Abstract
This study aggregated data from three randomized control trials to explore the differential efficacy of three forms of exposure therapy, namely, in vivo (iVET), virtual reality (VRET), and augmented reality (ARET), in the treatment of small animal phobia. Additionally, baseline patient characteristics were used to detect subgroups of patients who showed a differential response to certain treatment modalities. Primary measures were distance covered, anxiety during the behavioral avoidance test (BAT), and overall fear of small animals. A repeated-measures analysis of variance was used to explore the overall treatment effect across the exposure modalities. A cluster analysis and an analysis of moderation were conducted to explore differential response to treatments. The main study finding was that the three treatment conditions were similarly efficacious in the treatment of small animal phobia for all study outcomes. Only for distance covered, our results revealed a tendency for iVET to be more effective than VRET and ARET in participants with worse performance on the BAT before treatment. The present study findings provide further evidence for the comparable efficacy of the three forms of exposure. Our results also suggest that, overall, treatments are likely to be similarly effective, regardless of the individual baseline characteristics (i.e., fear, anxiety, and age), whereas pretreatment scores on distance covered in the avoidance test might be used to personalize treatments (iVET may be preferable when participants perform worse at pretreatment).
Keywords: virtual reality exposure therapy, augmented reality exposure therapy, in vivo exposure therapy, small animal phobia
Introduction
Animal phobia (i.e., insects, snakes, birds, or other animals) is one of the most prevalent forms of specific phobias, especially in women. Lifetime rates of this disease range from 5 percent to more than 12 percent, depending on the study, with the highest prevalence rates in young adults.1–4
In vivo exposure therapy (iVET) is the treatment of choice for specific phobias, including animal phobia, as it has been shown to outperform all other forms of nonexposure psychosocial treatment. However, other forms of exposure treatment, such as virtual reality exposure therapy (VRET), have been found to be just as efficacious as iVET,5 especially on posttreatment followup measures.6
Virtual reality and augmented reality are two technologies that can be used as alternatives to iVET. In the former, the patient is asked to interact with a computer-generated, three-dimensional environment or object. In the latter, a computer-generated virtual object is superimposed onto reality. Thus, while both technologies have the ability to enhance or enrich an experience using artificial, computer-generated elements, they differ in their purpose. Specifically, while virtual reality aims at transporting the user to a different location by substituting the existing physical environment with a virtual one, thus creating an immersive effect, in augmented reality the virtual elements are the ones that are transported into the user's location and added to the user's world, arguably resulting in a different quality of the experience.7,8
The use of these alternative forms of exposure (i.e., virtual reality and augmented reality) has been found to have some advantages over traditional iVET. For instance, some clinicians and patients are reluctant to use iVET because they find it cruel.9,10 In fact, there is evidence suggesting that virtual reality has a much lower refusal rate than in vivo treatment.11 In addition, VRET and augmented reality exposure therapy (ARET) offer ecological treatments when the availability of the feared stimuli is limited.12
There is currently extensive evidence for the effectiveness of VRET and ARET in the treatment of a wide range of mental disorders.13,14 For instance, their use is now supported in post-traumatic stress disorder15,16 and anxiety disorders,17–19 including panic disorder, social anxiety disorder, and specific phobias.
Despite the shared advantages of ARET and VRET over iVET (e.g., safety, control over the scenario and the stimuli, and easy use in repeated training), both technologies present an important difference that might influence the experience of exposure and, ultimately, the efficacy of interventions. Specifically, ecological validity is higher in augmented reality, as the feared element is embedded in the real environment of the individual. Consequently, the user is allowed to use his/her own body to interact with the virtual object as opposed to participating via a virtual representation of his/her body, as in virtual reality.7,20 Also importantly, augmented reality only requires a few elements to be developed, thus resulting in reduced production costs when compared with virtual reality, which makes it an attractive technology for research and clinical purposes.
The extent to which the aforementioned differences between ARET and VRET ultimately result in differential efficacy scores in small animal phobia is still unclear. There is extensive literature showing that VRET can effectively reduce symptoms and avoidance in small animal fear.21 There is also some recent evidence to suggest that ARET can effectively induce and reduce anxiety and fear and increase the ability to interact with the feared small animal (e.g., spider or cockroach).22–25 However, studies into small animal phobia have rarely compared the effectiveness of more than one treatment modality in the same investigation, so the differential effects of ARET and VRET, and the ability of both technology-based forms of exposure to become alternatives to traditional iVET in the treatment of small animal phobia require further investigation.
