Abstract
AIM:
In recent decades, despite various types of cancer inflicting many people worldwide, the existing therapies are not satisfactory and have many side effects. The present study was conducted to optimise the synthesis of novel alginate-CuO nanocomposite with utmost anticancer activity.
METHODS:
In this study, 9 nanocomposites were designed using Taguchi method and three factors including copper oxide nanoparticles, alginate biopolymer and stirring times were assessed at three different levels. The anticancer activity of the synthesised nanocomposites was evaluated on the MCF-7 cell line using the MTT method. Using the Qulitek-4 software, we determined the optimum conditions for the synthesis of alginate-CuO nanocomposite with the highest anticancer activity.
RESULTS:
The results indicated that all three factors (copper oxide, alginate and stirring time) were effective on the anticancer activity of the alginate-CuO nanocomposite. Also, the nanocomposite produced under the conditions of experiment 9 (8 mg/ml of copper oxide, 2 mg/ml of alginate and 60 min of stirring time) provided the highest growth inhibition rate as 75.63% against cancer cells.
CONCLUSION:
The synthesised alginate-copper oxide nanocomposites in this study showed a significant anticancer effect. Therefore, the synthesised nanocomposite under optimal conditions can be used in the design of new anticancer drugs.
Keywords: Anticancer, CuO nanoparticles, Alginate, Nanocomposite, Taguchi method
Introduction
Despite the synthesis of numerous new drug compounds in recent decades, no effective and definitive treatment has been still provided for some diseases such as autoimmune diseases [1], [2], microbial resistance [3], [4], AIDS [5], chronic pains [6], [7], [8] and cancer [9], [10]. Cancer is one of today’s common diseases, which is caused by the uninhibited proliferation of cells. The number of people affected by different types of cancer and the resulting deaths is increasing every year [11], [12]. Some of the factors involved in increasing the risk of cancer include new lifestyles, increased consumption of unhealthy foods, increased elderly population, and tobacco and alcohol consumption [13]. Despite the prevalence of various types of cancer, the current anticancer compounds are not responsive and have some disadvantages and problems. Increased drug resistance, lack of distinction between cancer cells and normal cells, severe side effects and high costs seem to be some of the current common anticancer drug problems. Hence, much effort is needed to find new anticancer compounds in the view of the increasing growth of this disease. The researchers have recently focused on the use of nanotechnology for the synthesis of various anticancer compounds and reported an optimal anticancer activity for a variety of nanoparticles.
Copper oxide nanoparticles have recently received much attention because of their biocompatibility and anticancer properties. Despite few studies reported in this regard, these studies have shown that copper oxide nanoparticles have a potential ability as an anticancer agent [14], [15]. In recent years, the use of metal nanoparticles in the structure of nanocomposites has expanded rapidly. Some of the advantages of employing nanoparticles in the form of nanocomposites include the easy control of the metals concentrations, low costs of the production process and most importantly the prevention of the agglomeration of nanoparticles. Different compounds are used as the substrate for the synthesis of nanocomposites. In this regard, polymers are one of the most important ones [16]. Due to biocompatibility and biodegradability, alginate biopolymer has come to the focus of attention for various biological applications. This biopolymer is a polysaccharide composed of two subunits of mannuronic acid and glucuronic acid [17]. Polymer alginate is usually synthesised in two forms; chemical and microbial. Microbial alginate can be synthesized by fungi and bacteria. Bacterial alginate is produced using Azotobacter and Pseudomonas. Also, bacterial biosynthesis of alginate can provide compounds with defined chemical structures and physical properties. Recent developments in the relative ease of modifying bacteria and regulating alginate biosynthesis in bacteria may empower the production of alginate with customised features and extensive biomedical applications [18].
Although much research has been done on the use of nanocomposites as antimicrobial agents, their anticancer properties have been less noticeable. The present study was conducted to use copper oxide nanoparticles in the form of alginate – copper oxides nanocomposite for the synthesis of novel nanocomposite with the most favourable anticancer activity using the Taguchi method.
Material and Methods
The copper oxide nanoparticles were synthesised using the coprecipitation method by an approach introduced in the previous study. Alginate biopolymer was synthesized using Azotobacter vinelandii IBRC 10786 prepared from Iranian Biological Resource Center [19].
