Abstract
BACKGROUND:
Ischemic stroke occurs due to the abrupt occlusion in the brain which leads to neuronal death. Neuronal death in ischemic stroke is due to increase production of reactive oxygen species (ROS). Neuronal death occurs via necrosis and apoptosis mechanisms. Apoptosis can either occur via extrinsic or intrinsic pathway. Meanwhile, the intrinsic pathway can be caspase-dependent or independent. Anthocyanin is a natural pigment with antioxidant, anti-inflammatory, anti-cancer, and neuroprotective properties. Balinese cultivate of purple potato extract contains a high level of anthocyanin and has been proven for its antioxidant activity.
AIM:
Antioxidant effect of Balinese cultivates purple potato extract has not been studied on an animal model with ischemic stroke. Accordingly, we would like to study the effect of antioxidant properties from Balinese cultivate of purple potato extract by assessing the neurological score, BNDF concentration, and caspase-independent apoptosis by measuring AIF concentration on Wistar rats with ischemic stroke.
METHODS:
This was an experimental study using male Wistar rats age between 12-14 weeks weigh between 200 to 250 g.
RESULTS:
This study demonstrated a significant difference of neurological score on day 3 among control versus treatment groups. Balinese cultivate of purple potato extract markedly reduced AIF, increased BDNF, and suppressed apoptosis among treatment group when compared with the control group.
CONCLUSION:
We have proven the efficacy of antioxidant activity of anthocyanin derived from Balinese cultivar of purple sweet potato by elevated AIF levels, lower apoptosis rate, improved neurological score on day-3 to day-7 post-stroke, as well as increased BDNF levels.
Keywords: Ischemic stroke, Balinese cultivate of purple potato extract, Neurological score, AIF, BDNF, Apoptosis
Introduction
Stroke is a degenerative disease leading to number one morbidity and the second cause of mortality worldwide. Ischemic stroke occurs due to the abruption of blood flow in a specific part of the brain which impairs neuronal homeostasis, leading to neuronal death. Neuronal death in ischemic stroke occurs due to increased production of reactive oxygen species (ROS) [1], [2]. Neuronal death in ischemic stroke occurs via either necrosis or apoptosis mechanism. Neuronal death can occur via extrinsic and intrinsic pathway.
Furthermore, the intrinsic pathway can be either caspase-dependent or independent. Also, mitochondria play an important role in apoptosis via extrinsic and intrinsic mechanisms. Intrinsic mechanism occurs via caspase activation, i.e. the release of cytochrome c from mitochondria, which in turn binds to apoptosis platelet activating factor (APAF) to form apoptosome, which then facilitates activation of procaspase 9 into caspase 9. Afterwards, caspase 9 activates procaspase 3 into caspase 3 which then becomes apoptosis executor. Also, mitochondria also play a role in caspase-independent apoptosis which releases proapoptotic proteins, including AIF, Diablo/Smac that triggers DNA fragmentation [3], [4].
Anthocyanin is a natural pigment that belongs to the flavonoid group. It exerts multiple colours to fruits, vegetables, and yams, while also possess intrinsic benefits as an antioxidant, anti-inflammation, anti-cancer, and neuroprotection [5], [6], [7].
Balinese cultivate of purple potato extract contains 209.8 mg/100 g of anthocyanin [8] and has been proven to possess intrinsic antioxidant activity [9]. It also induces intrinsic antioxidant activity [10]. Given the account of oxidative stress as the underlying cause of neuronal apoptosis in ischemic stroke, we would like to study the effects of anthocyanin derived from Balinese cultivate of purple potato extracts against the neurological score, BDNF, and AIF concentration as markers for caspase-independent apoptosis among Wistar rat with ischemic stroke model.
Material and Methods
The process of purple potato extraction was done in the Technological laboratory of farming products at Udayana University with the following described procedure.
Purple potatoes derived from the farmer were then washed with sterile water, peeled for its skin, and subsequently sliced transversally with a thickness of 2 to 2.5 cm. The sliced purple potato then mixed with sterile water with a ratio of 1 kg of purple potato to 1 L of water, and in turn was mixed and liquefied using a blender before filtered using three-layered bands. The obtained filtered water was heated until it boiled for 30 minutes for sterilisation purpose. The anthocyanin content from this sample was 147.0 mg/mL according to the letter from the Technological Laboratory of Farming Products Udayana University no. 145/AN/Lab/FTP/XII/2015.