To date, some previous evidence suggests that alternative forms of exposure and traditional iVET are equally effective,5 especially at followup.6 However, this latter meta-analysis did not report separate effect sizes for the different forms of alternative exposure treatments (i.e., imaginal exposure, cave automatic virtual environment (CAVE), and VRET), and neither of the two meta-analyses included data on augmented reality, so the benefits of using an arguably more ecological technology (i.e., augmented reality) in front of iVET and VRET remain speculative. Moreover, it is still unclear whether certain treatments might be more beneficial for a certain subset of patients (i.e., moderation). The goal of this study is twofold. First, we will compare the efficacy of three treatment modalities, namely, traditional iVET, VRET, and ARET, which have shown to be effective across investigations but have never been studied together in small animal phobia. Second, we will investigate differential treatment efficacy as a function of baseline patient characteristics, which would allow us to recommend a specific treatment for a given patient (i.e., personalized therapy). In the light of the reviewed research, we expect iVET and alternative treatments (VRET and ARET) to reduce fear, anxiety, and avoidance. We anticipate that differences in effectiveness across treatment modalities, if existent, will be small. Finally, the existence of subgroups of patients that preferably respond to one of the treatments is merely exploratory at this stage, so no hypotheses are provided.
Methods
Research design and procedure
In this study, three datasets from previously published randomized controlled trials from our team were compared.26–28 A summary of the studies included is shown in Table 1. All the studies included a VRET, an ARET, or an iVET group.
Table 1.
Description of Studies
| Measures | |||||
|---|---|---|---|---|---|
| Study | Clinical condition | Treatment conditions | Distance to feared animal (BAT) | Anxiety (BAT) | FSQ |
| Botella et al.26 | Cockroach or spider phobia | Augmented reality (n = 32) | Meters | 0–100 | 18 Items: 1–7 scale |
| In vivo exposure (n = 31) | |||||
| Hoffman et al.27 | Spider phobia | Virtual reality (n = 24) | Feet | 0–100 | 6 Items: 1–7 scale |
| Control (n = 12) | |||||
| García-Palacios et al.28 | Spider phobia | Virtual reality (n = 12) | Meters | 0–100 | 18 Items: 1–7 scale |
| Waiting list (n = 11) | |||||
BAT, behavioral avoidance test; FSQ, Fear of Spiders Questionnaire.
In all studies, participants were mostly university students either recruited via introductory Psychology classes or using advertisements around the university campus and mails to university community members.26–28 Once eligibility was ensured, which broadly included the presence of a small animal phobia and the physical and mental ability to participate (see each original study for a more detailed description of the inclusion criteria), participants were randomly assigned to an experimental condition by an independent researcher. Then, assessment was made before and after treatment (see the Measures and the Treatment sections below).
Measures
Behavioral avoidance test
The behavioral avoidance test (BAT) is an objective, observational test of the patient's behavior, to measure clinical progress in overcoming phobias through exposure to the feared object.29 In all the studies, a sealed container containing a live cockroach or a spider was placed on a table inside a room. Participants were asked to enter the room and approach the spider as much as they could. Then, the distance covered was measured, and participants were asked to rate their anxiety level during the test. In Botella et al.26 and García-Palacios et al.,28 the distance left to cover was measured in meters, whereas in the study by Hoffman et al.,27 the distance left to cover was measured in feet. In García-Palacios et al.28 and Hoffman et al.,27 a 101-point measure of anxiety was obtained, where 0 represented no anxiety, and 100 reflected extreme anxiety. The study by Botella et al.26 used an 11-point scale ranging from 0 (no anxiety) to 10 (extreme anxiety).
The BAT was performed in a separate room from the treatment room. During the test, the experimenter waited outside the door to minimize the impact of his/her presence. Participants were informed that the BAT was used as an objective measure of their fear, and not as part of the therapy.
Fear of Spiders Questionnaire
Items on the Fear of Spiders Questionnaire (FSQ) are designed to assess patients' anxiety about spiders. In the study,26 the instructions were changed so that participants with fear of cockroaches referred to their feared small animal (i.e., cockroaches).30 Two of the studies used the full version of the FSQ,26,28 which consists of 18 items, whereas Hoffman et al.27 used a reduced 6-item version. The same response scale, ranging from 1 (does not apply to me) to 7 (very much applies to me), was used in all cases. The FSQ has obtained excellent reliability and validity results in previous research.31 The FSQ was administered both before and after exposure treatment.
