Abstract
AIM:
This study was conducted to identify malondialdehyde (MDA) serum level, nerve growth factor (NGF) serum level, diabetic peripheral neuropathy score and the correlation between MDA and NGF serum level with diabetic peripheral neuropathy score.
METHODS:
A cross-sectional study was conducted to observe diabetic patients in the internal medicine department in Dr M. Djamil Hospital, Padang, Indonesia. The MDA serum level was measured using Beuge method with thiobarbituric acid. The NGF serum level was analysed using ELISA method. Diabetic peripheral neuropathy score was defined when history score in Michigan Neuropathy Screening Instrument (MNSI) ≥ 7 and physical assessment score in MNSI > 2.
RESULTS:
Thirty subjects with diabetes has diabetic peripheral neuropathy score 3.53 (± 0.91), MDA serum level 2.16 (± 2.89) nmol/ml, and NGF serum level 10.56 (± 2.89) pg/dl. There were significant correlations between the MDA serum level and the diabetic peripheral neuropathy score (r = 0.364, p = 0.048), and between the NGF serum level with the diabetic peripheral neuropathy score (r = -0.59, p = 0.001).
CONCLUSION:
There are high MDA serum level and low NGF serum level in patients with diabetic peripheral neuropathy. Low NGF serum level plays a bigger role than high MDA serum level in diabetic peripheral neuropathy.
Keywords: Malondialdehyde, Nerve growth factor, Diabetic peripheral neuropathy score
Introduction
Diabetes mellitus is a chronic disease that may cause a long term health problem and multiple quality of life depriving complications. One of the diabetic complications is diabetic peripheral neuropathy that can be assessed by Minnesota Neuropathy Screening Instrument (MNSI). The MNSI includes two separate assessments, a 15-item self-administered questionnaire and a lower extremity examination. Diabetic peripheral neuropathy is based on the existence of chronic hyperglycemia. Chronic hyperglycemia is associated with oxidative stress, endothelium damages, and it causes microvascular and hemorheological disturbances [1], [2].
Oxidative stress can be determined by several ways, such as MDA. MDA is one of recommended marker for oxidative stress. MDA, a product of the lipid peroxidation, has been accepted as one of the reliable biological markers for oxidative stress, based on BOS (Biomarker Oxidative Stress) Study in 2002 [3], [4], [5]. MDA has been documented as a primary biomarker of free radical-mediated lipid damage and oxidative stress. Increased MDA level in serum and may other tissues have been reported in diabetic patients. MDA may impact peripheral nerve among diabetic patients [6], [7].
NGF is an important protein to maintain life and survival of the neurons, and it works by increasing cell regeneration and decreasing the degeneration. NGF decline level is associated with the disturbances in Schwann cells regeneration because NGF produced by the neurons and the Schwann cells. NGF decline level induces peripheral nerve lesions in diabetic patients. The forms of lesions are axonal atrophy, demyelination, and reduced number of nerve fibres [8], [9]. Pittinger and Vinik (2003) found that a decrease of NGF serum level in diabetes correlates with the clinical symptoms of neuropathy [10]. The clinical symptoms of diabetic neuropathy can be assessed with diabetic peripheral neuropathy score by Minnesota Neuropathy Screening Instruments (MNSI) [11].
The objective of this study is to identify MDA serum level, NGF serum level, diabetic peripheral neuropathy score and the correlation between MDA and NGF serum level with diabetic peripheral neuropathy score.
Methods
This was a cross-sectional study, conducted in the internal medicine department in Dr M. Djamil Hospital, Padang, West Sumatera, Indonesia. This study was conducted from January 2017 until December 2017.
Inclusion criteria in this study are type 2 diabetes mellitus (T2DM) patients with peripheral neuropathy, aged between 18 to 59 years old that has agreed to participate and signed the informed consent form.
Exclusion criteria in this study are patients with chronic renal disease, liver cirrhosis, stroke, Alzheimer’s disease, allergic disease, rheumatoid arthritis, psoriatic arthritis, systemic lupus erythematosus, systemic sclerosis and other autoimmune disorder, leprosy, anemia, thrombocytosis, patients currently using antituberculosis drugs, cytostatic drug, steroid, alcohol, and patients with foot ulcer that is difficult to examine.
The diabetic peripheral neuropathy score was calculated by history score in MNSI score ‘7’ or higher and physical assessment score in MNSI more than two. The history score in MNSI is a self-fulfilled questionnaire. Physical assessment in MNSI was the appearance of feet, ulceration, ankle reflexes, vibration perception at great toe, and monofilament [6]. The appearance of feet was scored 1 when there was deformities, dry skin, callus, infection, fissure.
