Abstract
AIM:
To investigate immunomodulatory activities of Picria fel-terrae Lour herbs extract against inflammatory biomarkers by conducting cell culture experiments.
MATERIAL AND METHODS:
The herbs of Picria fel-terrae Lour were dried and extracted with n-hexane, ethyl acetate, 96% ethanol, followed by evaporation and freeze-drying. Phytochemicals screening were analysed with thin layer chromatography method. Cell viability was assessed with MTT assay. The genes of Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-6, interleukin (IL)-1β and inducible Nitric Oxide Synthase (iNOS), Cyclooxygenase (COX-2) in lipopolysaccharide (LPS)-induced macrophages were analysed by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) method.
RESULTS:
Phytochemicals screening showed the presence of steroids in n-hexane extract (ENPFH) and flavonoids, glycosides, saponins, tannins in ethyl acetate (EEAPFH) and ethanol (EEPFH) extracts. The Viability of RAW 264.7 cell toward ENPFH, EEAPFH, and EEPFH (1-200 μgmL-1) showed no toxicity effects. At the gene level, ENPFH; EEAPFH; EEPFH decreased the gene expression of TNF-α, IL-6, IL-1β, iNOS, and COX-2 which induced with LPS (1 μgmL-1).
CONCLUSIONS:
Our results suggest that extracts of Picria fel-terrae Lour Herbs possesses immunomodulatory activities by inhibiting selected inflammatory biomarkers at the gene levels in LPS-induced macrophages.
Keywords: Picria fel-terrae Lour, inflammatory biomarkers, Cell viability, MTT assay, Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Introduction
Among various immune system-related cells, macrophages are versatile cells that exist in almost all tissues and play necessary roles in immune responses. In particular, macrophages were recruited in infection sites where they are activated to perform many functions through increasing phagocytosis. This process is the first line of defence against microbial and parasitic infections and in removing senescent and dead cells, immune mediator secretion and antigen presentation. Activated macrophages also prevent the invasion of pathogens by secreting inflammatory mediators, including cytokines, such as TNF-α and interleukins (ILs) and protein inflammatories such as inducible nitric oxide synthase (iNOS) and COX-2. Recent data showed that natural compounds had been widely and safely consumed over centuries, and many studies have indicated that most natural compounds have a wide range of diverse biological activities but few side effects. These natural products could affect the development and progression of cancer in various ways, such as inhibiting tumour cell growth, angiogenesis, and metastasis, immunomodulating, and enhancing effects of chemotherapeutic drugs. Therefore, traditional herbal medicinal resources have been investigated extensively for their immunomodulatory potential for therapeutic agents in immune-related functions [1].
Poguntano (Picria fel-terrae Lour.) have been used a drug of colic, malaria, diuretic, fever, and skin disease [2]. Modern pharmacological investigations indicated that the extract of P. fel-terrae Lour exerts diuretic, antipyretic, hepatoprotective, cardioprotective, antidiabetic, antioxidant, anti-inflammatory, anthelmintic, and analgesic activities [3], [4], [5], [6], [7], [8], [9], [10], [11]. Moreover, P. fel-terrae inhibits hepatitis B (HB) e-antigen excreted by HepG2 2215 cell lines, suggesting having anti-HB virus activity [12]. It can be developed a co-chemotherapeutic regimen for breast cancer by inducing apoptosis, and cell cycle arrest and suppressing cyclin D1 and Bcl-2 expression based on the recent studies and it has antioxidant and antiproliferative activities of ethyl acetate fraction [13], [14], [15], and n-hexane, ethyl acetate, and ethanol fractions of P. fel-terrae Lour herbs have cytotoxic activity toward 4T1 and MCF-7 cell line [16].
Therefore in this study, we have examined the immunomodulatory activity of P. fel-terrae Lour herbs in RAW 264.7 mouse monocyte-macrophages.
Material and Methods
Fresh P. fel-terrae Lour herbs were collected from Tiga Lingga village, Dairi regency, Sumatera Utara province, Indonesia. RAW 264.7 cells were obtained from Parasitology Laboratory, Faculty of Medicine, Gadjah Mada University. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Fetal bovine serum and kept at 37°C with a CO2 supply of 5%. Lipopolysaccharides are from Escherichia coli O111.B4 (Sigma), Dexamethasone (Harsen), n-hexane, ethyl acetate, ethanol 96%. TLC Silica gel 60 F254 (Merck). All chemicals and reagents used in this work were of analytical grade. Total RNA Mini Kit (Geneaid), ReverTra Ace (Toyobo), GoTaq®Green (Promega), Nuclease-Free Water (Promega), TBE (Vivantis), agarose gel (Promega), Flurosafe (Smobio), DNA ladder 100 bp (Smobio).