Treatment
All studies used exposure therapy for treatment, which is the recommended and evidence-based treatment for small animal phobia.32 The treatment aims at exposing participants to phobic situations in a controlled, gradual, and planned manner so that the fear is tolerated and the negative thoughts associated with the feared object and its consequences are disconfirmed. Of course, the treatment was applied differently across conditions. Specifically, when there was an iVET group,26 these participants were exposed to real animals (i.e., live spiders or cockroaches), while those in the VRET and ARET condition26,27,28 were exposed to computer-generated animals using a virtual or an augmented system, respectively. The assessment of anxiety and distance covered with the BAT was performed using live animals irrespective of treatment condition, as recommended in guidelines.29
Therapists included expert clinicians and PhD students who were trained and supervised by senior clinicians to ensure the fidelity to the treatment requirements (e.g., sessions were recorded and weekly meetings were planned). As exposed above, before and after the treatment we administered the assessment protocol described in the Measures section.
Equipment
Patients in the VRET used a Silicon Graphics Octane MXE device with Octane Channel Option coupled with a 40° vertical × 105° horizontal field of view (40° overlap). The position of the head, hand, and virtual animal was measured with the Polhemus™ Fastrak tracking system.
Augmented reality was displayed using two devices: (a) the AR 5DT Head-Mounted Display with an 800 × 600 resolution, a 40° angle of view, and an embedded NX-Ultra video camera; and (b) Vuzix VR Goggles with a 640 × 480 resolution, a 30° field of view, and a camera attached to it.
Data analysis
First, all the variables were standardized to compare scores using different scales and numbers of items. The formula used was z = (X − μ)/σ, where z is the standardized score with a mean of 0 and standard deviation of 1, X is the score that has to be converted, μ is the sample mean, and σ is the standard deviation.
Baseline imbalance was explored for all study outcomes (i.e., fear of the small animal, anxiety, and distance left to cover), to decide whether covariates would be needed in the repeated-measures analysis.33 As Table 2 shows, baseline scores were comparable across conditions, and so there was no need to control for baseline ratings in the repeated-measures analysis of variance (ANOVA). In the ANOVA, time and treatment condition were the within- and between-subject factors, respectively. Fear, anxiety, and distance were the dependent variables.
Table 2.
Sociodemographic and Clinical Characteristics of the Sample
| Virtual reality | Augmented reality | In vivo | |
|---|---|---|---|
| No. of participants | 28 | 32 | 31 |
| Age (mean; SD) | 21.46 (8.81) | 31.03 (10.08) | 32.45 (11.50) |
| Gender (percent female) | 76.9 | 90.6 | 96.8 |
| Educational status (percent universitya) | 100 | 87.5 | 80.6 |
| Duration of fear (mean; SD) | 15.04 (9.30) | 17.68 (14.50) | 18.79 (12.87) |
Note: aUniversity students or completed university studies. Duration of the phobia is in years. The database in the study of Hoffman et al.27 was incomplete (8 participants in the virtual reality condition were missing), so the final sample comprised 28 participants.
Finally, a series of two-step cluster analyses were performed to explore whether there were groups of cases in the data (i.e., groups who differed in their responses to treatment). In each analysis, the condition was included as a categorical variable. Three cluster analyses were performed, one for each outcome variable. For each outcome, pretreatment and posttreatment ratings of that outcome were included as continuous variables.
Results
The final sample consisted of 91 participants, including 32 from the ARET condition, 28 from the VRET condition, and 31 from the iVET condition.
As seen in Table 2, which presents the sociodemographic and clinical characteristics of the sample, most individuals were highly educated, young, and female.
Table 3 shows the participants' median scores for all study outcomes across conditions, along with the results of the baseline imbalance test. The Kolmogorov–Smirnov test and Levene's test revealed that assumptions of parametric data were violated (i.e., normality and homogeneity of variance). Hence, baseline imbalance across the three conditions was explored by means of a Kruskal-Wallis test, which indicated that baseline scores were comparable across conditions for all outcomes [Hdistance (2) = 1.45, p = 0.485; Hanxiety (2) = 0.78, p = 0.676; Hfear (2) = 1.84, p = 0.399], and so there was no need to use baseline scores of study outcomes as covariates in the repeated-measures ANOVA.
Table 3.
Description of Participants' Baseline and Posttreatment Scores in Study Outcomes
| Virtual reality, n = 28 | Augmented reality, n = 32 | In vivo, n = 31 | |||
|---|---|---|---|---|---|
| Mdn | Mdn | Mdn | H | p | |
| Distance pretreatment | 0.64 | 0.63 | 0.63 | 1.45 | 0.485 |
| Distance posttreatment | −0.77 | −0.67 | −0.67 | ||
| Anxiety pretreatment | 0.37 | 0.54 | 0.90 | 0.78 | 0.676 |
| Anxiety posttreatment | −0.49 | −0.19 | −0.92 | ||
| FSQ pretreatment | 1.02 | 0.80 | 0.72 | 1.84 | 0.399 |
| FSQ posttreatment | −0.79 | −0.77 | −1.01 |
Note: All variables are standardized. Lower scores is distance reflect more distance covered (less distance left to cover). H, Kruskal-Wallis test.
