Abstract
BACKGROUND:
Pulmonary haemorrhage (PH) is a serious complication during Systemic Lupus Erythematosus (SLE).
AIM:
The aim was to present data on 12 patients of SLE with classic symptoms and signs of PH admitted throughout eleven years.
METHODS:
This retrospective study was carried out at King Abdul Aziz Specialist hospital in Taif-a tertiary care hospital in the western region of Saudi Arabia. The data was analysed from the case files of SLE patients who had episodes of PH throughout 11 years (January 2007 to December 2017).
RESULTS:
Twelve patients (10 females and 2 males) were found to have diffuse pulmonary haemorrhage during their SLE in the study period. Of 12 patients with confirmed pulmonary haemorrhage (hemoptysis, hypoxemia, new infiltrates on chest radiography, fall in haemoglobin and hemorrhagic returns of bronchoalveolar lavage with hemosiderin-laden macrophages) 4 patients had PH as the first presentation of SLE and 8 patients developed this complication during the disease. All patients presented with shortness of breath and hemoptysis. The most common extra-pulmonary involvement in the study cohort was renal (83%), which ranged from clinical nephritis, nephrotic syndrome to acute renal failure. All patients were managed in intensive care of the hospital, and of 12 patients, 9 (75%) required mechanical ventilation. All patients were uniformly treated with pulse Methylprednisolone; 9 received Cyclophosphamide, 6 received IVIG, and 4 received Plasmapheresis. Only 3 patients (25%) survived despite maximum possible support during their mean hospital stay of 18 ± 5 days.
CONCLUSION:
The requirement of mechanical ventilation and the association of renal and neuropsychiatric complications predicted mortality in patients with pulmonary haemorrhage.
Keywords: SLE, Nephritis, Neuropsychiatric manifestations, IVIG, Steroids, Mechanical ventilation, Pulmonary haemorrhages
Introduction
With its chronic and relapsing course, SLE can involve many organ systems, and pulmonary haemorrhage (PH) remains the devastating complication of this disease. The frequency of PH ranges from 0.63 to 5.4% in various cohorts of SLE [1]. While in admitted patients PH ranges from 0.5 to 9% [2], [3] of hospital admissions, the frequency of this complication steeps to 5.7% in an intensive care setting. Further, various autopsy series in SLE patients have demonstrated PH up to 12.3% connoting clinical PH to be the tip of the iceberg [4], [5]. The frequency of PH is higher in women as SLE is more common in this gender and the mean or median progression of SLE at the time of PH varies from 6 months to 14.1 years. The usual presentation of PH is shortness of breath with or without hemoptysis. However, the absence of hemoptysis in SLE patients doesn’t rule out PH in a given case. The presence of radiological evidence of infiltrates on CT scan with a corresponding drop in haemoglobin is the usual scenario among SLE cases with PH. The high-resolution CT scan is more sensitive than conventional radiography in detecting PH [6]. The characteristic features on imaging are diffuse bilateral alveolar infiltrates in most series and some researchers have reported alveolar-interstitial infiltrates as well. There is a paucity of data regarding the type of immune response that triggers PH in patients with SLE. In an animal model of PH pristane-induced SLE in susceptible mice, the involvement of the innate immune response was shown to have played a key role. The severity or recovery from the insult of PH is dependent on adaptive immunity with significant participation of B cells. The haemorrhage has been shown to be preceded by infiltration of macrophages and neutrophils [7].
Regarding lung biopsy, some of the first studies by Myers and Katzenstein [8], demonstrated the small vessel vasculitis or microangitis in 4 patients with lupus. The above study highlights the characteristic expression of PH in SLE and immune complex deposits. Capillaritis may have immune complexes associated with SLE [9]. We reiterate that many patients with PH in SLE described in the literature are reported with “soft bleeding” or without capillaritis.
We at this moment present data on 12 patients of SLE with classic symptoms and signs of PH admitted throughout eleven years.
Methods
This was a retrospective study conducted to assess the predictors of mortality due to pulmonary haemorrhage during SLE at King Abdul Aziz Specialist, Taif, Saudi Arabia. All patients with PH fulfilled the criteria of Systemic Lupus International Collaborating Clinics (SLICC) group [10].
Patients with signs of alveolar haemorrhage like hemoptysis, hypoxemia, new infiltrate on chest radiography fall in haemoglobin concentration, and hemorrhagic returns of bronchoalveolar lavage with hemosiderin-laden macrophages were included.
