the MFI of binding generated by using (a-c) Panel 1, (D-F) Panel 2, (g-i) Panel 3, and (j-l) Panel HLA anti-aNKR ligands reagents to stain to K562 (a, d, g, j), .221 (b, e, h, k) and CEM.NKr.CCR5 cells (c, f, i, l). White bars depict the MFI of staining with Panel A, B, C or HLA reagents while grey bars show the MFI of staining with an isotype control for ULBP-1 staining, fluorescence minus one controls for the other Panel A, Panel B and Panel 3 anti-ICAM-1 and anti-ICAM-2 antibodies and secondary antibody alone for all other aNKR ligand specific reagents. Bar height and error bars represent the median and range for the data set. Each data point represents one of three to six replicates. Significant differences are indicated by a line joining the observations being compared. (*) = p-values < 0.05 and (**) = p-values < 0.01
All the mAbs and chimeric proteins in the Panels 1, 2, 3 and HLA stained K562 at above background levels (Fig. 1a-d, top panels and Fig. 2a, d, g and j, p < 0.05 for all, Mann-Whitney tests). Many of these reagents also stained .221 at above background levels. Exceptions to this occurred for staining .221 cells with anti-ULBP-1, -ULBP-2/5/6, -ULPB-3 and -CD112 mAbs and with NKp30-Fc and NKp46-Fc chimeric proteins (Fig. 1a-d middle panels and Fig. 2b, e, h and k, p < 0.03 for all conditions that stained .221 cells at levels significantly above background, Mann-Whitney tests). The mAbs and chimeric proteins that stained CEM.NKr.CCR5 at levels above background were anti-ULBP-1, -ULBP-2, -MIC-B, -CD48, -CD112 and -CD155 mAbs and all the reagents in Panels 3 and HLA (Fig. 1a-d, lower panels and Fig. 2c, f, i and l, p < 0.05, Mann-Whitey tests).
Comparison of the background corrected MFI of staining of aNKR ligand expression on K562, .221 and CEM.NKr.CCR5 cells
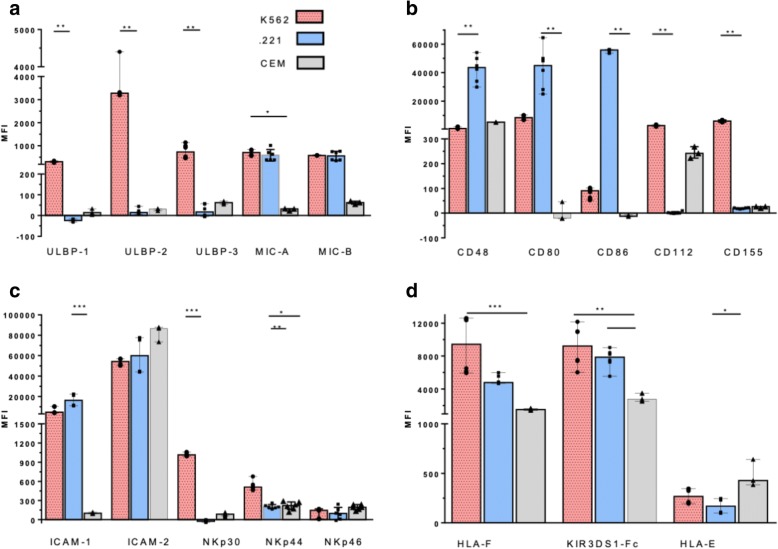
Figure 3 compares the background corrected MFI values generated by mAb/chimeric protein staining of K562, .221 and CEM.NKr.CCR5 cells. Most of the mAbs/chimeric proteins stained these three cell lines with MFIs that were significantly different from each other (p < 0.034 for all, Kruskal-Wallis tests). The only exceptions to this were for staining these three cell lines with anti-ICAM-2 and the NKp46-Fc chimeric protein.
Fig. 3.