As Table 4 shows, the repeated-measures ANOVA revealed a significant effect of time for all study outcomes, namely, fear, F(1, 88) = 529.26, p < 0.001, anxiety, F(1, 88) = 90.96, p < 0.001, and distance, F(1, 88) = 109.33, p < 0.001. There was a decrease in fear (Mpretreatment = 0.82, Mposttreatment = −0.82), anxiety (Mpretreatment = 0.52, Mposttreatment = −0.52), and distance left to cover (Mpretreatment = 0.56, Mposttreatment = −0.56) over time. The largest change was revealed for fear (ηp2 = 0.86), although all the effects of time should be interpreted as large.
Table 4.
Repeated Measures Analysis of Variance of Fear, Anxiety, and Distance
| Outcome | Source | df | SS | F | p | ηp2 |
|---|---|---|---|---|---|---|
| Fear | Time | 1 | 122.09 | 529.26 | <0.001 | 0.86 |
| Time × condition | 2 | 0.40 | 0.88 | 0.420 | 0.02 | |
| Anxiety | Time | 1 | 49.23 | 90.96 | <0.001 | 0.51 |
| Time × condition | 2 | 2.07 | 1.92 | 0.153 | 0.04 | |
| Distance | Time | 1 | 57.86 | 109.33 | <0.001 | 0.55 |
| Time × condition | 2 | 0.44 | 0.42 | 0.661 | <0.01 |
Table 4 shows that the time × condition effect was not significant for any of the study outcomes, indicating comparable effects for VRET, ARET, and iVET.
Cluster analysis
The same three-factor solution was obtained for anxiety (condition = categorical variable; pretreatment and posttreatment anxiety = continuous variables) and fear (condition = categorical variable; pretreatment and posttreatment fear = continuous variables). Each cluster corresponded to one treatment condition (1 = ARET, n1 = 32; 2 = VRET, n2 = 28; and 3 = iVET, n3 = 31). Median pretreatment anxiety scores were Mdn1 = 0.54, Mdn2 = 0.37, and Mdn3 = 0.90. Median posttreatment anxiety ratings were Mdn1 = −0.19, Mdn2 = −0.49, and Mdn3 = −0.92. Median pretreatment fear scores were Mdn1 = 0.80, Mdn2 = 1.02, and Mdn3 = 0.72. Average posttreatment ratings for fear were Mdn1 = −0.77, Mdn2 = −0.77, and Mdn3 = −0.79.
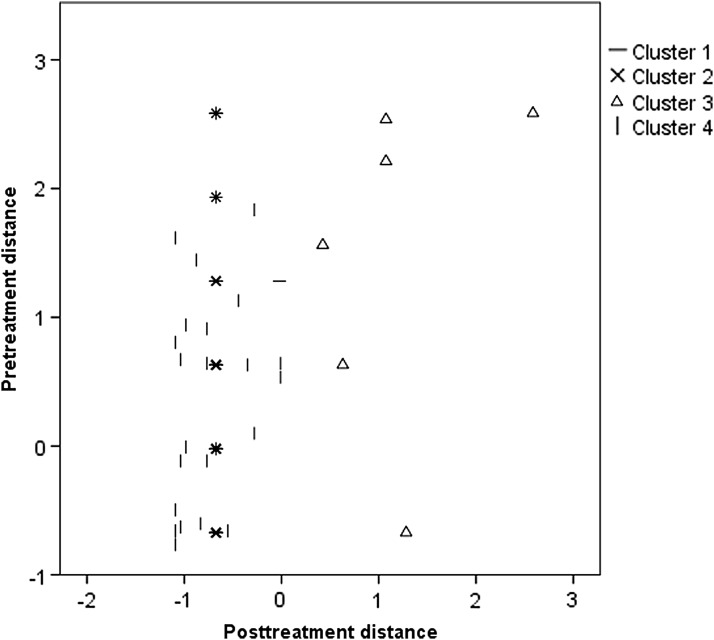
The cluster analysis for distance (condition = categorical variable; pretreatment and posttreatment distance = continuous variables) revealed a four-factor solution. Cluster 1 included all iVET participants (n1 = 31). Clusters 2 and 4 included the majority of the ARET (n2 = 29) or VRET participants (n4 = 25). The third cluster included six participants (10 percent of the total sample), three from the ARET condition and three from the VRET group. Median pretreatment distance scores for clusters 1–4 were Mdn1 = 0.63, Mdn2 = 0.63, Mdn3 = 1.89, and Mdn4 = 0.63. Median scores at posttreatment were Mdn1 = −0.68, Mdn2 = −0.67, Mdn3 = 1.07, and Mdn4 = −0.77. A graphical representation of this four-factor solution is shown in Figure 1. The graphical display suggested that the common feature of individuals in cluster 3 might be poor posttreatment performance. Indeed, the Kruskall-Wallis test revealed a difference between clusters in distance covered at posttreatment, H(3) = 31.59, p < 0.001, but not at pretreatment, H(3) = 3.63, p = 0.305. A post hoc Mann-Whitney test revealed a difference in posttreatment distance when comparing clusters 1 and 3 (U < 0.01, Z = −5.61, p < 0.001), 2 and 3 (U < 0.01, Z = −5.80, p < 0.001), and 3 and 4 (U < 0.01, Z = −3.76, p < 0.001). Effect sizes of these differences in posttreatment distance were calculated (r = Z/√n). All differences were found to be large (r13 = −0.92, r13 = −0.98, and r13 = −0.68).