The detailed history and thorough clinical examination were tabulated. Patients previously diagnosed to have SLE were studied regarding their disease manifestation and the treatment modalities before PH.
The laboratory investigations included complete blood counts, erythrocyte sedimentation rate (ESR) serum complement (C3, C4), autoimmune profile, urine analysis, coagulation, hepatic and renal functions were tabulated in the study cohort. Appropriate microbiological cultures data was tabulated as well.
The chest x-ray and CT scans were analysed, and patients with characteristic alveolar haemorrhages were included. Patients who were diagnosed with SLE previously, their records were reviewed.
Exclusion criteria: Pulmonary haemorrhage due to Pulmonary, renal syndromes Good pastures syndrome, Wegner’s Granulomatosis; Necrotising pneumonia;
Data were statistically described regarding frequencies (number of cases) and valid percentages for categorical variables. Mean, standard deviations, minimum and maximum were used to describe parametric numerical variable while median and inter-quartile range (IQR) were considered for non-parametric data. Comparison of categorical variables between the subgroups (cross-tabulation) was made using a Chi-square test. All statistical calculations were done using computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, USA) release 21 for Microsoft Windows.
Results
A total of 14 patients were found to have 15 episodes of pulmonary haemorrhage during the study period. The data on two patients were excluded as they had necrotising pneumonia. Finally, data on 12 SLE patients with PH was analysed.
The mean ± SD age of participants was 25.8 ± 8.3 years, and age ranged from 17-52 years. Of 12 patients, 8 patients 8/12 (66%) were known cases of SLE and 4 patients (33%) PH was the first presentation of SLE, as shown in Figure 1.
Figure 1.
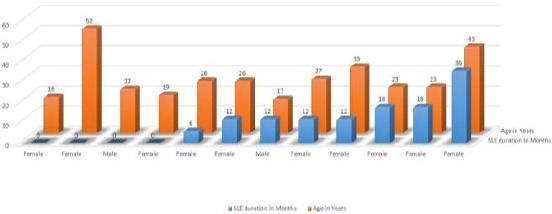
Clinical characteristics of SLE patients with Pulmonary haemorrhage
All the patients presented with hemoptysis (100%) in our series, 9 patients (75%) had shortness of breath, and 8 patients (66%) had coughed. Dyspnea at rest was mild, to begin with, and of 12 patients, the shortness of breath rapidly worsened necessitating mechanical ventilation in 9/12 (75%) patients. Only 2 patients (16%) had a fever and florid signs of sepsis. While as 7 patients (58%) had constitutional symptoms characteristic of SLE.
The characteristic rash of SLE was seen in 6 patients (50%) and musculoskeletal involvement was observed in 5 patients (40%) of patients in the study cohort. Five patients (40%) had serositis in the form of pericardial effusion. Of 12 patients, 3 (55%) had central nervous system involvement of SLE in the form of seizures. The details are shown in Figure 2.
Figure 2.
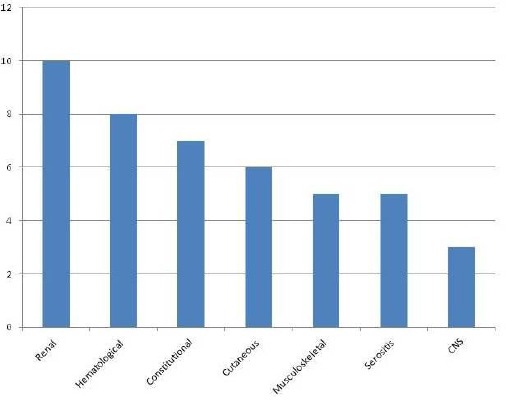
Extra Pulmonary involvement in PH patients
Severe disease activity according to SLE disease activity index was observed in 9, while moderate activity in 3 patients.
All patients in our study cohort were found to have severe anaemia, normal platelets and normal coagulation profiles as shown in Table 1. The chest radiographs and HRCT revealed diffuse alveolar infiltrates in all patients. Bronchoalveolar lavage in 5 patients revealed uniformly hemorrhagic fluid with hemosiderin-laden macrophages. In three patients BAL grew Acinetobacter on culture, Table 1.
Table 1.
