Abstract
AIM:
To determine the distribution of vancomycin MIC and the frequency of S. aureus strains with reduced vancomycin susceptibility among Methicillin-Resistant Staphylococcus aureus (MRSA) isolates.
METHODS:
MRSA isolates (n = 100) were tested for reduced susceptibility to vancomycin using MIC broth microdilution method (BMD), vancomycin screening agar with different vancomycin concentrations with and without casein, and Vitek 2 system.
RESULTS:
BMD detected (22%) vancomycin-intermediate S. aureus (VISA) and (78%) vancomycin-susceptible S. aureus (VSSA) but couldn’t detect nine (Heterogeneous VISA) (hVISA) isolates (9%) with MIC ≤ 2 µg/ml that grew on screening agar 4 µg/ml or 6 µg/ml. Adding casein to vancomycin screening agar increased detection rate of VISA by 4.5%. Screening agar with 6 µg/ml vancomycin overall detection rate for VISA was 95.45%. Probable ‘pre-hVISA’isolates (17%) showed growth on vancomycin screening agar 2 µg/ml with casein. Vitek 2 system failed to detect any VISA isolates.
CONCLUSION:
Vancomycin screening agar; 2 µg/ml and (4 and 6 µg/ml) were able to detect; probable “pre hVISA and (hVISA and VISA) isolates respectively based on their BMD MIC values. Decreased vancomycin susceptibility in MRSA isolates might be related to MIC creep. Analysis of vancomycin MIC values over longer periods is recommended to further study this phenomenon and its impact on vancomycin treatment failure.
Keywords: VISA, h-VISA, Vancomycin screening agar, BMD, Vancomycin MIC creep
Introduction
Staphylococcus aureus is a virulent microorganism responsible for many serious infections among the general population. The emergence of vancomycin resistance in S. aureus has been anticipated since vancomycin-resistant enterococci (VRE) has been recognised. Hiramatsu et al. in 1997 described the first documented case of infection caused by S. aureus with reduced susceptibility to vancomycin [1].
Reduced susceptibility could be either due to strains that showed intermediate resistance to vancomycin with MIC 4-8 µg/mL [vancomycin intermediate S. aureus (VISA)] or heteroresistant strains (Heterogeneous VISA) (h-VISA) that are defined as strains with minimal inhibitory concentrations (MICs) within the susceptible range (MIC ≤2 μg/ml), but containing subpopulations of cells in the vancomycin-intermediate range (VISA, MIC 4-8 μg/ml) These strains have been described for both MRSA and methicillin-susceptible S. aureus (MSSA) respectively [2].
There has been special interest in vancomycin MIC creep phenomenon that was associated with greater rates of complications, and vancomycin therapeutic failures with vancomycin MICs within the susceptible range (MICs of 1-2 mg/L) [3], [4], [5]. Unfortunately, there has been uncertainty regarding optimal laboratory detection of S. aureus with reduced susceptibility to vancomycin [6].
Center for Disease Control and Prevention (CDC) in 2012 recommended that screening of VISA should be done by MIC method plus vancomycin screening agar method with 6ug of vancomycin per ml, but this is not reliable to detect all VISA; some strains for which the vancomycin MICs are 4 ug/ml will fail to grow [7], [8].
Screening for h-VISA by the population analysis profile-area under the curve (PAP-AUC) method has been the most reliable and reproducible approach but is labour-intensive, costly, and unsuitable for routine use in clinical laboratories [9], [10].
Moreover, standardized reference methods for susceptibility testing, such as CLSI broth microdilution, agar dilution, and standard Etest methods, can detect VISA but fail to detect h-VISA due to several factors as small inoculum size, the relatively poor support of growth on Mueller-Hinton agar plates, the slow growth of h-VISA strains and its unique pleomorphic features, such as small-colony variant [9], [11].
Satola et al. showed that the use of BHI screen agar with 4 μg/ml vancomycin with increase incubation time to 48 hours and the addition of casein could increase the sensitivity and specificity for detection of VISA and may be useful for clinical detection of h-VISA [12].
The study aimed to determine the distribution of vancomycin MIC, and the frequency of S. aureus strains with reduced vancomycin susceptibility among Methicillin-Resistant Staphylococcus aureus (MRSA) isolates.
Materials and Methods
The study included 100 MRSA isolates analysed by Kirby Bauer disc diffusion method [13] recovered from different specimens referred to Central Microbiology Laboratory of Ain Shams University Hospitals for routine culture and sensitivity. Isolates were collected throughout four months from January till April 2017 and were preserved on tryptone soy broth with 15% glycerol at -80°C until use.