The powder of P. fel-terrae Lour herbs (1 kg) was repeatedly extracted by maceration with n-hexane (3 x 3 day, 10 L) (ENPFH). The powder was dried in the air and extracted with ethyl acetate (3 x 3 day, 10 L) (EEAPFH), and then the powder was dried in the air and extracted with ethanol (3 x 3 day, 10 L) (EEPFH) at 25-30°C with periodical stirring. The filtrate was collected, and then evaporated to obtain a viscous fraction and then freeze-dried to dry [13], [15].
The Phytochemicals: Alkaloids, Flavonoids, Glycosides, Saponins, Tannins, Steroids were determined using thin layer chromatography standard procedures [17], [18], [19].
RAW 264.7 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum, 100 units/mL of penicillin and 100 μg mL-1 of streptomycin as previously described by Chi et al., 2016. Cells were incubated in the presence of 5% CO2 at 37°C. The cells (passage 7-12) were seeded at a concentration of 3 x 103 cells mL-1 in 96-well plates and incubation 24 h. The effects of P. fel-terrae Lour herbs extracts on cell viability were evaluated with the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-Tetrazolium bromide (MTT) colourimetric assay (Sigma-Aldrich). Extracts of P. fel-terrae Lour herbs were dissolved in 100% DMSO, and the stock solution of the extract at a concentration of 50.000 μgmL-1 was prepared in 10% DMSO. The final concentrations of the extract ranged from 1-200 μgmL-1 in the culture media. Dexamethasone and lipopolysaccharides were used as positive and negative controls [20], [21].
The gene’s expression of TNF-α, IL-6, IL-1β, iNOS, and COX-2 was determined by RT-PCR. Total RNA from the control cell, LPS, positive control, and treatment groups were extracted using the Total RNA Mini Kit (Geneaid) according to the manufacturer’s protocol. The oligonucleotide primers for TNF-α, IL-6, IL-1β, iNOS, COX-2, and β-actin were designed according to a PCR primer selection program at the website of the Virtual Genomic Center from the GenBank database (Table 1).
Table 1.
Mouse oligonucleotide primers sequences used for RT-PCR (5-3’) and Annealing temperature
| Gen | Primer Sequences | Size (bp) | Temp (°C) | |
|---|---|---|---|---|
| TNF-α | F | 5’-TGTGCCGCCGCTGTCTGCTTCACGCT-3’ | 374 | 55 |
| R | 5’-GATGAGGAAAGACACCTGGCTGTAGA-3’ | |||
| IL-6 | F | 5’-GATGCTACCAAACTGGATATAATC-3’ | 269 | 55 |
| R | 5’-GGTCCTTAGCCACTCCTTCTGTG-3’ | |||
| IL-1β | F | 5’-CCCTGCAGCTGGAGAGTGTGGA-3’ | 447 | 62.5 |
| R | 5’-TGTGCTCTGCTTGTGAGGTGCTG-3’ | |||
| iNOS | F | 5’-CGAAACGCTTCACTTCCAA-3’ | 311 | 60 |
| R | 5’-TGAGCCTATATTGCTGTGGCT-3’ | |||
| COX-2 | F | 5’-CCTGTGTTCCACCAGGAGT-3’ | 249 | 55 |
| R | 5’-GTCCCTGGCTAGTGC TTCAG-3’ | |||
| β-actin | F | 5’- TGGAATCCTGTGGCATCCATGAAAC-3’ | 349 | 55 |
| R | 5’- TAAAACGCAGCTCAGTAACAGTCCG-3’ | |||
PCR consisted of 35 amplification cycles and each cycle carried out for 30 s at 95°C, 1 min at annealing temperature (55°C for TNF-α, IL-6, COX-2 and beta-actin and 60°C for iNOS) and 45 s at 95°C, 1 min at annealing temperature 62.5°C for IL-1β.) and 1 min at 72°C in a thermal cycler (ProFlex™ 3 x 32-well PCR System, Applied Biosystems). The β-actin was used as an internal control to standardise the relative expression levels for all biomarkers. PCR products were separated electrophoretically on a 2% agarose and fluorosafe (Smobio) with Tris-Borate-EDTA (Vivantis) 0,5x. The stained gel was visualised by using Gel-Doc Quantity One software (Syngene) [22].