Comparison of the mean fluorescence intensity (MFI) generated by monoclonal antibodies (mAbs) and chimeric protein binding to activating natural killer cell receptor (aNKR) ligands on K562, .221 cells and CEM.NKr.CCR5 cells. The MFI of aNKR ligand expression on K562 .221 and CEM.NKr.CCR5 cells detected by (a) Panel 1, (b) Panel 2, (c) Panel 3, and (d) Panel HLA anti-aNKR ligand reagents is represented on the y-axis. Bar height and error bars represent the median and range for the data set. Each data point represents one of three to six replicates. Significant differences are indicated by a line joining the observations being compared. (*) = p-values < 0.05, (**) = p-values < 0.01 and (***) = p-values < 0.001
Dunn’s post tests showed that K562 expressed ULBP-1, ULBP-2/5/6, ULBP-3 with higher background corrected MFIs than did .221 cells (p < 0.01 for all, Table 2 and Fig. 3a). The MFI of background corrected MIC-A and MIC-B cell surface staining was not significantly different on K562 and .221 cells, while K562 expressed the MIC-A ligand at a higher MFI than did CEM.NKr.CCR5 cells (p < 0.05, Table 2 and Fig. 3a). Staining with anti-CD48, -CD80 and -CD86 mAbs was highest on .221 cells and lowest on CEM.NKr.CCR5 cells. Observations that achieved statistical significance were lower expression of the CD48 ligand on K562 than .221 cells (p < 0.01, Table 2 and Fig. 3b). The CD112 and CD155 ligands were expressed at higher levels on K562 than .221 cells (p < 0.01 for both, Table 2 and Fig. 3b). The ICAM-1 ligand was expressed with a higher MFI on .221 than on CEM.NKr.CCR5 cells (p < 0.01) while the ICAM-2 ligand was expressed on all three cell lines at high levels that were not significantly different from each other (Table 2 and Fig. 3c). The ligands for NKp30 and NKp44 were expressed at a higher MFI on K562 than on .221 (p < 0.001 and p < 0.01, respectively) and the NKp44 ligand levels were higher on K562 than CEM.NKr.CCR5 cells (p < 0.05, Table 2 and Fig. 3c). KIR3DS1-Fc and mAb 3D11 bind HLA-F. HLA-F was present at a higher MFI on K562 than on CEM, NKr.CCR5 cells (p < 0.001, for both) and staining with KIR3DS1-Fc revealed a higher MFI of HLA-F expression on .221 than on CEM.NKr.CCR5 cells (p < 0.05). On the other hand, HLA-E was present at a higher corrected MFI on CEM.NKr.CCR5 cells than on .221 cells, while both K562 and .221 cells expressed HLA-E at background corrected MFIs that did not differ significantly from each other (Table 2 and Fig. 3d).
Together, these results show that K562 cells expressed higher levels of several of the ligands for the aNKRs NKG2D and NCRs than did .221 and CEM.NKr.CCR5 cells. K562 differed from .221 by having lower expression levels of the CD48 ligand. K562 also has lower levels of CD80 and CD86 ligand expression that did not achieve significance when Dunn’s post tests were applied. However, the CD80 and CD86 ligand expression levels were significantly higher on .221 cells than on either K652 or CEM.NKr.CCR5 cells when results for .221 and K562 were compared using Mann-Whitney tests (p = 0.002 and 0.005, respectively). The median MFI of background corrected aNKR ligand expression generated by staining CEM.NKr.CCR5 cells with the mAbs/chimeric proteins in panel 1, 2 and 3 was low for 4 of the ligands tested (MFI < 234). Only anti-CD48 and anti-ICAM-2, stained CEM.NKr.CCR5 cells with a high MFI that was readily distinguishable from background staining. This cell line also expressed higher levels of HLA-E than did K562 and .221 cells and expressed above background levels of HLA-F that were lower than those observed on K562 and .221 cells.
Discussion
In this report we screened three cell lines that are frequently used to test NK cell functionality for expression of a panel of aNKR ligands. K562 and .221 cells are both HLA-null cell lines that should have a similar ability to abrogate NK cell inhibitory signals mediated by HLA binding to their iNKR. iNKR-HLA interactions determine NK cell education status and functional potential [60, 61]. Previous work showed that these HLA-null cell lines induced differential patterns of IFN-γ secretion, CCL4 secretion and CD107a expression by NK cells, suggesting that expression patterns for aNKR ligands may differ between these 2 cell lines and explain differential activation patterns. We found that K562 and .221 expression levels did not differ significantly for MIC-A, MIC-B, ICAM-2, HLA-E and NKp46 ligands. K562 expressed significantly higher levels of the NKG2D ligands ULBP-1, ULBP-2/5/6 and ULBP-3 and the ligands for NCRs NKp30 and NKp44. K562 differed from .221 by having significantly lower expression levels of CD48 and lower levels of CD80, and CD86 that did not achieve statistical significance using Dunn’s post tests. On the other hand, the NK resistant cells line CEM.NKr.CCR5, often used as a target cell in ADCC assays, expressed most of the aNKR ligands tested at low expression levels, with the exception of the ligands for CD48, ICAM-2, KIR3DS1 (HLA-F) and NKG2A/C (HLA-E).