FIG. 1.
Graphical representation of the four-factor cluster solution for distance.
We compared differences in baseline measures between cluster 3 and the other three clusters together (n = 85) to better understand the poorer performance in posttreatment distance covered. We did not find group differences in age (U < 223.50, Z = −0.51, p = 0.613), duration of fear (U < 195.00, Z = −0.76, p = 0.764), pretreatment anxiety (U < 246.50, Z = −0.14, p = 0.891), or pretreatment fear (U < 183.00, Z = −1.15, p = 0.249). We found a nonsignificant trend for pretreatment distance (U < 143.50, Z = −1.80, p = 0.072).
Median distances covered at pretreatment in cluster 3 (the one with poor performance on distance after treatment) and the other three clusters were 1.89 and 0.63, respectively. In our sample, a standardized distance of 1.89 should be interpreted as ∼4 m away from the feared animal (from an initial distance of 5 m), whereas a standardized distance of 0.63 reflects a distance of 2 m left to cover (from an initial distance of 5 m).
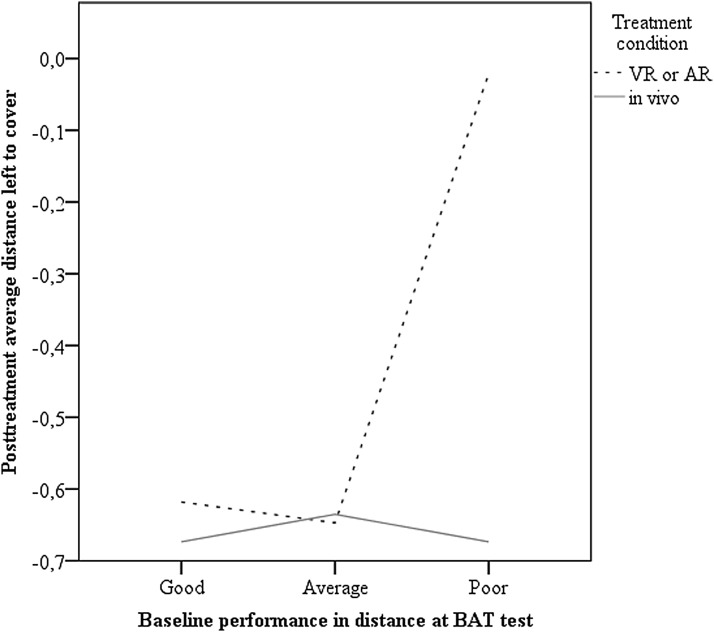
Because there were no individuals from the iVET condition in cluster 3, and we found a trend toward a significantly higher baseline distance left to cover in this cluster, we explored whether iVET was more effective than VRET and ARET for individuals with high baseline scores on distance left to cover. A multivariate regression was performed with treatment condition (1 = ARET or VRET; 2 = in vivo) in the first block, baseline distance in the second block, and the interaction between condition and baseline distance in the third block. Posttreatment distance covered was used as the dependent variable. The model explained 10.2 percent of the variance in posttreatment distance covered, and the effect of the interaction term was marginally significant, F(1) = 3.82, p = 0.054, change in R2 = 2.9 percent, B = −0.21 (−0.420 to 0.004), p = 0.054. A graphical representation of this marginally significant moderation effect is presented in Figure 2.