Triplicate experiments were performed throughout this study. All data were presented as the mean ± Standard Error Minimum (SEM), which were analysed using the SPSS 22 software. The significant difference between Lipopolysaccharide and treated groups were analyzed by the paired Turkey HSD (p < 0.05).
Results
The results of phytochemicals screening of ethyl acetate and ethanol extracts of Picria fel-terrae Lour. revealed that those extracts contained flavonoids, tannins, saponins, glycosides while n-hexane extract only contained steroids as seen in Table 2.
Table 2.
Phytochemicals Screening Result
| NO | Phytochemicals | ENPFH | EEAPFH | EEPFH |
|---|---|---|---|---|
| 1. | Alkaloids | - | - | - |
| 2. | Flavonoids | - | + | + |
| 3. | Tannins | - | + | + |
| 4. | Saponins | - | + | + |
| 5. | Glycosides | - | + | + |
| 6. | Steroids | + | - | - |
The results were showed the cell viability test on extracts of n-hexane, ethyl acetate and ethanol of Picria fel-terrae Lour Herbs and dexamethasone as the positive control. The best results were shown on an extract of n-hexane with the concentration of 12.5 and 25 μgmL-1 which the resulted in the highest viability percentage. Percentage of cell viability on RAW 264.7 cells for extract and Dexamethasone was showed in Figure 1 A and B.
Figure 1.
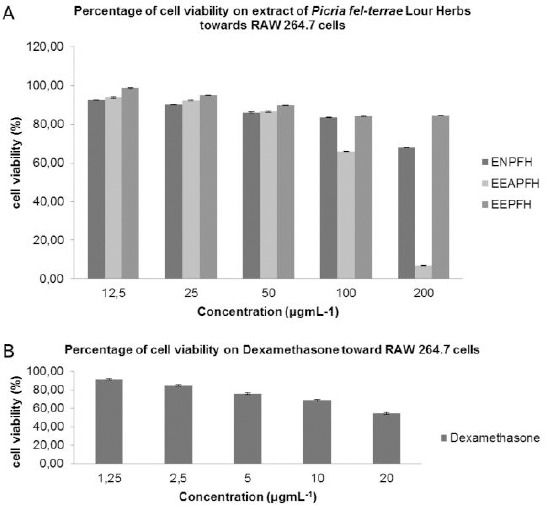
A) Percentage of cell viability on RAW 264.7 cells treated by ENPFH, EEAPFH and EEPFH; B) Percentage of cell viability on RAW 264.7 cells treated by dexamethasone as a positive control
The results expression of genes which treated with ENPFH, EEAPFH, EEPFH and dexamethasone were analysed using RT-PCR methods, and the results were shown in Figure 2.
Figure 2.
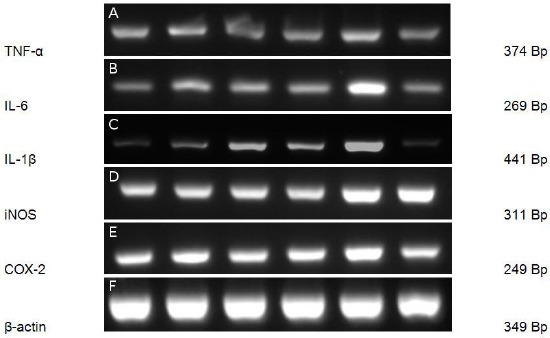
The effect of extracts on the expression of genes in RAW 264.7 cell which induced LPS 1 μgmL-1 for 6 hours. The total RNAs were isolated, and RT-PCR was performed using the indicated primers in Materials and Methods. Dexamethasone 2.5 μgmL-1(A); EEPFH 25 μgmL-1(B); EEAPFH 25 μgmL-1(C); ENPFH 25 μgmL-1(D); LPS (E); control cells (F). β-actin was used as the internal control. LPS, Lipopolysaccharide; RT-PCR, reverse transcription-PCR; iNOS, Inducible Nitric Oxide Synthase; IL, interleukin; COX-2, Cyclooxygenase-2; Bp, Base Pair
Furthermore, we were tested whether ENPFH; EEAPFH; EEPFH has potential immunomodulatory in the expression of inflammatory cytokines in LPS-induced macrophages. As shown in Figure 2, resulting treatment with ENPFH; EEAPFH; EEPFH (25 μgmL-1) were inhibited the expression of the gene of cytokines (TNF-α, IL-6, IL-1β), iNOS and COX-2 in macrophages treated with LPS.