Although prior studies have partially characterized the expression profiles of ligands to aNKRs on K562 and .221 cells, to our knowledge this has not been done for the CEM.NKr.CCR5 cell line that differs from its parent CEM cell line by being resistant to direct NK cell cytolysis [25, 62]. In this study, we included a larger and more comprehensive panel of mAbs/chimeric proteins detecting aNKR ligands than have previously been investigated.
The receptors implicated in triggering NK cell mediated cytolysis include NKG2D and the NCRs [62–65]. The ligands for NKG2D include ULBP-1, ULBP-2/5/6, ULBP-3, MIC-A and MIC-B [65]. NKG2D is a C type lectin receptor that associates with signaling molecules such as DAP10/KAP10 to initiate the cascade of events leading to cytolysis [17, 66–68]. The NCRs include NKp30, NKp44 and NKp46, among others [65]. They associate with different tyrosine kinase activating motifs bearing signal transducing polypeptides to mediate activating signals [31, 32]. The characterization of the ligands for these NCRs is incomplete, which is why chimeric proteins based on these receptors are used to probe for the presence of their ligands on target cells. Blocking the interaction of NCRs and NKG2D with these ligands also reduces cytolysis mediated by NK cells, highlighting the role of NKG2D engagement in NK cell killing [62].
In our previous studies comparing the functional profile induced in NK cells by K562 and .221 cell stimulation, we found that while both HLA null cell lines were able to induce CD107a expression, the functional NK subsets that included CD107a expression contributed to a higher proportion of the total NK cell response when stimulated by K562 than by .221 cells [16]. The higher expression level of NKG2D and NCR ligands on K562 than on .221 cells reported here and the lower levels of expression of other ligands for aNKR such as CD48, CD80 and CD86 would be consistent with an aNKR ligand profile that favors NK cell stimulation towards CD107a expression, which is a marker for degranulation, a step in the pathway toward cytolysis. NKG2D signaling has been shown to play a crucial role in NK cell cytotoxic granule polarization, degranulation, and cytotoxicity [69]. The ability of K562 to induce signaling through this pathway may explain why this cell line preferentially stimulates NK cell degranulation, rather than cytokine or chemokine secretion [16]. Unfortunately, no antibody currently exists that can discriminate between ULBP-2, − 5, and − 6 and we are incapable of determining which combinations of these ligands are expressed on K562. However, it is possible that K562 cells express ligands for all three of these receptors, in addition to ligands for ULBP-1 and -3. The consequence of this may be induction of more potent signaling through NKG2D of K652 than .221 cells, which only express MIC-A and MIC-B with a modest MFI. Moreover, expression of NKp30 on NK cells has been correlated with both perforin expression and degranulation. Engagement of this aNKR by K562 may also contribute to their induction of degranulation [70]. CD112 and CD155 bind to DNAM-1 [18, 28, 29]. Thus, K562 has a ligand profile that does not only stimulate NK cells through NKG2D, NKp30 and NKp44 but also through DNAM-1.
Our finding that .221 cells express the ULBP-1, ULBP-2/5/6 and ULBP-3 ligands for NKG2D at a low MFI that is not much above background levels is in line with a report by Pende et al. [62]. Our findings differ for MIC-A, which we found was expressed over background by .221 cells while Pende et al. found it not to be expressed over background. These discrepant results may be due to differences in the reagents used to detect the NKp30 ligand or to other technical issues relating to staining and analysis methods. These findings are consistent with previous reports that ULBP ligands are preferentially expressed on K562 and with the observation that K562 cells express the tumor ligand B7-H6, which is a ligand for NKp30 [21, 62].
The higher expression levels of CD48, CD80, CD86 and ICAM-1 on .221 than K562 cells may explain why .221 cells stimulated a higher frequency of functional NK cells, particularly those secreting IFN-γ and CCL4 than did K652 [16]. When K562 expressed a higher MFI of aNKR ligands than .221 cells, as was in the case of CD112, CD155 and HLA-F, the differences were more modest than were the between-cell differences in CD48, CD80, CD86 and ICAM-1 expression on .221 versus K562 cells. The elevated expression level of these ligands on .221 cells may provide these cells with a more potent activating signal. Engagement of the aNKR 2B4 by its ligand CD48 on .221 has been reported to induce low levels of IFN-γ production and concurrent signaling through 2B4; signaling through other aNKRs can drive high levels of cytokine and chemokine production [9, 20]. Although, the exact NKRs that recognize the co-stimulatory molecules CD80 and CD86 have not been identified, expression of these ligands on target cells can trigger NK cell-mediated cytolysis [50]. Together, the ligands that are expressed on .221 are capable of engaging receptors that are important for cytokine and chemokine production, which may explain why .221 cell stimulate larger numbers of IFN-γ and CCL4 secreting NK cells than do K562 cells [16].