Wistar rats age between 3-3.5 months weighing 200 to 250 g were obtained from Biopharma Laboratory, Bandung. The study was conducted at the Bioscience Laboratory of Brawijaya University, Malang after had been formally permitted by the Ethical Committee of Brawijaya University, Malang. Animals were acclimatised for a week before put into the bite-resistant cage: twenty rats, each 10 for positive control and treatment group which underwent cerebral artery ligation without and with the administration of purple potato extracts, respectively. AIF and BDNF levels and the number of cerebral cortex neurons which underwent apoptosis were measured from 33 Wistar rats, each of which consists of 11 rats of the negative and positive control, and treatment group. Negative controls underwent neck skin incision and subsequently stitched, while positive controls underwent cerebral artery ligation without purple potato extracts administration, whereas the treatment group underwent cerebral artery ligation with subsequent purple potato extract administration. All animal models were previously anaesthetised using 10 mg/kg body weight of ketamine given intravenously via caudal vein. Ischemic stroke upon Wistar rats was induced by the previously described method [11], i.e. by ligating the right common carotid artery, right external carotid artery, and internal carotid artery on its root to the middle cerebral artery using silk one med soak for 2 hours. The ligation was in turn released after 7 days for reperfusion. Three mL of anthocyanin per day was administered per orally among the treatment group, as soon as there were any neurologic deficits after reperfusion. This was in accordance to the dose used by Jawi et al., [9] for 7 days, only among treatment group. Neurological score among positive control and treatment group were evaluated on the first day at the first, second, and third hour, the third day, and the seventh day. On the eighth day, serum was obtained from animal models to evaluate BDNF and AIF levels, before decapitated using 10 mg per kg of body weight of ketamine using the caudal vein to evaluate the apoptosis.
Neurological score was assessed by well-trained doctoral students of the Veterinary Faculty, Brawijaya University. Neurological score was assessed by using Bederson criteria [12] as follows: rat was picked up by holding the distal tip of its tail and subsequently observed for the position of both of its feet. If both of their frontal feet were aligned, no neurological deficit was assumed. On the other hand, if the contralateral frontal foot from the ligated cerebral artery was in a flexed position, then a neurological deficit was assumed to occur. Rats were then put into a flat cover and given pressure on its back afterwards. Their reactions were then recorded (Table 1).
Table 1.
Neurological score
| Parameter | Scale | Interpretation |
|---|---|---|
| Normal | Level 0 | No neurological deficit |
| Moderate | Level 1 | Frontal foot flexion |
| Severe | Level 2 | Resistance toward lateral pressure was decreased with the absence of any rotation. |
| Level 3 | Identical to level 2, accompanied by rotation. |
Coating microplate with protein derived from blood serum was done undercoating buffer for 2 hours on room temperature, before being washed using washing buffer (PBST) 3 x 3 minutes, then put into 50 μL of 1% BSA for 1.5-hour incubation period.
The sample was subsequently washed using washing buffer thrice for each for 3 minutes and added with 100 μL of biotin-antibody BDNF and AIF (rabbit anti-rat BDNF and AIF) afterwards on each well with subsequent incubation for 1.5-hour period on 37°C. The samples were then washed with washing buffer thrice and added with TMB substrate before incubated for 20 minutes under room temperature and added NaOH. Samples were, in turn, read with ELISA reader on 450 nm wavelength.
Rat brain of those had been necropsied was soaked in 4% paraformaldehyde solution for 1-7 days before being dehydrated. The tissue was mixed with 70% alcohol thrice, and each process was left for 10 minutes while being agitated, with subsequent mixing with 80%, 90%, and 95% of alcohol, and absolute alcohol for 10 minutes each while being agitated. Samples were then added to xylol for clearing process which lasted overnight. Samples were then infiltrated by xylol paraffin and paraffin with a ratio of 1:1 for 30 minutes. Samples were then covered with paraffin to form paraffin block. Liquid paraffin was subsequently added to the paraffin block, which then followed by tissue addition to the block, and waited until the paraffin solidified. The next step was sectioning with 5 μm thickness. The ribbon-like tissue sections were then attached to the object glass which had been wiped with Mayer Albumin.
The next step was deparaffination, in which the paraffin was eliminated using bathing the slide in the xylol for 10 minutes. Samples were soaked into absolute ethanol twice before being put into 96-90-80-70% ethanol, respectively, each for 5 minutes, before rinsed with PBS pH 7.4, thrice, each for 5 minutes. Samples were then soaked in 3% hydrogen peroxide (inside DI water) for 5-10 minutes and subsequently rinsed with PBS pH 7.4, thrice, each for 5 minutes, then soaked into 5% BSA in PBS for 30 minutes on room temperature before finally rinsed again with PBS solution pH 7.4, thrice, each for 5 minutes.