Investigative profile of SLE patients with pulmonary haemorrhage
| No | Age | Hb | WBC | Platelets | INR | Create | 24 Hr UP | C3 /C4 | ANA dsDNA | ECHO | BAL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18/F | 7.9 | 3.8 | 145 | 1.2 | 1.5 | 1.2 | Low | Positive | Normal | Sterile |
| 2 | 52/F | 6.9 | 3.8 | 132 | 1.1 | 6 | 1.8 | Low | Positive | Moderate MS | Sterile |
| 3 | 22/M | 7.9 | 3.9 | 116 | 1.2 | 1.5 | 1.5gm | Low | Positive | Global hypokinesia | Sterile |
| 4 | 19/F | 6.8 | 7.3 | 150 | 1.1 | 2.9 | 3.9 | Low | Positive | Pericardial Effusion | Sterile |
| 5 | 26/F | 7.9 | 6.1 | 248 | 0.9 | 1.2 | 3.2 | Low | Positive | Pericardial Effusion | Sterile |
| 6 | 26/F | 5.3 | 2.4 | 204 | 1.4 | 2.2 | 8gm | Low | Positive | Marantic Endocarditis | Acinetobacter |
| 7 | 17/M | 6.5 | 8.2 | 136 | 1.3 | 3.7 | 3.7gm | Low | Positive | Normal | Sterile |
| 8 | 27/F | 6.2 | 2.3 | 154 | 1.4 | 3.2 | 6.6gm | Low | Positive | Pericardial Effusion | Acinetobacter |
| 9 | 33/F | 7.9 | 4.5. | 155 | 1.1 | 1.9 | 6.9gm | Low | Positive | Pericardial Effusion | Sterile |
| 10 | 23/F | 7 | 7.4 | 273 | 1.3 | 5.4 | 2-4gm | Low | Positive | Pericardial Effusion | Sterile |
| 11 | 23/F | 7.5 | 7.1 | 258 | 1.7 | 1.9 | 2.6gm | Low | Positive | Normal | Acinetobacter |
| 12 | 43/F | 6.9 | 6.9 | 153 | 1.1 | 7 | 3gm | Low | Positive | Normal | Sterile |
Echocardiography was normal in 4/12 (34%) while as pericardial effusion was seen in 5 patients, one patient had marantic endocarditis, and the other had global hypokinesia. The pericardial effusion improved in 3 without drainage. Moderate Mitral stenosis was seen in one of the patients. The details are shown in Table 1.
The patients with PH were managed in intensive care of the hospital. All patients received pulse Methylprednisolone, IVIG had been given to 6 (50%), and 4 (33%) had received plasmapheresis. Of 12 patients with PH 9 patients succumbed despite maximum available supportive treatment. Thus the mortality rate was 75% (9/12) in this study. The cause of death was respiratory failure compounded by polymicrobial sepsis in 3 patients and Varicella pneumonia after Immunosuppression therapy was observed in one patient who succumbed.
Three patients who survived episodes of PH didn’t require mechanical ventilation and had no sepsis. They were treated with pulse steroids and IVIG while as plasmapheresis was given to one as shown in Table 2. However, all the three had active disease, anaemia and renal derangements but none of the three patients had neuropsychiatric manifestations.
Table 2.
Management & outcome of SLE patients with pulmonary haemorrhage
| No | Age | BT | M.V | Steroids | IVIG | PF | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 18/F | 1unit | NO | YES | YES | NO | SURVIVED |
| 2 | 52/F | 2units | YES | YES | NO | NO | DIED |
| 3 | 22/M | 0 | YES | YES | YES | NO | DIED |
| 4 | 19/F | 2units | YES | YES | NO | NO | DIED |
| 5 | 26/F | 1 | NO | YES | YES | NO | SURVIVED |
| 6 | 26/F | 2units | YES | YES | YES | YES | DIED |
| 7 | 17/M | 1unit | YES | YES | NO | NO | DIED |
| 8 | 27/F | 0 | YES | YES | NO | NO | DIED |
| 9 | 33/F | 2units | YES | YES | YES | YES | DIED |
| 10 | 23/F | 0 | NO | YES | NO | YES | SURVIVED |
| 11 | 23/F | 2 | YES | YES | NO | NO | DIED |
| 12 | 43/F | 2 | YES | YES | YES | YES | DIED |
Abbreviation used BT = Blood transfusion; M.V = Mechanical ventilation; IVIG = Intranvenous immunoglobulins; PF = Plasmapheresis.
All the three patients who survived are on our follow up for the last two years now. Two patients are in remission, and one patient is on maintenance hemodialysis. Details are shown in Table 3.
Table 3.