Aimed at determining the optimal conditions for the synthesis of nanocomposites with the highest anticancer activity, 9 experiments were designed by Taguchi method with different amounts of biopolymer and nanoparticles at different stirring times using the Qulitek-4 software (Table 1). Thus, solutions of copper oxides nanoparticles (2, 4, and 8 mg/ml) and alginate biopolymer (0.5, 1, and 2 mg/ml) were prepared at 3 levels separately. The solution containing the nanoparticles was then added to the biopolymer solution drop by drop and combined with the in situ synthesis method at stirring times of 30, 60 and 90 min. After stirring, the solutions containing the nanocomposite were placed in a 40 °C oven as long as the white powder of the nanocomposite is formed.
Table 1.
Taguchi design of experiments and results of anticancer activity of alginate-copper oxide bionanocomposites
| Experiment | CuO (mg/ml) | Alginate (mg/ml) | Stirring time (min) | Cell growth inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 0.5 | 1 | 2 | 30 | 60 | 90 | ||
| 1 | 2 | 0.5 | 30 | 42.62 | ||||||
| 2 | 2 | 1 | 60 | 55.39 | ||||||
| 3 | 2 | 2 | 90 | 46.11 | ||||||
| 4 | 4 | 0.5 | 60 | 66.38 | ||||||
| 5 | 4 | 1 | 90 | 72.30 | ||||||
| 6 | 4 | 2 | 30 | 68.36 | ||||||
| 7 | 8 | 0.5 | 90 | 55.14 | ||||||
| 8 | 8 | 1 | 30 | 70.42 | ||||||
| 9 | 8 | 2 | 60 | 75.63 | ||||||
The MTT method was used to determine the anticancer activity of 9 nanocomposites synthesised in this study. To do so, the cells of the Michigan Cancer Foundation-7 (MCF-7) line were cultured in the Dulbecco’s Modified Eagle’s Medium (DMEM) medium in the presence of 5% CO2 and 95% moisture at 37°C. Then, 180 μl of cell suspension containing 5 × 104 cells were added to each well plate to be cultured for 24 h. About 20 μl of the synthesised nanocomposites in 9 experiments was added to each well, and the cells were treated for 72 h. After adding 20 μl of the MTT solution to each well and performing MTT test steps, the absorption rate of each plate was determined by ELISA reader. All experiments of this study included three tests carried out in triplicate. Finally, the inhibition rate of MCF-7 cancer cells growth by the studied nanocomposites was determined using the proposed relation [20].
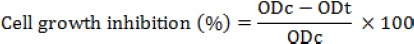
ODt: optical density of treated cells
ODc: optical density of control cells
Results
Based on Taguchi method, nine nanocomposites were synthesised using factors including the content of copper oxide nanoparticles, alginate biopolymer and different stirring times. Synthesised nanocomposites were added to the MCF-7 cancer cells to identify the most potent anticancer compound (Table 1). The nanocomposite produced using 8 mg/ml of copper oxide, 2 mg/ml of alginate and the stirring time of 60 min (Test 9) showed the highest inhibitory growth rate of 75.63% against the cancer cells. The lowest inhibition of cell growth was related to the nanocomposite synthesized under the conditions of experiment 1 (2 mg/ml of copper oxide, 0.5 mg/ml of alginate, and the stirring time of 30 min), which was 42.62%.
The effects of the factors of copper oxide nanoparticles, alginate biopolymer and different stirring times at different levels on the growth inhibition rate of the MCF-7 cells by alginate-copper oxide nanocomposites are presented in Table 2. The second level in all three examined factors showed the highest effect in inhibiting the growth of cancer cells. The effects of factors, including copper oxide nanoparticles, alginate biopolymer and the stirring time in the structure of alginate-copper oxide nanocomposite on the inhibitory effect on the cancer cell growth were 69.01%, 66.04% and 65.80%, respectively.
Table 2.
Effect of different levels of factors on the anticancer activity of alginate-copper oxide bionanocomposite
| Factors | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| CuO | 48.04 | 69.01 | 67.06 |
| Alginate | 54.71 | 66.04 | 63.37 |
| Stirring time | 60.47 | 65.80 | 57.85 |
Table 3 shows the interactions among the studied factors (copper oxide nanoparticles, alginate biopolymer and the stirring time) at different levels. The third level of alginate biopolymer and the second level of the stirring time had the highest interaction effect on inhibiting the growth of cancer cells by a value of 58.57%. The copper oxide nanoparticles at the third level and the stirring time at the second level showed a significant interaction in inhibiting the cells growth by 22.34%. The interaction between the third levels of copper oxide nanoparticles and alginate biopolymer by 10.37% revealed the lowest severity of the effect on reducing the growth of MCF-7 cells.
Table 3.