Detection of MRSA with reduced susceptibility to vancomycin was performed using vancomycin screening agar with different vancomycin concentrations 2, 4, and 6 µg/ml with and without casein and compared to MIC broth microdilution method for vancomycin (BMD) [13]. Finally, MIC susceptibility testing for MRSA isolates was performed by Vitek 2 automated system (Biomerieux, France).
Brain heart infusion (BHI) agar without casein (Oxoid, UK) was prepared according to the manufacturer’s instructions and BHI agar with casein was similarly prepared but with the addition of eight gram pancreatic digest of casein (Sigma Aldrich, USA) to every 500 ml of media [12]. A stock solution of vancomycin was prepared by dissolving 500 mg of vancomycin powder in 10 ml of sterile distilled water (final concentration was 50 mg/ml). At the time of media preparation, further dilution of 1:10 was done twice to produce a working solution of 0.5 mg/ml vancomycin. For the final preparation of vancomycin screening agar with and without casein; six ml, four ml and two ml of 500 ml prepared media were removed under complete aseptic precautions and replaced by 6 ml, 4 ml and 2 ml of working solution of vancomycin to prepare Vancomycin screening agar with 6 μg/ml, 4 μg/ml and 2 μg/ml respectively.
All isolates were subcultured by taking a small piece of a frozen organism with a sterile loop and plated twice onto blood agar plates. The used vial was returned immediately to the deep freezer to be used if needed as repeated thawing and re-freezing can reduce the viability of the organism. The cultivated plates were incubated aerobically at 35°C for 24 hours. Two to three colonies were picked up by the sterile loop and adjusted to 0.5 McFarland standards in 5 ml sterile tubes. Quadruplicate technique was performed (i.e. four droplets, 10ul each, from 0.5 McFarland MRSA suspension, was dropped by a pipette onto the 2, 4, and 6 vancomycin screening agars) and the plates were incubation for a full 48 hours at 35°C to enhance the sensitivity of detection of MRSA with vancomycin reduced susceptibility [12]. Plates were examined at 24 and 48 hours. Vancomycin-Resistant Enterococcus Faecalis ATCC 51299 and Staphylococcus aureus ATCC 25923 (NAMRU-3) were used as positive and negative controls respectively.
No growth in any of the four droplets was denoted as sensitive to vancomycin. Growth in any of the four droplets was considered as MRSA with vancomycin reduced susceptibility.
The broth microdilution method was used for determination of the MICs of vancomycin [14]. Vancomycin suspension used was prepared by dissolving 500 mg of vancomycin powder in 10 ml of sterile distilled water (50 mg/ml), then further dilution 1:10 was done twice (0.5 mg/ml). From the prepared dilution, 640 μl was added to 10 ml of D.W to reach a final concentration of 32 μg/ml vancomycin.
Serial two-fold dilution of the prepared vancomycin concentration was carried in a 96 well plate. Fifty microliters of double-strength Muller Hinton Broth (MHB), 50 μl of the antibiotic dilutions, and (5 μl of the organism suspension adjusted to 0.5 McFarland standards and then diluted 1:20) were mixed and incubated at 35°C for 24 hours. MICs; ≤ 2 µg/ml is considered as sensitive, 4-8 µg/ml as VISA and ≥ 16 µg/ml as vancomycin resistant S. aureus (VRSA) (Figure 1).
Figure 1.
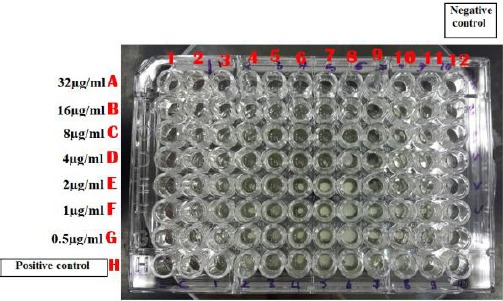
Broth microdilution plate for detection of vancomycin susceptibility in MRSA isolates; A12 to G12 are negative growth control wells. H1 to H11 wells are positive growth control wells. MIC of vancomycin for isolate; 4 (well E4) is 2 µg/ml, 5 (well E5) is 2 µg/ml (VSSA), 6 (well D6) is 4 µg/ml and for isolate 8 (well C8) is 8 µg/ml (VISA)
Susceptibility testing on Vitek 2 system was performed with AST PG 76 cards according to the manufacturer’s instructions, and susceptibility breakpoints of Staphylococcus aureus were interpreted per CLSI 2015 [13].