Clinical and investigative profile of patients who survived
| No | Age | SLE duration | Hb | WBC | Platelets | INR | Create | 24 Hr UP | C3 &C4 | ANA dsDNA | ECHO | Treatment +No MV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18/F | First | 7.9 | 3.8 | 145 | 1.2 | 1.5 | 1.2 | Low | Positive | Normal | S+IVIG |
| 2 | 26/F | 6 M | 7.9 | 6.1 | 248 | 0.9 | 1.2 | 3.2 | Low | Positive | Pericardial Effusion | S+IVIG+ |
| 3 | 23/F | 18M | 7 | 7.4 | 273 | 1.3 | 5.4 | 2.4 | Low | Positive | Pericardial Effusion | S+IVIG+PF |
Hb = gm/dl; S-STEROIDS; IVIG = Intravenous immunoglobulin; PF = Plasmapheresis; MV = Mechanical ventilation; Seizures: None; Sepsis: None; Blood transfusion: None; Patients 1 and 2 are in remission while as patient number 3 is on maintenance Hemodialysis.
Discussion
The classical triad of hemoptysis, abrupt fall in haemoglobin level and new lung infiltrate on CT scan chest were observed in all the patients in this study. However, overt hemoptysis may not be present in all SLE patients presenting with PH as reported by Zamora et al., [9], which means thereby that a physician needs to have a high degree of clinical suspicion in a given case of SLE presenting with shortness of breath and radiological changes. Even at high altitudes PH should not be confused with high altitude pulmonary oedema as reported by Lis et al., [11]. PH is one of the rare but catastrophic complications in the course of SLE, and the mortality continues to be high as was observed in this study. We observed that patients requiring mechanical ventilation had a poor outcome in line with other workers [1], [9]. Mechanical ventilation (MV) per se predisposes patients to many complications, and one of the life-threatening complications of MV is Ventilator-associated pneumonia (VAP). The risk of VAP is highest immediately after intubation and steadily increases after that. Even though broad-spectrum antibiotics had been given to all patients but keeping in view concomitant immunosuppressant treatment all patients who received MV succumbed.
Contrary to these three patients who didn’t receive MV, despite their active disease survived after PH episodes were aggressively managed. PH was the first presentation of SLE in four patients in our study and 3 of them had seizures in addition to PH. Their shortness of breath progressively deteriorated and required mechanical ventilation meaning thereby that PH can present irrespective of the duration of SLE as described in the literature [12], [13]. Even pulmonary haemorrhage in SLE can present without extra pulmonic manifestation of SLE as reported by Bajantari et al., [14].
Patients with the associated neuropsychiatric manifestation of SLE had a poor outcome in this study. The data on 21 Korean patients by Kwok et al., [15], revealed that SLE patients with neuropsychiatric manifestation were at an increased risk for developing PH. Our results are in coherence with their study. Renal impairment was associated with the majority of patients with PH in this study. Similar results were demonstrated by Chang et al., [16].
The profile of autoantibodies in our study including anti-DNA, lupus anticoagulant, anticardiolipin, anti-Sm, anti-Ro and anti-RNP were similar in patients with PH compared with SLE patients without PH. Hence these may not serve as biomarkers to predict PH in SLE. None of our patients had thrombocytopenia or deranged coagulation. However, all our patients had hypocomplementemia, and overwhelming sepsis contributed towards mortality in 3 patients. In a study data on 50 patients with pulmonary haemorrhage in SLE the bivariate analysis, factors associated with mortality were high Acute Physiology and Chronic Health Evaluation II scores, the requirement of mechanical ventilation, infections, renal failure and thrombocytopenia [17].
All patients in our study cohort received pulse Methylprednisolone which has been shown to provide a survival advantage compared to prednisone 1 mg/kg [1]. In our study 4 patients (33%) were given plasmapheresis and one of them survived, but it is difficult to assess its utility alone. Intravenous immunoglobulin was given to 6patients (50%) in our study and of these, a 26-year-old female patient survived as shown in Table 2, and 3. This is not sufficient to prove the efficacy of IVIG but in a retrospective study by Shen et al., [17], the data on 29 SLE patients with PH, authors advocated early aggressive management with high-dose steroids, intravenous immunoglobulin and cyclophosphamide.
Infections have been reported by different series as an important factor associated with PH [18], [19]. In our study bronchoalveolar lavage (BAL) in three patients grew Acinetobacter species and BAL was sterile in rest of the patients. It is difficult to separate whether the infections occurred at the time of diagnosis of PH or after this complication. In a study by Rojas-Serrano et al., [18] data on 14 events of PH in SLE, evaluated during the first 48h with bronchoscopy and bronchoalveolar lavage, showed the presence of infection in 57%; of the patients leading to the observation that an infection could be a precipitating or contributing factor. Thus it is prudent to mention that all efforts must be utilised to identify and treat infection in a given case of PH.