Samples were then mixed with rabbit anti-rat apoptosis and were incubated overnight at 4°C temperature. It was then rinsed again with PBS thrice, each for 3 minutes, and were in turn mixed with the secondary antibody of anti-goat IgG Biotin conjugated. Incubation was done for an hour under ambient temperature and was then rinsed using PBS, thrice, each for 3 minutes, and subsequently, SA-HRP (Strep Avidin-Horseradish Peroxidase) was added to the mixture for 10-20 minutes before rinsed again using PBS, thrice, each for 3 minutes. DAB chromogen substrate (3,3-diaminobenzidine tetrahydrochloride) was added and incubated for 5-10 minutes on ambient temperature and rinsed again using PBS. Counterstain was done using Hematoxylin for 5 minutes on ambient temperature and subsequently washed with flowing water, before dried. It was then mounted with entellan and observed under light microscope BX-53 with 600 times magnification. Calculation method was done using Axio-vision ratio, of which can be seen at http://153.1.200.58:8080/immunoratio/.
Normal data distribution of all research variables included in the study was tested. After data had been known to distribute normally, a mean difference of BDNF, AIF levels, as well as the difference between apoptosis rate between treatment, negative control, and positive control groups were measured and analysed by using one way ANOVA. Meanwhile, the neurological score was analysed in two phases, i.e. the first phase was to evaluate the mean difference of neurological score between control and treatment group over time (head-to-head) which was analysed using independent t-test. The second phase was to evaluate the mean difference of neurological score over time in one group (treatment and control group were analysed separately) by using one way ANOVA test. All data were tested under 95% confidence interval with a p-value of less than 0.05 considered being significant.
Results
In the control group, there were differences of the neurological score between the first day (first, second, and third hour), second, and seventh day (table 2). There was no observable difference of neurological score between the first, second, and third hour in the first day (mean difference = 0). However, neurological score differed significantly between the first and third day by 1.4 (95% CI 1.11 – 1.69; p < 0.001). Similarly, the mean neurological score day 1 also differed significantly from day 7 by 3 (95% CI 2.71 – 3.29; p < 0.001). This trend was also observed among a mean neurological score of day 3 when compared with day 7 which differed by 1.6 (95% CI 1.31 – 1.89; p < 0.001) (Table 2).
Table 2.
Mean Difference and Significance of Neurological Score based on Timing
| Parameter | Time Indicator | Mean Difference | P Value | 95% Ci |
|---|---|---|---|---|
| Control Group | ||||
| Day-1 (1st Hour) | Day-1 (2nd Hour) | 0 | 1.0 | -0.29 – 0.29 |
| Day-1 (3rd Hour) | 0 | 1.0 | -0.29 – 0.29 | |
| Day-3 | 1.4 | < 0.001 | 1.11 – 1.69 | |
| Day-7 | 3 | < 0.001 | 2.71 – 3.29 | |
| Day-3 | Day-7 | 1.6 | < 0.001 | 1.31 – 1.89 |
| Treatment Group | ||||
| Day-1 (1st Hour) | Day-1 (2nd Hour) | 0 | 1.0 | -1.06 – 1.06 |
| Day-1 (3rd Hour) | 0.6 | 0.50 | -0.46 – 1.66 | |
| Day-3 | 2 | < 0.001 | 0.94 – 3.06 | |
| Day-7 | 2.6 | < 0.001 | 1.54 – 3.66 | |
| Day-3 | Day-7 | 0.6 | 0.50 | -0.46 – 1.66 |
Neurological score of the first hour vs. third hour on the first day did not differ significantly among treatment group (mean: 0.6; 95% CI 0.46-1.66; p = 0.50). Nevertheless, neurological score of day-1 vs day-3 differed markedly by 2 (95% CI 0.94-3.06; p < 0.001). Meanwhile, neurological score on day 1 also differed significantly when compared with day 7 by 2.6 (95% CI 1.54-3.66; p < 0.001). Neurological score between day 3 and 7 also differed though statistically insignificant by 0.6 (95% CI -1.66-0.46; p < 0.50) (Table 2).
Table 3.
