Abstract
Azoxystrobin is a strobilurin of growing concern in aquatic environments because it is the most sold fungicide worldwide, however, the information available about its effect on aquatic non-target organisms is scarce. The objective of the present study was to evaluate potential physiological, biochemical, and genetic effects at environmentally relevant (1–10 μg/L) and elevated (100–500 μg/L) concentrations in the aquatic macrophyte Myriophyllum quitense exposed to the commercial formulation AMISTAR®. Following an acute 24-h exposure, there were no effects of AMISTAR® on photosynthetic pigments at any of the concentrations evaluated. Glutathione-S-transferase activity was significantly elevated at 1 and 10 μg/L AZX. Significant decrease of catalase and guaiacol peroxidase activities in plants exposed to 500 μg/L, and to 100 and 500 μg/L, respectively, and an increase in glycolate oxidase activity at 500 μg/L was observed. DNA damage at 100 and 500 μg/L was observed. These data indicate that although environmentally relevant levels of AMISTAR® did not result cytotoxic, this fungicide was genotoxic, affecting the physiological process of photorespiration and caused oxidative damage at high concentrations. In this sense, it is necessary to explore sub-lethal responses in non-target organisms because some effects could promote further potential long-term biological consequences in a context of repeated pulses of exposure.
Keywords: Azoxystrobin, Myriophyllum quitense, Antioxidant enzymes, “Comet” assay, Chlorophyll
Introduction
Globally, agricultural producers apply around USD 40 billion worth of pesticides per annum (Popp et al. 2013). Consequently, their presence in the environment has grown in the past few years and has become an intensive and burning issue of discussion (Ghosh and Singh 2009). Particularly, the consumption of agrochemicals in Argentina increased from 73 to 236 million kg per year over the last 10 years (De Gerónimo et al. 2014). Fungicides are widely applied mostly in arable crops, which are more sensitive to fungal infections (Hocinat and Boudemagh 2016). The strobilurins are an important class of agricultural fungicides (Bartlett et al. 2001). Azoxystrobin (AZX) was the first strobilurin compound launched in the market in 1996 under the commercial name of AMISTAR® (Bartlett et al. 2002). The fungicidal activity is generated by binding Q0 site of cytochrome b blocking the electron transfer (Huang et al. 2007). Nowadays AZX is marketed in 100 countries and applied for approximately 120 crops (SYNGENTA 2017). According to international authorities, it is the principal fungicide sold in the world (Royal Society of Chemistry 2017). The information available about AZX concentration in water bodies is scarce. Concentrations from 0.06 µg/L in streams and ponds from the US (Reilly et al. 2012) to 29.7 µg/L in streams from Germany (Berenzen et al. 2005) have been reported. In addition, Edwards et al. (2016) showed the horizontal movement with agricultural runoff as vector for transport of AZX, which increases the likelihood of exposure to non-target aquatic species. Therefore, the increase in the volume applied due to the resistance of fungi and its massive use worldwide suggest that the detections of AZX in waterbodies near agricultural fields will be more frequent in the future. In 2010, the European Food Safety Authority (EFSA) determined a value of 3.3 μg/L as the regulatory acceptable concentration (RAC) (Rodrigues et al. 2013). However, recent data suggest a Maximum Acceptable Concentration-Environmental Quality Standard (MAC-EQS) of 1.8 µg/L as a limit value protective in acute exposure (Rodrigues et al. 2017). It is well recognized that there is a gap in the ecotoxicological information concerning fungicides in the aquatic environment and these fact limits result interpretations (Battaglin et al. 2011). In this sense, the impact on non-target organisms can be toxic, but it is not fully known and can lead to ecosystem alterations (Deb et al. 2010). Fungicides, like most of the pesticides, are always used with emulsifiers, carrying solvents, and other additives. Accordingly, other authors highlighted about the higher potency of commercial formulations than their corresponding active ingredients for several pesticides (Pereira et al. 2009).
Effects of AZX have been detected in terrestrial plants. In long-term exposures, a delay in senescence in winter wheat (Triticum aestivum) have been observed (Wu and von Tiedemann 2001; Zhang et al. 2010). This fact is an advantage from the agronomic point of view because it enhances the grain yield of wheat. Although it could be considered a good characteristic to producers, non-target organisms that occur in natural water bodies could suffer negative effects. Indeed, perturbation of ascorbate synthesis, fat acid metabolism, and RNA translation have been recently demonstrated in the green algae Chlorella pyrenoidosa exposed to 0.5–10 mg/L AZX for 10 days, suggesting that AZX inhibits algal cell growth through multiple pathways (Lu et al. 2018). On the other hand, AZX also affects mitochondrial respiration and mechanisms controlling cell growth and proliferation in fish (Olsvik et al. 2010). Adult zebrafish (Danio rerio) showed reduced egg production and fertilization rates, as well as other reproductive effects during exposures at 200 μg/L AZX for 21 days (Cao et al. 2016). Toxic effects of AZX on brackish water copepods has also been described, and these effect could trigger a cascade effect affecting ecosystem functioning e.g. indirect effects on phytoplankton community composition (Gustafsson et al. 2010).
Aquatic macrophytes play a key role in aquatic ecosystems as primary producers, shelter, oxygen contributor, sediment stabilizer, and potential detoxifier. In order to maintain ecosystem structure and functions, the aquatic macrophytes, as other aquatic organisms, must be protected from adverse chemical effects (Arts et al. 2010). Macrophytes could be employed as biomonitor organisms, because they are easy to see and identify in the field, sedentary and, depending on the pollutant characteristics, they concentrate it in their tissues reflecting the environmental contamination (Nimptsch et al. 2005). In 2008, a panel of experts from the Society of Environmental Toxicology and Chemistry (SETAC)—The Aquatic Macrophyte Ecotoxicology Group (AMEG)—published guidance and recommendations to evaluate different substances in aquatic macrophyte risk assessment. They proposed Myriophyllum as a recommended genus because of the considerable knowledge and experience that researchers have about it. Indeed, the Organization for Economic Cooperation and Development (OECD) adopted M. spicatum in the guideline TG 239 (2014) as new test system for rooted aquatic plants (OECD 2014). This genus has been used to evaluate the toxicity of different substances such as chlorodifluoroacetic acid (Popp et al. 2013), Cd, Cu (Ngayila et al. 2009), Ni, Pb, Zn (Nimptsch et al. 2005), ammonia (Nimptsch and Pflugmacher 2007) and the insecticide endosulfan (Menone et al. 2008).