The results were shown from the value of genes expression from ENPFH, EEAPFH, EEPFH toward LPS showed a significant difference with P < 0.05.
Table 3.
The value of genes expression in RAW 264.7 cells which induced LPS
| Gene | Mean ± SEM | |||||
|---|---|---|---|---|---|---|
| Dexamethasone | EEPFH | EEAPFH | ENPFH | LPS | Control cell | |
| TNF-α | 1,23 ± 0,01 | 1,26 ± 0,01 | 1,03 ± 0,01 | 1,08 ± 0,02 | 1,46 ± 0,03 | 1,00 ± 0,00 |
| IL-6 | 0,78 ± 0,02 | 1,33 ± 0,01 | 1,29 ± 0,02 | 1,27 ± 0,02 | 2,61 ± 0,02 | 1,00 ± 0,00 |
| COX-2 | 1,02 ± 0,01 | 1.23 ± 0,02 | 1,05 ± 0,01 | 1,16 ± 0,01 | 1,50 ± 0,02 | 1,00 ± 0,00 |
| IL-1β | 1,06 ± 0,02 | 1,80 ± 0,03 | 2,78 ± 0,03 | 2,33 ± 0,04 | 4,02 ± 0,04 | 1,00 ± 0,00 |
| iNOS | 0,65 ± 0,03 | 0,69 ± 0,01 | 0,75 ± 0,01 | 0,67 ± 0,01 | 1,03 ± 0,02 | 1,00 ± 0,00 |
| β-actin | 1,02 ± 0,01 | 1,05 ± 0,01 | 1,06 ± 0,02 | 1,09 ± 0,02 | 1,04 ± 0,01 | 1,00 ± 0,00 |
Discussion
The scientific evidence of P. fel-terrae Lour has been widely investigated, but the immuno-modulatory effects extract of P. fel-terrae Lour Herbs is rarely to be explored. In this present study, previously we were examined of phytochemicals screening from ENPFH, EEAPFH, EEPFH. Cells viability was performed to determine the toxicity of that extracts of P. fel-terrae Lour herbs towards RAW 264.7 cells. RAW 264.7 cells in culture media were treated with ENPFH, EEAPFH, EEPFH with various concentrations. After 24 hours of incubation, the culture medium was aspirated, and cell viability was measured using an MTT solution [23]. Cell viability was showed that extracts did not cause toxicity to RAW 264.7 cells. As shown in Figure 1 (cell viability > 85%) were selected for further analysis [21], and we were determined at concentration 25 μgmL-1, which exhibited immunomodulatory activity on decreasing the production of various inflammatory cytokines (i.e., TNF-α, IL-6, IL-1β), inducible enzyme iNOS and COX-2 in macrophages treated with LPS.
Meanwhile, LPS is widely recognised as the major inducer for the production of inflammatory cytokines which in turn stimulates iNOS induction during the inflammatory process in macrophages [24], [25]. These cytokines can be produced from macrophages in response to bacterial LPS, infection, and inflammatory stimulation. They also play an important role in the immune system by aiding cytotoxic and cytostatic effects on infected or malignant cells. Among them, TNF-α is one of the earliest factors to be induced or activated in macrophages for eliciting tumour immunity. TNF-α plays as a key mediator of T lymphocyte, and macrophage activation. Similarity, IL-1β and IL-6 are produced by various immune cells, including macrophages [1]. Our results suggest that ENPFH; EEAPFH; EEPFH may reduce the expression of cytokines TNF-α, IL-1β, and IL-6, subsequently leading to the blockade of inflammatory enzyme induction (iNOS) and COX-2, and the value of gene expression significant difference with P < 0.05, which indicates that it has an immunomodulatory effect on RAW 264.7 macrophages. It has been recognised that the blockade of inflammatory cytokines by natural anti-inflammatory products is a potent strategy for the management of various inflammatory diseases, immune system upset [22].