FIG. 2.
Graphical representation of the relationship between distance covered at baseline and posttreatment for in vivo exposure and alternative forms of exposure treatment (virtual reality and augmented reality).
No moderation effect was found when the same analysis was performed for anxiety, F(1) = 0.19, p = 0.667, change in R2 < 0.1 percent, B = −0.12 (−0.682 to 0.439), p = 0.667, and fear, F(1) = 1.61, p = 0.208, change in R2 < 0.1 percent, B = −0.38 (−0.981 to 0.217), p = 0.208.
Discussion
This study aimed to study the differential efficacy of VRET, ARET, and iVET for the treatment of small animal phobia. Research had revealed that other exposure treatments, including VRET, were good alternatives to iVET for the treatment of specific phobias,5,6,17 including small animal phobia.27,28 However, to the best of our knowledge, no study had compared the efficacy of the three treatment modalities together. This study is the first to include ARET.
As expected, the results from this study suggest that the three approaches may be equally efficacious in the treatment of behavioral (e.g., how close patients were willing to approach the feared object, namely, a cockroach or a spider, during the BAT) and psychological (i.e., anxiety during the BAT and fear) features of the disease. In our study, this similarity in the results was observed right after treatment, coinciding with the most recent meta-analysis on specific phobias.5 A previous meta-analysis only indicated the comparability of traditional iVET and alternative forms of exposure at followup.6 It is important to note, however, that alternative forms of exposure in this study included a mixture of different procedures (i.e., imaginal exposure, CAVE, and VRET), but no study on augmented reality, which might help to understand the differences found in our investigation and in the more recent meta-analysis.5
This study used cluster analysis and moderation analysis to explore whether subgroups of patients showed a differential response to the treatments. This is important because the detection of subgroups would provide evidence about how to maximize treatment effectiveness (i.e., personalized treatments) by selecting the treatment that has the most impact on each subset of individuals. Overall, our results suggest that the three treatment modalities are effective across different levels of patient characteristics, including age, fear, and anxiety during the BAT. The cluster analyses only revealed differences in the response to treatment for distance. Specifically, one group of individuals (n = 6) was found to differ from the other groups in distance covered after treatment. None of the poor-responding patients belonged to the iVET group, three received VRET treatment, and three participated in the ARET condition. Accordingly, a moderation analysis revealed a marginally significant trend toward a superior efficacy of iVET when less distance was covered in the baseline BAT. Although the implications of these results should be interpreted with caution, it is possible that iVET is more effective when individuals show worse performance on distance covered during the BAT before treatment onset. Further research should explore the reliability of this finding, which might be important in personalizing treatments, but could be problematic for institutions planning on implementing virtual or augmented reality for animal phobia treatment.
One of the strengths of the present investigation is that, after aggregating the three datasets used, the sample size was large compared to previous research. In fact, the most recent meta-analysis comparing VRET and iVET for the treatment of specific phobias revealed that the mean sample size for the 14 studies included was only 18.64 participants.5 Our investigation intended to overcome this limitation of small sample sizes by aggregating data from three studies. However, this study is not free of limitations. First, there was very low variability in age and sex, and so generalization of the results to men and older samples should be done with caution. However, it is important to note that small animal phobia is more frequent in women and younger adults, so that these study findings may be relevant for a large percentage of patients. In addition, although cluster analyses are useful tools to identify groups of cases with a shared characteristic, it is also true that they are atheoretical, and their solution is not generalizable because it depends on the variables used. Hence, the results of our cluster analyses should be considered exploratory and should be interpreted with caution. Thus, replication is needed. Also, while standardization of all variables was required due to the use of different scales and numbers of items, this might have made the results difficult to interpret. Using a homogeneous set of measures will be important in future replication studies. Finally, although the aggregation of studies led to a considerable sample size, even larger sample sizes are needed in future studies to explore whether certain treatments are more effective for a subset of patients, which is a key to personalizing psychological treatments. For example, our cluster and moderation analyses did not reveal different subgroups of patients as a function of baseline and posttreatment anxiety and fear. However, larger sample sizes with greater variability, for example in sex, could test different moderators and grouping variables.
Despite these shortcomings, our study findings may have important clinical implications because they support previous literature indicating that VRET and ARET are useful alternatives to traditional iVET treatments for small animal phobia. Considering that iVET treatments are more frequently refused by patients than VRET,11 these findings are important because they provide clinicians with two equally efficacious alternatives to iVET.