The xenobiotic stress in plants is manifested firstly at the biochemical level, before its manifestation at organism level, therefore this biochemical effects can work as early warning systems (Brain and Cedergreen 2009). For this reason, these type of biomarkers were chosen in the present study to evaluate the potential sub-lethal effects of AMISTAR® in acute bioassays. Particularly, in ecotoxicological studies, the oxidative stress caused by oxiradicals and the potential cellular oxidative damage is widely applied to determine effects of several xenobiotics (Valavanidis et al. 2006). Exposure to some xenobiotics may result in decrease of antioxidant defenses, leading to tissue damage, and different processes that ultimately cause cellular aging and diseases (Sohal et al. 2002), moreover the nuclear DNA is sensitive to oxidative damage by ROS. Genetic alterations can have a number of immediate effects upon the cells involved, including cell death or transformation into malignancy (Bickham et al. 2000). The most advantage method to measure DNA strand breaks is the “Comet assay”. The DNA damage is measured quantitatively by single-cell gel electrophoresis technique which is widely applied because of its rapidity and sensitivity in aquatic organisms, although scarcely applied in plant tissues (Valavanidis et al. 2006).
Considering that there is not enough available data on the effects of AZX commercial formulations on non-target aquatic organisms, its increasing application worldwide and the sensitivity of Myriophyllum, the objective of the present study was to evaluate potential physiological, biochemical and genetic effects at environmentally relevant (1–10 μg/L) and elevated (100–500 μg/L) concentrations in the aquatic macrophyte M. quitense exposed to the commercial formulation AMISTAR®.
Materials and methods
Plant material and exposure conditions
Myriophyllum quitense plants were obtained from a natural reservoir, placed in the Estación Experimental Agropecuaria Balcarce (INTA), Argentina. Species identification was done according to Orchard (1981). Plants were acclimated for 15 days in 30 L tanks, the light was provided by fluorescence lamps with an irradiance of 8000 lux at a light/dark cycle of 12:12 h. Room temperature was maintained at 20–22 °C, and the plants were submerged in Hoagland’s medium (pH 5) (Arts et al. 2010).
Two experiments were carried out to evaluate oxidative biomarkers and genotoxicity, respectively. Four concentrations of AZX were evaluated, two environmental concentrations, and two concentrations that exceed the actual environmental values, but would be expected if the consumption of AZX continues increasing. Plants of M. quitense (six independent replicates per treatment, n = 6) were exposed to AMISTAR® Syngenta® equivalent to 0 (Co-), 1, 10, 100 and 500 μg/L of AZX, in a volume of 350 mL each, for 24 h, under constant light and temperature conditions. AMISTAR® was dissolved in bidestillated water in a stock solution of 2000 mg/L. Each exposure concentration was prepared by diluting the appropriate amount of AMISTAR® in Hoagland medium to a final volume of 350 mL. In the negative control treatment (Co-), AMISTAR® was omitted from the medium. One positive mutagenic control (Co +) consisting of 7.14 g/L of hydrogen peroxide was also included. After exposures, plants were frozen with liquid N2 before being stored at − 80 °C until processing for analyzing enzymes activities, H2O2 and MDA contents, or directly submerged in extraction buffer for the “Comet” assay.
Azoxystrobin quantification
Water samples (250 ml) were extracted with 20 ml chloroform. 50 μl of 5 ppm 1-bromo-2-nitrobenzene in toluene was added as internal standard. The method was based on extraction with chloroform, followed by a clean-up with QuEChErs (AOAC Methods 2003, 2007). Sample preparation, based on QuEChErs method, combined a liquid–liquid extraction with acetonitrile and a dispersive-SPE clean-up. The organic phase was transferred into a beaker (250 mL) and evaporated under nitrogen stream at 40 °C ± 2 °C in a water bath to a volume of about 0.3–0.5 ml. The residue was taken up with toluene to a volume of about 1 ml and transferred to a chromatography vial refrigerated until use.
Chromatographic conditions: An Agilent 7890 chromatograph was used for the chromatographic run, associated with a 5975 Series Mass Selective Detector (MSD). Oven temperatures were 70 °C (2 min), 25 °C up to 150 °C (0 min), 3 °C/min up to 200 (0 min), and 8 °C/min up to 280 °C (10 min). Injection was in pulsed splitless mode with an automatic injector. Helium was used as carrier gas. The mass detector was used in SIM Mode, Source, Quadrupole, Temperature of Transfer Line mode: 230, 150, and 280, respectively, Solvent delay: 5.00 min, Multiplier voltage: Gain = 25. After the run was completed, AZX was quantified with the chromatograph (Agilent G1701 EA GC/MSD ChemStation) software. An analyte recovery percentage between 82 and 110% was obtained with a coefficient of variation ≤ 14%. The detection limits were generally 10 ng/L. To evaluate the method performance, calibration curves at concentrations of 10, 20, 50, 100, 200 ng/L were constructed. Injections of the standards were repeated at concentrations of 50 ng/L in matrix to assure system stability. The limits of detection and quantification were 1 μg/L (signal-to-noise ratio > 3).