Mean difference and Significance of Neurological Score between Control and Treatment Group over time (head-to-head)
| Parameter | Control Group | Treatment Group | Mean difference | 95% CI | P value |
|---|---|---|---|---|---|
| 1st hour score | 3 | 2.60 | 0.40 | -0.16 – 0.96 | 0.15 |
| 2nd hour score | 3 | 2.60 | 0.40 | -0.16 – 0.96 | 0.17 |
| 3rd hour score | 3 | 2 | 1 | 0.17 – 1.83 | 0.023 |
| Day 3 score | 1.6 | 0.6 | 1 | 0.33 – 1.67 | 0.006 |
| Day 7 score | 0 | 0 | 0 | 0 | 0 |
Neurological score difference from day to an hour between control and treatment group demonstrated that 1st-hour score on day 1 between control and treatment group differed by 0.4, although statistically insignificant (95% CI -0.16-0.96; p = 0.15). Whereas 2nd-hour score of both groups also differed by 0.4 but statistically insignificant (95% CI -0.16-0.96; p = 0.17). In contrast, neurological sore on the 3rd hour began to show gap by 1 and was statistically significant (95% CI 0.17-1.83; p = 0.023) (Figure 1).
Figure 1.
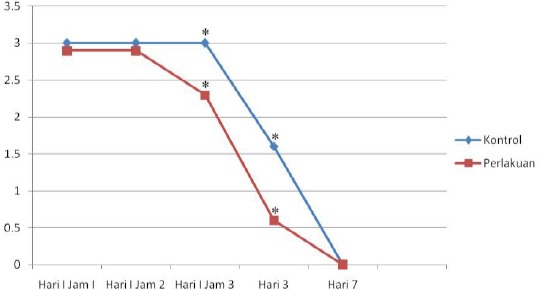
Mean neurological score difference between treatment and control group over time (* indicates significance, p < 0.05)
Mean BDNF levels between treatment and positive control differed markedly by 965.45 (95% CI 909.44 – 1,021.46; p < 0.001).
Table 4.
BDNF, AIF, and Apoptosis
| Parameter | Reference Indicator | Comparator | Mean difference | P value | 95% CI |
|---|---|---|---|---|---|
| BDNF | Treatment | Positive control | 965.45 | < 0.001 | 909.44 – 1,021.46 |
| Negative control | -916.99 | < 0.001 | -973.00 – -860.99 | ||
| Negative control | Positive control | 1.882.45 | < 0.001 | 1,826.44 – 1,938.46 | |
| AIF | Treatment | Positive control | -4.02 | < 0.001 | -4.06 – -3.98 |
| Negative control | 2.30 | < 0.001 | 2.26 – 2.34 | ||
| Negative control | Positive control | -6.32 | < 0.001 | -6.36 – -6.28 | |
| Apoptosis | Treatment | Positive control | -17.86 | < 0.001 | -26.04 – -9.69 |
| Negative control | 1.99 | 0.821 | -6.18 – 10.16 |
Meanwhile, average BDNF levels between treatment and negative control group were also found to differ significantly by -916.99 (95% CI -973.00 – -860.99; p < 0.001). Mean BDNF levels between positive and negative control group also differed significantly by 1,882.45 (95% CI 1,826.44 – 1,938.46) [Figure 2 (left)].
Figure 2.
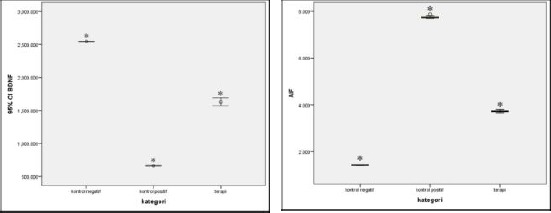
Mean difference of BDNF levels between treatment, negative and positive control group (* indicates significant results at p < 0.05) (left); Mean difference of AIF levels between treatment, negative and positive control group (* indicates significant results at p < 0.05) (right)
According to the one-way ANOVA test, AIF levels differed markedly between negative control, positive control, and treatment group. The mean AIF levels between treatment and positive control differed markedly by -4.02 (95% CI -4.06 – 03.98; p < 0.001), while AIF levels between treatment and negative control group differed significantly by 2.30 (95% CI 2.26 – 2.34; p < 0.001). In addition, mean AIF levels between negative and positive control group also differed by -6.32 (95% CI -6.36 – 6.28; p < 0.001) [Figure 2 (right)].
The number of cells which underwent apoptosis between treatment and negative control group was found to differ insignificantly with mean of 1.99 (95% CI -6.18 – 10.16; p = 0.82), whereas the mean apoptosis rate between treatment and control group was 17.86 and significant statistically (95% CI -26.04 – -9.69; p < 0.001) (Figure 3).