Results
Broth microdilution (BMD) method (Figure 1) revealed that, out of 100 MRSA isolates, 22/100 (22%) were VISA (14/22 VISA with MIC = 8 µg/ml and 8/22 VISA with MIC = 4 µg/ml) and 78/100 (78%) were VSSA (VSSA MIC ≤ 2 µg/ml).
In vancomycin screening agar method, h-VISA was reported if one or two colonies on at least one droplet showed growth on screening agar with 4 µg/ml or 6 µg/ml [12]. Among MRSA isolates that showed MIC ≤ 2 µg/ml by BMD; 9 isolates (9%) grew on screening agar 4 µg/ml or 6 µg/ml and were designated as h-VISA (Figure 2).
Figure 2.
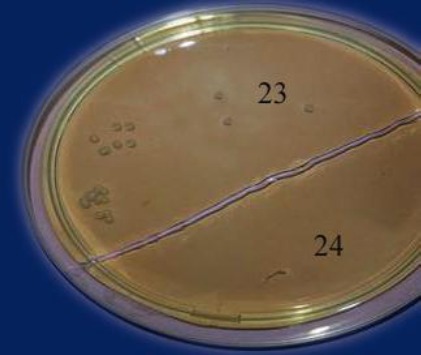
Vancomycin screening agar 6 µg/ml with casein; isolate [23] shows the growth of hVISA. Isolate [24] shows no growth (VSSA)
Seventeen isolates with susceptible MIC by BMD (17%) showed growth on vancomycin screening agar 2 µg/ml, six out of them with MIC of 2 µg/ml by BMD. These isolates were considered as probable ‘pre-hVISA’, which represent small subpopulations of cells capable of growth in the presence of 2-4 mg/L vancomycin [14] (Table 1 and Figure 3).
Table 1.
Detection rate of MRSA with reduced susceptibility to vancomycin among 100 tested isolates
| Vancomycin screening Agar | BMD MIC | |
|---|---|---|
| VSSA (MIC ≤ 2 μg/ml) | VISA (MIC 4-8) | |
| No growth | 52 (52%) | 0 |
| 2 μg/ml (probable pre-hVISA) | 17 (17%) | 0 |
| 4 μg/ml or 6 μg/ml | 9 (9%) hVISA | 22 (22%) |
| Total | 78 (78%) | 22 (22%) |
Figure 3.
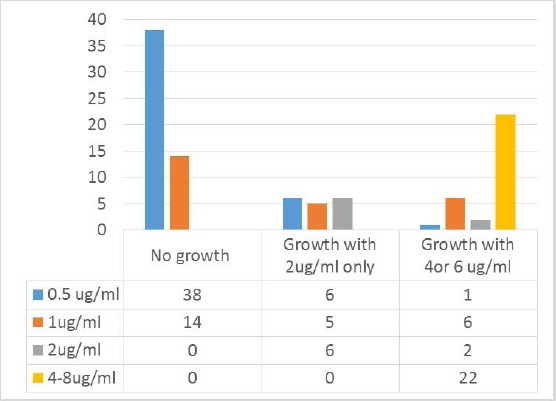
Results of Broth microdilution and vancomycin screening agar among 100 tested isolates
All of the results of screening agar with and without casein were similar except for two isolates; one isolate showed growth on screening agar with 4 µg/ml, with casein but not in that without casein, and one more isolate grew on screening agar 2 µg/ml without casein only after 48 hours. So, adding casein to vancomycin screening agar increased detection rate of VISA by 4.5% (only one VISA out of 22). Screening agar with 6 µg/ml vancomycin (with and without casein showed similar results) detected 7 out of 8 VISA with BMD MIC equal to 4 µg/ml (87.5%) and 14 out of 14 with BMD MIC equal to 8 µg/ml (100%), with overall detection rate of VISA 95.45% (Table 2).
Table 2.