Battery of biomarkers
Chlorophyll content
Chlorophyll content was measured according to Inskeep and Bloom (1985). Leaf samples of 0.2 g each were put into vials containing 2 ml of N, N-dimethylformamide (DMF). The vials were kept closed for 72 h, at 4 °C in darkness until the complete loss of green color. The absorbance values of the supernatant were recorded at 647 and 664 nm using a spectrophotometer. For calculation of quantitative values of pigments, the following equations were applied:
Chl a = (12.70A664.5 − 2.79A647)/g Fw−1
Chl b = (20.70A647 − 4.62A664.5)/g Fw−1
Total Chl = (17.90A647 + 8.08A664.5)/g Fw−1
Oxidative stress biomarkers
Preparation of guaiacol peroxidase (POD), glutathione-S-transferase (GST) and catalase (CAT) enzymes was performed according to Pflugmacher (2004). Approximately 10 g fresh weight of plant leaves were homogenized with sodium-phosphate buffer 0.1 M (pH 6.5), containing glycerol 20%, dithioerythritol (DTE) 14 mM and ethylenediaminetetraacetic acid (EDTA) 1 mM. The homogenate was centrifuged at 10,000 g for 10 min. The enzyme glycolate oxidase (GOX) was extracted with a buffer containing sodium-phosphate buffer 100 mM (pH 6.8), with EDTA 1 mM, BSA 0.1% and Triton 0.1%. Protein determination was done according to Bradford (1976) using bovine serum albumin (BSA) as the standard. Enzyme activity was calculated in nanokatals per milligram of protein from percent substrate conversion. Measurement of the POD activity using guajacol as the substrate was performed as described by Drotar et al. (1985). Determination of GST activity with the model substrate 1-chloro-2, 4-dinitrobenzene (CDNB) was done according to Habig et al. (1974). CAT activity was assayed according to Claiborne (1985). GOX activity was measured following the method of Archer and Ting (1996). All measurements were made in duplicate.
H2O2 content
The level of cell internal H2O2 was determined calorimetrically by monitoring the formation of titanium peroxide according to Jana and Choudhuri (1982). H2O2 was extracted by homogenizing 0.4 g of leaf tissue with 3 ml of 50 mM sodium phosphate buffer (pH 7.0). The homogenate was spun down at 10,000 g for 2 min at 4 °C and the transparent supernatant was collected. 750 μl of supernatant was mixed with 250 μl of 0.1% titanium sulphate (TiSO4) in 20% (v/v) sulfuric acid (H2SO4). Then, the intensity of red–orange colour of the mixture was measured at 410 nm after 1 min. All measurements were made in triplicate with each extract from 6 independent replicates. The content of H2O2 was calculated using the extinction coefficient 0.28 l/mmol cm and expressed as μmol g/FW.
Lipid peroxidation
Malondialdehyde (MDA), the principal product of polyunsaturated fatty acid peroxidation, was evaluated according to Shi et al. (2006). A leaf sample of 0.3 g was homogenized in 3 mL of trichloroacetic acid (TCA) 0.1% (w/v), centrifuged at 10,000 g for 10 min, and 3 mL of TCA 20% containing thiobarbituric acid (TBA) 0.5% (w/v) was added to 1 mL of supernatant. The mixture was heated at 95º C for 30 min, placed into an ice-bath and centrifuged at 10,000g for 10 min, and the absorbance of the supernatant was read at 532 and 600 nm. After subtracting the non-specific absorbance at 600 nm, the TBARS concentration was calculated by its extinction coefficient of 155 mM/cm.
DNA fragmentation
The isolation of nucleoids and the alkaline “Comet” assay was made according to Garanzini and Menone (2015). Briefly, the isolation was made through a sucrose cushion and the electrophoresis was run in denaturing solution at 0.72 V/cm for 30 min. Gels were silver stained according to Nadin et al. (2001) for optic microscope observation using a microscope OLYMPUS CX31. The quantification of the level of DNA damage was made by measuring the relative length of the tail of the “Comet” in 200 nuclei per sample. Where each nucleoid was classified into five classes according to tail size (from undamaged, class 0, to maximally damaged, class 4), resulting in a single DNA damage score (Damage Index, DI) for each slide. The DI was calculated as follows: DI = n1 + 2n2 + 3n3 + 4n4; where n1, n2, n3 and n4 are the number of cells in class 1, 2, 3 and 4 of damage respectively according to Poletta et al. (2009).
Statistical analysis
Normality and homogeneity of variances were verified by Shapiro–Wilk and Levene tests, respectively. Photosynthetic pigment data showed normality, therefore a one-way ANOVA test was applied followed by the post hoc Dunnett´s test. For the other parameters, the median was used as the measure of central tendency, and non-parametric tests were applied, because data did not fit with a normal distribution and variance homogeneity. Kruskal–Wallis and, a posteriori, Dunn test for testing differences among treatments were applied (Zar 1999). In all cases, statistical analyses were determined at a 0.05% significance level. A canonical discriminant analysis of principal coordinates (CAP) using 9999 permutations (Anderson and Robinson 2003, Anderson and Willis 2003) were performed in order to determine to what extent the set of 11 variables can actually explain treatment differences. For this, Euclidean distances was applied (Anderson and Willis 2003).
Results
Azoxystrobin quantification
Analytical measurements of AZX were carried out to corroborate the expected concentration according to the nominal concentrations in AMISTAR®. Measured concentrations of 1.1, 9.8, 99.7 and 503.2 µg/L AZX corresponding to nominal concentrations of 1, 10, 100 and 500 µg/L AZX were obtained.
Battery of biomarkers
Chorophyll content
None of the concentrations evaluated exerted effects in total chlorophyll, chlorophyll a, chlorophyll b and chlorophyll a/b ratio as none differed significantly from the control (p > 0.05) (Table 1).
Table 1.