Figure 3.
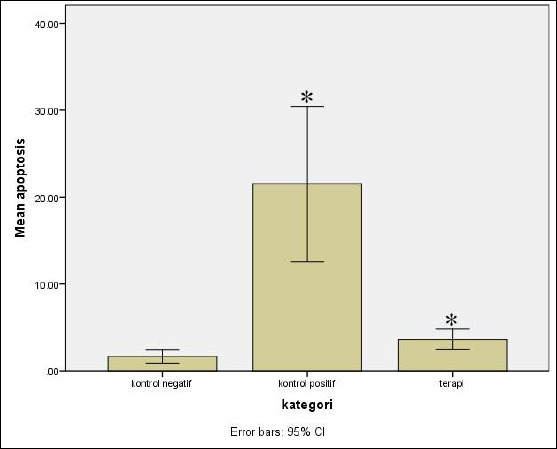
Mean rate of apoptosis between negative and positive control, and treatment group (* indicates significant results at p<0.05)
Discussion
Middle cerebral artery occlusion leads to the neurological deficit, infarction, and apoptosis which were regarded as important indicators for brain damage after ischemia [13]. Cerebral artery occlusion induces cerebral ischemia with subsequent mitochondrial damage that stimulates ROS production. ROS is a key factor in the underlying pathophysiology of ischemia, trauma, and degenerative disease. ROS induces macromolecular damage, including lipid peroxidation, protein oxidation, and DNA oxidation, all of which lead to cellular damage and death (necrosis and apoptosis) [14]. Apoptosis occurs both via extrinsic and intrinsic pathway. The intrinsic mechanism involves mitochondria after receiving stimulus from Bcl-2 protein family, BIH only protein, such as Bid, Ba, and Bim. Stimuli from these proteins will induce oligomerisation of pro-apoptotic proteins, including Bax and Bak. Bak oligomerisation and Bax will induce changes in the outer mitochondrial membrane permeability which in turn releases proapoptotic protein, including cytochrome c, endonuclease G, apoptosis induction factor (AIF), a second mitochondrial activator of caspase/direct IAP binding protein with low pl (Smac/diablo). Cytochrome c will bind to apoptosis platelet activating factor (APAF) which then forms apoptosome which further activates procaspase 9 into caspase 9, and subsequently activates caspase-3 as an executor caspase. AIF can induce caspase-independent apoptosis which spontaneously triggers DNA condensation and fragmentation [3], [4].
A pharmacological drug with antioxidant properties that can inhibit ROS production has a role in preventing brain tissue destruction) [15]. Anthocyanin belongs to polyphenolic class compound easily found in fruits, leaves, and vegetables. Both epidemiological data and clinical studies have confirmed anthocyanin efficacy in preventing multiple types of diseases. For instance, individuals who consume high levels of anthocyanin were linked with lower arterial stiffness rate [16]. Also, young women who consume high anthocyanin concentration also has a lower risk of getting a myocardial infarction [16] and Parkinson’s disease [17]. Anthocyanin can be commonly derived from purple sweet potato and possess a neuroprotective effect by inhibiting ASK1-JNK/p38 and neutralise ROS, thus preventing apoptosis [7]. Balinese cultivar of purple sweet potato possesses anthocyanin and thus antioxidant and anti-inflammatory properties [9]. Antioxidant activity will suppress ROS production, hence minimising mitochondrial damage and apoptotic cascade. This was proven by the significantly lower AIF levels (p < 0.05) among the treatment group. Lower AIF levels also responsible for the significantly lower apoptosis rate among the treatment group (p < 0.05) (Figure 4). The lower rate of apoptosis also reduces neurological score among anthocyanin-treated mice (Figure 1).