Vancomycin MIC results using Broth microdilution and vancomycin screening agar for detection of MRSA with reduced susceptibility to vancomycin
| Vancomycin screening agar MIC | ||||||||
|---|---|---|---|---|---|---|---|---|
| BMD MIC | No growth (< 2 μg/ml) with and without casein | > 2 μg/ml (< 4 μg/ml) | > 4 μg/ml-< 6 μg/ml | > 6 μg/ml | ||||
| With casein* | Without casein | With* casein | Without* casein | With* casein | Without* casein | |||
| Sensitive | MIC ≤ 1 | 52 | 11 | 10 + 1• | 0 | 0 | 7 | 7 |
| MIC 2 | 0 | 6 | 6* | 0 | 0 | 2 | 2 | |
| Interme-diate | MIC 4 | 0 | 0 | 0 | 1 | 0 | 7 | 7 |
| MIC 8 | 0 | 0 | 0 | 0 | 0 | 14 | 14 | |
| Total | 52 | 17 | 16 + 1• | 1 | 0 | 30 | 30 | |
No difference between 24 and 48 hours;
the Only one showed no growth at 24 hours but detected at 48h.
Increasing incubation time did not increase the detection rate for vancomycin with reduced susceptibility among screening agar with casein and only affected one isolate grown on 2 µg/ml screening agar without casein (Table 2).
Table 2 shows vancomycin MIC results using Broth microdilution and vancomycin screening agar. It is noted that Broth microdilution method was not able to detect nine (9) h-VISA isolates.
All of MRSA isolates (100%) were susceptible for both vancomycin and linezolid by VITEK 2 system. (Figure 4) shows the result of susceptibility testing for MRSA isolates on the Vitek 2 system.
Figure 4.
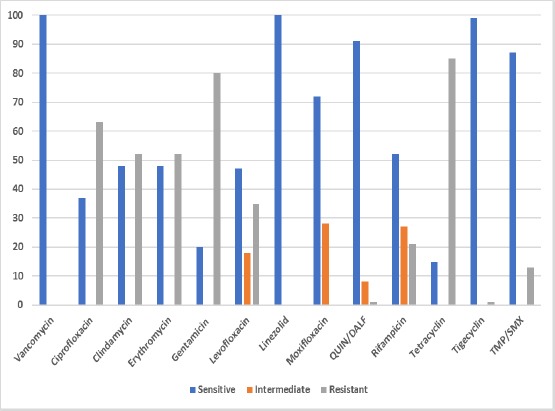
Result of susceptibility testing for 100 MRSA isolates using AST GP 76 cards on Vitek 2 system [14]
Discussion
Overuse of vancomycin has led to the development of a selective pressure over time with the result of the emergence of S. aureus with reduced vancomycin susceptibility. The emergence of MRSA with reduced susceptibility to vancomycin is worrisome as the available drugs for MRSA treatment are limited [6].
We report 22% VISA isolates by broth microdilution method. Vaudaux et al. reported that Broth microdilution assay led to under detection of the vancomycin-intermediate S. aureus VISA) phenotype, yielding only three VISA isolates, for which vancomycin MICs were 4 μg/ml compared to 8 and 19 VISA isolates detected by microdilution and agar testing, respectively [14].
In the present study, among the MRSA isolates that showed MIC less than or equal to 2 µg/ml by BMD; (9%) of h-VISA isolates showed growth on screening agar 4 µg/ml or 6 µg/ml. Whereas, (17%) of isolates with susceptible MIC by BMD showed growth on vancomycin screening agar 2 µg/ml (probable ‘pre-hVISA’) [15]. The pre-hVISA strains may be correlated with the ‘MIC creep’ phenomenon observed in hospitals where anti-MRSA chemotherapy is frequently implemented [15].
Lodise et al. observed that patients with MRSA bloodstream infections with elevated vancomycin MICs but within the susceptible range (≥ 1.5 mg/mL) had higher probabilities of recurrent bacteremia and longer hospital stays [16]. Sakoulas et al. reported that the likelihood of treatment success is significantly lower in patients with MRSA isolate with a vancomycin MIC of 1-2 mg/mL compared with patients infected by isolates with a vancomycin MIC ≤ 1.5 mg/Ml [17]. Edwards et al. suggested lowering vancomycin breakpoints further, to avoid clinical failure and the increased risk of mortality [4].
Satola et al., tested 140 MRSA blood isolates with vancomycin MICs 2 µg/ml by reference broth microdilution and screened for reduced susceptibility to vancomycin using PAP-AUC as the reference method, where they detected 15% h-VISA. They evaluated brain heart infusion (BHI) screen agar containing 16 g/liter casein and 4 mg/liter vancomycin for the detection of h-VISA, revealing 90% and 95% sensitivity and specificity with a 0.5 McFarland inoculum and 100% and 68% sensitivity and specificity with a 2.0 McFarland inoculum respectively [12].