Despite aggressive treatment the outcome in our study was poor as only 3 patients (25%) survived which is, however higher than reported by Marino et al., [19]. Various experimental attempts to treat this devastating complication have been attempted recently in which Umbilical cord-derived mesenchymal stem cell transplantation (UC-MSCT) has been shown to be effective in the treatment of four SLE patients with PH. Authors concluded that UC-MSCT could be applied as a salvage strategy for SLE patients with PH [20]. However, the data is too small, and the availability of this technique is not universal. In another study data on 21 patients from China, Bronchoalveolar lavage in combination with high-dose pulse therapy of methylprednisolone was found to be superior to methylprednisolone alone. The authors observed a statistically significant difference between the two groups. Broncho alveolar lavage alleviated hypoxemia and dyspnea in patients with PH [21].
To conclude, PH is a devastating complication of SLE, and despite aggressive treatment mortality remains high. Our study showed that the requirement of mechanical ventilation during the disease and association of neuropsychiatric manifestations and renal impairment predicted mortality in patients with PH. Further studies may be carried out to confirm the association.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Barile LA, Jara LJ, Medina-Rodriguez F, Garcia-Figueroa JL, Miranda-Limón JM. Pulmonary hemorrhage in systemic lupus erythematosus. Lupus. 1997;6(5):445–8. doi: 10.1177/096120339700600506. https://doi.org/10.1177/096120339700600506 PMid: 9229363. [DOI] [PubMed] [Google Scholar]
- 2.Chang MY, Fang JT, Chen YC, Huang CC. Diffuse alveolar hemorrhage in systemic lupus erythematosus: a single center retrospective study in Taiwan. Renal failure. 2002;24(6):791–802. doi: 10.1081/jdi-120015681. https://doi.org/10.1081/JDI-120015681 PMid: 12472201. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Martinez MU, Abud-Mendoza C. Predictors of mortality in diffuse alveolar haemorrhage associated with systemic lupus erythematosus. Lupus. 2011;20(6):568–74. doi: 10.1177/0961203310392430. https://doi.org/10.1177/0961203310392430 PMid: 21558137. [DOI] [PubMed] [Google Scholar]
- 4.Ca-as C, Tobón GJ, Granados M, Fernández L. Diffuse alveolar hemorrhage in Colombian patients with systemic lupus erythematosus. Clinical rheumatology. 2007;26(11):1947–9. doi: 10.1007/s10067-007-0576-3. https://doi.org/10.1007/s10067-007-0576-3 PMid: 17377738. [DOI] [PubMed] [Google Scholar]
- 5.Mintz G, Galindo LF, Fernandez-Diez J, Jimenez FJ, Robles-Saavedra E, Enriquez-Casillas RD. Acute massive pulmonary hemorrhage in systemic lupus erythematosus. The Journal of rheumatology. 1978;5(1):39–50. PMid:147942. [PubMed] [Google Scholar]
- 6.Martínez-Martínez MU, Abud-Mendoza C. Predictors of mortality in diffuse alveolar haemorrhage associated with systemic lupus erythematosus. Lupus. 2011;20(6):568–74. doi: 10.1177/0961203310392430. https://doi.org/10.1177/0961203310392430 PMid: 21558137. [DOI] [PubMed] [Google Scholar]
- 7.Barker TT, Lee PY, Kelly-Scumpia KM, Weinstein JS, Nacionales DC, Kumagai Y, Akira S, Croker BP, Sobel ES, Reeves WH, Satoh M. Pathogenic role of B cells in the development of diffuse alveolar hemorrhage induced by pristane. Laboratory Investigation. 2011;91(10):1540. doi: 10.1038/labinvest.2011.108. https://doi.org/10.1038/labinvest.2011.108 PMid: 21808234 PMCid: PMC3184182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers JL, Katzenstein AL. Microangiitis in lupus-induced pulmonary hemorrhage. American journal of clinical pathology. 1986;85(5):552–6. doi: 10.1093/ajcp/85.5.552. https://doi.org/10.1093/ajcp/85.5.552. [DOI] [PubMed] [Google Scholar]