BNDF is a neurotrophic factor which plays a central role in the recovery phase after stroke. BDNF is a signal molecule which is crucial in neuronal adaptation and plasticity post-stroke [18]. BNDF is involved in neuronal viability, synaptic plasticity, learning, memory, and neuronal plasticity. BDNF treatment in animal models or rehabilitation has been shown to increase BDNF and improve neuronal recovery after injury/trauma. Neuronal improvement after cerebral trauma depends on BDNF levels because it is a critical neuroprotectant against cerebral ischemia which on in vivo study acts as anti excitotoxicity and inhibits inflammation, thus reducing apoptosis [19]. BDNF maintains neuronal viability via activating 2 surface receptors, i.e. tropomyosin-related kinase B (TrkB) and p75 neurotrophin (p75NTR). BDNF will, in turn, activates intracellular signalling pathway such as PI3K/Akt using TrkB receptor, which influences central nervous system development and function [20]. PI3K/Akt signalling pathway promotes cellular growth and viability by suppressing the activities of proapoptotic protein family of Bcl-2 [21]. Also, Yao et al., [22] found an increase of BDNF expression via TrkB and Akt activities, thus ameliorating apoptotic rate among mice models with focal cerebral ischemia treated with one of the flavonoid groups, i.e. quercetin. It is known that pharmacological therapy can modify the BDNF signalling pathway, including cAMP response-element binding protein (CREB) which facilitates cortical reorganisation and functional recovery after ischemic stroke [23]. We found significantly higher BDNF levels among treatment compared to positive control groups (p < 0.05), thus in line with lower apoptosis levels found among treatment groups and better neurological score on day-3 among treatment as opposed to control groups (p < 0.05).
AIF is a proapoptotic protein released from mitochondria in the cytosolic and nucleus along with EndoG. Its released level is highly dependent on familial proapoptotic protein activity Bcl2 on mitochondria. Also, AIF is capable of inducing caspase-independent apoptosis by directly binds with DNA chromosome and induce chromatin condensation and remodelling, all of which facilitates DNA fragmentation by nuclease enzyme such as Endo G [24]. Apoptosis via mitochondria is induced by increased ROS production and inhibiting ROS as a representative of oxidative stress using antioxidant (such as anthocyanin in the form of cyanidin-3-O-glucoside) has been proven to inhibit mitochondrial AIF release, thus ameliorate apoptosis among mice with focal cerebral ischemia [25]. Furthermore, AIF was found significantly different between negative and positive controls (p < 0.01). AIF levels between positive controls and treatment group were also found to be significantly different (p < 0.001).
Apoptosis can occur via both extrinsic and intrinsic pathway. Apoptosis in ischemic stroke is one mechanism of cellular death among others, as a consequence of excessive ROS production which impairs mitochondrial outer membrane permeability, thus triggering Bax translocation from the cytoplasm to mitochondria and subsequent release of cytochrome c into the cytoplasm. Proapoptotic protein translation is governed by the proapoptotic protein family of Bcl-2 [26], [27].
Apoptosis via the intrinsic pathway consists of two mechanisms, i.e. caspase-dependent and caspase-independent. Caspase-dependent apoptosis occurs due to Bcl-2 proapoptotic protein family signalling, e.g. BH3 only, which then activates Bax and Bak protein to form oligomer in the mitochondrial outer membrane, thus changing its permeability which triggers the release of proapoptotic proteins including AIF and Endo G to the cytoplasm which then activates apoptotic cascade [28].
Anthocyanin is capable of suppressing excessive ROS production, thus ameliorating ROS damaging effects, including the activation of apoptosis cascade [29], [30]. Anthocyanin derived from Balinese cultivar of purple sweet potato also possesses antioxidant properties as well as inducing endogenous antioxidant production [9], [10], thus limiting apoptosis cascade as demonstrated by lower AIF levels among treatment group (p < 0.05), Figure 4.