In the present work, adding casein to vancomycin screening agar increased detection rate of VISA by 4.5% (only one VISA out of 22). The base medium of the screening agar might be as important as the vancomycin concentration. Enhancement of detection of h-VISA by screen agar methods could be obtained by the addition of supplements to the agar. Willey et al. reported that the addition of pancreatic digest of casein to BHI agar and 4 g/ml vancomycin improved the detection of VISA on screen agars, as 97.7% of VISA strains in their study were successfully detected with high specificity within 24 h [18]. Other supplement suggested differentiating between h-VISA and VSSA was the addition of 20% horse serum to BHI [19].
Riederer et al., tested 485 MRSA blood isolates with vancomycin MICs 0.5 to 4 µg/ml using BHI-V3, BHI-V4 and other methods. The modified PAP/AUC was measured for all isolates revealing seven VISA and 33 h-VISA phenotypes. The sensitivity and specificity for detecting VISA were 100% and 94.6% for BHI-V3, 100% and 99.2%, for BHI-V4 respectively [20]. These observations differ from those of Burnham et al., who reported 100% sensitivity and 65% specificity for detecting VISA with BHI-V3 [21]. The reason for the difference is unclear but might be related to isolates selection as Burnham et al., selected their isolates based on MIC results and did not perform PAP/AUC [21].
In the present study, screening agar with 6 µg/ml vancomycin detected 7 out of 8 VISA with BMD MIC equal to 4 μg/ml (87.5%) with overall detection rate of VISA 95.45%. CDC 2015 stated that growth of more than one colony on screening agar with 6 μg/ml vancomycin is considered a positive result for VISA [13]. All S. aureus isolates for which the vancomycin MIC ≥ 8 µg/ml grow on these plates and some isolates for which the vancomycin MIC = 4 μg/ml will also grow [22].
As a vancomycin MIC of 4 to 8 µg/ml is considered an intermediate susceptibility, the use of an agar medium such as BHI-V6 as a means to screen for vancomycin-intermediate strains of S. aureus (VISA) is not adequate for this purpose, as those strains having a vancomycin MIC greater than 2 but less than 6 µg/ml could not be detected by this method [23].
Swenson et al. reported that BHI-V6 agar failed to detect 33% (12 of 36) of VISA isolates with MIC 4 µg/ml [24]. Similarly, Walsh et al. reported low sensitivity (22%) for the agar screening method using brain heart infusion agar (6 mg of vancomycin per litre), and 97% specificity [23].
In the present study, the VITEK 2 system failed to detect any isolates with reduced susceptibility to vancomycin. Swenson et al. reported that the Vitek 2 system tended to categorise VISA isolates as susceptible [24]. This was justified by Edwards et al., who demonstrated that MICs from automated systems and the E-test were significantly lower after cryopreservation if compared with those from the E-test analysis, at the time of isolation [4]. Also, Mason et al. pointed out that the prevalence of vancomycin MIC creeps may be underestimated because of the cryopreservation effect [25].
On the other hand, the study performed by Burnham et al., showed that Vitek2 using card GP67 had the worst sensitivity (7.7%), detecting only one out of the 13 VISA isolates compared to Microscan system which had the highest sensitivity (92%), followed by Etest (85% sensitive) and then Sensititre (54% sensitive). Thereby, they suggested that laboratories using the GP67 AST card for vancomycin susceptibility testing of S. aureus should consider additional testing to rule out VISA when MIC 2 µg/ml is generated and/or the concomitant use of a screening medium such as BHI-V3 to ensure detection of VISA isolates [21]. Also, Kruzel et al. stated that it became evident that the automated susceptibility testing methods are inappropriate for the detection of VISA [26].
All of our MRSA isolates were susceptible to vancomycin using VITEK 2 system. They were also sensitive to linezolid (100%) followed by tigecycline (99%) then Quinupristin-dalfopristin (91%). A study by Cook et al. described the successful treatment of a ventriculoperitoneal shunt infection caused by h-VISA with linezolid due to its tolerability and excellent blood-brain barrier penetration [27]. High-dose of Quinupristin-dalfopristin (Synercid) significantly reduced the number of bacteria detected in the VISA hematogenous infection in murine models [28].
Since the first reports of hVISA/VISA, their prevalence differed among geographic regions: the incidence of h-VISA was 6.81% in Asia and 5.60% in Europe/America, and that of VISA was 3.42% and 2.75%, respectively. Several factors may be responsible for such condition; i) high public hygiene standards and meticulous antimicrobial treatments in most European and American countries [29], [30], [31], ii) the control of nosocomial infections is more successful in European and American countries [32, 33], iii) Asia is the most populous region of the world, susceptible to microbial transmission, and iv) more MRSA infections occur in Asian countries [34].