Based on the results of phytochemical screening ENPFH contained triterpenoid/steroid compounds. Steroids were a group of compounds which have a basic framework of cyclopentane perhydro phenanthrene, having four integrated rings. These compounds have certain physiological effects. In previous studies, steroids can inhibit TNF-α, IL-6, IL-1β, COX-2 and iNOS gene expression [26], [27]. In phytochemical screening, EEAPFH and EEPFH contained flavonoid, saponin, tannin, glycoside compounds. Flavonoids were a group of natural compounds in the polyphenol group found in plants. Flavonoids are composed of two aromatic rings which can or cannot form a third ring with a C6-C3-C6 arrangement [28]. Saponin is a complex glycoside compound, which is the result of a sugar condensation compound with an organic hydroxyl compound which when hydrolysed will produce sugar (glycone) and non-sugar (aglycone). In previous studies, flavonoids can inhibit TNF-α, IL-6, IL-1β, COX-2, and iNOS gene expression [29], [30], [31], [32], Saponins can also inhibit TNF-α, IL-6, IL-1β, COX-2 and iNOS gene expression [33], [34], [35].
Based on the immunomodulatory profile exposed through various assays, we summarised that P. fel-terrae Lour. Herb extracts significantly decreased genes expression of TNF-α, IL-6, IL-1β, iNOS, and COX-2, in LPS-induced macrophages in vitro. The extract of P. fel-terrae Lour Herbs could be potentially used as a herbal medicine to immunomodulatory. Further study on molecular mechanisms by which extract of P. fel-terrae Lour herbs modulated the expression of inflammatory cytokines and proteins in macrophages in response to LPS is still needed. Also, in vivo study using animal models is needed to determine the exact immunomodulatory potential of that.
Footnotes
Funding: This research was funding by PDUPT 2018 from the ministry of research technology and higher education
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Da Hye Kwon JM, Choi EO, Jeong JW, Lee KW, Kim KY, Kim SG, Kim S, Hong SH, Park C, Hwang HJ, Choi YH. The immunomodulatory activity of Mori folium, the leaf of Morus alba L., in RAW 264.7 macrophages in vitro. Journal of cancer prevention. 2016;21(3):144. doi: 10.15430/JCP.2016.21.3.144. https://doi.org/10.15430/JCP.2016.21.3.144 PMid: 27722140 PMCid: PMC5051588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry LM, Metzger J. MIT Press; 1980. Medicinal plants of east and southeast Asia: attributed properties and uses. [Google Scholar]
- 3.Dalimunthe A, Urip H, Rosidah G, Pandapotan NM. Evaluation of diuretic activity of Picria fel-terrae (Lour.) leaves extracts. Asian J Pharm Clin Resc. 2015;8:204–5. [Google Scholar]
- 4.Huang Y, Cimanga K, Lasure A, Poel VB. Biological activities of Picria fel-terrae Lour. Pharm World Sci. 1994;16:18. [Google Scholar]
- 5.Thuan ND, Thuong PT, Na MK, Bae K, Lee JP, Lee JH, Seo HW, Min BS, Kim JC. A phenylpropanoid glycoside with antioxidant activity from Picria tel-ferae. Archives of pharmacal research. 2007;30(9):1062–6. doi: 10.1007/BF02980238. https://doi.org/10.1007/BF02980238 PMid: 17958321. [DOI] [PubMed] [Google Scholar]
- 6.Zhong SQ, Zhang BN, Huang FX. An anti-tumor herb Cucao. China: Chin Tradit Herb Drugs Lett. 1979;3:45–6. [Google Scholar]
- 7.Zou JM, Wang LS, Niu XM, Sun HD, Guo YJ. Phenylethanoid glycosides from Picria felterrae Lour. Journal of Integrative Plant Biology. 2005;47(5):632–6. https://doi.org/10.1111/j.1744-7909.2005.00082.x. [Google Scholar]
- 8.Harfina F, Bahri S, Saragih A. Pengaruh serbuk daun puguntano (Curanga fel-terrae Merr.) pada pasien diabetes mellitus. Journal of Pharmaceutics and Pharmacology. 2012;1(2):112–8. [Google Scholar]
- 9.Sitorus P, Harahap U, Barus T. Isolation of β-sitosterol from n-hexane extract of Picria fel-terrae Lour. leave and study of its antidiabetic effect in alloxan induced diabetic mice. 2014 [Google Scholar]