In conclusion, anthocyanins derived from Balinese cultivar of purple sweet potato possesses antioxidant activity, thus able to inhibit ROS damaging effects among ischemic stroke model of Wistar rats. We have proven the efficacy of antioxidant activity of anthocyanin derived from Balinese cultivar of purple sweet potato by elevated AIF levels, lower apoptosis rate, improved neurological score on day-3 to day-7 post-stroke, as well as increased BDNF levels.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. International journal of stroke. 2009;4(6):461–70. doi: 10.1111/j.1747-4949.2009.00387.x. https://doi.org/10.1111/j.1747-4949.2009.00387.x PMid: 19930058. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee D, and Patil CG. Epidemiology and global burden of stroke. World Neurosurgery. 2011;76:885–90. doi: 10.1016/j.wneu.2011.07.023. https://doi.org/10.1016/j.wneu.2011.07.023 PMid: 22182277. [DOI] [PubMed] [Google Scholar]
- 3.Niizuma K, Endo H, Chan PH. Oxidative stress and mitochindria dysfunction as determinants of ischemic neuroanal death and survival. J Neurochem. 2009;109(1):133–8. doi: 10.1111/j.1471-4159.2009.05897.x. https://doi.org/10.1111/j.1471-4159.2009.05897.x PMid: 19393019 PMCid: PMC2679225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Circu M, Aw TY. Reactive Oxygen Species, Celluler redox system and apoptosis. Free Radical Biology and Medicine. 2010;48:749–62. doi: 10.1016/j.freeradbiomed.2009.12.022. https://doi.org/10.1016/j.freeradbiomed.2009.12.022 PMid: 20045723 PMCid: PMC2823977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutrition research reviews. 2000;13(1):79–106. doi: 10.1079/095442200108729016. https://doi.org/10.1079/095442200108729016 PMid: 19087434. [DOI] [PubMed] [Google Scholar]
- 6.Hwang YP, Choi JH, Choi JM, Chung YC, Jeomg HG. Protective mechanisms of anthocyanin from purple sweet potato against tert-butyl hydroperoxie induced hepatotoxicity. Food Chem Toxicol. 2011;49:93–9. doi: 10.1016/j.fct.2011.05.021. https://doi.org/10.1016/j.fct.2010.10.002 PMid: 20934476. [DOI] [PubMed] [Google Scholar]
- 7.Kim SM, Chung MJ, Ha TJ, Choi HN, Jang SJ, Kim SO, et al. Neuroprotective effects of black soybean anthhocyanin via inactivation ASK1-JNK/p38 pathways and mobilization of celluler sialic acids. Life Sciences. 2012;90:874–82. doi: 10.1016/j.lfs.2012.04.025. https://doi.org/10.1016/j.lfs.2012.04.025 PMid: 22575822. [DOI] [PubMed] [Google Scholar]
- 8.Suprapta DN, Antara M, Arya N, Sudana M, Duniaji AS, Sudarma M. Review of plantation cultivation aspects and the application of yams as an alternative food source. Report of joint research cooperation BAPEDA Bali and Faculty of Agriculture UNUD. 2004 [Google Scholar]
- 9.Jawi IM, Sutirta-Yasa IWP, Suprapta DN, Mahendra AN. Hypoglycemic and Antioxidant Activities of Balinese Purple Sweet Potato (Ipomea Batatas L) in Induced Diabetic Rats. CIBTeech Journal of Pharmaceutical Sciences. 2012;1(2-3):1–6. [Google Scholar]
- 10.Jawi IM, Wita IW, Suprapta DN. Aqueous Extract of Purple Sweet Potato Tuber Increases SOd-2 and decrease VCAM-1 Expression by increasing Nrf2 Expression in The Aortic Endothelia of Hypercholetrolemic rabbits. Journal of Biology, Agriculture and Healthcare. 2014;4(10):76–84. [Google Scholar]
- 11.Adnyana IMO, Sudewi AAR, Samatra DPGP, Suprapta DN, Aulanni'am Aulanni'am. A simple method to stimulate ischemic stroke in Wistar rat for animal testing. Bali Medical Journal. 2016;1:145–9. [Google Scholar]
- 12.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-Triphenyltetrazolium Chloride as a Stain for Detection and Quantification of Experimental Cerebral Infarction Rats. Stroke, 1986. 1986;17(3):472–6. doi: 10.1161/01.str.17.6.1304. https://doi.org/10.1161/01.STR.17.3.472 PMid: 3715945. [DOI] [PubMed] [Google Scholar]
- 13.Rizk NN, Rafols J, Dunbar JC. Cerebral ischemia and induced apoptosis and necrosis in normal and diabetic rats. Brain Res. 2005;1(2):1–9. doi: 10.1016/j.brainres.2005.05.036. https://doi.org/10.1016/j.brainres.2005.05.036 PMid: 16038884. [DOI] [PubMed] [Google Scholar]
- 14.Chan P. Reactive oxygen radical in signaling and damage in the ischemic brain.J. Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. https://doi.org/10.1097/00004647-200101000-00002 PMid: 11149664. [DOI] [PubMed] [Google Scholar]
- 15.Rajanikant GK, Zemke D, Senut MC, Frenkel MB, Chen AF, Gupta R, et al. Carnosine is neuroprotective againts permanent focal cerebral ischemia in mice. Stroke. 2007;38:3023–31. doi: 10.1161/STROKEAHA.107.488502. https://doi.org/10.1161/STROKEAHA.107.488502 PMid: 17916766. [DOI] [PubMed] [Google Scholar]