Our findings confirm that HLA-E, the ligand to the aNKRs NKG2E and NKG2C and the iNKR, NKG2A, on NK cells, is expressed by K562 and .221 cells with a low MFI that was nevertheless above background staining levels [71]. On the other hand, CEM.NKr.CCR5 cells express cell surface MHC-I antigens and higher HLA-E levels than do K562 and .221. Stable cell-surface expression of HLA-E requires binding to one of a set of nonamer peptides derived from the leader sequences of MHC-Ia molecules or HLA-G, which are absent on the HLA-null .221 cell surface [72, 73]. It is unlikely that HLA-F expression by K562 and .221 cell lines contributes to HLA-E expression as the signal sequence of HLA-F does not have a nonamer peptide able to bind HLA-E [55]. However, the HLA-E peptidome was recently shown to be less restricted than previously thought. HLA-E can also bind to an array of self-peptides in the absence of HLA class I signal peptides, permitting its stable expression and induction of NK cell cytotoxicity [74, 75]. In addition, HLA-E is also capable of presenting EBV-derived peptides, such as BZLF1, which would be expected to be present in the EBV transformed .221 cell line [76, 77]. HLA-E was recently shown to present a cytomegalovirus derived signal peptide important in driving the expansion of adaptive-like NKG2C+ NK cells [78]. HLA-E/BZLF1 complexes are poorly recognized by NK cells and it is likely that HLA-E molecules presenting non-canonical self, make greater contributions to .221-induced NK cell activation [76].
The non-classical MHC-I antigen, HLA-F, is a KIR3DS1 ligand, which is expressed by K562, .221 and CEM.NKr.CCR5 cells [56–58]. HLA-F has also been reported to interact with KIR3DL2 and KIR2DS4 [79]. Although the interaction of HLA-F with KIR3DL2 was confirmed by others, its interaction with KIR2DS4, which is structurally related to KIR3DL2 due to a gene conversion event has not been confirmed [56, 80]. HLA-F is also expressed on HIV infected cells [56]. Other investigators did not observe HLA-F on K562 [56, 57]. Here, we used both the mAb 3D11 and KIR3DS1-Fc to stain K562 cells for cell surface HLA-F. Both reagents generated concordant results for the presence of HLA-F on this cell line.
KIR3DS1 homozygotes were more frequent in a population of HIV exposed seronegative than in HIV susceptible individuals and KIR3DS1 homozygotes remained uninfected for longer time intervals despite HIV exposure than those with other KIR3DL1/S1 genotypes, suggesting that KIR3DS1 HLA-F interactions may provide protection from HIV infection [81, 82]. The global distribution of KIR3DS1 varies from one population to another [83, 84]. For example, it is rare in sub-Saharan African populations [83]. It is interesting to speculate on whether HLA-F/KIR3DS1 or /KIR3DL2 or possibly /KIR2DS4 combinations can influence HIV control mediated by NK cells and whether this could account for between-individual or -population differences in HIV susceptibility or the rate of HIV disease progression.
For the purpose of this study, the ligands analyzed were included on the basis of their ability to stimulate NK cell responses through the engagement of aNKRs. However, it is important to consider that several of these ligands are capable of engaging both aNKRs and iNKRs. CD112 and CD155, which signal through the activating DNAM-1, can also bind to the iNKR, T cell immunoreceptor with immunoglobulin and ITIM motifs (TIGIT) [85, 86]. While both DNAM-1 and TIGIT are widely expressed on NK cells, the affinity of CD155 for TIGIT is greater than for DNAM-1 and TIGIT expression can reduce DNAM-1/CD155 interactions in a dose-dependent manner [87–89]. TIGIT has also been shown to compete with DNAM-1 for the binding of CD112. Furthermore, when transfected into the NK cell line YTS, TIGIT greatly limits NK-mediated cytotoxicity by disrupting cytotoxic granule polarization [89, 90]. Considering this, it is possible that CD112, which is exclusively expressed on K562, and CD155 which is expressed at higher levels on K562 than .221 cells contributes more to NK cell inhibition than activation and may be an additional reason why K562 activates a smaller fraction of NK cells, compared to .221 [16]. Another aNKR ligand, HLA-E, similarly contributes to both NK cell activation and inhibition. HLA-E binds to the CD94/NKG2 family of NK cell receptors, which includes the activating NKG2E and -C and the inhibitory NKG2A and -B NKRs [53, 54]. Interactions between NKG2A, which is expressed on the majority of NK cells, and HLA-E have been shown to predominate over interactions with NKG2C and surface expression of HLA-E is sufficient to rescue those cells from lysis by NKG2A+ NK cells [53, 54, 91]. Despite this, work assessing NK cell stimulation by autologous HIV-infected CD4 T cells, which express HLA-E, found that expression of NKG2A on NK cells was associated with improved activation [92]. It is plausible that, while interactions between HLA-E on .221 and NKG2A on NK cells can tune down NK cell activation, signaling through the other aNKR engaged by .221 ligands can compensate for this inhibitory input.