In the present work, Vancomycin screening agar; 2 µg/ml and (4 and 6 µg/ml) were able to detect; probable “pre hVISA and (hVISA and VISA) isolates respectively based on their broth microdilution MIC values. We believe that decreased vancomycin susceptibility in MRSA isolates might be related to MIC creep, but we could not indicate this phenomenon since an earlier data from our lab was not available for comparison. Similar factors as that found in Asia could be responsible for the occurrence of MRSA with reduced susceptibility to vancomycin in our country. Further studies on a large scale are needed to determine the prevalence of VISA and h-VISA and also to study the phenomenon of vancomycin MIC creep.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. The Journal of antimicrobial chemotherapy. 1997;40(1):135–6. doi: 10.1093/jac/40.1.135. https://doi.org/10.1093/jac/40.1.135 PMid: 9249217. [DOI] [PubMed] [Google Scholar]
- 2.Di Gregorio S, Perazzi B, Ordonez AM, De Gregorio S, Foccoli M, Lasala MB, García S, Vay C, Famiglietti A, Mollerach M. Clinical, microbiological, and genetic characteristics of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia in a teaching hospital. Microbial Drug Resistance. 2015;21(1):25–34. doi: 10.1089/mdr.2014.0190. https://doi.org/10.1089/mdr.2014.0190 PMid: 25535825 PMCid: PMC4367492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadarajah R, Post LR, Liu C, Miller SA, Sahm DF, Brooks GF. Detection of vancomycin-intermediate Staphylococcus aureus with the updated Trek-Sensititre System and the MicroScan System: comparison with results from the conventional Etest and CLSI standardized MIC methods. American journal of clinical pathology. 2010;133(6):844–8. doi: 10.1309/AJCPMV1P0VKUAZRD. https://doi.org/10.1309/AJCPMV1P0VKUAZRD PMid: 20472841. [DOI] [PubMed] [Google Scholar]
- 4.Edwards B, Milne K, Lawes T, Cook I, Robb A, Gould IM. Is vancomycin MIC “creep” method dependent? Analysis of methicillin-resistant Staphylococcus aureus susceptibility trends in blood isolates from North East Scotland from 2006 to 2010. Journal of clinical microbiology. 2012;50(2):318–25. doi: 10.1128/JCM.05520-11. https://doi.org/10.1128/JCM.05520-11 PMid: 22135252 PMCid: PMC3264194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojas L, Bunsow E, Munoz P, Cercenado E, Rodríguez-Créixems M, Bouza E. Vancomycin MICs do not predict the outcome of methicillin-resistant Staphylococcus aureus bloodstream infections in correctly treated patients. Journal of antimicrobial chemotherapy. 2012;67(7):1760–8. doi: 10.1093/jac/dks128. https://doi.org/10.1093/jac/dks128 PMid: 22556382. [DOI] [PubMed] [Google Scholar]
- 6.Devi Y, Punithavathy PM, Thomas S, Veeraraghavan B. Challenges in the Laboratory Diagnosis and Clinical Management of Heteroresistant Vancomycin Staphylococcus aureus (hVISA) [Open Access. 2015];Clinical Microbiology. [Google Scholar]
- 7.CDC. 2012. https://www.cdc.gov/hai/settings/lab/visa_vrsa_lab_detection.html .