Photosynthetic parameters (mean ± SD) in the aquatic macrophyte Myriophyllum quitense exposed to AMISTAR®
| AZX Concentrations (μg/L) | |||||
|---|---|---|---|---|---|
| Co− | 1 | 10 | 100 | 500 | |
| Chl a (mg/g Fw−1) | 430.1 ± 376.0 | 468.7 ± 118.3 | 696.1 ± 103.2 | 595.0 ± 122.5 | 526.1 ± 130.6 |
| Chl b (mg/g Fw−1) | 2226 ± 873.2 | 1812 ± 687.8 | 1575 ± 231.2 | 1499 ± 134.3 | 1443 ± 604.2 |
| Total Chl (mg/g Fw−1) | 2691 ± 637.8 | 2291 ± 608.6 | 1978 ± 638.2 | 2071 ± 133.6 | 1979 ± 519.6 |
| Ratio Chl a/b | 0.16 ± 0.13 | 0.31 ± 0.19 | 0.45 ± 0.02 | 0.36 ± 0.01 | 0.46 ± 0.31 |
Biochemical biomarkers
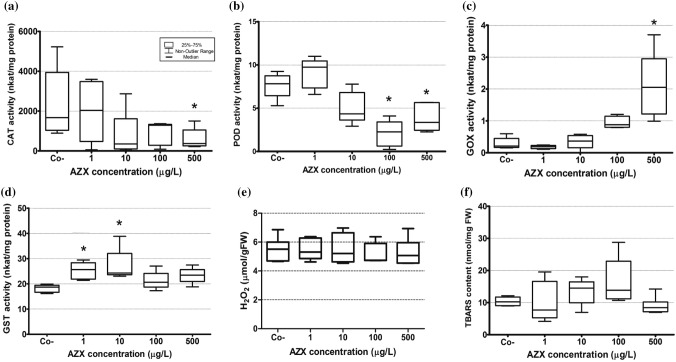
The activity of GST increased in plants exposed at 1 and 10 μg/L (p < 0.05) (Fig. 1 D). The antioxidant enzymes CAT and POD showed a decrease in comparison to controls at 500 μg/L, and at 100 and 500 μg/L, respectively (p < 0.05) (Fig. 1a, b). The activity of GOX was increased compared to controls at 500 μg/L (p < 0.05) (Fig. 1c). Levels of H2O2 as well as lipid peroxidation measured as TBARS were not different in plants exposed to AMISTAR® with respect to control plants (p > 0.05) (Fig. 1e, f).
Fig. 1.
Oxidative stress parameters in Myriophyllum quitense exposed to AMISTAR® (μg/L AZX). Co−: negative control. a Catalase (CAT) activity; b guaiacol peroxidase activity (POD) activity; c glycolate oxidase (GOX) activity; d glutathione-S-transferase (GST) activity; e H2O2 content (mmol/gFW) and f lipid peroxidation as TBARs content (nmol/mgFW). *Significantly different from the control (Kruskall–Wallis with post hoc Dunn’s test) (p < 0.05). In all cases, box limits show 25–75 percentiles and whiskers represent the non-outlier range
DNA fragmentation
Figure 2 shows a significant increment of DNA fragmentation in plants exposed to the positive control with respect to the negative control. Increased DNA damage was observed at 100 and 500 μg/L compared to the negative controls (p < 0.05).
Fig. 2.
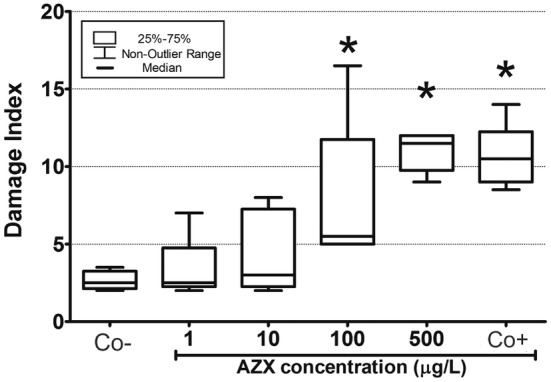
DNA fragmentation measured through the alkaline “Comet” assay in the aquatic macrophyte Myriophyllum quitense exposed to AMISTAR®. Co−: negative control, CO+: positive control (7.14 g/L H2O2). *Significantly different from the control (Kruskall–Wallis with post hoc Dunn’s test) (p < 0.05). In all cases, box limits show 25–75 percentiles and whiskers represent the non- outlier range
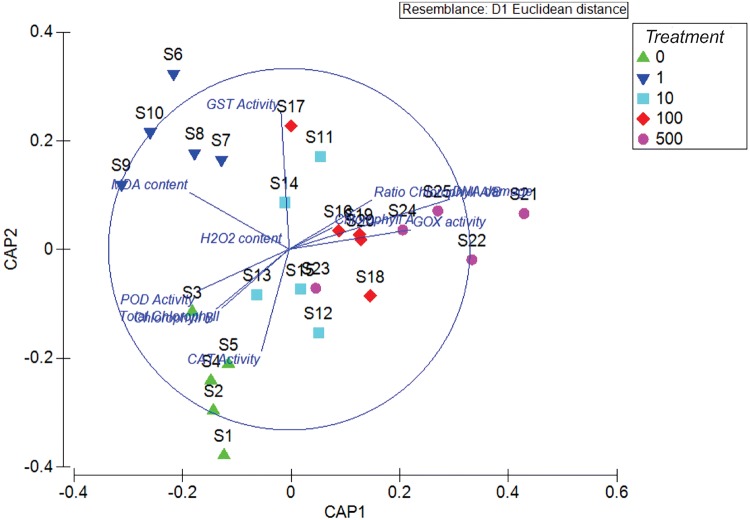
Canonical discriminant analysis of principal coordinates (CAP)
The CAP analysis showed the same patterns of response observed in the individual statistical analysis. The plot produced by visualizing the values obtained on the new canonical axes (Fig. 3) graphically separated the different treatments, mainly controls from plants exposed to AMISTAR ®. The cross validation of allocation of observations to groups showed a value of 80% (p < 0.0001).