CEM.NKr.CCR5 cells expressed few aNKR ligands. Ligands for CD48 and ICAM-2 were present on this cell line as well as low levels of ligands for NCRs. The interaction of ICAM-2 with its ligand may contribute to the formation of NK cell CEM.NKr.CCR5 conjugates [65]. Less is known regarding the consequences of CD48 ligand expression. It is interesting to speculate that the presence of few other cell surface ligands for aNKR contributes to the resistance of this cell line to direct NK cell cytolysis in the absence of an antibody bridging target and effector cells [25]. Pende et al., tested the CEM.NKr.CCR5 parental cell line, CEM, for cell surface expression of ULBP-1. ULBP-2, ULBP-3 and MIC-A and found that CEM cells expressed ULBP-2 and ULBP-3 [62]. The absence of these aNKR on CEM.NKr.CCR5 is suggestive that the process of selecting for CEM.NKr resistance to direct NK cell cytolysis led to loss of several ligands for aNKR [25].
Conclusion
The two HLA-null cell lines K562, .221 and the ADCC target cell CEM.NKr.CCR5 differed in their expression of ligands to aNKR. The data presented here provide a systematic assessment the stimulatory potential of three cell types commonly used to study NK cell activation. The different aNKR ligand expression profiles of K562, .221 and CEM.NKr.CCR5 are associated with the induction of qualitative differences in the NK cell responses to these cell lines. CEM.NKr.CCR5 cells, that are resistant to direct NK cell killing express few ligands for aNKR. This work provides a basis for examining the specific contribution of each ligand-aNKR pair to different stages of NK cell activation.
Additional file
Figure S1. Gating strategy. Gating strategy identifying live, singlet, (A) K562 and (B) .221 and (C) CEM.NKr.CCR5 cells. Forward and side scatter plots were used to gate on cells as indicated by the outlined area of each left-hand plot. From these cells, singlets were gated on as shown in the outlined area of the middle panels. From the singlet population live cells were gated on as indicated by the outlined area in the right-hand plots. (TIF 411 kb)
Acknowledgments
We would like to acknowledge the generosity of Dr. Galit Alter, who provided the 721.221 cell line, Ms. Xiaoyan Ni and Ms. Tsoarello Mabanga for expert technical assistance, and Drs. Irene Lisovsky and Gamze Isitman for advice on protocol development.
Funding
This study received support from the Canadian Institutes for Health Research [grant # MOP-142494]. ATM was supported by the Canadian Association for HIV Research Abbvie Master’s Award in Basic Science. NFB is a member of the Research Institute of the McGill University Health Centre, an institution funded in part by the Fond de Recherche de Quebec-Santé. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All data is presented in the manuscript. All raw data are kept in experimental notebooks or as electronic files such as those acquired by flow cytometry. These are available on request.
Abbreviations
- 221
721.221
- ADCC
Antibody dependent cellular cytotoxicity
- aNKR
Activating natural killer cell receptors
- CD
Cluster of differentiation
- DNAM-1
Leukocyte adhesion molecule DNAX accessory molecule-1
- FBS
Fetal bovine serum
- HLA
Human leukocyte antigen
- ICAM
Intercellular adhesion molecule
- IFN-γ
Interferon-γ
- ILC
Innate lymphoid cell
- iNKR
Inhibitory natural killer cell receptor
- IU
International unit
- KIR
Killer immunoglobulin-like receptors
- LFA-1
Lymphocyte function-associated antigen 1
- mAb
Monoclonal antibody
- MFI
Mean fluorescence intensity
- MHC-I
Major histocompatibility complex class I
- MIC
Major histocompatibility complex class I related chain
- NCR
Natural cytotoxicity receptors
- NK
Natural killer
- NTB-A
Natural killer-T-B cell antigen
- RT
Room temperature
- TIGIT
T cell immunoreceptor with immunoglobulin and ITIM motifs
- ULBP
UL-16 binding protein
Authors’ contributions
ATM and NFB were responsible for study design, ATM, SC, ZK, FPD and NFB were responsible data analysis, and manuscript preparation. Data collection and statistical analysis were performed by ATM, SC and NFB. All authors have read and approved the manuscript. I, Nicole Bernard, as the corresponding author ensure that this is the case.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexandra Tremblay-McLean, Email: alexandra.tremblay-mclean@mail.mcgill.ca.