Ulceration was score 1 when present, and scored 0 if absent.
The ankle reflexes, also known as the Achilles reflex, examined by tapped the Achilles tendon while the foot is dorsiflexed. When there was no reflex, they would get score 1, when the reflex which appeared only after assisted by Jendrassic manoeuvre, they would get score 0.5.
Vibration perception at great toe was examined by placing the fork on the projection of distal interphalangeal joint of the hallux. Subjects would tell the examiner whether they felt the vibration when it disappeared with closed eyes. When subjects did not report any vibration, the score was zero. When the subjects felt the vibration less than 10 seconds, they would get score 0.5.
The monofilament testing was done on ten points in each foot. The monofilament was perpendicular and pressed for two seconds only, while the subjects closed their eyes. All points would be tested for three times. Score zero would be given for eight right answers or more, and a half point for one to seven right answers.
MDA and NGF serum level were measured by laboratory methods. About 3 mL blood samples were taken from the cubital vein using an anticoagulant-free container to examine MDA and NGF serum levels. MDA serum level was measured by using Beuge method with thiobarbituric acid, while NGF serum level was measured by using the ELISA method for human nerve growth factor-β.
The numerical data would be presented as mean and standard deviation. Data were analysed using Statistical Package for Social Sciences (SPSS) 22.0. The normality test was performed only for the numerical data using the Kolmogorov-Smirnov test. Correlation between MDA and NGF serum levels with peripheral diabetic neuropathy score were examined using the Spearman correlation test. The coefficient correlation was calculated and the level of significance for the correlation was counted. The level of statistical significance was 5%.
Results
Patient’s age in this study ranged from 41 to 59 years old. The range of the MDA serum level in this study was between 1.6 nmol/mL to 3.01 nmol/ml while the NGF serum level ranged from 6.8 pg/dl up to 15.7 pg/dl. The peripheral diabetic neuropathy score in this study is 3.53 (± 0.91)
Table 1.
Characteristics of the subjects
| Variable | n (%) | mean (SD) |
|---|---|---|
| Age (years) | 53.9 (5.7) | |
| Sex | ||
| Male | 13 (43) | |
| Female | 17 (57) | |
| Body Mass Index (Kg/m2) | 24.6 (3.7) | |
| HbA1c (%) | 8.8 (2.0) | |
| Neuropathy score | 3.53 (0.91) | |
| MDA (nmol/mL) | 2.16 (0.28) | |
| NGF (pg/dL) | 10.56 (2.89) | |
There was a positive correlation (r = 0.364, p = 0.048) proved between the MDA serum level with diabetic peripheral neuropathy score (Figure 1). The correlation between NGF serum level and diabetic peripheral neuropathy score was r = -0.59 and p = 0.001 (Figure 2).
Figure 1.
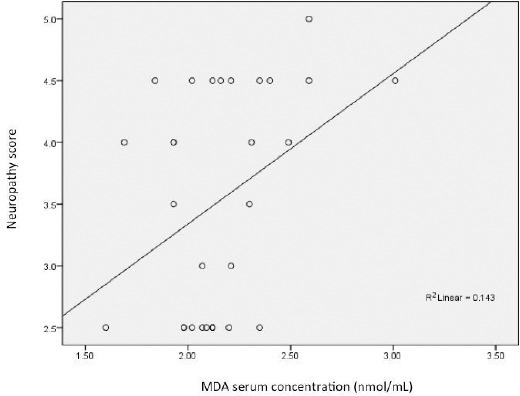
Correlation between the MDA serum level and the diabetic peripheral neuropathy score (r = 0.364 and p = 0.048)
Figure 2.
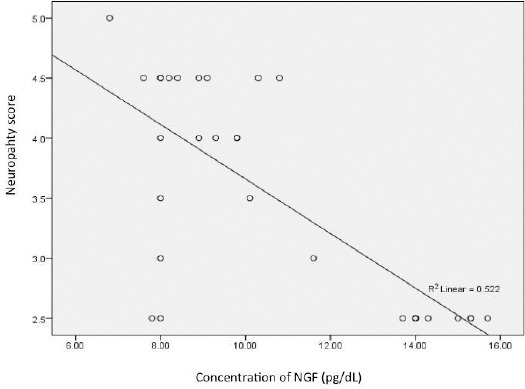
Correlation between NGF serum level and diabetic peripheral neuropathy score (r = -0.59 and p = 0.001)
Discussion
The mean MDA serum level of this study was higher than normal. Maitreyess (2011) and Martin-Gallan (2003) has also found that MDA serum level on T2DM with complication was higher than the no complication group [12], [13]. Bhutia (2011) and Jalees (2017) found that the MDA serum level was significantly elevated in patients with diabetes compared to those in normal subjects [14], [15]. Zavar-Reza (2014) observed significant increased MDA serum level in T2DM patients with complications [16]. Increased MDA serum level can be explained by increasing oxidative stress in diabetic patients. Increased level of oxidative stress associated with free radical-mediated lipid peroxidation. MDA serum level increased because of free oxygen radicals in diabetes that cause peroxidative breakdown of phospholipids.