It is important to note, however, that the implementation of VRET and ARET in clinical practice is still difficult. Research has revealed some resistance to implementing new digital technologies in clinical practice.34 Moreover, virtual reality devices have traditionally been expensive, which might have restricted their use. Fortunately, this situation is already starting to change, and new trials are being carried out with inexpensive commercial devices.35 It is important to make virtual reality devices accessible in terms of ease of use and price if their benefits are to be transferred from research to clinical practice. In this regard, it is likely that bigger sample sizes will be recruited for future studies, and, thus, it will be easier to establish differential responses to VRET and ARET. In fact, as exposed previously, the reduced costs of augmented reality when compared with virtual reality (i.e., less elements need to be created) might help achieve this goal.7 The remaining benefits of using ARET as opposed to VRET (e.g., the former is argued to be more ecological), however, did not result in greater treatment efficacy in the present investigation, so further research is required.
Acknowledgments
This study was partially supported by two governmental grants: PROMETEOII/2013/003 (Conselleria de Educación, Cultura y Deporte; Generalitat Valenciana) and PSI2014-54172-R (Ministerio de Economía y Competitividad; Gobierno de España). The European Union also partially supported the elaboration of this work under the Marie Sklodowska-Curie grant agreement number 722022 (Horizon 2020). CIBERobn is a initiative of ISCIII.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Becker ES, Rinck M, Türke V, et al. . Epidemiology of specific phobia subtypes: findings from the Dresden Mental Health Study. European Psychiatry 2007; 22:69–74 [DOI] [PubMed] [Google Scholar]
- 2. Curtis GC, Magee WJ, Eaton WW, et al. . Specific fears and phobias. Epidemiology and classification. British Journal of Psychiatry 1998; 173:212–217 [PubMed] [Google Scholar]
- 3. Depla MFIA, ten Have ML, van Balkom AJLM, et al. . Specific fears and phobias in the general population: results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Social Psychiatry and Psychiatric Epidemiology 2008; 43:200–208 [DOI] [PubMed] [Google Scholar]
- 4. Fredrikson M, Annas P, Fischer H, et al. . Gender and age differences in the prevalence of specific fears and phobias. Behaviour Research and Therapy 1996; 34:33–39 [DOI] [PubMed] [Google Scholar]
- 5. Morina N, Ijntema H, Meyerbröker K, Emmelkamp PMG. Can virtual reality exposure therapy gains be generalized to real-life? A meta-analysis of studies applying behavioral assessments. Behaviour Research and Therapy 2015; 74:18–24 [DOI] [PubMed] [Google Scholar]
- 6. Wolitzky-Taylor KB, Horowitz JD, Powers MB, et al. . Psychological approaches in the treatment of specific phobias: a meta-analysis. Clinical Psychology Review 2008; 28:1021–1037 [DOI] [PubMed] [Google Scholar]
- 7. Baus O, Bouchard S. Moving from virtual reality exposure-based therapy to augmented reality exposure-based therapy: a review. Frontiers in Human Neuroscience 2014; 8:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chicchi Giglioli IA, Pallavicini F, Pedroli E, et al. . Augmented reality: a brand new challenge for the assessment and treatment of psychological disorders. Computational and Mathematical Methods in Medicine 2015; 2015:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becker C, Zayfert C, Anderson E. A survey of psychologists' attitudes towards and utilization of exposure therapy for PTSD. Behaviour Research and Therapy 2004; 42:277–292 [DOI] [PubMed] [Google Scholar]
- 10. Olatunji BO, Deacon BJ, Abramowitz JS. The cruelest cure? Ethical issues in the implementation of exposure-based treatments. Cognitive and Behavioral Practice 2009; 16:172–180 [Google Scholar]
- 11. García-Palacios A, Botella C, Hoffman H, et al. . Comparing acceptance and refusal rates of virtual reality exposure vs. in vivo exposure by patients with specific phobias. CyberPsychology & Behavior 2007; 10:722–724 [DOI] [PubMed] [Google Scholar]
- 12. Maples-Keller JL, Bunnell BE, Kim S-J, et al. . The use of virtual reality technology in the treatment of anxiety and other psychiatric disorders. Harvard Review of Psychiatry 2017; 25:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valmaggia LR, Latif L, Matthew J, et al. . Virtual reality in the psychological treatment for mental health problems: an systematic review of recent evidence. Psychiatry Research 2016; 236:189–195 [DOI] [PubMed] [Google Scholar]