- 10.Sihotang Y, Silalahi J, Hadisahputra S, Hasibuan PA, Satria D. Cardioprotective effect of ethylacetate extract of poguntano (Picria fel-terrae Lour.) against doxorubicin-induced cardiotoxicity in rats. Cardioprotective Effect of Ethylacetate Extract of Poguntano (Picria fel-terrae Lour.) Against Doxorubicin-Induced Cardiotoxicity in Rats. 2016 PMCid: PMC4896251. [Google Scholar]
- 11.Patilaya P, Husori DI. Preliminary study on the anthelmintic activity of the leaf ethanolic extract of Indonesian Curanga fel-terrae (Lour.) Merr. Int J Pharmtech Res. 2015;8(3):347–51. [Google Scholar]
- 12.Zhang Y, Seeram NP, Lee R, Feng L, Heber D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. Journal of agricultural and food chemistry. 2008;56(3):670–5. doi: 10.1021/jf071989c. https://doi.org/10.1021/jf071989c PMid: 18211028. [DOI] [PubMed] [Google Scholar]
- 13.Satria D, Furqan M, Hadisahputra S. Rosidah. Combinational Effects Of Ethylacetate Extract Of Picria Fel-Terrae Lour and Doxorubicin On T47d Breast Cancer Cells. International Journal of Pharmacy and Pharmaceutical Sciences. 2015;7(7):73. [Google Scholar]
- 14.Lestari P. Thesis: Medan. Faculty of Pharmacy. University of Sumatera Utara; 2013. The effect of a combination of active extracts of poguntano leaves (Picria fel-terrae Lour.) With doxorubicin on breast cancer cells in vitro. [Google Scholar]
- 15.Satria D, Silalahi J, Haro G, Ilyas S, Hsb PA. Antioxidant and Antiproliferative Activities of an Ethylacetate Fraction of Picria Fel-Terrae Lour. Herbs. Asian Pacific journal of cancer prevention: APJCP. 2017;18(2):399. doi: 10.22034/APJCP.2017.18.2.399. PMid:28345821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harahap U, Hasibuan PAZ, Sitorus P, Satria S. Cytotoxicity Activity of Picria fel-terrae Lour. Herbs againt 4T1 and MCF-7 Breast Cancer Cells. Asian J Pharm Clin Res. 2018;11(1):194–195. https://doi.org/10.22159/ajpcr.2018.v11s1.26608. [Google Scholar]
- 17.Wagner H, Bladt S. Plant drug analysis: a thin layer chromatography atlas. Springer Science & Business Media. 1996 https://doi.org/10.1007/978-3-642-00574-9. [Google Scholar]
- 18.Kemkes RI. Indonesian herbal pharmacopeia. Edition I. Jakarta: Ministry of health the Republic of Indonesia; 2013. Supplement III; p. 28. [Google Scholar]
- 19.Musa AY. Phytochemical Constituents, Thin Layer Chromatography and Antimicrobial Activity of Methanol Extract of the Stem and Leave of Citrus Limon (L) International Journal of Biochemistry, Biophysics & Molecular Biology. 2017;2(4):31–35. https://doi.org/10.11648/j.ijbbmb.20170204.12. [Google Scholar]
- 20.Ahamed T, Rahman SKM, Shohael AM. Thin layer chromatographic profiling and phytochemical screening of six medicinal plants in Bangladesh. International Journal of Biosciences. 2017;11(1):131–140. https://doi.org/10.12692/ijb/11.1.131-140. [Google Scholar]
- 21.Chi C, Giri SS, Jun JW, Kim HJ, Yun S, Kim SG, Park SC. Immunomodulatory effects of a bioactive compound isolated from Dryopteris crassirhizoma on the grass carp Ctenopharyngodon idella. Journal of immunology research. 2016;2016 doi: 10.1155/2016/3068913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanti PT, Nuriasari N, Juliana K. Lemon pepper fruit extract (Zanthoxylum acanthopodium DC.) suppresses the expression of inflammatory mediators in lipopolysaccharide-induced macrophages in vitro. American Journal of Biochemistry and Biotechnology. 2011;7(4):190–5. https://doi.org/10.3844/ajbbsp.2011.190.195. [Google Scholar]