The interactions effects of studied factors on anticancer activity of alginate-copper oxide bionanocomposite
| Interacting factor pairs | Column | Severity Index (%) | Optimum conditions |
|---|---|---|---|
| Alginate × Stirring time | 2 × 3 | 58.75 | [3, 2] |
| CuO × Stirring time | 1 × 3 | 22.34 | [3, 2] |
| CuO × Alginate | 1 × 2 | 10.37 | [3, 3] |
Analysis of the variance of copper oxide nanoparticles, alginate biopolymer and the stirring time on the inhibition of growth of the MCF-7 cancer cells is shown in Table 4. Copper oxide nanoparticles (68.75%) and the stirring time (6.60%) showed the highest and lowest effect on inhibiting the cells growth, respectively. Alginate biopolymer was also effective in reducing the growth of cancer cells by 16.42%.
Table 4.
Analysis of variance for anticancer activity of alginate-copper oxide bionanocomposite
| Factors | DOF | Sum of Squares | Variance | F-Ratio (F) | Pure Sum | Per cent (%) |
|---|---|---|---|---|---|---|
| CuO | 2 | 805.57 | 402.78 | 34.44 | 782.18 | 68.75 |
| Alginate | 2 | 210.23 | 105.11 | 8.99 | 186.84 | 16.42 |
| Stirring time | 2 | 98.49 | 49.25 | 4.21 | 75.10 | 6.60 |
The optimal conditions for the synthesis of alginate-copper oxide nanocomposites with the highest anticancer activity were predicted using the Taguchi method (Table 5). Based on the obtained results, the second levels of the factors of copper oxide nanoparticles, alginate biopolymer and the stirring time had the highest role in improving the inhibition of cancer cell growth by 7.64%, 4.66% and 4.43% contribution, respectively. Due to the mean growth inhibition rate of MCF-7 cells under different conditions (61.37%) and the reduced growth rate at optimal conditions (16.73%), it is expected that the growth of cancer cells will be inhibited as 78.10% under the optimal conditions recommended for the synthesis of nanocomposites.
Table 5.
Prediction of optimal conditions for the synthesis of alginate-copper oxide bionanocomposite with maximum anticancer activity
| Factors | Level | Contribution |
|---|---|---|
| CuO | 2 | 7.64 |
| Alginate | 2 | 4.66 |
| Stirring time | 2 | 4.43 |
| The total contribution from all factors | 16.73 | |
| Current grand average of performance | 61.37 | |
| Cell growth inhibition at optimum condition | 78.10 |
Discussion
The results indicated that all synthesised nanocomposites had a desirable anticancer activity. Also, all the studied factors (copper oxide nanoparticles, alginate biopolymer and stirring time) were effective on the anticancer activity of alginate-copper oxide nanocomposite. In agreement with the results obtained, previous studies have also reported the anticancer activity of copper oxide nanoparticles and the nanocomposites containing those [21], [22], [23]. Jeronsia et al., [22] synthesised the copper oxide nanoparticles using the coprecipitation method and evaluated their anticancer activity at different levels against the MCF-7 cell line. According to their reports, with increasing dose, the anticancer activity of nanoparticles would increase.
The placement of nanoparticles in the nanocomposite structure improves their structural properties, prevents their agglomeration and increases their contact surface [19]. The sum of the factors mentioned above can be effective in improving the anticancer activity of nanocomposites. The mechanism of the effect of nanoparticles and the nanocomposites containing them on cancer cells and how to differentiate them from other cells is not exactly known. In this regard, Azizi et al., [24] synthesised a biocompatible nanocomposite using albumin and copper oxide nanoparticles. The anticancer properties of synthesised nanocomposites against breast cancer cells were studied by MTT method. They indicated that anticancer activity of albumin-copper oxide nanocomposite is significantly improved compared to the copper oxide nanoparticles. Also, ROS production was significantly higher in nanocomposite treated cells.
ROS may provide an appropriate explanation for the selective toxicity of nanoparticles against cancer cells [25]. ROS and various signalling molecules are mainly found more in cells with a high proliferation such as cancerous cells because of their high metabolic rate compared to the normal cells. In the treatment of cancer cells, the nanoparticles react with chemicals and the signalling molecules surrounding them with a high rate, leading to the increased production of the ROS. By increasing the amount of ROS, the intensity of oxidative stress increases in the cell, which ultimately leads to apoptosis. However, ROS is also produced in the treatment of normal cells using the nanoparticles, but their production rate is relatively low in the normal cells compared to cancer cells since the normal cells have lower amounts of ROS and less signalling molecules. Therefore, the oxidative stress may not be sufficient to kill the cell, and as a result, they show a relatively low response to toxicity. Hence, the mentioned mechanism may be the main contributor to the selective toxicity of nanoparticles for cancer cells [26].