Sita Coenraads, Email: sitacoenraads@gmail.com.
Zahra Kiani, Email: Zahra.kiani@mail.mcgill.ca.
Franck P. Dupuy, Email: dura007@hotmal.fr
Nicole F. Bernard, Email: nicole.bernard@mcgill.ca
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. 2016;17(7):758–764. doi: 10.1038/ni.3482. [DOI] [PubMed] [Google Scholar]
- 3.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 4.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 5.Lettau M, Schmidt H, Kabelitz D, Janssen O. Secretory lysosomes and their cargo in T and NK cells. Immunol Lett. 2007;108(1):10–19. doi: 10.1016/j.imlet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–1558. doi: 10.1016/S1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 7.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115(11):2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 11.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12(4):239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein E, Ben-Bassat H, Neumann H, Ralph P, Zeuthen J, Polliack A, et al. Properties of the K562 cell line, derived from a patient with chronic myeloid leukemia. Int J Cancer. 1976;18(4):421–431. doi: 10.1002/ijc.2910180405. [DOI] [PubMed] [Google Scholar]
- 13.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- 14.Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989;142(9):3320–3328. [PubMed] [Google Scholar]
- 15.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisovsky I, Isitman G, Bruneau J, Bernard NF. Functional analysis of NK cell subsets activated by 721.221 and K562 HLA-null cells. J Leukoc Biol. 2015;97(4):761–767. doi: 10.1189/jlb.4AB1014-499R. [DOI] [PubMed] [Google Scholar]
- 17.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 18.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalupny NJ, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophys Res Commun. 2003;305(1):129–135. doi: 10.1016/S0006-291X(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 20.Sandusky MM, Messmer B, Watzl C. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur J Immunol. 2006;36(12):3268–3276. doi: 10.1002/eji.200636146. [DOI] [PubMed] [Google Scholar]
- 21.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90(1):109–115. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 23.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol. 1999;73(11):8966–8974. doi: 10.1128/jvi.73.11.8966-8974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyerly HK, Reed DL, Matthews TJ, Langlois AJ, Ahearne PA, Petteway SR, Jr, et al. Anti-GP 120 antibodies from HIV seropositive individuals mediate broadly reactive anti-HIV ADCC. AIDS Res Hum Retrovir. 1987;3(4):409–422. doi: 10.1089/aid.1987.3.409. [DOI] [PubMed] [Google Scholar]
- 25.Howell DN, Andreotti PE, Dawson JR, Cresswell P. Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. J Immunol. 1985;134(2):971–976. [PubMed] [Google Scholar]
- 26.Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141(10):3478–3485. [PubMed] [Google Scholar]
- 27.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123–133. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 29.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 30.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, et al. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186(7):1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187(12):2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190(10):1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 34.Bloushtain N, Qimron U, Bar-Ilan A, Hershkovitz O, Gazit R, Fima E, et al. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173(4):2392–2401. doi: 10.4049/jimmunol.173.4.2392. [DOI] [PubMed] [Google Scholar]
- 35.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31(9):2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::AID-IMMU2680>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 36.Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol. 2014;92(3):221–229. doi: 10.1038/icb.2013.98. [DOI] [PubMed] [Google Scholar]
- 37.Hecht ML, Rosental B, Horlacher T, Hershkovitz O, De Paz JL, Noti C, et al. Natural cytotoxicity receptors NKp30, NKp44 and NKp46 bind to different heparan sulfate/heparin sequences. J Proteome Res. 2009;8(2):712–720. doi: 10.1021/pr800747c. [DOI] [PubMed] [Google Scholar]
- 38.Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe. 2013;14(4):387–397. doi: 10.1016/j.chom.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Li SS, Mody CH. NKp46 is an NK cell fungicidal pattern recognition receptor. Trends Microbiol. 2016;24(12):929–931. doi: 10.1016/j.tim.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Li SS, Ogbomo H, Mansour MK, Xiang RF, Szabo L, Munro F, et al. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat Commun. 2018;9(1):751. doi: 10.1038/s41467-018-03014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188(11):2083–2090. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pende D, Bottino C, Castriconi R, Cantoni C, Marcenaro S, Rivera P, et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42(4):463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 43.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, et al. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16(4):533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 44.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4(6):573–581. doi: 10.1016/S1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 45.Shibuya K, Lanier LL, Phillips JH, Ochs HD, Shimizu K, Nakayama E, et al. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 1999;11(5):615–623. doi: 10.1016/S1074-7613(00)80136-3. [DOI] [PubMed] [Google Scholar]