Fig. 3.
Canonical discriminant analysis of principal coordinates (CAP) indicating effects of AMISTAR® in the aquatic macrophyte Myriophyllum quitense. Treatments: 0 = negative control, 1 = 1 μg/L AZX, 10 = 10 μg/L AZX, 100 = 100 μg/L AZX and 500 = 500 μg/L AZX
Discussion
Azoxystrobin quantification
The AZX measured concentrations were highly concordant with the nominal concentrations. These results are in agreement with Singh et al. (2010) as they found that this fungicide is hydrolytically fairly stable between pH 4 and 9.
Battery of biomarkers
The results indicated that the fungicide AMISTAR® did not disturb the photosynthetic pigments at any of the concentrations evaluated, from environmentally low to high. Kozlowski et al. (2009) also found no differences in chlorophyll content in the terrestrial plant Phaseolus vulgaris exposed to AMISTAR® during 21 days. Indeed, previous studies, for other fungicides like tebuconazole have shown the same results, in the green algae Pseudokirchneriella subcapitata and Nannochloris oculata (Martinez et al. 2015). In all cases, this lack of response was interpreted as a non-cytotoxic effect. On the other hand, a decrease in the net photosynthetic rate, measured through the gas exchange and intercellular carbon dioxide concentration, in Triticum aestivum L., Hordeum vulgare L. and Glycine max exposed to the individual active ingredient AZX for at least 24 h have been detected (Nason et al. 2007). This disagreement with our data could be due to excipients of the commercial formulation or to differences among species.
GST activity is sensitive to acute exposures to xenobiotics (< 24 hs), and this response allows its application as an early warning biomarker (Brain and Cedergreen 2009). This enzyme system could be important in a protective role catalyzing the conjugation and detoxification of many types of pesticides. For example, conjugation of herbicides with reduced glutathione (7-glutamyl cysteinyl glycine, GSH) is an important and irreversible mode of detoxification (Cole 1994). However, in addition to its detoxifying function in plants and animals (conjugation with gluthation), GSTs conjugate GSH with a variety of electrophilic substrates to produce less toxic forms, which results in their detoxification, and reduce oxidative stress (Marrs 1996; Liu et al. 2015). The latter may be the process that takes place in M. quitense exposed to AMISTAR® where the GST activity increased in plants exposed to environmentally relevant concentrations. Taking into account that the most abundant metabolite of AZX in plants is the product of the ether bond cleavage, cyanophenoxy-pyrimidinol, which is readily converted as N-glucoside conjugate (Balba 2007); the response in GST observed in M. quitense could be related to its role as an antioxidant enzyme by conjugating membrane intermediate products of lipid peroxidation in a direct manner (Marrs 1996; Cummins et al. 1999) instead to biotransformation.
The decrease in CAT activity observed could indicate oxidative damage, as proposed by Kono and Fridovich (1982) who observed that CAT activity may decrease when there is an excess of radical superoxide anion. Our results were obtained immediately after an exposure of 24 h, simulating a runoff event; but in long-term exposures, increase of these enzymes in plants of Tritricum aestivum after 37–80 days (Wu and von Tiedemann 2001; Zhang et al. 2010); and in the algae Chlorella pyrenoidosa after 10 days (Lu et al. 2018) was observed. A peak of H2O2 would be expected when peroxidases activities were decreased, however no changes in this biomarker were found. Our results are in agreement with data reported by Liang et al. (2016) in ginseng stem (Panax ginseng) exposed to AZX.
The activity of GOX generates H2O2 as a result of the transformation of glycolate to glyoxylate in the process of photorespiration (McCarthy et al. 2001). AMISTAR® would deregulate the photorespiration process evidenced by the increased activity observed in this enzyme, that may generate H2O2 which could contribute to its total content (Jana and Choudhuri 1982). However, no changes in H2O2 observed would indicate that it might be confined to glioxisomes where it was quickly detoxified.
DNA fragmentation
It has been demonstrated that ROS induce oxidation of DNA, breakage of DNA chains, and other deleterious changes of the nucleotide bases (Cooke et al. 2003). Han et al. (2014) observed that an excess of ROS was likely a cause of DNA damage observed in the earthworm Eisenia fetida, especially when the animals were exposed to 0.1 mg AZX/Kg soil for 7 days. The same authors observed increased DNA damage levels in zebrafish (Danio rerio) livers, reporting DNA strand breaks under exposure to AZX in a concentration and exposure time dependent manner (Han et al. 2016).
Integration of biomarkers
The significant inhibition of CAT and POD, as well as the increased DNA damage observed at high concentrations of AMISTAR® coincided with the breakdown of the GST enzyme at 100 and 500 μg/L, indicating an acute stress condition. Exposure to AMISTAR® may produce oxidative damage in proteins and DNA. The results obtained only showed GST as an early warning signal, since it was the only biomarker that responded at low concentrations of AMISTAR®.
The canonical discriminant analysis of principal coordinates allowed to correlate all the effects observed in the different biomarkers evaluated. This analysis showed the integrated responses in plants exposed to AMISTAR®, where plants unexposed to AMISTAR® were significantly different to the exposed plants in the two principal axes evaluated. In addition, the different levels of exposition generated different responses in the biomarkers, while plants exposed to low concentrations showed mainly differences in GST activity, plants exposed to high concentrations behaved similarly between them, particularly in DNA damage and GOX activity. Overall, the discriminant analysis allowed the integration of all biomarkers used, and helped us to identify the oxidative stress effect exerted by AMISTAR® on M. quitense, more difficult to understand when individual biomarkers were analyzed.