In this study, the Taguchi method was used to determine the optimum conditions for the synthesis of alginate-copper oxide nanocomposite with the highest anticancer ability considering its applied advantages in reducing the cost, time and the optimal predictive power. The findings of this study suggested that the alginate-copper oxide nanocomposite has an anticancer effect on the MCF-7 cancer cells at low concentrations. Moreover, these findings provided a new perspective on the use of nanocomposites in cancer treatment. Thus, they can be used to combat all types of cancers by performing more thorough studies. Also, animal and clinical studies of alginate-copper oxide nanocomposite and its mechanisms of action are recommended for further studies.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Mozaffari HR, Sharifi R, Sadeghi M. Prevalence of oral lichen planus in diabetes mellitus: a meta-analysis study. Acta Inform Med. 2016;24(6):390–393. doi: 10.5455/aim.2016.24.390-393. https://doi.org/10.5455/aim.2016.24.390-393 PMid: 28077900 PMCid: PMC5203753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffari HR, Sharifi R, Sadeghi M. Interleukin-6 levels in the serum and saliva of patients with oral lichen planus compared with healthy controls: a meta-analysis study. Centr Eur J Immunol. 2018;43(1):103–108. doi: 10.5114/ceji.2018.74880. https://doi.org/10.5114/ceji.2018.74880 PMid: 29731693 PMCid: PMC5927179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safaei M, Taran M. Fabrication, characterization, and antifungal activity of sodium hyaluronate-TiO2 bionanocomposite against Aspergillus niger. Mater Lett. 2017;207:113–116. https://doi.org/10.1016/j.matlet.2017.07.038. [Google Scholar]
- 4.Taran M, Etemadi S, Safaei M. Microbial levan biopolymer production and its use for the synthesis of an antibacterial iron (II, III) oxide–levan nanocomposite. J Appl Polym Sci. 2017;134(12):44613. https://doi.org/10.1002/app.44613. [Google Scholar]
- 5.Bhatti AB, Usman M, Kandi V. Current scenario of HIV/AIDS, treatment options, and major challenges with compliance with antiretroviral therapy. Cureus. 2016;8(3):515. doi: 10.7759/cureus.515. https://doi.org/10.7759/cureus.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharifi R, Nazari H, Bolourchi P, Khazaei S, Parirokh M. The most painful site of maxillary anterior infiltrations. Dent Res J (Isfahan) 2016;13(6):539–543. doi: 10.4103/1735-3327.197030. https://doi.org/10.4103/1735-3327.197030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravarthy KV, Boehm FJ, Christo PJ. Nanotechnology: A Promising New Paradigm for the Control of Pain. Pain Med. 2017;19(2):232–243. doi: 10.1093/pm/pnx131. https://doi.org/10.1093/pm/pnx131 PMid: 29036629. [DOI] [PubMed] [Google Scholar]
- 8.Sharifi R, Khazaei S, Mozaffari HR, Amiri SM, Iranmanesh P, Mousavi SA. Effect of massage on the success of anesthesia and infiltration injection pain in maxillary central incisors: Double-blind, crossover trial. Dent Hypotheses. 2017;8(3):61–64. https://doi.org/10.4103/denthyp.denthyp_52_16. [Google Scholar]
- 9.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12(1):39–50. doi: 10.1038/nrc3180. https://doi.org/10.1038/nrc3180 PMid: 22193407. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffari HR, Payandeh M, Ramezani M, Sadeghi M, Mahmoudiahmadabadi M, Sharifi R. Efficacy of palifermin on oral mucositis and acute GVHD after hematopoietic stem cell transplantation (HSCT) in hematology malignancy patients: a meta-analysis of trials. Wspolczesna Onkol. 2017;21(4):299–305. doi: 10.5114/wo.2017.72400. https://doi.org/10.5114/wo.2017.72400 PMid: 29416437 PMCid: PMC5798422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalyanaraman B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017;12:833–842. doi: 10.1016/j.redox.2017.04.018. https://doi.org/10.1016/j.redox.2017.04.018 PMid: 28448945 PMCid: PMC5406543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Brawley OW, Wender RC. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68(4):297–316. doi: 10.3322/caac.21446. https://doi.org/10.3322/caac.21446 PMid: 29846940. [DOI] [PubMed] [Google Scholar]