- 23.Yuandani Jantan I, Husain K. 4,5,4′-rihydroxychalcone, 8,8′-(ethene-1,2- diyl)-dinaphtalene-1,4,5-triol and rutin from Gynura segetum inhibit phagocytosis, lymphocyte proliferation, cytokine release and nitric oxide production from phagocytic cells. BMC Complementary and Alternative Medicine. 2017;17:211. doi: 10.1186/s12906-017-1726-z. https://doi.org/10.1186/s12906-017-1726-z PMid: 28399868 PMCid: PMC53∏7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joo T, Sowndhararajan K, Hong S, Lee J, Park SY, Kim S, Jhoo JW. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi journal of biological sciences. 2014;21(5):427–35. doi: 10.1016/j.sjbs.2014.04.003. https://doi.org/10.1016/j.sjbs.2014.04.003 PMid: 25313277 PMCid: PMC4191610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang LP, Lai YS, Wu CJ, Chou TC. Liquid perfluorochemical inhibits inducible nitric oxide synthase expression and nitric oxide formation in lipopolysaccharide-treated RAW 264.7 macrophages. Journal of pharmacological sciences. 2009;111(2):147–54. doi: 10.1254/jphs.09043fp. https://doi.org/10.1254/jphs.09043FP PMid: 19834286. [DOI] [PubMed] [Google Scholar]
- 26.Checker R, Sandur SK, Sharma D, Patwardhan RS, Jayakumar S, Kohli V, Sethi G, Aggarwal BB, Sainis KB. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PloS one. 2012;7(2):e31318. doi: 10.1371/journal.pone.0031318. https://doi.org/10.1371/journal.pone.0031318 PMid: 22363615 PMCid: PMC3282718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatesha SH, Dudics S, Astry B, Moudgil KD. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Fems Pathogens and Disease. 2016;74(6):ftw059. doi: 10.1093/femspd/ftw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markham KR. InThe flavonoids. Boston, MA: Springer; 1988. Distribution of flavonoids in the lower plants and its evolutionary significance; pp. 427–468. [Google Scholar]
- 29.Lee SH, Kim YJ, Kwon SH, Lee YH, Choi SY, Park JS, Kwon HJ. Inhibitory effects of flavonoids on TNF-α-induced IL-8 gene expression in HEK 293 cells. BMB reports. 2009;42(5):265–70. doi: 10.5483/bmbrep.2009.42.5.265. https://doi.org/10.5483/BMBRep.2009.42.5.265 PMid: 19470239. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Jia L, Gao Y, Li B, Tu Y. Anti-inflammatory activity of total flavonoids from seeds of Camellia oleifera Abel. Acta Biochim Biophys Sin. 2014;46(10):920–2. doi: 10.1093/abbs/gmu071. https://doi.org/10.1093/abbs/gmu071 PMid: 25189429. [DOI] [PubMed] [Google Scholar]
- 31.Fachinan R, Fagninou A, Nekoua MP, Amoussa AM, Adjagba M, Lagnika L, Lalèyè A, Moutairou K, Yessoufou A. Evidence of Immunosuppressive and Th2 Immune Polarizing Effects of Antidiabetic Momordica charantia Fruit Juice. BioMed research international. 2017:2017. doi: 10.1155/2017/9478048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018;5(3):93. doi: 10.3390/medicines5030093. https://doi.org/10.3390/medicines5030093 PMid: 30149600 PMCid: PMC6165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu GJ, Choi IW, Kim GY, Kim BW, Park C, Hong SH, Moon SK, Cha HJ, Chang YC, Paek KY, Kim WJ. Anti-inflammatory potential of saponins derived from cultured wild ginseng roots in lipopolysaccharide-stimulated RAW 264.7 macrophages. International journal of molecular medicine. 2015;35(6):1690–8. doi: 10.3892/ijmm.2015.2165. https://doi.org/10.3892/ijmm.2015.2165 PMid: 25847675. [DOI] [PubMed] [Google Scholar]
- 34.Ahn S, Siddiqi MH, Noh HY, Kim YJ, Kim YJ, Jin CG, Yang DC. Anti-inflammatory activity of ginsenosides in LPS-stimulated RAW 264.7 cells. Science bulletin. 2015;60(8):773–84. https://doi.org/10.1007/s11434-015-0773-4. [Google Scholar]
- 35.Jang KJ, Choi SH, Yu GJ, Hong SH, Chung YH, Kim CH, Yoon HM, Kim GY, Kim BW, Choi YH. Anti inflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide activated RAW 264.7 macrophages. Experimental and therapeutic medicine. 2016;11(3):1109–15. doi: 10.3892/etm.2015.2965. https://doi.org/10.3892/etm.2015.2965 PMid: 26998045 PMCid: PMC4774435. [DOI] [PMC free article] [PubMed] [Google Scholar]