- 46.Gross CC, Brzostowski JA, Liu D, Long EO. Tethering of intercellular adhesion molecule on target cells is required for LFA-1-dependent NK cell adhesion and granule polarization. J Immunol. 2010;185(5):2918–2926. doi: 10.4049/jimmunol.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schleinitz N, March ME, Long EO. Recruitment of activation receptors at inhibitory NK cell immune synapses. PLoS One. 2008;3(9):e3278. doi: 10.1371/journal.pone.0003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Culley FJ, Johnson M, Evans JH, Kumar S, Crilly R, Casasbuenas J, et al. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 2009;7(7):e1000159. doi: 10.1371/journal.pbio.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luque I, Reyburn H, Strominger JL. Expression of the CD80 and CD86 molecules enhances cytotoxicity by human natural killer cells. Hum Immunol. 2000;61(8):721–728. doi: 10.1016/S0198-8859(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 50.Wilson JL, Charo J, Martin-Fontecha A, Dellabona P, Casorati G, Chambers BJ, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immunol. 1999;163(8):4207–4212. [PubMed] [Google Scholar]
- 51.Costa C, Barber DF, Fodor WL. Human NK cell-mediated cytotoxicity triggered by CD86 and gal alpha 1,3-gal is inhibited in genetically modified porcine cells. J Immunol. 2002;168(8):3808–3816. doi: 10.4049/jimmunol.168.8.3808. [DOI] [PubMed] [Google Scholar]
- 52.Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, Gaken J, et al. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol. 1999;163(1):62–70. [PubMed] [Google Scholar]
- 53.Kaiser BK, Barahmand-Pour F, Paulsene W, Medley S, Geraghty DE, Strong RK. Interactions between NKG2x immunoreceptors and HLA-E ligands display overlapping affinities and thermodynamics. J Immunol. 2005;174(5):2878–2884. doi: 10.4049/jimmunol.174.5.2878. [DOI] [PubMed] [Google Scholar]
- 54.Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 55.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, et al. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A. 1998;95(9):5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Beltran WF, Holzemer A, Martrus G, Chung AW, Pacheco Y, Simoneau CR, et al. Open conformers of HLA-F are high-affinity ligands of the activating NK-cell receptor KIR3DS1. Nat Immunol. 2016;17(9):1067–1074. doi: 10.1038/ni.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burian A, Wang KL, Finton KA, Lee N, Ishitani A, Strong RK, et al. HLA-F and MHC-I open conformers bind natural killer cell Ig-like receptor KIR3DS1. PLoS One. 2016;11(9):e0163297. doi: 10.1371/journal.pone.0163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiani Z, Dupuy FP, Bruneau J, Lebouche B, Zhang CX, Jackson E, et al. HLA-F on HLA-null 721.221 cells activates primary NK cells expressing the activating killer Ig-like receptor KIR3DS1. J Immunol. 2018;201(1):113–123. doi: 10.4049/jimmunol.1701370. [DOI] [PubMed] [Google Scholar]
- 59.Isitman G, Lisovsky I, Tremblay-McLean A, Parsons MS, Shoukry NH, Wainberg MA, et al. Natural killer cell education does not affect the magnitude of granzyme B delivery to target cells by antibody-dependent cellular cytotoxicity. AIDS. 2015;29(12):1433–1443. doi: 10.1097/QAD.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 60.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 62.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, et al. Major histocompatibility complex class I-related chain a and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62(21):6178–6186. [PubMed] [Google Scholar]
- 63.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21(9):420–422. doi: 10.1016/S0167-5699(00)01673-X. [DOI] [PubMed] [Google Scholar]
- 64.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29(5):1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang C, Dietrich J, Harpur AG, Lindquist JA, Haude A, Loke YW, et al. Cutting edge: KAP10, a novel transmembrane adapter protein genetically linked to DAP12 but with unique signaling properties. J Immunol. 1999;163(9):4651–4654. [PubMed] [Google Scholar]
- 67.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 68.Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31(4):1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::AID-IMMU1076>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 69.Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci U S A. 2007;104(15):6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52(5):1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lo Monaco E, Tremante E, Cerboni C, Melucci E, Sibilio L, Zingoni A, et al. Human leukocyte antigen E contributes to protect tumor cells from lysis by natural killer cells. Neoplasia. 2011;13(9):822–830. doi: 10.1593/neo.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maier S, Grzeschik M, Weiss EH, Ulbrecht M. Implications of HLA-E allele expression and different HLA-E ligand diversity for the regulation of NK cells. Hum Immunol. 2000;61(11):1059–1065. doi: 10.1016/S0198-8859(00)00190-7. [DOI] [PubMed] [Google Scholar]
