Abstract
BACKGROUND:
Melanoma appears to be a malignant disease, whose development can be potentiated by different drug groups. More and more data are in favour of the claim that commonly used antihypertensive drugs also contain the risk of developing melanoma. The most evidence is that angiotensin receptor blockers may be carcinogenic. Two representatives from this group, valsartan and irbesartan, produced by certain pharmaceutical companies are being withdrawn from the market due to finding content of NDMA and NDEA, which are believed to be potent carcinogens. Another representative of this group, losartan, according to in vitro data, potentiates cell adhesion and invasion of human melanoma cells.
CASE REPORT:
We present a 45-year-old man with arterial hypertension. For year and a half/two years, the patient is on systemic therapy with Aspirin and Irbesartan/Hydrochlorothiazide. The patient also reported about the presence of a pigmented lesion in the abdominal area, which occurred 5-6 years ago, before the onset of cardiac therapy. According to him, there was a change in the colour and size of the lesion within the framework of cardiac therapy (from 1.5-2 years). Innovative one step melanoma surgery was performed, and the lesion was radically removed with a 1 cm operational safety margin in all directions within one operative session. The subsequent histological verification found the presence of thin melanoma.
CONCLUSION:
Drug-induced melanoma turned out to be a problem of significant importance. The group of angiotensin receptor blockers should be investigated more thoroughly and in detail on the probability of potentiating carcinogenesis. We describe an interesting case showing the progression of pigment lesion to melanoma as a possible result of irbesartan therapy, i.e. we share a theory that differs from that of drug-induced de novo melanomas. It should not be overlooked the fact that another widely used drug-Aspirin, is also likely to potentiate the development of melanoma. Furthermore, the case is indicative of the use of one step melanoma surgery in a melanoma patient with a thickness less than 1 mm.
Keywords: Losartan, Valsartan, Melanoma, Surgery procarcinogenic effect
Introduction
The number of studies, according to which different groups of antihypertensive drugs are likely to potentiate the development of melanoma, increases significantly [1], [2], [3]. According to the literature, angiotensin receptor blockers (ARBs), which are widely used as antihypertensive drugs, carry an increased risk of developing cancer [4]. The latest data suggest that the occurrence of cutaneous cancer, including cutaneous melanoma, is also associated with this group of drugs [2], [5]. Experimental in vitro data show that losartan (ARB) stimulates cell adhesion and invasion of human melanoma cells [3].
Case report
We present a 45-year-old man in good general condition and concomitant arterial hypertension, controlled through medication with Aspirin 100 mg (0-0-1) and Irbesartan/ Hydrochlorothiazide 150 mg/12.5 mg (1-0-0). The patient was hospitalised at the clinic on the occasion of worsening, for several months, of psoriasis vulgaris, diagnosed from 20 years. During the dermatological examination, despite the psoriatic skin changes, in the area of regio abdominalis dextra, was visualised as finding a hyperpigmented macula with uneven pigmentation and uneven edges (Figure 1a and 1b). According to the patient, the pigmented lesion appeared 5-6 years ago. Medication therapy with Aspirin and Irbesartan/Hydrochlorothiazide dates from 1.5-2 years. According to anamnestic data, within the framework of cardiac therapy, the patient observed a change in the colour and size of the lesion. Clinically and dermatoscopically, the pigmented macula met the requirements for a malignant melanocytic lesion. Also, clinical and dermatoscopic data spoke in favour of melanoma less than 1 mm thick. Based on these data, one step melanoma surgery (OSMS) was performed. The lesion was removed by elliptical excision, under local anaesthesia, with a surgical field of 1 cm in all directions (Figure 1b and 1c). The surgical defect was closed by a single interrupted sutures (Figure 1d). Histological examination confirmed the diagnosis: superficial malignant melanoma, Clark II level, Breslow’s thickness below 1 mm, no ulceration, low mitotic activity, well-expressed lymphocytic stromal reaction, clear resection lines. The staging was performed according to which it was found to be melanoma Stage I (T1aN0M0).
Figure 1.