In the aquatic environment, AZX short-term pulses of few days would be expected in surface waters, because fungicide occurrence is related to its use in the associated drainage watersheds (Battaglin et al. 2011). AZX has a water solubility of 6 mg/L and a log Kow = 2.5 (Bartlett et al. 2002), therefore, it can remain partitioned to water in the environment. However, in this matrix, a short half-life of only 14 days in natural conditions due to the photolytic degradation has been described (Boudina et al. 2007). As it was mentioned before, there are few reports of AZX toxicological data in non-target organisms, even less in aquatic ones acutely exposed. In this sense, Liu et al. (2013) determined a LC50 value of 549 μg/L in early life stage of grass carp (Ctenopharyngodon idella) and also an increase in CAT and POD activities, and inhibited expression of several genes related to growth, energy pathways and up-regulation of stress. In a study with tadpoles of Rana temporaria, the exposure to AZX resulted as the more toxic among six pesticides tested, exerting negative effect in the growth (Johansson et al. 2006). Recent studies of chronic toxicity of AZX on the amphipod Hyalella azteca, the midge Chironomus dilutus, the cladoceran Ceriodaphnia dubia and the unionid mussel Lampsilis siliquoidea exposed to environmentally relevant concentrations (4.2–12 μg/L) showed negative effects in reproduction and survival (Kunz et al. 2017).
Although environmentally relevant levels of AMISTAR® did not result cytotoxic in M. quitense, this fungicide was genotoxic, affected the physiological process of photorespiration and caused changes in antioxidant enzymes mostly at high concentrations. In this sense, it is necessary to explore sub-lethal responses in aquatic non-target organisms because some effects could promote further potential long-term biological consequences in a context of repeated pulses of exposure.
Acknowledgements
This work was supported by FONCYT (PICT 2011 1597 and PICT 2013 1348) and UNMdP (EXA 795/16). This work is part of the Ph D. Thesis of D. Garanzini. We thanks very much Verónica Taglioretti and Gastón Iturburu for helping us with the statistical analysis of the data.
References
- Anderson MJ, Robinson J. Generalized discriminant analysis based on distances. Aust N Z J Stat. 2003;45:301–318. doi: 10.1111/1467-842X.00285. [DOI] [Google Scholar]
- Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84:511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2. [DOI] [Google Scholar]
- Archer EK, Ting BL. A virescent plastid mutation in tobacco decreases peroxisome enzyme activities in seedlings. J Plant Physiol. 1996;149:520–526. doi: 10.1016/S0176-1617(96)80328-2. [DOI] [Google Scholar]
- Arts G, Davies J, Dobbs M, Ebke P, Hanson M, Hommen U, Knauer K, Loutseti S, Maltby L, Mohr S, Poovey A, Poulsen V. AMEG: the new SETAC advisory group on aquatic macrophyte ecotoxicology. Environ Sci Pollut Res. 2010;17:820–823. doi: 10.1007/s11356-010-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balba H. Review of strobilurin fungicide chemicals. J Environ Sci Health, Part B. 2007;42:441–451. doi: 10.1080/03601230701316465. [DOI] [PubMed] [Google Scholar]
- Bartlett DW, Clough JM, Godfrey CR, Godwin JR, Hall AA, Heaney SP, Maund SJ. Understanding the strobilurin fungicides. Pestic Outlook. 2001;12:143–148. doi: 10.1039/b106300f. [DOI] [Google Scholar]
- Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B. The strobilurin fungicides. Pest Manag Sci. 2002;58:649–662. doi: 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- Battaglin WA, Sandstrom MW, Kuivila KM, Kolpin DW, Meyer MT. Occurrence of Azoxystrobin, Propiconazole, and selected other fungicides in US streams, 2005–2006. Water Air Soil Pollut. 2011;218:307–322. doi: 10.1007/s11270-010-0643-2. [DOI] [Google Scholar]
- Berenzen N, Lentzen-Godding A, Probst M, Schulz H, Schulz R, Liess M. A comparison of predicted and measured levels of runoff-related pesticide concentrations in small lowland streams on a landscape level. Chemosphere. 2005;58:683–691. doi: 10.1016/j.chemosphere.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R. Effects of chemical contaminants on genetic diversity in natural populations: implications for biomonitoring and ecotoxicology. Mutat Res. 2000;463:33–51. doi: 10.1016/S1383-5742(00)00004-1. [DOI] [PubMed] [Google Scholar]
- Boudina A, Emmelin C, Baaliouamer A, Paissé O, Chovelon JM. Photochemical transformation of azoxystrobin in aqueous solutions. Chemosphere. 2007;68:1280–1288. doi: 10.1016/j.chemosphere.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brain RA, Cedergreen N. Biomarkers in aquatic plants: selection and utility. Rev Environ Contam Toxicol. 2009;198:49–109. doi: 10.1007/978-0-387-09647-6_2. [DOI] [PubMed] [Google Scholar]
- Cao F, Zhu L, Li H, Yu S, Wang C, Qiu L. Reproductive toxicity of azoxystrobin to adult zebrafish (Danio rerio) Environ Pollut. 2016;219:1109–1121. doi: 10.1016/j.envpol.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC handbook of methods in oxygen radical research. Boca Raton: CRC Press; 1985. [Google Scholar]