- 13.Pacheco SO, Pacheco FJ, Zapata GM, Garcia JM, Previale CA, Cura HE, Craig WJ. Food habits, lifestyle factors, and risk of prostate cancer in Central Argentina: a case control study involving self-motivated health behavior modifications after diagnosis. Nutrients. 2016;8(7):419. doi: 10.3390/nu8070419. https://doi.org/10.3390/nu8070419 PMid: 27409631 PMCid: PMC4963895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sankar R, Maheswari R, Karthik S, Shivashangari KS, Ravikumar V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater Sci Eng C. 2014;44:234–239. doi: 10.1016/j.msec.2014.08.030. https://doi.org/10.1016/j.msec.2014.08.030 PMid: 25280701. [DOI] [PubMed] [Google Scholar]
- 15.Sivaraj R, Rahman PK, Rajiv P, Narendhran S, Venckatesh R. Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A. 2014;129:255–258. doi: 10.1016/j.saa.2014.03.027. https://doi.org/10.1016/j.saa.2014.03.027 PMid: 24747845. [DOI] [PubMed] [Google Scholar]
- 16.Safaei M, Taran M. Optimal conditions for producing bactericidal sodium hyaluronate-TiO2 bionanocomposite and its characterization. Int J Biol Macromol. 2017b;104:449–456. doi: 10.1016/j.ijbiomac.2017.06.016. https://doi.org/10.1016/j.ijbiomac.2017.06.016 PMid: 28619641. [DOI] [PubMed] [Google Scholar]
- 17.Raveendran S, Rochani AK, Maekawa T, Kumar DS. Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers. Materials. 2017;10(8):929. doi: 10.3390/ma10080929. https://doi.org/10.3390/ma10080929 PMid: 28796191 PMCid: PMC5578295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remminghorst U, Rehm BH. Bacterial alginates: from biosynthesis to applications. Biotechnol Lett. 2006;28(21):1701–1712. doi: 10.1007/s10529-006-9156-x. https://doi.org/10.1007/s10529-006-9156-x PMid: 16912921. [DOI] [PubMed] [Google Scholar]
- 19.Safaei M, Taran M. Optimized synthesis, characterization, and antibacterial activity of an alginate–cupric oxide bionanocomposite. J Appl Polym Sci. 2018;135(2):45682. https://doi.org/10.1002/app.45682. [Google Scholar]
- 20.Rezaei R, Mostafaie A, Gorgin Karaji A, Mansouri K. The effect of standardized extract of Echinacea Purpurea on cytotoxicity and proliferation of rat splenocytes. J Appl Biol Sci. 2015;9(2):19–22. [Google Scholar]
- 21.Jan T, Iqbal J, Farooq U, Gul A, Abbasi R, Ahmad I, Malik M. Structural, Raman and optical characteristics of Sn doped CuO nanostructures: a novel anticancer agent. Ceram Int. 2015;41(10):13074–13079. https://doi.org/10.1016/j.ceramint.2015.06.080. [Google Scholar]
- 22.Jeronsia JE, Raj DV, Joseph LA, Rubini K, Das SJ. In vitro antibacterial and anticancer activity of copper oxide nanostructures in human breast cancer Michigan Cancer Foundation-7 cells. J Med Sci. 2016;36(4):145–151. https://doi.org/10.4103/1011-4564.188899. [Google Scholar]
- 23.Sriram K, Maheswari PU, Ezhilarasu A, Begum KM, Arthanareeswaran G. CuO-loaded hydrophobically modified chitosan as hybrid carrier for curcumin delivery and anticancer activity. Asia-Pac J Chem Eng. 2017;12(6):858–871. https://doi.org/10.1002/apj.2124. [Google Scholar]
- 24.Azizi M, Ghourchian H, Yazdian F, Dashtestani F, AlizadehZeinabad H. Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB 231. PloS one. 2017;12(11):e0188639. doi: 10.1371/journal.pone.0188639. https://doi.org/10.1371/journal.pone.0188639 PMid: 29186208 PMCid: PMC5706725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009;2(11):882–890. https://doi.org/10.1007/s12274-009-9089-5. [Google Scholar]
- 26.Bisht G, Rayamajhi S. ZnO Nanoparticles: A promising anticancer agent. Nanobiomedicine. 2016;3:9. doi: 10.5772/63437. https://doi.org/10.5772/63437 PMid: 29942384 PMCid: PMC5998263. [DOI] [PMC free article] [PubMed] [Google Scholar]