- 8.Clinical and Laboratory Standards Institute. Twenty-eighth informational supplements. M100-S28. Wayne, PA: CLSI; 2018. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 9.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clinical microbiology reviews. 2010;23(1):99–139. doi: 10.1128/CMR.00042-09. https://doi.org/10.1128/CMR.00042-09 PMid: 20065327 PMCid: PMC2806658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clinical Infectious Diseases. 2007;44(9):1208–15. doi: 10.1086/513203. https://doi.org/10.1086/513203 PMid: 17407040. [DOI] [PubMed] [Google Scholar]
- 11.Yusof A, Engelhardt A, Karlsson Å, Bylund L, Vidh P, Mills K, Wootton M, Walsh TR. Evaluation of a new Etest vancomycin-teicoplanin strip for detection of glycopeptide-intermediate Staphylococcus aureus (GISA), in particular, heterogeneous GISA. Journal of clinical microbiology. 2008;46(9):3042–7. doi: 10.1128/JCM.00265-08. https://doi.org/10.1128/JCM.00265-08 PMid: 1↔146 PMCid: PMC2546754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satola SW, Farley MM, Anderson KF, Patel JB. Comparison of detection methods for heteroresistant vancomycin-intermediate Staphylococcus aureus, with the population analysis profile method as the reference method. Journal of clinical microbiology. 2011;49(1):177–83. doi: 10.1128/JCM.01128-10. https://doi.org/10.1128/JCM.01128-10 PMid: 21048008 PMCid: PMC3020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Twenty-fifth informational supplements. M100-S25. Wayne, PA: 2015. [Google Scholar]
- 14.Vaudaux P, Huggler E, Bernard L, Ferry T, Renzoni A, Lew DP. Underestimation of vancomycin and teicoplanin MICs by broth microdilution leads to underdetection of glycopeptide-intermediate isolates of Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2010;54(9):3861–70. doi: 10.1128/AAC.00269-10. https://doi.org/10.1128/AAC.00269-10 PMid: 20547791 PMCid: PMC2934981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiramatsu K, Kayayama Y, Matsuo M, Aiba Y, Saito M, Hishinuma T, Iwamoto A. Vancomycin-intermediate resistance in Staphylococcus aureus. Journal of global antimicrobial resistance. 2014;2(4):213–24. doi: 10.1016/j.jgar.2014.04.006. https://doi.org/10.1016/j.jgar.2014.04.006 PMid: 27∠79. [DOI] [PubMed] [Google Scholar]
- 16.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrobial agents and chemotherapy. 2008;52(9):3315–20. doi: 10.1128/AAC.00113-08. https://doi.org/10.1128/AAC.00113-08 PMid: 18591266 PMCid: PMC2533486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. Journal of clinical microbiology. 2004;42(6):2398–402. doi: 10.1128/JCM.42.6.2398-2402.2004. https://doi.org/10.1128/JCM.42.6.2398-2402.2004 PMid: 15184410 PMCid: PMC427878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willey BM, Kreiswirth N, Gelosia A, Porter C, Alqhahtani M, Mazzulli T, Pong-Porter S, Larocque C, Pike K, Kreiswirth NNB, Wong K, Poutanen S, Low DE. (ICAAC) Infect Dis Soc Am. (IDSA) 46th Annu Meet American Society for Microbiology and Infectious Diseases Society of America. Washington, DC: 2008. Screening for vancomycin-intermediate Staphylococcus aureus (VISA): does casein make a difference?, abstr. D-2210. Abstr. 48th Annu Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 19.Howden BP, Ward PB, Xie S, Wang JL, Johnson PD, Charles PG, Grayson ML. A new agar dilution screening method for the accurate detection of heterogenous-vancomycin intermediate Staphylococcus aureus (hVISA) ASA Newsl. 2004;19:9–10. [Google Scholar]
- 20.Riederer K, Shemes S, Chase P, Musta A, Mar A, Khatib R. Detection of vancomycin-intermediately susceptible and heterogeneous Staphylococcus aureus isolates: comparison of Etest and agar screening methods. Journal of clinical microbiology. 2011 doi: 10.1128/JCM.01435-10. https://doi.org/10.1128/JCM.01435-10 PMid: 21490190 PMCid: PMC3122762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnham CA, Weber CJ, Dunne WM. Novel screening agar for detection of vancomycin-nonsusceptible Staphylococcus aureus. Journal of clinical microbiology. 2010;48(3):949–51. doi: 10.1128/JCM.02295-09. https://doi.org/10.1128/JCM.02295-09 PMid: 20089765 PMCid: PMC2832426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC (2015): Investigation and Control of Vancomycin- Resistant Staphylococcus aureus (VRSA), 2015. Update. https://www.cdc.gov/hai/pdfs/vrsa-investigation-guide-05_12_2015.pdf .