- Cole DJ. Detoxification and activation of agrochemicals in plants. Pest Manag Sci. 1994;42:209–222. doi: 10.1002/ps.2780420309. [DOI] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Cummins I, Cole DJ, Edwards R. A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black grass. The Plant J. 1999;18:285–292. doi: 10.1046/j.1365-313X.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- De Gerónimo E, Aparicio VC, Bárbaro S, Portocarrero R, Jaime S, Costa JL. Presence of pesticides in surface water from four sub-basins in Argentina. Chemosphere. 2014;107:423–431. doi: 10.1016/j.chemosphere.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Deb D, Engel BA, Harbor J, Hahn L, Jae Lim K, Zhai T. Investigating potential water quality impacts of fungicides used to combat soybean rust in Indiana. Water Air Soil Pollut. 2010;207:273–288. doi: 10.1007/s11270-009-0135-4. [DOI] [Google Scholar]
- Drotar A, Phelps P, Fall R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985;42:35–40. doi: 10.1016/0168-9452(85)90025-1. [DOI] [Google Scholar]
- Edwards PG, Murphy TM, Lydy MJ. Fate and transport of agriculturally applied fungicidal compounds, azoxystrobin and propiconazole. Chemosphere. 2016;146:450–457. doi: 10.1016/j.chemosphere.2015.11.116. [DOI] [PubMed] [Google Scholar]
- Garanzini DS, Menone ML. Azoxystrobin causes oxidative stress and DNA damage in the aquatic macrophyte Myriophyllum quitense. Bull Environ Contam Toxicol. 2015;94:146–151. doi: 10.1007/s00128-014-1428-x. [DOI] [PubMed] [Google Scholar]
- Ghosh RK, Singh N. Effect of organic manure on sorption and degradation of azoxystrobin in soil. Eff Org Manure Sorpt Degrad Azoxystrobin Soil. 2009;57:632–636. doi: 10.1021/jf802716f. [DOI] [PubMed] [Google Scholar]
- Gustafsson K, Blidberg E, Karlsson Elfgren I, Hellstrom A, Kylin H, Gorokhova E. Direct and indirect effects of the fungicide azoxystrobin in outdoor brackish water microcosms. Ecotoxicology. 2010;19:431–444. doi: 10.1007/s10646-009-0428-9. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Han Y, Zhu L, Wang J, Wang J, Xie H, Zhang S. Integrated assessment of oxidative stress and DNA damage in earthworms (Eisenia fetida) exposed to azoxystrobin. Ecotoxicol Environ Saf. 2014;107:214–219. doi: 10.1016/j.ecoenv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Han Y, Liu T, Wang J, Wang J, Zhang C, Zhu L. Genotoxicity and oxidative stress induced by the fungicide azoxystrobin in zebrafish (Danio rerio) livers. Pestic Biochem Physiol. 2016;133:13–19. doi: 10.1016/j.pestbp.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocinat A, Boudemagh A. Biodegradation of commercial Ortiva fungicide by isolated actinomycetes from the activated sludge. Desalin Water Treat. 2016;57:6091–6097. doi: 10.1080/19443994.2015.1022799. [DOI] [Google Scholar]
- Huang X, Rogers RB, Orr N, Sparks TC, Gifford JM, Loso MR, Zhu Y, Meade T (2007) U.S. Patent Application No. 11/704,824
- Jana S, Choudhuri MA. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat Bot. 1982;12:345–354. doi: 10.1016/0304-3770(82)90026-2. [DOI] [Google Scholar]
- Johansson M, Piha H, Kylin H, Merila H. Toxicity of six pesticides to common frog (Rana temporaria) tadpoles. Environ Toxicol Chem. 2006;25:3164–3170. doi: 10.1897/05-685R1.1. [DOI] [PubMed] [Google Scholar]
- Kono Y, Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982;257:5751–5754. [PubMed] [Google Scholar]
- Kozlowski LA, Simões DFM, Souza C. Efeito fisiológico de estrobilurina F 500® no crescimento e rendimento do feijoeiro. Rev Acadêmica Ciênc Agrár E Ambient. 2009;7:41–54. [Google Scholar]
- Kunz JL, Ingersoll CG, Smalling KL, Elskus AA, Kuivila KM. Chronic toxicity of azoxystrobin to freshwater amphipods, midges, cladocerans, and mussels in water- only exposures. Environ Toxicol Chem. 2017;36:2308–2315. doi: 10.1002/etc.3764. [DOI] [PubMed] [Google Scholar]
- Liang S, Xu X-W, Zhao X-F, Hou Z-G, Wang X-H, Lu Z-B. Two new fatty acids esters were detected in ginseng stems by the application of azoxystrobin and the increasing of antioxidant enzyme activity and ginsenosides content. Pestic Biochem Physiol. 2016;134:63–72. doi: 10.1016/j.pestbp.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Liu L, Jiang C, Wu Z-Q, Gong Y-X, Wang G-X. Toxic effects of three strobilurins (trifloxystrobin, azoxystrobin and kresoxim-methyl) on mRNA expression and antioxidant enzymes in grass carp (Ctenopharyngodon idella) juveniles. Ecotoxicol Environ Saf. 2013;98:297–302. doi: 10.1016/j.ecoenv.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhu B, Wang G-X. Azoxystrobin-induced excessive reactive oxygen species (ROS) production and inhibition of photosynthesis in the unicellular green algae Chlorella vulgaris. Environ Sci Pollut Res. 2015;22:7766–7775. doi: 10.1007/s11356-015-4121-7. [DOI] [PubMed] [Google Scholar]
- Lu T, Zhu Y, Xu J, Ke M, Zhang M, Tan C, Fu Z, Qian H. Evaluation of the toxic response induced by azoxystrobin in the non-target green alga Chlorella pyrenoidosa. Environ Pollut. 2018;234:379–388. doi: 10.1016/j.envpol.2017.11.081. [DOI] [PubMed] [Google Scholar]
- Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- Martinez RS, Di Marzio WD, Sáenz ME. Genotoxic effects of commercial formulations of Chlorpyrifos and Tebuconazole on green algae. Ecotoxicology. 2015;24:45–54. doi: 10.1007/s10646-014-1353-0. [DOI] [PubMed] [Google Scholar]
- McCarthy I, Romero-Puertas MC, Palma JM, Sandalio LM, Corpas FJ, Gómez M, Del Rio LA. Cadmium induces senescence symptoms in leaf peroxisomes of pea plants. Plant, Cell Environ. 2001;24:1065–1073. doi: 10.1046/j.1365-3040.2001.00750.x. [DOI] [Google Scholar]