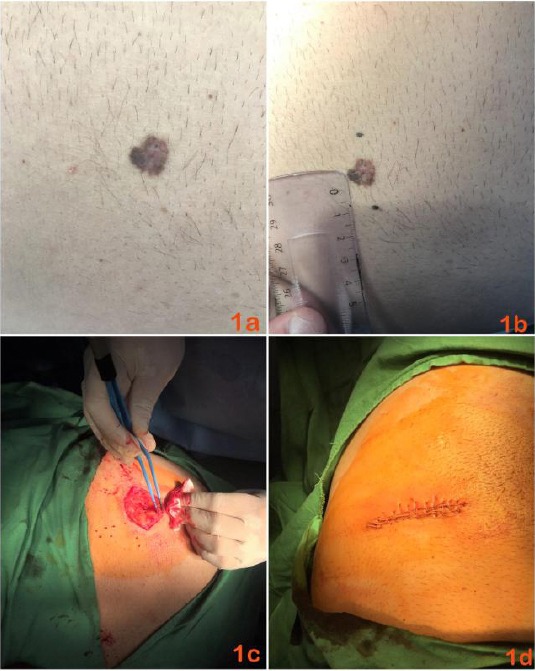
a) Clinical picture of primary cutaneous melanoma located in regio abdominalis dextra with uneven pigmentation; b) Outlining the 1 cm operational security boundaries in all directions, preoperative finding; c) Intraoperative picture of the lesion removed by elliptical excision; d) Postoperative clinical picture of surgical defect closed by single interrupted sutures
Discussion
Antihypertensive drugs are the most common and widely used drugs among the population; however, according to very new data, they may be associated with an increased risk of melanoma occurrence [1]. As possibly potentiating the development of this type and another type of cutaneous tumours, different groups of drugs for the treatment of arterial hypertension are mentioned [1], [2], [5]. Studies of the carcinogenic effect of angiotensin receptor blockers (ARBs) predominate [2], [3], [4], [5], [5]. It is believed that this group of antihypertensive drugs generally carries a risk of developing cancer [4], but the individual risk of different agents is not yet known [4]. The prevailing number of data, however, is in the direction of claiming that ARBs are highly associated with melanoma development [2], [3], [5]. In vitro, experimental data show that losartan inhibits NHE1 (Na + /H + exchanger isoform 1) activity and migration of human melanoma cells (MV3), but at the same time stimulates MV3 cell adhesion and invasion [3].
Some events in 2018 lead to important analyses and conclusions. In June 2018, US manufacturer Prinston Pharmaceuticals Inc. stopped producing products containing valsartan because it detected traces of N-nitrosodimethylamine (NDMA) in the active pharmaceutical ingredient of valsartan (API) provided by a Chinese manufacturer (Zhejiang Huahai Pharmaceutical Co) [6]. NDMA is defined as a chemical substance in the group of potent carcinogens and, according to the US Department of Health and Human Services, exposure to high doses of NDMA may cause liver damage [7], [8]. This is confirmed by animal studies that indicate that NDMA can cause tumours in the liver, kidneys and the respiratory tract, making it a likely human carcinogen [8]. Subsequently, the withdrawal of valsartan expands, following the detection of NDMA in drugs manufactured by a second Chinese manufacturer (Zhejiang Tianyu Pharmaceuticals of Taizhou) and by a manufacturer in India (Hetero Labs Limited, called Camber Pharmaceuticals), followed by voluntary withdrawal of valsartan products from several companies (Major Pharmaceuticals, Solco Healthcare and Teva Pharmaceuticals Industries, as well as valsartan/hydrochlorothiazide from Solco and Teva) [9], [10].
In September 2018, information about a second potential carcinogen in the product valsartan-N-nitrosodiethylamine (NDEA) was published [11].
In November 2018, SciGen Pharmaceuticals voluntarily withdrew ARB-irbesartan due to NDEA content and according to the US Food and Drug Administration (FDA): „This is the first non-valsartan drug product the agency has found to contain the NDEA impurity” (Aurobindo Pharma Ltd manufactures the active pharmaceutical ingredient (API) for ScieGen’s irbesartan products) [12].
The case we have described supports the thesis that, in the presence of pigment lesion, irbesartan probably promotes carcinogenesis in the direction of cutaneous melanoma. This statement differs from that shared in other articles, according to which valsartan are a possible inducer of de novo melanomas, i.e., without the presence of precursor lesions.
Also, the FDA shares the likelihood of cancer being dose-dependent (daily intake of the highest dose of valsartan (320 mg) throughout 4 years, 1/8000 patients are likely to develop cancer) [13].
According to the EMA (European Medicines Agency) (as a result of an electronic correspondence), there have been 9 reported cases of melanoma in patients receiving valsartan. None of them has been formalised and suggestions remain as to whether products are contaminated with NDMA/NDEA, or it is possible that the carcinogenic effect comes directly from the generic substance of valsartan as well as from the presence of a potential another carcinogen [3], [11]. The same open question remains about the carcinogenic effect of irbesartan.