In this study, the mean serum level of NGF was lower than normal. Mahmoud et al. in 2009 found a significant lower NGF serum level among patients with peripheral neuropathy [17]. Obrosova et al., (2001) had shown that NGF serum level in the diabetic patient was lower than the control [18]. Tosaki et al., (2008) reported that the Schwann cell planted on high-glucose culture media had a low concentration of NGF [19]. Yilmaz et al., (2013) obtained the result of lower NGF production by the Schwann cell of diabetic subjects [20]. NGF decline level is associated with the disturbances in Schwann cells regeneration because NGF produced by the neurons and the Schwann cells. The oxidative stress that occurs in diabetic patients will diminish the nutrient and oxygen supply for the nerve cells, resulting in interfering the life of the nerve and the Schwann cells and increasing the cell degeneration [2], [21].
In this study, we found a positive correlation between MDA serum level with diabetic peripheral neuropathy score. Aziza (2014) found that an increase of MDA serum level in diabetic neuropathy rats [22]. Martinez-Hevaz (2017) reported that altered in MDA values is associated with polyneuropathy in diabetic patients [23].
We found a significant negative correlation between NGF serum level with diabetic peripheral neuropathy score. Yasuda et al., (2003) discovered a positive correlation between decreasing NGF serum level and the neuron regeneration. NGF regulates intraneural homeostasis during development by providing neurotrophic and regulating intracellular pathways to promote neuronal sprouting [11]. Li (2017) showed that NGF has a protective effect in diabetic peripheral neuropathy [24].
In conclusion, there is a high MDA serum level and low NGF serum level in patients with diabetic peripheral neuropathy. Low NGF serum level play a bigger role than high MDA serum level in diabetic peripheral neuropathy.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. https://doi.org/10.1038/nrendo.2017.151 PMid: 29219149. [DOI] [PubMed] [Google Scholar]
- 2.Ollendorf DA, Kotsanos JG, Wishner WJ, Friedman M, Cooper T, Bittoni M, et al. Potential economic benefits of lower-extremity amputation prevention strategies in diabetes. Diabetes Care. 1998;21:1240–5. doi: 10.2337/diacare.21.8.1240. https://doi.org/10.2337/diacare.21.8.1240 PMid: 9702427. [DOI] [PubMed] [Google Scholar]
- 3.Vincent AM, Russel JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25(4):612–28. doi: 10.1210/er.2003-0019. https://doi.org/10.1210/er.2003-0019 PMid: 15294884. [DOI] [PubMed] [Google Scholar]
- 4.Park Y. Oxidative Stress and Diabetic Neuropathy. InDiabetes: Oxidative Stress and Dietary Antioxidants. 2014:3–13. [Google Scholar]
- 5.Donne ID, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarker of oxidative damage in human disease. Clin Chem. 2006;52(4):601–23. doi: 10.1373/clinchem.2005.061408. https://doi.org/10.1373/clinchem.2005.061408 PMid: 16484333. [DOI] [PubMed] [Google Scholar]
- 6.Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24:250–6. doi: 10.2337/diacare.24.2.250. https://doi.org/10.2337/diacare.24.2.250 PMid: 11213874. [DOI] [PubMed] [Google Scholar]
- 7.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9. doi: 10.2337/diacare.17.11.1281. https://doi.org/10.2337/diacare.17.11.1281 PMid: 7821168. [DOI] [PubMed] [Google Scholar]
- 8.Subekti I. Neuropati perifer. In: Sudoyo AW, Setiyohadi B, Alwi I, Simadibrata M, Setiati S, editors. Buku ajar ilmu penyakit dalam. 5th eds. Jakarta: Pusat Penelitian Ilmu Penyakit Dalam FKUI; 2010. pp. 1947–51. [Google Scholar]
- 9.Tesfaye S. Neuropathy in diabetes. Medicine. 2010;38(12):649–55. https://doi.org/10.1016/j.mpmed.2010.08.012. [Google Scholar]