- 23.Walsh TR, Bolmström A, Qwärnström A, Ho P, Wootton M, Howe RA, MacGowan AP, Diekema D. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. Journal of clinical microbiology. 2001;39(7):2439–44. doi: 10.1128/JCM.39.7.2439-2444.2001. https://doi.org/10.1128/JCM.39.7.2439-2444.2001 PMid: 11427551 PMCid: PMC88167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swenson JM, Anderson KF, Lonsway DR, Thompson A, McAllister SK, Limbago BM, Carey RB, Tenover FC, Patel JB. Accuracy of commercial and reference susceptibility testing methods for detecting vancomycin-intermediate Staphylococcus aureus. Journal of clinical microbiology. 2009;47(7):2013–7. doi: 10.1128/JCM.00221-09. https://doi.org/10.1128/JCM.00221-09 PMid: 19420170 PMCid: PMC2708520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason EO, Lamberth LB, Hammerman WA, Hulten KG, Versalovic J, Kaplan SL. Vancomycin MICs for Staphylococcus aureus vary by detection method and have subtly increased in a pediatric population since 2005. Journal of Clinical Microbiology. 2009;47(6):1628–30. doi: 10.1128/JCM.00407-09. https://doi.org/10.1128/JCM.00407-09 PMid: 19403769 PMCid: PMC2691127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruzel MC, Lewis CT, Welsh KJ, Lewis EM, Dundas NE, Mohr JF, Armitige LY, Wanger A. Determination of vancomycin and daptomycin MICs by different testing methods for methicillin-resistant Staphylococcus aureus. Journal of clinical microbiology. 2011;49(6):2272–3. doi: 10.1128/JCM.02215-10. https://doi.org/10.1128/JCM.02215-10 PMid: 21450951 PMCid: PMC3122735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook AM, Ramsey CN, Martin CA, Pittman T. Linezolid for the treatment of a heteroresistant Staphylococcus aureus shunt infection. Pediatric neurosurgery. 2005;41(2):102–4. doi: 10.1159/000085165. https://doi.org/10.1159/000085165 PMid: 15942282. [DOI] [PubMed] [Google Scholar]
- 28.Yanagihara K, Okada M, Fukuda Y, Imamura Y, Kaneko Y, Ohno H, Higashiyama Y, Miyazaki Y, Tsukamoto K, Hirakata Y, Tomono K. Efficacy of quinupristin-dalfopristin against methicillin-resistant Staphylococcus aureus and vancomycin-insensitive S. aureus in a model of hematogenous pulmonary infection. Chemotherapy. 2004;50(5):260–4. doi: 10.1159/000081948. https://doi.org/10.1159/000081948 PMid: 15528893. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz FJ, Krey A, Geisel R, Verhoef J, Heinz HP, Fluit AC SENTRY Participants Group. Susceptibility of 302 methicillin-resistant Staphylococcus aureus isolates from 20 European university hospitals to vancomycin and alternative antistaphylococcal compounds. European Journal of Clinical Microbiology and Infectious Diseases. 1999;18(7):528–30. doi: 10.1007/s100960050340. https://doi.org/10.1007/s100960050340 PMid: 10482037. [DOI] [PubMed] [Google Scholar]
- 30.Aucken HM, Warner M, Ganner M, Johnson AP, Richardson JF, Cookson BD, Livermore DM. Twenty months of screening for glycopeptide-intermediate Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 2000;46(4):639–40. doi: 10.1093/jac/46.4.639-a. https://doi.org/10.1093/jac/46.4.639-a PMid: 11020266. [DOI] [PubMed] [Google Scholar]
- 31.Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. Journal of Antimicrobial Chemotherapy. 2001;47(4):399–403. doi: 10.1093/jac/47.4.399. https://doi.org/10.1093/jac/47.4.399 PMid: 11266410. [DOI] [PubMed] [Google Scholar]
- 32.Denis O, Nonhoff C, Byl B, Knoop C, Bobin-Dubreux S, Struelens MJ. Emergence of vancomycin-intermediate Staphylococcus aureus in a Belgian hospital: microbiological and clinical features. Journal of antimicrobial chemotherapy. 2002;50(3):383–91. doi: 10.1093/jac/dkf142. https://doi.org/10.1093/jac/dkf142 PMid: 12205063. [DOI] [PubMed] [Google Scholar]
- 33.Pierard D, Vandenbussche H, Verschraegen I, Lauwers S. Screening for Staphylococcus aureus with a reduced susceptibility to vancomycin in: a Belgian hospital. Pathologie Biologie. 2004;52(8):486–8. doi: 10.1016/j.patbio.2004.07.016. https://doi.org/10.1016/j.patbio.2004.07.016 PMid: 15465269. [DOI] [PubMed] [Google Scholar]
- 34.Chen CJ, Huang YC. New epidemiology of S taphylococcus aureus infection in A sia. Clinical Microbiology and Infection. 2014;20(7):605–23. doi: 10.1111/1469-0691.12705. https://doi.org/10.1111/1469-0691.12705 PMid: 24888414. [DOI] [PubMed] [Google Scholar]