According to a study of 2015, angiotensin II therapy potentiates melanogenesis (increases melanin content and increases tyrosinase activity) through angiotensin type 1 receptors, and losartan eliminates this effect [13]. This, however, indicates that ARBs directly affect melanogenesis and the carcinogenic effect may be related to another, unknown pathogenetic chain.
In conclusion, the case presented is an interesting example due to 1) the possibility of Irbesartan induced cutaneous melanoma in the presence of precursor pigmented lesion and 2) the perfect therapeutic result due to perform one step melanoma surgery.
The content of probable carcinogens, in representatives of the group of angiotensin receptor blockers, suggests the need for further studies on the individual carcinogenic risk for particular drugs. Interestingly, one of the most widely used drugs, i.e. the antihypertensive drugs and acetylsalicylic acid, are pointed out as probably triggering the development of melanoma.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Tang H, Zhai S, Song Y, Han J. Use of Antihypertensive Drugs and Risk of Malignant Melanoma: A Meta-analysis of Observational Studies. Drug Saf. 2018;41(2):161–169. doi: 10.1007/s40264-017-0599-x. https://doi.org/10.1007/s40264-017-0599-x PMid: 28905299. [DOI] [PubMed] [Google Scholar]
- 2.Nardone B, Orrell K, Vakharia P, West D. Skin cancer associated with commonly prescribed drugs: tumor necrosis factor alpha inhibitors (TNF-αIs), angiotensin-receptor blockers (ARBs), phosphodiesterase type 5 inhibitors (PDE5Is) and statins -weighing the evidence. Expert Opin Drug Saf. 2018;17(2):139–147. doi: 10.1080/14740338.2018.1400530. https://doi.org/10.1080/14740338.2018.1400530 PMid: 29103328. [DOI] [PubMed] [Google Scholar]
- 3.Olschewski D, Hofschröer V, Nielsen N, Seidler D, Schwab A, Stock C. The Angiotensin II Type 1 Receptor Antagonist Losartan Affects NHE1-Dependent Melanoma Cell Behavior. Cell Physiol Biochem. 2018;45:2560–2576. doi: 10.1159/000488274. https://doi.org/10.1159/000488274 PMid: 2955∨. [DOI] [PubMed] [Google Scholar]
- 4.Sipahi I, Debanne S, Rowland D, Simon D, Fang J. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11(7):627–36. doi: 10.1016/S1470-2045(10)70106-6. https://doi.org/10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt S, Schmidt M, Mehnert F, Lemeshow S, Sørensen H. Use of antihypertensive drugs and risk of skin cancer. J Eur Acad Dermatol Venereol. 2015;29(8):1545–54. doi: 10.1111/jdv.12921. https://doi.org/10.1111/jdv.12921 PMid: 25640031. [DOI] [PubMed] [Google Scholar]
- 6.D'Arrigo T. FDA issues statement as valsartan recalls grow. AphA, September 4, 2018. Retrieved from https://www.pharmacist.com/article/fda-issues-statement-valsartan-recalls-grow .
- 7.Christensen J. FDA expands recall of blood pressure drug valsartan due to cancer concern. CNN, August 10, 2018. Retrieved from https://edition.cnn.com/2018/08/08/health/valsartan-recall-fda-expanded/index.html .
- 8.WJZ, CBS Baltimore. FDA Recalls Common Blood Pressure Medicine Due To Cancer Concerns. July 17, 2018 [Google Scholar]
- 9.Wendling P. More Drug Makers Tagged as Valsartan Recall Grows. WebMD, August 13, 2018. Retrieved from https://www.webmd.com/heart-disease/news/20180813/more-drug-makers-tagged-as-valsartan-recall-grows .
- 10.Howard J. Valsartan recall:4 things patients should know. CNN, August 28, 2018. Retrieved from https://edition.cnn.com/2018/07/19/health/valsartan-recall-explainer/index.html .
- 11.Herman A. Another Potential Carcinogen Found in Valsartan. NEJM Journal Watch, September 17, 2018. Retrieved from https://www.jwatch.org/fw114583/2018/09/17/another-potential-carcinogen-found-valsartan .
- 12.Brooks M. FDA Issues Alert on Irbesartan Due to Contamination. Medscape Medical News, November 01, 2018. Retrieved from https://www.medscape.com/viewarticle/904270 .
- 13.Liu L, Fan X, Li H, An X, Yang R. Angiotensin II promotes melanogenesis via angiotensin II type 1 receptors in human melanocytes. Mol Med Rep. 2015;12(1):651–6. doi: 10.3892/mmr.2015.3438. https://doi.org/10.3892/mmr.2015.3438 PMid: 25760379. [DOI] [PubMed] [Google Scholar]