- 10.Pittenger G, Vinik A. Nerve growth factor and diabetic neuropathy. Exp Diabesity Res. 2003;4(4):271–85. doi: 10.1155/EDR.2003.271. https://doi.org/10.1155/EDR.2003.271 PMid: 14668049 PMCid: PMC2478610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, et al. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69(4):229–85. doi: 10.1016/s0301-0082(03)00034-0. https://doi.org/10.1016/S0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 12.Maitreyess DS. Study of free iron, superoxide dismutase, malondialdehyde and glycated hemoglobin in type 2 diabetes mellitus with and without microvascular complications (dissertation) Bangalore: Rajiv Gandhi University of Health Sciences; 2011. [Google Scholar]
- 13.Martin-Gallan P, Carrascosa A, Gussinye M, Dominguez C. Biomarkers of diabetes- associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med. 2003;34(12):1563. doi: 10.1016/s0891-5849(03)00185-0. https://doi.org/10.1016/S0891-5849(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 14.Bhutia Y, Ghosh A, Sherpa ML, Pal R, Mohanta PK. Serum malondialdehyde level: surrogate stress marker in the Sikkimese diabetics. J Nat Sci Biol Med. 2011;2(1):107–12. doi: 10.4103/0976-9668.82309. https://doi.org/10.4103/0976-9668.82309 PMid: 22470243 PMCid: PMC3312689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalees SS, Rosaline M. Study of malondialdehyde and estimation of blood glucose levels in patients with diabetes mellitus with cataract. International Journal of Clinical Biochemistry and Research. 2017;4(3):319–23. [Google Scholar]
- 16.Zavar-Reza J, Shahmoradi H, Mohammadyari A, Mohammadbeigi M, Hosseini R, Vakili M, et al. Evaluation of malondialdehyde in type 2 diabetic patients with coronary artery disease. J Biol Today's World. 2014;3(6):129–32. https://doi.org/10.15412/J.JBTW.01030602. [Google Scholar]
- 17.Mahmoud ME, Doria AEF, Heba AS, Fawzy AEM, Nashwa MA. Assessment of nerve growth factor and nerve conduction velocity in diabetic patients with neuropathy. Egypt J Neurol Psychiatr Neurosurg. 2009;46(1):101–9. [Google Scholar]
- 18.Obrosova IG, Fathallah L, Stevens MJ. Taurine counteracts oxidative stress and nerve growth factor deficit in early experimental diabetic neuropathy. Exp Neurol. 2001;172:211–9. doi: 10.1006/exnr.2001.7789. https://doi.org/10.1006/exnr.2001.7789 PMid: 11681853. [DOI] [PubMed] [Google Scholar]
- 19.Tosaki T, Kamiya H, Yasuda Y, Naruse K, Kato K, Kozakae M, et al. Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons. Exp Neurol. 2008;213:381–7. doi: 10.1016/j.expneurol.2008.06.017. https://doi.org/10.1016/j.expneurol.2008.06.017 PMid: 18675804. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz M, Aktug H, Oltulu F, Erbas O. Neuroprotective effects of folic acid on experimental diabetic peripheral neuropathy. Toxicology Industrial Health. 2013:1–10. doi: 10.1177/0748233713511513. [DOI] [PubMed] [Google Scholar]
- 21.Harsono Neuropati diabetika. Buku ajar neurologi klinis. Yogyakarta: Perhimpunan Dokter Spesialis Syaraf Indonesia - Gadjah Mada University Press; 2005. [Google Scholar]
- 22.Aziza SAH, El-Haggar M, Abo-Zaid OA, Hassanien MR, El-Shawarby R. Biomarkers of oxidative stress of sciatic nerve tissues in experimental diabetic neuropathy. Journal of Medical Sciences. 2014;14(1):12–20. https://doi.org/10.3923/jms.2014.12.20. [Google Scholar]
- 23.Martinez-Hervaz S, Mendez MM, Folgado J, Tormos C, Ascaso P, Peiro M, et al. Altered Semmes-Weinstein monofilament test results are associated with oxidative stress markers in type 2 diabetic subjects. J Transl Med. 2017;15:187–94. doi: 10.1186/s12967-017-1291-8. https://doi.org/10.1186/s12967-017-1291-8 PMid: 28∥61 PMCid: PMC55↝9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R, Ma J, Wu Y, Nangle M, Zou S, Li Y, et al. Dual delivery of NGF and bFGF coacervater ameliorates diabetic peripheral neuropathy via inhibiting Schwann cells apoptosis. Int J Biol Sci. 2017;13:640–51. doi: 10.7150/ijbs.18636. https://doi.org/10.7150/ijbs.18636 PMid: 28539836 PMCid: PMC5441180. [DOI] [PMC free article] [PubMed] [Google Scholar]