- 73.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998;160(10):4951–4960. [PubMed] [Google Scholar]
- 74.Kraemer T, Celik AA, Huyton T, Kunze-Schumacher H, Blasczyk R, Bade-Doding C. HLA-E: presentation of a broader peptide repertoire impacts the cellular immune response-implications on HSCT outcome. Stem Cells Int. 2015;2015:346714. doi: 10.1155/2015/346714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Celik AA, Kraemer T, Huyton T, Blasczyk R, Bade-Doding C. The diversity of the HLA-E-restricted peptide repertoire explains the immunological impact of the Arg107Gly mismatch. Immunogenetics. 2016;68(1):29–41. doi: 10.1007/s00251-015-0880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jorgensen PB, Livbjerg AH, Hansen HJ, Petersen T, Hollsberg P. Epstein-Barr virus peptide presented by HLA-E is predominantly recognized by CD8(bright) cells in multiple sclerosis patients. PLoS One. 2012;7(9):e46120. doi: 10.1371/journal.pone.0046120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romagnani C, Pietra G, Falco M, Millo E, Mazzarino P, Biassoni R, et al. Identification of HLA-E-specific alloreactive T lymphocytes: a cell subset that undergoes preferential expansion in mixed lymphocyte culture and displays a broad cytolytic activity against allogeneic cells. Proc Natl Acad Sci U S A. 2002;99(17):11328–11333. doi: 10.1073/pnas.172369799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hammer Q, Ruckert T, Borst EM, Dunst J, Haubner A, Durek P, et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018;19(5):453–463. doi: 10.1038/s41590-018-0082-6. [DOI] [PubMed] [Google Scholar]
- 79.Goodridge JP, Burian A, Lee N, Geraghty DE. HLA-F and MHC class I open conformers are ligands for NK cell Ig-like receptors. J Immunol. 2013;191(7):3553–3562. doi: 10.4049/jimmunol.1300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Graef T, Moesta AK, Norman PJ, Abi-Rached L, Vago L, Older Aguilar AM, et al. KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A*11 while diminishing avidity for HLA-C. J Exp Med. 2009;206(11):2557–2572. doi: 10.1084/jem.20091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, et al. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS. 2008;22(5):595–599. doi: 10.1097/QAD.0b013e3282f56b23. [DOI] [PubMed] [Google Scholar]
- 82.Tallon BJ, Bruneau J, Tsoukas CM, Routy JP, Kiani Z, Tan X, et al. Time to seroconversion in HIV-exposed subjects carrying protective versus non protective KIR3DS1/L1 and HLA-B genotypes. PLoS One. 2014;9(10):e110480. doi: 10.1371/journal.pone.0110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Norman PJ, bi-Rached L, Gendzekhadze K, Korbel D, Gleimer M, Rowley D, et al. Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nat Genet. 2007;39(9):1092–1099. doi: 10.1038/ng2111. [DOI] [PubMed] [Google Scholar]
- 84.Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006;203(3):633–645. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(42):17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu X, Harden K, C Gonzalez L, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 87.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 88.Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol. 2015;45(10):2886–2897. doi: 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- 89.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol. 2013;43(8):2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu S, Zhang H, Li M, Hu D, Li C, Ge B, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013;20(3):456–464. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vales-Gomez M, Reyburn HT, Erskine RA, Lopez-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-a and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999;18(15):4250–4260. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lisovsky I, Isitman G, Song R, DaFonseca S, Tremblay-McLean A, Lebouche B, et al. A higher frequency of NKG2A+ than of NKG2A− NK cells respond to autologous HIV-infected CD4 cells irrespective of whether they co-express KIR3DL1. J Virol. 2015;89(19):9909–19. doi: 10.1128/JVI.01546-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy. Gating strategy identifying live, singlet, (A) K562 and (B) .221 and (C) CEM.NKr.CCR5 cells. Forward and side scatter plots were used to gate on cells as indicated by the outlined area of each left-hand plot. From these cells, singlets were gated on as shown in the outlined area of the middle panels. From the singlet population live cells were gated on as indicated by the outlined area in the right-hand plots. (TIF 411 kb)
Data Availability Statement
All data is presented in the manuscript. All raw data are kept in experimental notebooks or as electronic files such as those acquired by flow cytometry. These are available on request.