- 9.Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine. 1997;76(3):192–202. doi: 10.1097/00005792-199705000-00005. https://doi.org/10.1097/00005792-199705000-00005 PMid: 9193454. [DOI] [PubMed] [Google Scholar]
- 10.Petri M, Orbai AM, Alarcón GS, Gordon C, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–86. doi: 10.1002/art.34473. https://doi.org/10.1002/art.34473 PMid: 22553077 PMCid: PMC3409311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Wang Y, Huang X, Cao J, Yang D. Diffuse alveolar hemorrhage from systemic lupus erythematosus misdiagnosed as high altitude pulmonary edema. High Alt Med Biol. 2015;16(1):67–70. doi: 10.1089/ham.2014.1094. https://doi.org/10.1089/ham.2014.1094 PMid: 25803143. [DOI] [PubMed] [Google Scholar]
- 12.Cucuzza ME, Marino SD, Schiavone L, Smilari P, Filosco F, Barone P. Diffuse alveolar haemorrage as initial presentation of systemic lupus erythematosus: a case report. Lupus. 2018;27(3):507–510. doi: 10.1177/0961203317713144. https://doi.org/10.1177/0961203317713144 PMid: 28592199. [DOI] [PubMed] [Google Scholar]
- 13.Porres-Aguilar M, Mendez-Ramirez J, Eraso LH, Porres-Munoz M, Pema K. Diffuse alveolar hemorrhage as an initial presentation of systemic lupus erythematosus. J Natl Med Assoc. 2008;100(12):1485–7. doi: 10.1016/s0027-9684(15)31553-4. https://doi.org/10.1016/S0027-9684(15)31553-4. [DOI] [PubMed] [Google Scholar]
- 14.Bajantri B, Sapkota B, Venkatram S. Diffuse Alveolar Hemorrhage without Extrapulmonary Manifestations: A Rare Presentation of Lupus. Am J Case Rep. 2018;19:218–223. doi: 10.12659/AJCR.907148. https://doi.org/10.12659/AJCR.907148 PMid: 29487279 PMCid: PMC5839422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok SK, Moon SJ, Ju JH, Park KS, Kim WU, Cho CS, Kim HY, Park SH. Diffuse alveolar hemorrhage in systemic lupus erythematosus: risk factors and clinical outcome: results from affiliated hospitals of Catholic University of Korea. Lupus. 2011;20(1):102–7. doi: 10.1177/0961203310381511. https://doi.org/10.1177/0961203310381511 PMid: 20956464. [DOI] [PubMed] [Google Scholar]
- 16.Chang MY, Fang JT, Chen YC, Huang CC. Diffuse alveolar hemorrhage in systemic lupus erythematosus: a single center retrospective study in Taiwan. Ren Fail. 2002;24(6):791–802. doi: 10.1081/jdi-120015681. https://doi.org/10.1081/JDI-120015681 PMid: 12472201. [DOI] [PubMed] [Google Scholar]
- 17.Shen M, Zeng X, Tian X, Zhang F, Zeng X, Zhang X, Xu W. Diffuse alveolar hemorrhage in systemic lupus erythematosus: a retrospective study in China. Lupus. 2010;19(11):1326–30. doi: 10.1177/0961203310373106. https://doi.org/10.1177/0961203310373106 PMid: 20647253. [DOI] [PubMed] [Google Scholar]
- 18.Rojas-Serrano J, Pedroza J, Regalado J, Robledo J, Reyes E, Sifuentes-Osornio J, Flores-Suárez LF. High prevalence of infections in patients with systemic lupus erythematosus and pulmonary haemorrhage. Lupus. 2008;17(4):295–9. doi: 10.1177/0961203307086930. https://doi.org/10.1177/0961203307086930 PMid: 18413410. [DOI] [PubMed] [Google Scholar]
- 19.Marino CT, Pertschuk LP. Pulmonary hemorrhage in systemic lupus erythematosus. Arch Intern Med. 1981;141(2):201–3. https://doi.org/10.1001/archinte.1981.00340020063018. [PubMed] [Google Scholar]
- 20.Shi D, Wang D, Li X, Zhang H, Che N, Lu Z, Sun L. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol. 2012;31(5):841–6. doi: 10.1007/s10067-012-1943-2. https://doi.org/10.1007/s10067-012-1943-2 PMid: 22302582. [DOI] [PubMed] [Google Scholar]
- 21.Liu GY, Yang HJ, Tong SQ, Li YJ, Guo AR, Pang HW. [Value of bronchoalveolar lavage as a rescue measurement for systemic lupus erythematosus complicated with diffuse alveolar hemorrhage] Zhonghua Yi Xue Za Zhi. 2011;91(27):1917–9. PMid:22093849. [PubMed] [Google Scholar]