- 16.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. A high anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127(2):188–96. doi: 10.1161/CIRCULATIONAHA.112.122408. https://doi.org/10.1161/CIRCULATIONAHA.112.122408 PMid: 23319811 PMCid: PMC3762447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao T, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A. Habitual intake of dietary falvonoids and risk of Parkinson disease. Neurology. 2012;78(15):138–45. doi: 10.1212/WNL.0b013e31824f7fc4. https://doi.org/10.1212/WNL.0b013e31824f7fc4 PMid: 22491871 PMCid: PMC3320056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gate keeper of neural plasticity. Curr Mol Pharmacol. 2010;3(1):12–29. doi: 10.2174/1874467211003010012. https://doi.org/10.2174/1874467211003010012 PMid: 20030625. [DOI] [PubMed] [Google Scholar]
- 19.Ploughman M, Windle V, Crystal L, MacLellan, White N, Dore JJ, et al. Brain-Drived neurotrophic Factor Contributes to recovery of skilled Reaching After Focal ischemia in rats. Stroke. 2009;(40):1490–5. doi: 10.1161/STROKEAHA.108.531806. https://doi.org/10.1161/STROKEAHA.108.531806 PMid: 19164786. [DOI] [PubMed] [Google Scholar]
- 20.Patapoutian A, Reichart LF. Trk receptors: mediators of neurontrophin action. Curr Opin in Neurobiol. 2001;11(3):272–80. doi: 10.1016/s0959-4388(00)00208-7. https://doi.org/10.1016/S0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. https://doi.org/10.1016/j.cell.2007.06.009 PMid: 17604717 PMCid: PMC2756685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao R-Q, Qi D-S, Yu H-L, Liu J, Yang L-H, Wu X-X. Quercetin Attenuates Cell Apoptosis in Focal Cerebral Ischemia Rat Brain Via Activation of BDNF–TrkB–PI3K/Akt Signaling Pathway. Neurochemical Research. 2012;37(12):2777–86. doi: 10.1007/s11064-012-0871-5. https://doi.org/10.1007/s11064-012-0871-5 PMid: 22936120. [DOI] [PubMed] [Google Scholar]
- 23.McDonald E, Van der Lee H, Pocock D, Cole C, Thomas N, VandenBerg PM, Bourtchouladze R, et al. A novel phosphodieterase type 4 inhibitor, HT-0712, enhance rehabilitation-dependent motor recovery and cortical reorganization after focal cortical ischemia. Neurorehabil Neurla repair. 2007;21:486–96. doi: 10.1177/1545968307305521. https://doi.org/10.1177/1545968307305521 PMid: 17823313. [DOI] [PubMed] [Google Scholar]
- 24.Brass M, Queenan B, Susin SA. Program cell death via mitochondria: Different mode of dying. Biochemistry. 2005;70:231–9. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 25.Min J, Yu SW, Baek SH, Nair KM, Baw OK, Bhatt A, et al. Neuroprotective effect of cyanidine-3-o-glucoside anthocyanin in mice with focal cerebral iascemia. Neuroscience letters. 2011;500:157–61. doi: 10.1016/j.neulet.2011.05.048. https://doi.org/10.1016/j.neulet.2011.05.048 PMid: 21651957. [DOI] [PubMed] [Google Scholar]
- 26.Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorder and ischemic brain diseases. Apoptosis. 2001;6:69–81. doi: 10.1023/a:1009676112184. https://doi.org/10.1023/A:1009676112184 PMid: 11321043. [DOI] [PubMed] [Google Scholar]
- 27.Kowaltowski AJ, Katilho RF, Vercesi AE. Mitochondria permeability transtition and oxidative stres. FEBS Letter. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. https://doi.org/10.1016/S0014-5793(01)02316-X. [DOI] [PubMed] [Google Scholar]
- 28.Grasso S, Menedez-Guitierresz P, Carrasco-Garcia E, Mayor-Lopez L, Tristance E, Rocamora-Reverte L, et al. Cell death and cancer. Novel therapeutic strategies. In Apoptosis and medicine, Intek open science. 2012:67–110. [Google Scholar]
- 29.Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF. Purple sweet potato color alleviates D galactose-indeuced brain aging in old mice by promoting survival of neurons via PI3K pathway and inhibting cytochrome c-mediated apoptosis. Brain pathology. 2010;20:598–612. doi: 10.1111/j.1750-3639.2009.00339.x. https://doi.org/10.1111/j.1750-3639.2009.00339.x PMid: 19863544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ullah I, Park HY, Kim M. Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathwy in hipocampal neurons. CNS Neuroscience & Therapeutic. 2014;20:327–38. doi: 10.1111/cns.12218. https://doi.org/10.1111/cns.12218 PMid: 24393263. [DOI] [PMC free article] [PubMed] [Google Scholar]