- 14. Turner WA, Casey LM. Outcomes associated with virtual reality in psychological interventions: where are we now? Clinical Psychology Review 2014; 34:634–644 [DOI] [PubMed] [Google Scholar]
- 15. Rizzo A, Cukor J, Gerardi M, et al. . Virtual reality exposure for PTSD due to military combat and terrorist attacks. Journal of Contemporary Psychotherapy 2015; 45:1–10 [Google Scholar]
- 16. Botella C, Serrano B, Baños R, et al. . Virtual reality exposure-based therapy for the treatment of post-traumatic stress disorder: a review of its efficacy, the adequacy of the treatment protocol, and its acceptability. Neuropsychiatric Disease and Treatment 2015; 11:2533–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Opris D, Pintea S, García-Palacios A, et al. . Virtual reality exposure therapy in anxiety disorders: a quantitative meta-analysis. Depression and Anxiety 2012; 93:85–93 [DOI] [PubMed] [Google Scholar]
- 18. Parsons TD, Rizzo AA. Affective outcomes of virtual reality exposure therapy for anxiety and specific phobias: a meta-analysis. Journal of Behavior Therapy and Experimental Psychiatry 2008; 39:250–261 [DOI] [PubMed] [Google Scholar]
- 19. Powers MB, Emmelkamp PMG. Virtual reality exposure therapy for anxiety disorders: a meta-analysis. Journal of Anxiety Disorders 2008; 22:561–569 [DOI] [PubMed] [Google Scholar]
- 20. Riva G, Baños RM, Botella C, et al. . Transforming experience: the potential of augmented reality and virtual reality for enhancing personal and clinical change. Frontiers in Psychiatry 2016; 7:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Botella C, Fernández-Álvarez J, Guillén V, et al. . Recent progress in virtual reality exposure therapy for phobias: a systematic review. Current Psychiatry Reports 2017; 19:42. [DOI] [PubMed] [Google Scholar]
- 22. Botella CM, Juan MC, Baños RM, et al. . Mixing realities? An application of augmented reality for the treatment of cockroach phobia. CyberPsychology & Behavior 2005; 8:162–171 [DOI] [PubMed] [Google Scholar]
- 23. Juan MC, Alcaniz M, Monserrat C, et al. . Using augmented reality to treat phobias. IEEE Computer Graphics and Applications 2005; 25:31–37 [DOI] [PubMed] [Google Scholar]
- 24. Wrzesien M, Botella C, Bretón-López J, et al. . Treating small animal phobias using a projective-augmented reality system: a single-case study. Computers in Human Behavior 2015; 49:343–353 [Google Scholar]
- 25. Botella C, Bretón-López J, Quero S, et al. . Treating cockroach phobia with augmented reality. Behavior Therapy 2010; 41:401–413 [DOI] [PubMed] [Google Scholar]
- 26. Botella C, Pérez-Ara MÁ, Bretón-López J, et al. . In vivo versus augmented reality exposure in the treatment of small animal phobia: a randomized controlled trial. PLoS One 2016; 11:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoffman HG, Garcia-Palacios A, Carlin A, et al. . Interfaces that heal: coupling real and virtual objects to treat spider phobia. International Journal of Human-Computer Interaction 2003; 16:283–300 [Google Scholar]
- 28. García-Palacios A, Hoffman H, Carlin A, et al. . Virtual reality in the treatment of spider phobia: a controlled study. Behaviour Research and Therapy 2002; 40:983–993 [DOI] [PubMed] [Google Scholar]
- 29. Öst L-G, Salkovskis PM, Hellström K. One-session therapist-directed exposure vs. self-exposure in the treatment of spider phobia. Behavior Therapy 1991; 22:407–422 [DOI] [PubMed] [Google Scholar]
- 30. Szymanski J, O'Donohue W. Fear of Spiders Questionnaire. Journal of Behavior Therapy and Experimental Psychiatry 1995; 26:31–34 [DOI] [PubMed] [Google Scholar]
- 31. Muris P, Merckelbach H. A comparison of two spider fear questionnaires. Journal of Behavior Therapy and Experimental Psychiatry 1996; 27:241–244 [DOI] [PubMed] [Google Scholar]
- 32. Nathan PE, Gorman JM. (2007) A guide to treatments that work. 3rd ed. New York, NY: Oxford University Press [Google Scholar]
- 33. Egbewale BE, Lewis M, Sim J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: a simulation study. BMC Medical Research Methodology 2014; 14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dauphin B. Therapists' resistance to understanding the importance of technology for child and adolescent psychotherapy. Journal of Infant, Child, and Adolescent Psychotherapy 2013; 12:45–50 [Google Scholar]
- 35. Powers MB, Carlbring P. Technology: bridging the gap from research to practice. Cognitive Behaviour Therapy 2016; 45:1–4 [DOI] [PubMed] [Google Scholar]