- Menone ML, Pesce SF, Díaz MP, Moreno VJ, Wunderlin DA. Endosulfan induces oxidative stress and changes on detoxication enzymes in the aquatic macrophyte Myriophyllum quitense. Phytochemistry. 2008;69:1150–1157. doi: 10.1016/j.phytochem.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Nadin SB, Vargas-Roig LM, Ciocca DR. A silver staining method for single-cell gel assay. J Histochem Cytochem. 2001;49:1183–1186. doi: 10.1177/002215540104900912. [DOI] [PubMed] [Google Scholar]
- Nason MA, Farrar J, Bartlett D. Strobilurin fungicides induce changes in photosynthetic gas exchange that do not improve water use efficiency of plants grown under conditions of water stress. Pest Manag Sci. 2007;63:1191–1200. doi: 10.1002/ps.1443. [DOI] [PubMed] [Google Scholar]
- Ngayila N, Botineau M, Baudu M, Basly J-P. Myriophyllum alterniflorum DC. Effect of low concentrations of copper and cadmium on somatic and photosynthetic endpoints: a chemometric approach. Ecol Indic. 2009;9:307–312. doi: 10.1016/j.ecolind.2008.05.006. [DOI] [Google Scholar]
- Nimptsch J, Pflugmacher S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere. 2007;66:708–714. doi: 10.1016/j.chemosphere.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Nimptsch J, Wunderlin DA, Dollan A, Pflugmacher S. Antioxidant and biotransformation enzymes in Myriophyllum quitense as biomarkers of heavy metal exposure and eutrophication in Suquía River basin (Córdoba, Argentina) Chemosphere. 2005;61:147–157. doi: 10.1016/j.chemosphere.2005.02.079. [DOI] [PubMed] [Google Scholar]
- OECD 239 (2014) Test No. 239: water-sediment Myriophyllum Spicatum toxicity test
- Olsvik PA, Kroglund F, Finstad B, Kristensen T. Effects of the fungicide azoxystrobin on Atlantic salmon (Salmo salar L.) smolt. Ecotoxicol Environ Saf. 2010;73:1852–1861. doi: 10.1016/j.ecoenv.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Orchard AE. A revision of South American Myriophyllum (Haloragaceae) and its repercussions on some Australian and North American species. Aust Syst Bot. 1981;4:27–65. [Google Scholar]
- Pereira JL, Antunes SC, Castro BB, Marques CR, Gonçalves AMM, Gonçalves F, Pereira R. Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: commercial formulation versus active ingredient. Ecotoxicology. 2009;18:455–463. doi: 10.1007/s10646-009-0300-y. [DOI] [PubMed] [Google Scholar]
- Pflugmacher S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat Toxicol. 2004;70:169–178. doi: 10.1016/j.aquatox.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Poletta GL, Larriera A, Kleinsorge E, Mudry MD. Genotoxicity of the herbicide formulation Roundup®(glyphosate) in broad-snouted caiman (Caiman latirostris) evidenced by the Comet assay and the Micronucleus test. Mutat Res. 2009;672:95–102. doi: 10.1016/j.mrgentox.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Popp J, Pet Ho K, Nagy J. Pesticide productivity and food security. A review. Agron Sustain Dev. 2013;33:243–255. doi: 10.1007/s13593-012-0105-x. [DOI] [Google Scholar]
- Reilly TJ, Smalling KL, Orlando JL, Kuivila KM. Occurrence of boscalid and other selected fungicides in surface water and groundwater in three targeted use areas in the United States. Chemosphere. 2012;89:228–234. doi: 10.1016/j.chemosphere.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Rodrigues ET, Lopes I, Pardal MÂ. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: a review. Environ Int. 2013;53:18–28. doi: 10.1016/j.envint.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Rodrigues ET, Pardal MÂ, Gante C, Loureiro J, Lopes I. Determination and validation of an aquatic Maximum Acceptable Concentration-Environmental Quality Standard (MAC-EQS) value for the agricultural fungicide azoxystrobin. Environ Pollut. 2017;221:150–158. doi: 10.1016/j.envpol.2016.11.058. [DOI] [PubMed] [Google Scholar]
- Royal Society of Chemistry (n.d.) Organic chemistry contributing to food production [WWW Document], 2017. http://www.rsc.org/Membership/Networking/InterestGroups/OrganicDivision/organic-chemistry-case-studies/organic-chemistry-food-production.asp. Accessed 6.9.17
- Shi Q, Zhu Z, Xu M, Qian Q, Yu J. Effect of excess manganese on the antioxidant system in Cucumis sativus L. under two light intensities. Environ Exp Bot. 2006;58:197–205. doi: 10.1016/j.envexpbot.2005.08.005. [DOI] [Google Scholar]
- Singh N, Singh SB, Mukerjee I, Gupta S, Gajbhiye VT, Sharma PK, Goel M, Dureja P. Metabolism of 14C-azoxystrobin in water at different pH. J Environ Sci Health, Part B. 2010;45:123–127. doi: 10.1080/03601230903471910. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/S0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- SYNGENTA [WWW Document] (2017) https://www.sec.gov/Archives/edgar/data/1123661/000095010315001201/dp52748_20f.htm. Accessed 6.9.17
- Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf. 2006;64:178–189. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wu Y-X, von Tiedemann A. Physiological effects of azoxystrobin and epoxiconazole on senescence and the oxidative status of wheat. Pest Biochem Physiol. 2001;71:1–10. doi: 10.1006/pest.2001.2561. [DOI] [Google Scholar]
- Zar JH. Biostatistical analysis. London: Pearson Education; 1999. [Google Scholar]
- Zhang Y-J, Zhang X, Chen C-J, Zhou M-G, Wang H-C. Effects of fungicides JS399-19, Azoxystrobin, Tebuconazloe, and Carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pestic Biochem Physiol. 2010;98:151–157. doi: 10.1016/j.pestbp.2010.04.007. [DOI] [Google Scholar]